Abstract
Xenotransplantation is a potential solution to the organ donor shortage. Immunosuppression is required for successful application of xenotransplantation but may lead to infection and cancer. Thus, strategies for immune tolerance induction need to be developed. Polyclonal regulatory T cells (Treg) play a central role in the induction and maintenance of immune tolerance and have been shown to protect against islet xenograft rejection in vivo. However, global immune suppression may be mediated by polyclonal Treg immunotherapy and a simple method for in vitro expansion of xenoantigen-specific Treg for efficient Treg application becomes necessary. Human Treg isolated from peripheral blood mononuclear cells (PBMCs) were initially cultured with anti-CD3/CD28 beads, rapamycin and IL-2 for 7 days as polyclonal expansion. Expanded Treg were then cocultured with irradiated porcine PBMC as xenoantigen stimulation for three subsequent cycles with 7 days for each cycle in the presence of IL-2 and anti-CD3/CD28 beads. Treg phenotype and suppressive capacity were assessed after each cycle of xenoantigen stimulation. Treg expanded with one cycle of xenoantigen stimulation retained Treg suppressive phenotype but acquired no xenoantigen specificity along with poor expansion efficiency, whereas expansion with two-cycle xenoantigen stimulation resulted in not only more than 800-fold Treg expansion but highly suppressive xenoantigen-specific Treg with effector Treg phenotype. However further increase of stimulation cycles resulted in reduced Treg suppressive potency. This study provides a simple approach to obtain high numbers of xenoantigen-specific Treg for immune tolerance induction in xenotransplantation.
Keywords: Regulatory T cells, Xenoantigen-specificity, In vitro expansion, Xenotransplantation
Introduction
The shortage of organs and cells from deceased donors continues to restrict allotransplantation. Xenotransplantation (e.g., from pigs to people) provides a potential solution to this issue (Buhler 2012). However, xenogeneic rejection mediated by T cell response is one of major barriers to be overcome for clinical application of xenotransplantation. Although life-long systemic immunosuppression decreases the incidence of xenograft rejection, this is inevitably accompanied by increased infection and cancer due to its nonspecific effects (Lechler et al. 2005). Thus, clinically applicable strategies for immunomodulation need be developed to achieve tolerance to transplants by reducing or eliminating the requirement of immunosuppression. Regulatory T cells (Treg) have emerged as critically important for the control of autoimmunity and for the maintenance of allograft tolerance (Bluestone et al. 2008; Brusko et al. 2008; Wood and Sakaguchi 2003; Golshayan et al. 2007). Recent studies have shown that human Treg are capable of suppressing CD4+CD25− effector T cell-mediated anti-pig cellular response in vitro (Porter and Bloom 2005; Sun et al. 2010a, b; Wu et al. 2008) and that adoptive transfer with in vitro polyclonally expanded human Treg prevents islet xenograft rejection by suppressing effector T cell responses (Yi et al. 2012). This raises the possibility that Treg may be used therapeutically at the time of xenotransplantation to reduce the requirement of systemic immunosuppression (Muller et al. 2012; O’Connell et al. 2010; Muller et al. 2009). However, the infused polyclonal Treg may also deliver pan-immunosuppressive effects, such as opportunistic infections and tumor-growth. Studies with transplantation animal models and immune monitoring of transplant patients indicate that these side effects could be avoided by specific suppression of the expansion of donor-alloreactive effector T cells after transplantation, which can be achieved by using donor allospecific Treg that are more effective at preventing allograft and improving clinical outcome than polyclonal Treg (Masteller et al. 2006; Peters et al. 2008; Sagoo et al. 2011).
For the xenotransplantation setting, little is known on how to generate sufficient numbers of xenoantigen-specific human Treg ex vivo from naturally occurring Treg. In this study we developed a strategy to obtain human Treg with xenoantigen specificity in large scale for use in xenotransplantation. These Treg demonstrated the superior efficacy of xenoantigen-specific suppression of xenoreactive responder cells when compared to polyclonal Treg.
