Abstract
To design an estrogen and phenol red free medium for cell culture and check its effectiveness and safety on osteoblast growth it is necessary to maintain the estrogen receptors free for tests. For this purpose, we tested some modifications of the traditional culture media: estrogen depleted fetal bovine serum; estrogen charcoal stripped fetal bovine serum and phenol red free α-MEM. The aim of this work is to examine the effects of its depletion in the proliferation, differentiation, and toxicity of mesenchymal stromal cells differentiated into osteoblasts to obtain an effective interference free culture medium for in vitro studies, focused on non-previously studied estrogen receptors. We performed viability tests using the following techniques: MTT, alkaline phosphatase specific activity, formation of mineralized matrix by Alizarin technique and analysis of SEM/EDX of mineralized nodules. The results showed that the culture media with estrogen free α-MEM + phenol red free α-MEM did not impact viability, alkaline phosphatase activity and mineralization of the osteoblasts culture compared to control. In addition, its nodules possess Ca/P ratio similar to hydroxyapatite nodules on the 14th and 21st day. In conclusion, the modified culture medium with phenol red free α-MEM with estrogen depleted fetal bovine serum can be safely used in experiments where the estrogen receptors need to be free.
Keywords: Osteoblasts, α-MEM, Phenol red free, Fetal bovine serum, Estrogen depleted
Introduction
Cellular tests are an important manner to ensure the effectiveness and safety of medicines, drugs, and supplies in general. However, it is necessary to guarantee that the cellular environment is free from interferences (substances that compete for the same cellular receptor) that may lead to false (both positive and negative) results. This competition occurs mainly when there is structural similarity between the molecules under study (Berthois et al. 1986; Welshons et al. 1988).
Several cellular systems are used to study the interaction between estrogen and phytoestrogens and receptors in cultures. The use of these systems is affected by the interaction between the phenol red, a pH indicator present in most cell culture media, and the estrogen receptors (ER) (Welshons et al. 1988; Liu et al. 2013). Literature reports that phenol red acts as a weak estrogen that may stimulate some cells sensitive to estrogen (Liu et al. 2013). The impact of phenol red on the different cell lineages is controversial. It is responsible for a higher responsiveness of the human breast cancer-derived MCF-7 cells and stimulation of primary cultures of immature pituitary cells and primary cultures of rat uterine cells and a low responsiveness of the human breast cancer-derived T47D cells (Welshons et al. 1988). Another effect reported in literature is the suppressive effect on the epileptiform burst activity in primary hippocampal culture (Liu et al. 2013). Estrogens, phytoestrogens, and phenol red in their acid and base form display similar structures and both have affinity to ER. Although the affinity of phenol red for the ER is considered rather weak (binding affinity around 0.001 % of the estradiol), its concentration in the culture medium is considered high, which leads to the occupation of ER (Liu et al. 2013; Welshons et al. 1988).
Another interference found in these systems is the estrogen that is already present in the fetal bovine serum (FBS), since this hormone passes through the placenta to the fetus’ circulation. The FBS is widely used in the supplementation of culture media because it contains a large amount of biological components, such as fatty acids, growth factors, aminoacids, vitamins, and hormones, including estrogen (Hemeda et al. 2014; Tekkatte et al. 2011). The cell growth is promoted by these components, but when an estrogen-free culture is needed, the estrogen depletion in the FBS is essential (Simoncini et al. 2005). The changes in the culture medium must not affect the proliferation and/or cell differentiation.
Steroid hormones and molecules with similar structure and charge can penetrate the membrane of target cells and the simple interaction with the ERs allows the hormone-receptor complex migration into the cell nucleus (Osborne and Schiff 2005). Two ER subtypes of the estrogen receptor are well known: ERα and ERβ, which are encoded by different genes and are located on chromosomes 6 and 14, respectively (Foster 2012; Saji et al. 2000). The ERβ is expressed in the tissues such as endothelium lungs, prostate, ovary, urogenital tissue, and some bone tissue cells, such as osteoblasts (Imamov et al. 2005; Saji et al. 2000). Studies indicate that estrogen has a protective effect on bone by decreasing its reabsorption. This protective effect is due to the increase of the osteoprotegerin delivery and decrease of the RANKL (Receptor Activator of Nuclear Factor Kappa B Ligand) on osteoblasts. These molecules regulate the maturation and proliferation of osteoclasts on bone tissue (Somjen et al. 2011). It prevents bone loss and reduces the risk of fractures (Zhao et al. 2013; Lee et al. 2013; Whedon 1981). Estrogen also improves calcium absorption in the intestinal tract and reduces the loss of calcium through urine (Terauchi 2011; Heaney et al. 1987), raises the active form of D vitamin, stimulates the circulation and production of calcitonin, which prevents the removal of calcium from bone (Lerchbaum 2014; Simonelli et al. 2005; Richart and Lindsay 1984). Therefore, the aim of this study was to examine the effects of estrogen and phenol red depletion on the proliferation, differentiation, and toxicity of mesenchymal stromal cells differentiated into osteoblasts to obtain an effective interference free culture medium for in vitro studies, focused on non-previously studied ER.
Materials and methods
The reagents 3[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), β-glycerophosphate, 2-amino-2-methyl-propan-1-ol (AMPOL), Tris hydroxymethyl-amino-methane (Tris), p-nitrophenylphosphate (PNPP), alizarin red, Bovine Serum Albumin (BSA), and dexamethasone were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Ascorbic acid, α-MEM, FBS, estrogen depleted FBS (ES FBS), gentamicin, fungizone, and trypsin were purchased from Gibco-Invitrogen Technologies (Grand Island, NY, USA). All aqueous solutions were prepared using ultra-pure apyrogenic water (Millipore Direct-Q, Millipore, Billerica, MA, USA). The analytical grade reagents were used without further purification.
Cell lineage
The mesenchymal stromal cells (MSC) were isolated, cultured, and differentiated into osteoblasts as described by Simão et al. (2007a). β-glycerophosphate, ascorbic acid, and dexamethasone are responsible for the differentiation process.
Culture media
The control (Ct) culture medium of MSCs for differentiation into osteoblasts is composed by α-MEM, gentamicin, fungizone, dexamethasone, ascorbic acid, β-glycerophosphate, and FBS.
The modified culture medium includes: phenol red free α-MEM (PRF α-MEM) or α-MEM, estrogen depleted FBS (ES FBS) or estrogen charcoal stripped FBS (CS FBS).
The CS FBS estrogen depletion was performed by the methodology described by Simoncini et al. (2005). Briefly, 500 mL of heat-inactive FBS was treated with 8 g of active charcoal at room temperature during 24 h, then 5–6 times centrifuged at 4,000g per 15 min, and filtrated through 0.22 μm of pore size filter to separate the charcoal from the serum.
All tests were performed using the Ct; α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS.
Cell viability assays
The classic MTT assay was used for cell viability in order to evaluate survival and proliferation of osteoblasts on different supplementations of culture media. The tetrazolium salt [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] produced the highly colored formazan dye upon nicotinamide adenine dinucleotide hydride reduction (NADH), which reflected a living cellular dehydrogenase (Mosmann 1983; Simioni et al. 2006). After the color reaction is finished, the absorbance of each well was measured at 560 and 690 nm by means of the Spectronic device (Genesys 2). To investigate the toxicity of the alterations to the culture medium, a suspension of 2 × 104 osteoblastic cells in 1 mL of media (Ct; α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS) was added into each well in a 24-well microplate. The monolayer cultures of osteoblasts were incubated for 1, 7, and 14 days. The cell viability results were expressed as the percentage of the average of experiments performed in triplicate compared with the control at the first day.