Materials and methods
Cell isolation
Human PBMC were obtained from healthy donors (60–100 ml blood, healthy donors were from Center for Transplant and Renal Research, Westmead Hospital) using density gradient centrifugation over Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) for isolation of human Treg. Crucial to this process is the identification of markers that permit the isolation of pure Treg population for further expansion. The inverse correlation between the IL-7 receptor-α (CD127) subunit and the suppressive capacity of human CD4+CD25+ Treg provides an additional potential target to isolate and expand human Treg with increased suppressive function ex vivo (Liu et al. 2006; Nadig et al. 2010). Thus, CD4+CD25+CD127lo Treg and CD4+CD25− T cells were isolated from PBMC using CD4+CD25+CD127dim/− Regulatory T Cell Isolation Kit (MiltenyiBiotec, Bergisch Gladbach, Germany). The purity of CD4+CD25+CD127lo Treg and CD4+CD25− T cells was greater than 98 and 99 %, respectively. Porcine PBMC were isolated from heparinized whole blood from adult Westran pigs (an inbred syngeneic strain with high genetic homology) (O’Connell et al. 2005) and used as xenogeneic stimulator cells for human Treg expansion and their suppression assay. Landrace cross with Large White pigs obtained from a local commercial pig farm (The University of Sydney Farms, Camden, NSW, Australia) were used for isolation of porcine PBMC as a third party xenogeneic stimulator cells for Treg suppression assay. Purified CD4+CD25− T cells from different individual donors were used as autologous effector cells. Human and animal studies were approved by the Sydney West Area Health Service Human and Animal research Ethics communities.
Xenoantigen-specific Treg expansion
To obtain high numbers of functionally xenoantigen-specific Treg, 5 × 104 isolated CD4+CD25+CD127lo Treg were initially cultured in each well of 96-well round bottom plates with 200 μl RPMI 1640 (Invitrogen, San Diego, CA, USA) containing 10 % human AB serum (Invitrogen), 2 mM glutamine, 25 mM HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 50 μM 2-mercaptoethanol (Sigma, St. Louis, MO, USA), and 100 nM rapamycin (Sigma) at 37 °C and 5 % CO2, in the presence of 400 U/ml IL-2 (Chiron, Emeryville, CA, USA) and T cell expander beads (CD3/CD28 Dynabeads; Invitrogen Dynal) at a ratio of 4 beads per cell for 7 days as polyclonal stimulation. The polyclonally stimulated Treg were then expanded with three subsequent cycles of xenoantigen stimulation (7 days per cycle) using irradiated (30 Gy) pig PBMC at a 4:1 ratio of xenoPBMC:Treg in the presence of 400 U/ml IL-2 and T cell expander beads at a 1:1 ratio of beads:Treg (Fig. 1). Cells along with their culture medium were split into three wells of 96-well round bottom plates every 2 or 3 days when they reached to 5 × 105, and fresh RPMI 1640 medium was added to each well up to 200 μl. After each stimulation cycle expanded Treg were harvested for the subsequent experiments. Treg expanded as described above but in the absence of pig PBMC were used as polyclonally expanded Treg in this study (Fig. 1).
Fig. 1.
Overview of Treg expansion strategies. Treg were initially expanded with polyclonal stimulation for 7 days followed by expansion with either polyclonal or xenoantigen stimulation for three subsequent cycles with 7 days for each cycle
Flow cytometry
Expanded Treg collected at the end of each stimulation cycle were used for flow cytometric analysis of Treg phenotype. Cells were surfaced stained with fluorescently coupled antibodies specific for human antigens CD4, CD127, CD62L (purchased from BD, San Jose, CA, USA), CD25, CD45RA, CD45RO (purchased from eBioscience, San Diego, CA, USA), and glucocorticoid-induced TNFR-related protein (GITR) (purchased from R&D System, Minneapolis, MN, USA), respectively in staining buffer (1 % bovine serum albumin and 0.1 % sodium azide in phosphate-buffered saline) at 4 °C for 30 min followed by fixation and permeabilization (Fix/Perm buffer, eBioscience) and intracellular staining with fluorescently coupled anti Foxp3 (eBioscience) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) (BD) antibodies respectively in permeabilization buffer (eBioscience) for 60 min at 4 °C. Flow cytometric data were acquired using an LSRII flow cytometer (BD) and analysed using FlowJo software version 7.5.5 (Treestar, Ashland, OR, USA).
Real-time PCR
Expanded Treg collected at the end of each stimulation cycle were used to extract RNA for Real-time PCR (Sun et al. 2010b). PCR primers specific for human IL-10, TGF-β and GAPDH are summarized in Table 1.
Table 1.
Sequence of primers used for real-time PCR
| Gene | Sequence |
|---|---|
| IL-10 | Sense: 5′-GCC TAA CAT GCT TCG AGA TC-3′ |
| AntiSense: 5′-TGA TGT CTG GGT CTT GGT TC-3′ | |
| TGF-β | Sense: 5′-TGGAAACCCACAACGAAATC-3′ |
| AntiSense: 5′-GGGTTCAGGTACCGCTTCTC-3′ | |
| GAPDH | Sense: 5′-TGCACCACCAACTGCTTAGC-3′ |
| AntiSense: 5′-GGCATGGACTGTGGTCATGAG-3′ |
Treg suppression assay
Treg in vitro suppressive activity was assessed by mixed lymphocyte reaction (MLR) assay. Human CD4+CD25− T cells were labeled with 5 μM 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen). 5 × 104 CFSE-labeled CD4+CD25− T cells were stimulated with 5 μg/ml anti-human CD3 mAb and 2 μg/ml anti-human CD28 mAb (BD) or 1 × 105 irradiated (30 Gy) xenogeneic PBMC either from the same pig used to expand Treg or from a different breed of pig in the presence or absence of serial dilutions of polyclonally or xenoantigen expanded autologous Treg for polyclonal or xenogeneic MLR assay, respectively, in each well of round-bottom 96-well plates with RPMI 1640 medium (Invitrogen) containing 10 % human AB serum for 7 days. After MLR assay, cells were stained with APC-conjugated anti-CD25 mAb prior to analysis of CFSE dilution by flow cytometry. Proliferation of CD4+CD25− T cells (CD25− and CFSE+ cells) was evaluated based on the percent proliferating CD4+CD25− T cells cultured in the absence of Treg compared with the percent proliferating CD4+CD25− T cells cultured in the presence of Treg. The percent proliferating CD4+CD25− T cells in the absence of Treg was taken as 100 % of proliferation and 0 % of suppression. Percent suppression of proliferating CD4+CD25−T cells was determined as % suppression = (percent proliferating CD4+CD25− T cells in the presence of Treg/percent proliferating CD4+CD25− T cells) × 100 %.
Statistics
Results were analyzed using Student’s two-tailed t test and were presented as mean ± SD. P < 0.05 was considered as significant.
Results
High numbers of human Treg are obtained after expansion with porcine xenoantigen stimulation
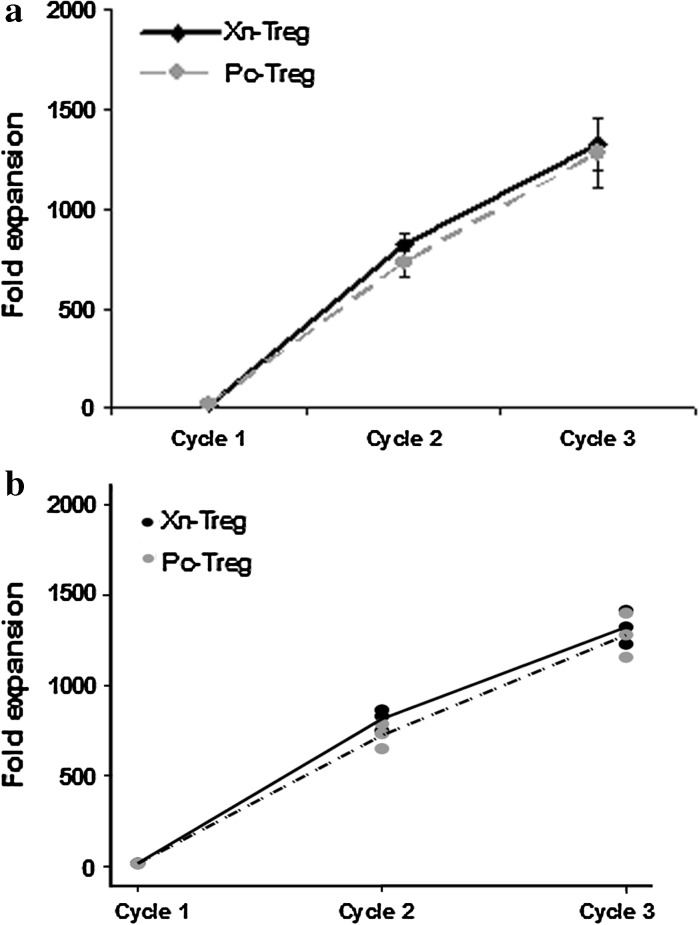
Stimulation with CD3/CD28 beads has been widely used for large scale in vitro expansion of polyclonal human Treg (Wu et al. 2008; Hoffmann et al. 2004; Ruitenberg et al. 2011; Qian et al. 2011; Yi et al. 2012). Without using CD3/CD28 beads lower numbers of human Treg could be expanded by specific antigen stimulation alone than that expanded with polyclonal stimulation (Fan et al. 2012). This finding was also supported by our preliminary experiments, showing that human CD4+CD25+CD127lo Treg stimulated in vitro with porcine xenoantigen alone for 3 weeks were expanded less than 50-fold (data not shown). In order to obtain high numbers of xenoantigen specific Treg, we tested an ex vivo Treg expansion strategy in this study by combining irradiated pig PBMC and CD3/CD28 beads to stimulate human Treg for three subsequent cycles and evaluated the effect of each stimulation cycle on Treg expansion. Fluorescence-activated cell sorting analysis of expanded Treg purity was performed at the end of each stimulation cycle. Treg purity was presented as the percentage of CD4+CD25+CD127lo cells in Treg populations examined after each cycle of expansion. The percentage of cell purity after each stimulation cycle was 98.6 ± 1.7, 96.1 ± 3.0 and 92.4 ± 3.3, respectively for xenoantigen expanded Treg, and 98.9 ± 1.1, 95.8 ± 3.8 and 96.0 ± 3.3, respectively for polyclonally expanded Treg. Cell numbers were counted by trypan blue exclusion method at the end