Alkaline phosphatase (ALP) activity
Monitoring the biomineralization process was followed by quantification of the levels of ALP. This enzyme is considered a phenotypic biomarker of the biomineralization process (Ge et al. 2006; Millán 2006b). ALP is a nonspecific enzyme and its enzymatic activity was determined by its action on the substrate p-nitrophenylphosphate (PNPP). The p-nitrophenylphosphatase (PNPPase) activity was determined discontinuously at 37 °C, in the supernatant after treatment of homogenized cells suspension with phospholipase C (PIPLC) and ultracentrifugation at 36,000 rpm. The activity was measured via formation of the p-nitrophenolate ion (PNP−) (ε = 17,600 M−1 cm−1, pH 13), at 410 nm, in 50 mM of the 2-amino-2-methyl-1-propanol (AMPOL) buffer, pH 10, containing 2 mM MgCl2 and 10 mM (PNPP), in a final volume of 1 mL, according to the procedure described by Camolezi et al. (2002). The assays were performed on the 7th, 14th, and 21st day of plating. All determinations were carried out in triplicate. Controls without added enzyme were included in each experiment to determine non-enzymatic substrate hydrolysis. One enzyme unit (1 U) is defined as the amount of enzyme hydrolyzing 1.0 nmol of substrate per min at 37 °C per mg or mL of protein.
Mineralized matrix formation
The mineralized matrix formation was assessed at the days 14th and 21st, when the formation of the nodules of hydroxyapatite crystal in the extracellular matrix is evident. For this purpose, two techniques were used: (i) The Alizarin Red S method, in which the nodules were fixed with 10 % formalin for 24 h, at room temperature. After fixation, the specimens were dehydrated through a graded series of alcohol, and processed for staining with Alizarin red that blushes red the mineralization nodules rich in calcium. Subsequently, the contents of the wells were solubilized with 10 % acetic acid, processed, and neutralized with 10 % ammonium hydroxide. The absorbance of the samples was read at 405 nm in a spectrophotometer, as described by Gregory et al. (2004); (ii) SEM/EDX: The surface morphology of the hydroxyapatite crystals deposited on plastic discs was investigated by scanning electron microscopy (SEM) using a Zeiss-EVO50 microscope (Souza et al. 2014). The Ca/P molar ratio of the films was evaluated by X-ray dispersive energy (EDX) (IXRF system 500 Digital Processing spectrometer).
Statistical analysis
Statistical comparisons were accomplished by two-way ANOVA followed by Bonferroni’s test for all data sets. P values >0.05 are considered significant.
Results
Cell viability assays
Figure 1 presents the results concerning the viability of osteoblasts. The cell culture containing α-MEM + ES FBS; PRF α-MEM + FBS, and PRF α-MEM + ES FBS increased the osteoblasts viability with culture time. But, the comparison between these media reveals that there was not any statistical difference during cell proliferation after 1, 7, and 14 days. The α-MEM + CS FBS and PRF α-MEM + CS FBS resulted in a decrease of the osteoblasts viability at the first, 7th and 14th day. Table 1 shows the results expressed as percent viability compared to the Ct at the first day.
Fig. 1.
Cell viability of osteoblasts cultures incubated during 1, 7 and 14 days with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS. Osteoblasts (2 × 104 cells/well) were cultured in 24-well plates, and the results were expressed as percentage of the average of three experiments performed in triplicate compared to control. *p value < 0.05 versus control. The error bars represent the standard deviation of the data set
Table 1.