of each stimulation cycle. The percentage of cell viability after each stimulation cycles was 97.8 ± 2.3, 94.9 ± 4.5 and 87.5 ± 4.4, respectively for xenoantigen expanded Treg, and 98.2 ± 1.1, 94.8 ± 2.0 and 84.5 ± 6.9, respectively for polyclonally expanded Treg. Average Treg expansion values are shown in Fig. 2a, and individual experiments in dots are displayed in Fig. 2b. After two cycles of Treg expansion a similar large average expansion fold was achieved with or without xenoantigen stimulation (814-fold expansion of xenoantigen stimulated Treg vs. 723-fold expansion of polyclonally stimulated Treg). Again, a similarly increased cell yield of Treg was detected after three cycles of expansion regardless of xenoantigen stimulation (1323-fold expansion of xenoantigen stimulated Treg vs. 1279-fold expansion of polyclonally stimulated Treg). These data showed that xenoantigen stimulated Treg could be readily expanded in vitro in the presence of CD3/CD28 beads and IL-2 within 2–3 weeks, thereby ensuring a large number of xenoantigen stimulated Treg available for any future clinical application.
Fig. 2.
Treg expansion efficiency with xenoantigen or polyclonal stimulation. Treg were expanded with xenoantigen (Xn-Treg) or polyclonal (Pc-Treg) stimulation as described in Fig. 1. Numbers of expanded Xn- and Pc-Treg were counted at the end of each stimulation cycle. Treg expansion efficiency was determined as fold expansion as below. Fold expansion = number of expanded Treg at the end of each stimulation cycle divided by number of Treg at the time of culture for initial polyclonal expansion. Data are represented as mean ± SD of three independent experiments (a); individual experiment of three different experiments in dots are displayed in b
Human Treg expanded with xenoantigen stimulation acquires activated Treg phenotype
Next, we examined the impact of in vitro Treg expansion with xenoantigen stimulation on the phenotypic characteristics of human Treg by flow cytometry. Fresh isolated Treg (Table 2), as a purity control, expressed high-level of CD4, CD25, Foxp3 and low-level of CD127 (Fig. 3a). Similar to Treg expanded with polyclonal stimulation (Pc-Treg), Treg expanded with xenoantigen stimulation (Xn-Treg) retained Treg regulatory phenotype, with high level of expression of CD25, Foxp3, GITR, CTLA-4, CD62L while maintaining a very low expression of CD127 after the primary and second stimulation cycles (Fig. 3b). However, after the third stimulation Xn-Treg expressed reduced levels of Foxp3 (60.1 % of expanded Treg expressed Foxp3 after three cycles of xenoantigen stimulation vs. 96.8 % expanded Treg expressed Foxp3 after two cycles of xenoantigen stimulation) and CTLA-4 (55.4 % of expanded Treg expressed CTLA-4 after three cycles of xenoantigen stimulation vs. 93.4 % of expanded Treg expressed CTLA-4 after two cycles of xenoantigen stimulation) as compared to their counterparts with two cycles of xenoantigen stimulation, though they showed unchanged low level of CD127 expression (Fig. 3b). Interestingly, Treg expanded with xenoantigen stimulation for both two and three cycles acquired activated Treg phenotypic characteristics, evidenced by upregulated expression of activated and effector Treg markers CD45RO (74.4 and 87.7 % of xenoantigen stimulated Treg expressed CD45RO after two and three expansion cycles, respectively vs. 52.5 and 70.5 % of polyclonally expanded Treg expressed CD45RO after two and three stimulation cycles, respectively) and HLA-DR (52.1 and 83.8 % of xenoantigen