Percentages of cell viability of osteoblasts cultures incubated during 1, 7 and 14 days with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS
| Conditions | Day 1 | Day 7 | Day 14 |
|---|---|---|---|
| Ct (α-MEM + FBS) | 100 ± 5.0 | 123.5 ± 6.2 | 182.0 ± 9.1 |
| α-MEM + ES FBS | 106.5 ± 5.3 | 118.3 ± 5.9 | 168.1 ± 8.4 |
| α-MEM + CS FBS | 15.4 ± 0.8a | 28.9 ± 1.4a | 26.1 ± 1.3a |
| PRF α-MEM + FBS | 96.5 ± 4.8 | 121.7 ± 6.1 | 178.3 ± 8.9 |
| PRF α-MEM + ES FBS | 106 ± 5.3 | 115.4 ± 5.8 | 171.7 ± 8.6 |
| PRF α-MEM + CS FBS | 8.8 ± 0.44a | 16.4 ± 0.8a | 17.1 ± 0.9a |
aResults with statistical significance when compared at the same day of growth
ALP activity
The ALP activity was studied on the 7th, 14th, and 21st day of culture. A maximum in activity was reached at the 14th day of culture for the systems containing α-MEM + ES FBS; PRF α-MEM + FBS, and PRF α-MEM + ES FBS; and decreased on the 21st day (Fig. 2). There is a natural relation between the increase in the number of active osteoblasts and the high production of enzymes and proteins, such as ALP and collagen type 1, on the 14th day to initiate the biomineralization process (Simão et al. 2007a). The decreased activity on the 21st day is related to death of osteoblasts and mineralization process, where large amounts of hydroxyapatite (HAP) were deposited in the collagen matrix. The ALP activity decreased atypically on the 7th, 14th and 21st days for both α-MEM + CS FBS, and PRF α-MEM + CS FBS on the 14th and 21st day. The values of ALP activity on the 14th and 21st day are shown in Table 2.
Fig. 2.
Levels of alkaline phosphatase activity in osteoblast cultures after 7, 14 and 21 days of incubation with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS. Osteoblasts (2 × 104 cells/well) were cultured in 24-well plates, and the results were expressed as percentage of the average of three experiments performed in triplicate compared to control. *p value < 0.05 versus control. The error bars represent the standard deviation of the data set
Table 2.
Mean values of alkaline phosphatase activity and absorbances of mineralized matrix formation in osteoblasts cultures after 7, 14 and 21 days, and 14 and 21 days (respectively) of incubation with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS
| Conditions | ALP specific activity (U/mg) | Absorbance of mineralized matrix (405 nm) | |||
|---|---|---|---|---|---|
| Day 7 | Day 14 | Day 21 | Day 7 | Day 14 | |
| Ct (α-MEM + FBS) | 664.6 ± 33.2 | 1001.3 ± 50.1 | 414.6 ± 20.7 | 0.175 ± 0.009 | 0.206 ± 0.010 |
| α-MEM + ES FBS | 611.3 ± 30.6 | 987.2 ± 49.4 | 399.9 ± 19.9 | 0.157 ± 0.008 | 0.197 ± 0.010 |
| α-MEM + CS FBS | 99.4 ± 4.9a | 56.5 ± 2.8a | 40.6 ± 2.0a | 0.045 ± 0.002a | 0.066 ± 0.003a |
| PRF α-MEM + FBS | 625.9 ± 31.3 | 943.7 ± 47.2 | 375.6 ± 18.8 | 0.178 ± 0.009 | 0.206 ± 0.010 |
| PRF α-MEM + ES FBS | 608.4 ± 30.4 | 932.3 ± 46.6 | 350.4 ± 17.5 | 0.169 ± 0.008 | 0.183 ± 0.009 |
| PRF α-MEM + CS FBS | 76.4 ± 3.8a | 70.2 ± 3.5a | 20.3 ± 1.0a | 0.033 ± 0.002a | 0.034 ± 0.002a |
aResults with statistical significance when compared at the same day of growth
Mineralized matrix formation
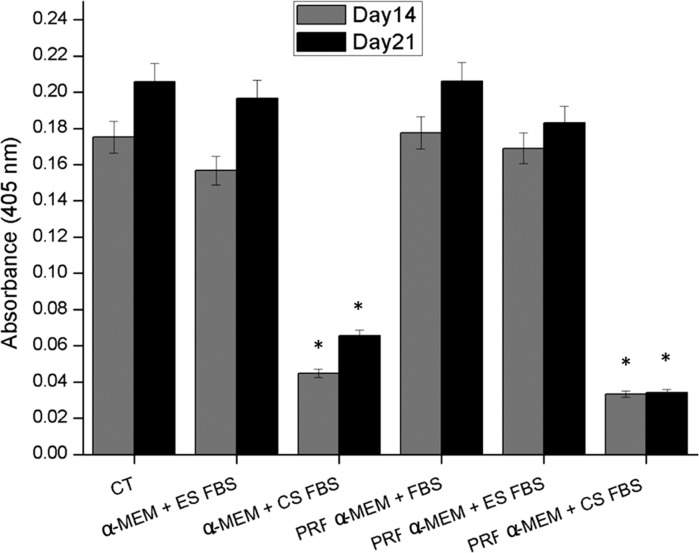
The absolute reading of the results of mineralized matrix formation at 405 nm showed an increase in osteoblast mineralization from the 14th day to the 21st for both Ct; α-MEM + ES FBS; and PRF α-MEM + ES FBS (Fig. 3) on the day, and there was no statistical difference between the three mentioned cultures. A decrease in the formation of mineral nodules was observed with α-MEM + CS FBS; and PRF α-MEM + CS FBS on the 14th and 21st day. The values of absorbance on the 14th and 21st day are shown in Table 2.