stimulated Treg expressed HLA-DR after two and three expansion cycles, respectively vs. 41.6 and 69.0 % of polyclonally expanded Treg expressed HLA-DR after two and three stimulation cycles, respectively) (Booth et al. 2010; Kleinewietfeld et al. 2005; Baecher-Allan et al. 2006; Schaier et al. 2012; Sakaguchi et al. 2010) along with reduced expression of naïve Treg marker CD45RA (19.5 and 15.5 % of xenoantigen stimulated Treg expressed CD45RA after two and three expansion cycles, respectively vs. 46.9 and 30.8 % of polyclonally expanded Treg expressed CD45RA after two and three stimulation cycles, respectively) (Sakaguchi et al. 2010) when compared to their polyclonal counterparts (Fig. 3b). Then mRNA expression of Treg cytokines interleukin (IL)-10, transforming growth factor (TGF)-β in Xn-Treg and Pc-Treg after each round of stimulation were assessed by real-time PCR. Figure 4 shows that expanded Treg stimulated under either polyclonal or xenoantigen condition expressed a slightly elevated level of TGF-β after the second stimulation cycle than that after the primary and third stimulation cycle, respectively (Fig. 4b), though no significant difference in TGF-β gene expression was found between Xn-Treg and Pc-Treg. However, after the second stimulation cycle, IL-10 gene expression was upregulated in xenoantigen but not polyconally stimulated Treg (Fig. 4a, P < 0.01), and the higher level of IL-10 gene expression in Xn-Treg was still detectable after three cycles of stimulation when compared to their Pc-Treg counterparts (3.2-fold higher IL-10 gene expression in Xn-Treg vs. Pc-Treg) (Fig. 4a). Collectively, these data demonstrated that Treg expanded in vitro with two or three cycles of xenoantigen—but not polyclonal-stimulation possessed the phenotypic characteristic of effector Treg, an important indicator for enhanced Treg function in immune suppression (Taams et al. 2002; Dieckmann et al. 2001; Baecher-Allan et al. 2006). Furthermore, the increased level of Treg effector cytokine IL-10 gene expression by xenoantigen-stimulated Treg suggested that Xn-Treg may acquire potent suppressive activity in xenogeneic responses both in vitro and in vivo via IL-10-involved mechanisms (Sun et al. 2010a, b; Yi et al. 2012).
Table 2.
Numbers and purity of fresh isolated Treg
| Donors | Blood volume (ml) | Isolated Treg numbers | Fresh Treg purity (%) |
|---|---|---|---|
| 1 | 60 | 6.5 × 105 | 98.5 |
| 2 | 100 | 1.2 × 106 | 98.0 |
| 3 | 80 | 8 × 105 | 99.1 |
Fig. 3.
Phenotypical characterization of expanded Treg. Phenotype of Xn-Treg and Pc-Treg expanded as described in Fig. 1 were analyzed by flow cytometry. Gates were set on CD4+CD25+ cells. Cell surface expression of CD25, CD127, CD62L, GITR, CD45RA, CD45RO and HLA-DR and intracellular expression of Foxp3 and CTLA-4 by Xn-Treg (black open histograms) or Pc-Treg (grey filled histograms) were presented as the percentage of CD4+CD25+ cells co-expressing individual molecules examined. Grey open histograms represented isotype controls. Data presented in brackets represent the ranges of percentage of CD4+CD25+ cells co-expressing individual Treg markers tested. Fresh isolated Treg, as a purity control, expressed high-level of CD4, CD25, Foxp3 and low-level of CD127 is displayed in a. b presents expression of CD25, CD127, CD62L, GITR, CD45RA, CD45RO and HLA-DR and intracellular expression of Foxp3 and CTLA-4 of Xn-Treg, Pc-Treg and isotype controls after each round of stimulation. Data are representative of three independent experiments
Fig. 4.
Quantitative analysis of IL-10 and TGF-β gene expression by real-time PCR. Quantitative analysis of IL-10 (a) and TGF-β (b) gene expression by real-time PCR using RNA samples isolated from expanded Treg with xenoantigen (Xn-Treg) and polyclonal (Pc-Treg) stimulation after each expansion round, respectively. Data are mean ± SD of three independent experiments with Treg from three individual donors. **P < 0.01, Xn-Treg versus Pc-Treg
Two cycles of xenoantigen stimulation leads to expansion of highly xenoantigen-specific human Treg
We hypothesized that xenoantigen-specific Treg should have stronger suppressive capacity than that polyclonal Treg would demonstrate in xenogeneic but not non-specific (e.g., polyclonal stimulation) immune response. The capacity and xenoantigen specificity of expanded Treg in suppression of xenogeneic and non-specific responses were assessed by MLR assay using CFSE-labelled CD4+CD25− T cells as responder cells. Both Pc- and Xn-Treg collected at the end of each expansion cycle demonstrated similar potency in suppression of CD4+CD25− T cell proliferation in polyclonally stimulated MLR (Poly MLR) in a Treg number dependent manner (Fig. 5a), suggesting that xenoantigen stimulation did not enhance Treg suppressive capacity in polyclonally stimulated response. However compared with two cycles of stimulation, three cycles of stimulation resulted in reduced suppressive potency of both Pc- and Xn-Treg at all responder cells:Treg ratios in polyclonally stimulated response (Fig. 5a), indicating that increase of stimulation cycles to three for either polyclonal or xenoantigen expansion did not further increase, but reduced Treg suppressive potency in antigen nonspecific immune response. By contrast, in xenoantigen driven MLR (Xeno MLR) Treg expanded with two cycles of xenoantigen stimulation showed very strong suppressive capacity in xenogeneic response, evidenced by >50 and >40 % suppression of responder cell proliferation at high responder cells:Treg ratios of 8:1 and 16:1, respectively (Fig. 5b), whereas only 28 % (P < 0.01) suppression of xenoreactive responder cell proliferation was achieved at a 8:1 ratio of responder cells:Treg by using Pc-Treg expanded with two-cycle stimulation in xeno MLR, and at least, a fourfold more Pc-Treg (responder cells:Treg ratio of 4:1) were required to achieve a similar level of >50 % suppression shown by Xn-Treg (Fig. 5b). These results suggest that Treg with enhanced suppressive capacity and high xenoantigen specificity were obtained after two cycles of expansion with xenoantigen stimulation. Increase of xenoantigen stimulation cycles further enriched xenoantigen specificity in Treg expanded with three cycles of xenoantigen stimulation, showing enhanced xenoantigen-specific suppression of xenogeneic response not only at high ratios of responder cells:Treg, but also at a low ratio (4:1) of responder cells:Treg when compared to those expanded with three cycles of polyclonal stimulation (Fig. 5b, P < 0.05). Nevertheless, similar to that seen in polyclonally stimulated MLR (Fig. 5a) both Pc-and Xn-Treg expanded with three-cycle stimulation demonstrated impaired suppressive capacity at all ratios of responder cells:Treg tested in xenoMLR when compared to that expanded with two-cycle stimulation (Fig. 5b). Taken together, our results suggest that multiple expansion cycles, regardless of the mode of stimulation, may lead to reduced suppressive capacity due to cell exhaustion and subsequent cell death (Peters et al. 2008). The xenoantigen specificity in suppression of xenogeneic response shown by xenoantigen stimulated Treg was not detected when Treg expanded with one cycle of xenoantigen stimulation were assessed in xenoantigen-stimulated MLR, as indicated by a similar suppressive potency revealed by both Pc- and Xn-Treg at all ratios tested (Fig. 5b), suggesting that one cycle of xenoantigen stimulation was not efficient to induce xenoantigen specificity in expanded Treg. Moreover, compared with suppression of the response to the same Westran pig PBMC, Xn-Treg could also but in a twofold less efficient manner, suppress the proliferation response against xenogeneic PBMC stimulator cells obtained from a third party pig differing from Westran pig used in the expansion of protocol (Fig. 5c).
Fig. 5.
Suppressive capacity of expanded Treg in MLR assays. After each stimulation cycle expanded Xn-Treg and Pc-Treg were used for assessment of suppressive capacity in polyclonally stimulated (Poly MLR) (a) and xenogeneic (Xeno MLR) (b) responses, respectively. CFSE labeled CD4+CD25− T cells were stimulated with irradiated xenogeneic pig PBMC as xenogeneic stimulation or with anti-human CD3 mAb and anti-human CD28 mAb as polyclonal stimulation, in the absence or presence of serial dilutions of Xn-Treg or Pc-Treg for 7 days prior to measurement of CD4+CD25− T cell proliferation by CFSE dilution. Irradiated xenogeneic PBMC from Landrace and Large White cross pigs were used to stimulate CFSE labeled CD4+CD25− T cells in the absence or presence of serial dilutions of Xn-Treg after each expansion cycle for a third party xeno MLR assay (Third Party Xeno MLR) (c). The percent proliferating CD4+CD25− T cells in the absence of Treg was taken as 100 % of proliferation and 0 % of suppression. Percent suppression of proliferating CD4+CD25− T cells was determined as % suppression = (percent proliferating CD4+CD25− T cells in the presence of Treg/percent proliferating CD4+CD25− T cells) × 100 %. Data are shown as mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01
Collectively, our results indicated that a large number of porcine xenoantigen specific Treg with highly suppressive capacity was generated from human CD4+CD25+CD127lo Treg by in vitro expansion with two cycles of xenoantigen stimulation, and the enhanced suppression of the xenogeneic response by xenoantigen specific Treg was associated with their acquisition of effector Treg phenotype.