Fig. 3.
Formation of mineralized matrix in osteoblast cultures after 14 and 21 days of incubation with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS. Osteoblasts (2 × 104 cells/well) were cultured in 24-well plates, and the results were expressed as Alizarin Red absorbances’ average at 405 nm (a.u.) of three experiments performed in triplicate compared to control. *p value < 0.05 versus control. The error bars represent the standard deviation of the data set
SEM/EDX
The EDX analysis of the surface was performed on the 14th and 21st day of culture in order to follow the growth and mineralization of osteoblasts. The results reveal Ca/P molar ratio of Ct; α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS on the 14th day and on the 21st day and are shown in Table 3. The deviation of the EDX quantitative analysis is close to 10 %.
Table 3.
Ca/P molar ratio of mineralized surfaces after 14 and 21 days of incubation with Ct (α-MEM + FBS); α-MEM + ES FBS; α-MEM + CS FBS; PRF α-MEM + FBS; PRF α-MEM + ES FBS and PRF α-MEM + CS FBS
| Conditions | Day 14 | Day 21 |
|---|---|---|
| Ct (α-MEM + FBS) | 1.55 ± 0.08 | 1.53 ± 0.08 |
| α-MEM + ES FBS | 1.53 ± 0.08 | 1.57 ± 0.08 |
| α-MEM + CS FBS | 1.46 ± 0.07 | 1.67 ± 0.08 |
| PRF α-MEM + FBS | 1.36 ± 0.07 | 0.96 ± 0.01a |
| PRF α-MEM + ES FBS | 1.45 ± 0.07 | 1.40 ± 0.07 |
| PRF α-MEM + CS FBS | 0.73 ± 0.04a | 1.48 ± 0.07 |
aResults with statistical significance when compared at the same day of growth
The SEM images at the magnification of 1000× (Fig. 4) and at the magnification of 3000× (Fig. 5) on 14th day, showed that mineralized nodules were formed in the different media. The nodules formed after 21 days are bigger in size, but exhibit similar morphology compared to the nodules formed after 14 days of culture.
Fig. 4.

SEM images at the magnification of 1000× of mineralized surfaces after 14 (a–f) and 21 (g–I) days of incubation with Ct (α-MEM + FBS) (Fig. 4a, g, respectively); α-MEM + ES FBS (Fig. 4b, h, respectively); α-MEM + CS FBS (Fig. 4c, i, respectively); PRF α-MEM + FBS (Fig. 4d, j, respectively); PRF α-MEM + ES FBS (Fig. 4e, k, respectively) and PRF α-MEM + CS FBS (Fig. 4f, l, respectively). Osteoblasts (2 × 104 cells/well) were cultured in 24-well plates
Fig. 5.