Discussion
Large scale ex vivo Treg expansion is required to obtain high numbers of functional human Treg for effective Treg immunotherapy to induce tolerance to xenografts. Selection and use of antigen-specific Treg has been proposed to improve Treg therapeutic efficiency and lower the risk for unwanted non-specific immune suppression caused by transferring large numbers of polyclonal Treg (Koenecke et al. 2009; Hall et al. 2009; Peters et al. 2008; Veerapathran et al. 2011). Recent studies have shown that ex vivo expanded alloantigen specific Treg obtained enhanced suppressive capacity in allogeneic responses in vitro (Peters et al. 2008; Golshayan et al. 2007) and were more potent than polyclonal Treg in protecting against alloimmune-mediated injury of human skin grafts in a humanized mouse model (Yamano et al. 2011; Sagoo et al. 2011). However, strategies for large scale expansion of xenoantigen-specific human Treg remain to be developed. To develop a successful strategy for obtaining large numbers of xenoantigen-specific human Treg, we hypothesized that it might be practical to combine polyclonal stimulation with CD3/CD28 beads to enhance expansion, and xenoantigen specific stimulation with pig PBMC to selectively boost xenoantigen-specific Treg. Our results show that by applying this protocol high numbers of Treg with enhanced suppressive capacity and xenoantigen-specificity were obtained in only two cycles of expansion, and the acquisition of xenoantigen-specificity was associated with the upregulated expression of effector Treg markers, CD45RO and HLA-DR (Booth et al. 2010; Schaier et al. 2012; Sakaguchi et al. 2010; Baecher-Allan et al. 2006; Kleinewietfeld et al. 2005) by xenoantigen expanded Treg. However, Treg expanded with one cycle of xenoantigen stimulation were not enriched for xenoantigen-specificity as xenogeneic response was suppressed equally well by both polyclonally- and xenoantigen-stimulated Treg, which was due, at least in part to their unchanged CD45RO expression in comparison with their counterparts with one cycle of polyclonal stimulation. To further enhance xenoantigen-specificity, it might be an option to repeat xenoantigen stimulation for multiple cycles, which in theory would progressively enrich for strictly antigen-specific Treg. Despite that their xenoantigen-specificity was further enriched Treg expanded with three stimulation cycles, regardless of the stimulation mode used, lost their potent suppression in both polyclonally- and xenoantigen-stimulated responses. The reduction of Treg suppressive capacity was most likely to be due to downregulated expression of Treg functional markers Foxp3 and CTLA-4 (Jain et al. 2010; Wing et al. 2008; Tai et al. 2012; Marson et al. 2007; Yamaguchi et al. 2011) detected in Treg expanded with three stimulation cycles. Thus, these findings indicate that the protocol applying a combination of one initial polyclonal expansion cycle and two subsequent cycles of xenoantigen stimulation would become a practical strategy to obtain high numbers of porcine xenoantigen-specific human Treg. Indeed, the use of two expansion cycles has been reported to be more efficient than any other cycles for expansion of highly suppressive alloantigen-specific human Treg in large numbers (Peters et al. 2008; Fan et al. 2012).
Here we have provided a simple and feasible method to generate high numbers of xenoantigen-specific human Treg for immunotherapeutic purpose in xenotransplantation, aiming at inducing xenograft tolerance while minimizing non-specific immunosuppression.
Acknowledgments
This study was supported by grants from the National Health and Medical Research Council of Australia (NH&MRCA), the Juvenile Diabetes Research Foundation International (JDRFI), the Key Program of National Nature Science Foundation of China (No. 30930088) and National Natural Science Foundation of China (No. 30872382).
Conflict of interest
The authors declare no conflicts of interest.