SEM images at the magnification of 3000× of mineralized surfaces after 14 (a–f) and 21 (g–I) days of incubation with Ct (α-MEM + FBS) (Fig. 5a, g, respectively); α-MEM + ES FBS (Fig. 5b, h, respectively); α-MEM + CS FBS (Fig. 5c, i, respectively); PRF α-MEM + FBS (Fig. 5d, j, respectively); PRF α-MEM + ES FBS (Fig. 5e, k, respectively) and PRF α-MEM + CS FBS (Fig. 5f, l, respectively). Osteoblasts (2 × 104 cells/well) were cultured in 24-well plates
Discussion
The studies on the interaction of estrogens and phytoestrogens in cellular systems are necessary to ensure that there are no interferences that may lead to false results. Our study proposed changes in the standard cultivation of MSCs in an environment free of estrogens and phenol red, where there is no impairment in growth and differentiation of osteoblasts.
The results concerning cell viability showed that α-MEM PRF is effective on growth of osteoblasts as much α-MEM. Also, the results indicated that the ES FBS supplementation is effective in the culture media (Fig. 1).
For the modification in the culture medium proposed (α-MEM PRF + ES FBS), the cell viability was statistically similar to the control, which shows that despite the depletion of interfering agents, the changes were effective in the maintenance of the cell culture viability. Some decreases (not significant) in cell viability on the 7th and 14th day were observed when the ES FBS was used. This find highlights the relevance of estrogen in the growth and maintenance of bone tissue in long-term, as shown by Somjen et al. (2011) and Whedon (1981). However, this modification is required for the ER total binding by molecules with affinity for this receptor on osteoblast, and did not show significant influence in the experiments.
All culture media supplemented with CS FBS showed no effective supplementation in both (α-MEM + CS FBS and PRF α-MEM + CS FBS) culture media, as evidenced by significant cell death. Even though the process of depletion of estrogen described by Simoncini et al. (2005) is a widely used technique in research, it was not effective for the growth of osteoblasts. The active charcoal removes non-polar compounds, such as lipophilic materials as virus, some hormones, growth factors, and cytokines; it may have depleted other important molecules for the growth and differentiation of osteoblasts.
The ALP activity of α-MEM PRF + ES FBS was kept at its normal rate compared to the Ct, α-MEM + ES FBS, and PRF α-MEM + FBS. It evidenced that the differentiation into osteoblasts was maintained despite modifications in the culture medium. In fact, this enzyme is expressed during development of the osteoblast phenotype and it is used as a biochemical marker of bone metabolism in cell cultures (Millán 2006a, b). Moreover, it is considered an indicator of osteoblastic differentiation and it is also a representative marker of presence of mature osteoblasts (Ge et al. 2006).
ALP is responsible for the biomineralization process of osteoblasts: it is a nonspecific phosphomonohydrolase capable of hydrolyzing in alkaline pH phosphate monoesters (ATP, ADP, AMP, pyrophosphate, glucose-6-phosphate, glucose-1-phosphate, glyceraldehyde-3-phosphate), and pyrophosphate diester phosphate, releasing organic phosphate crystals for the synthesis of the extracellular matrix hydroxyapatite. The ALP levels are directly related to the rate of cultured osteoblast mineralization (Millán 2013; Simão et al. 2007b; 2010). The results presented herein showed that the decrease in the activity of ALP by α-MEM + CS FBS and PRF α-MEM + CS FBS was confirmed by impaired mineralization.
The mineralization is a natural process that occurs in osteoblasts. For this, hydroxyapatite crystals formed within the osteoblast matrix vesicles are deposited together with the collagen to form the rigid matrix of mineralized bone. The mineralization in vitro begins around the 14th day, when the ALP activity is at its maximum, and exhibits a maximum in cell cultures on the 21st day. Our results showed an natural increase in biomineralization of osteoblasts from the 14th day to the 21st day to the Ct, α-MEM + ES FBS, α-MEM PRF + FBS, and α-MEM PRF + ES FBS, thus showing the effectiveness of fully modified culture medium (α-MEM PRF + ES FBS) to differentiate MSCs and stimulate osteoblast mineralization.
The Ca/P molar ratio from hydroxyapatite (HAP) crystals possess stoichiometric ratio [(Ca10(PO4)6(OH)2)] Ca/P = 1.67. Table 3 shows the Ca/P ratio of the samples on the 14th and 21st day, obtained by EDX technique. Except for the PRF α-MEM + CS FBS on the 14th day, the values attested that the nodules of mineralized matrix formed using the different culture media are composed by calcium phosphate crystals in the HAP crystalline form.
Figures 4 and 5 show the SEM images of the samples at 1000× and 3000×, respectively on the 14th and 21st day. The images reveal that the PRF α-MEM + ES FBS mineralization nodules are morphologically similar to the crystals obtained for the Ct. It is an evidence of the effectiveness of modifications in culture media. In turn, the CS FBS showed lower mineral formations around the hydroxyapatite nodules. Some few nodules may be formed, but the propagation of the mineralization is incomplete. The inefficiency of this medium for cell culture supplementation is evidenced by the empty space around the nodules.
Conclusion
The results of this study showed that it is possible to obtain a culture medium free of estrogens and phenol red that does not affect the viability, ALP activity, and biomineralization of osteoblasts. The influence of the phenol red in the culture medium cannot be discarded because it influences various cell lines, like human breast cancer-derived MCF-7, human breast cancer-derived T47D cells, primary cultures of rat uterine cells and primary cultures of immature pituitary cells. The culture medium free of phenol red and estrogens may be used for experiments involving different types of estrogens, phytoestrogens, and molecules that have some similarity with ERs.
Contributor Information
A. N. de Faria, Phone: +55 (16) 3602-4377, Email: amandabioquimica@gmail.com, Email: amandafaria@usp.br
P. Ciancaglini, Email: pietro@ffclrp.usp.br
References
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS (1986) Phenol red in tissue culture media is a weak estrogen:implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 83:2496–2500 [DOI] [PMC free article] [PubMed]
- Camolezi FL, Daghastanli KPR, Magalhães PP, Pizauro JM, Ciancaglini P. Construction of an alkaline phosphatase-liposome system: a tool for biomineralization study. Int J Biochem Cell Biol. 2002;1282:1–11. doi: 10.1016/s1357-2725(02)00029-8. [DOI] [PubMed] [Google Scholar]
- Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Chen D, Xie L, Zhang R. Enhancing effect of daidzein on the differentiation and mineralization in mouse osteoblast-like MC3T3-E1 cells. Yakugaku Zasshi. 2006;126:651–656. doi: 10.1248/yakushi.126.651. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Gunn WG, Peister A, Prockop DJ. An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Recker RR, Saville PD. Menopausal changes in calcium balance performace. J Lab Clin Med. 1987;92:953–963. [PubMed] [Google Scholar]
- Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–871. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- Lee WL, Cheng MH, Tarng DC, Yang WC, Lee FK, Wang PH. The benefits of estrogen or selective estrogen receptor modulator on kidney and its related disease-chronic kidney disease-mineral and bone disorder: osteoporosis. J Chin Med Assoc. 2013;76:365–371. doi: 10.1016/j.jcma.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Lerchbaum E. Vitamin D and menopause-A narrative review. Maturitas. 2014;14:S0378–S5122. doi: 10.1016/j.maturitas.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen B, Chen L, Ren WT, Liu J, Wang G, Fan W, Wang X, Wang Y. U-Shape suppressive Effect of phenol red on the epileptiform burst Activity. PLoS one. 2013;8:e60189. doi: 10.1371/journal.pone.0060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán JL. Mammalian alkaline phosphatases from biology to applications in medicine and biotechnology. Hoboken: Wiley; 2006. pp. 1–322. [Google Scholar]
- Millán JL. Alkaline phosphatases. Structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal. 2006;2:335–341. doi: 10.1007/s11302-005-5435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millán JL. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif Tissue Int. 2013;93:299–306. doi: 10.1007/s00223-012-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Richart RM, Lindsay R. Osteoporosis and its relationship to estrogen. Contemporary Ob/Gyn. 1984;24:201–224. [Google Scholar]
- Saji S, et al. Estrogen receptors α and β in the rodent mammary gland. Proc Natl Acad Sci USA. 2000;97:337–342. doi: 10.1073/pnas.97.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão AMS, Beloti MM, Rosa AL, De Oliveira PT, Granjeiro JM, Pizauro JM, Ciancaglini P. Culture of osteogenic cells from human alveolar bone: a useful source of alkaline phosphatase. Cell Biol Int. 2007;31:1405–1413. doi: 10.1016/j.cellbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Simão AM, Beloti MM, Cezarino RM, Rosa AL, Pizauro JM, Ciancaglini P. Membrane-bound alkaline phosphatase from ectopic mineralization and rat bone marrow cell culture. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:679–687. doi: 10.1016/j.cbpa.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Simão AM, Yadav MC, Ciancaglini P, Milla´n JL (2010) Pro-teoliposomes as matrix vesicles’ biomimetics to study the initiation of skeletal mineralization. Braz J Med Biol Res 43:234–241 [DOI] [PMC free article] [PubMed]
- Simioni AR, Martins OP, Lacava ZG, Azevedo RB, Lima EC, Lacava BM, Morais PC, Tedesco AC. Cell toxicity studies of albumin-based nanosized magnetic beads. J Nanosci Nanotechnol. 2006;6:2413–2415. doi: 10.1166/jnn.2006.511. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Fornari L, Mannella P, Caruso A, Garibaldi S, Baldacci C, Genazzani AR. Activation of nitric oxide synthesis in human endothelial cells by red clover extracts. Menopause. 2005;12:69–77. doi: 10.1097/00042192-200512010-00013. [DOI] [PubMed] [Google Scholar]
- Simonelli C, Weiss TW, Morancey J, Swanson L, Chen YT. Prevalence of vitamin D inadequacy in a minimal trauma fracture population. Curr Med Res Opin. 2005;7:1069–1074. doi: 10.1185/030079905X50598. [DOI] [PubMed] [Google Scholar]
- Somjen D, Katzburg S, Sharon O, Grafi-Cohen M, Knoll E, Stern N. The effects of estrogen receptors α- and β-specific agonists and antagonists on cell proliferation and energy metabolism in human bone cell line. J Cel Bioch. 2011;112:625–632. doi: 10.1002/jcb.22959. [DOI] [PubMed] [Google Scholar]
- Souza ID, Cruz MAE, Faria AN, Zancanela DC, Simão AMS, Ciancaglini P, Ramos AP (2014) Formation of carbonated hydroxyapatite films on metallic surfacesusing dihexade-cyl phosphate–LB film as template. Colloids Surf B 118:31–40 [DOI] [PubMed]
- Tekkatte C, Gunasingh GP, Cherian KM, Sankaranarayanan K. “Humanized” stem cell culture techniques: the animal serum controversy. Stem Cells Int. 2011;2011:504723. doi: 10.4061/2011/504723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi M. Bone and calcium metabolism in menopause transition. Clin Calcium. 2011;21:1353–1359. [PubMed] [Google Scholar]
- Welshons WV, Wolf MF, Murphy CS, Jordan VC. Estrogenic activity of phenol red. Mol Cell Endocrinol. 1988;57:169–178. doi: 10.1016/0303-7207(88)90072-X. [DOI] [PubMed] [Google Scholar]
- Whedon GD. Osteoporosis. N Engl J Med. 1981;6:397–398. doi: 10.1056/NEJM198108133050709. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Liu X, Zhang L, Shen X, Qi J, Wang J, Qian N, Deng L. Bone selective protective effect of a novel bone-seeking estrogen on trabecular bone in ovariectomized rats. Calcif Tissue Int. 2013;93:172–183. doi: 10.1007/s00223-013-9739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]