References
- Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28:677–684. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- Buhler LH. Xenotransplantation: the near future. Xenotransplantation. 2012;19:1. doi: 10.1111/j.1399-3089.2011.00689.x. [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Yang J, Hao J, Ren Y, Chen L, Li G, Xie R, Yang Y, Gao F, Liu M. Comparative study of regulatory T cells expanded ex vivo from cord blood and adult peripheral blood. Immunology. 2012;136:218–230. doi: 10.1111/j.1365-2567.2012.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–835. doi: 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- Hall BM, Tran G, Hodgkinson SJ. Alloantigen specific T regulatory cells in transplant tolerance. Int Immunopharmacol. 2009;9:570–574. doi: 10.1016/j.intimp.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]
- Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, Huehn J, Ganser A, Forster R, Prinz I. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39:3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation—how much of the promise has been realized? Nat Med. 2005;11:605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells—ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Muller YD, Golshayan D, Ehirchiou D, Wekerle T, Seebach JD, Buhler LH. T regulatory cells in xenotransplantation. Xenotransplantation. 2009;16:121–128. doi: 10.1111/j.1399-3089.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- Muller YD, Ehirchiou D, Golshayan D, Buhler LH, Seebach JD. Potential of T-regulatory cells to protect xenografts. Curr Opin Organ Transplant. 2012;17:155–161. doi: 10.1097/MOT.0b013e3283508e17. [DOI] [PubMed] [Google Scholar]
- Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, Schiopu A, Taggart DP, Wood KJ. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell PJ, Hawthorne WJ, Simond D, Chapman JR, Chen Y, Patel AT, Walters SN, Burgess J, Weston L, Stokes RA, Moran C, Allen R. Genetic and functional evaluation of the level of inbreeding of the Westran pig: a herd with potential for use in xenotransplantation. Xenotransplantation. 2005;12:308–315. doi: 10.1111/j.1399-3089.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- O’Connell PJ, Yi S, Carrington EM, Lew AM. Role of regulatory T cells in xenotransplantation. Curr Opin Organ Transplant. 2010;15:224–229. doi: 10.1097/MOT.0b013e3283373c27. [DOI] [PubMed] [Google Scholar]
- Peters JH, Hilbrands LB, Koenen HJ, Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4(pos)CD25(high) T cells for immunotherapy. PLoS One. 2008;3:e2233. doi: 10.1371/journal.pone.0002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter CM, Bloom ET. Human CD4+CD25+ regulatory T cells suppress anti-porcine xenogeneic responses. Am J Transplant. 2005;5:2052–2057. doi: 10.1111/j.1600-6143.2005.00972.x. [DOI] [PubMed] [Google Scholar]
- Qian X, Wang K, Wang X, Zheng SG, Lu L. Generation of human regulatory T cells de novo with suppressive function prevent xenogeneic graft versus host disease. Int Immunopharmacol. 2011;11:630–637. doi: 10.1016/j.intimp.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg JJ, Boyce C, Hingorani R, Putnam A, Ghanekar SA. Rapid assessment of in vitro expanded human regulatory T cell function. J Immunol Methods. 2011;372:95–106. doi: 10.1016/j.jim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Schaier M, Seissler N, Schmitt E, Meuer S, Hug F, Zeier M, Steinborn A. DR(high+)CD45RA(−)-Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLoS One. 2012;7:e34208. doi: 10.1371/journal.pone.0034208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yi S, O’Connell PJ. Foxp3 regulates human natural CD4+CD25+ regulatory T-cell-mediated suppression of xenogeneic response. Xenotransplantation. 2010;17:121–130. doi: 10.1111/j.1399-3089.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Yi S, O’Connell PJ. IL-10 is required for human CD4(+)CD25(+) regulatory T cell-mediated suppression of xenogeneic proliferation. Immunol Cell Biol. 2010;88:477–485. doi: 10.1038/icb.2009.117. [DOI] [PubMed] [Google Scholar]
- Taams LS, Vukmanovic-Stejic M, Smith J, Dunne PJ, Fletcher JM, Plunkett FJ, Ebeling SB, Lombardi G, Rustin MH, Bijlsma JW, Lafeber FP, Salmon M, Akbar AN. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–1630. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tai X, Van Laethem F, Pobezinsky L, Guinter T, Sharrow SO, Adams A, Granger L, Kruhlak M, Lindsten T, Thompson CB, Feigenbaum L, Singer A. Basis of CTLA-4 function in regulatory and conventional CD4+ T cells. Blood. 2012;119:5155–5163. doi: 10.1182/blood-2011-11-388918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–5680. doi: 10.1182/blood-2011-02-337097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Wu J, Yi S, Ouyang L, Jimenez E, Simond D, Wang W, Wang Y, Hawthorne WJ, O’Connell PJ. In vitro expanded human CD4+CD25+ regulatory T cells are potent suppressors of T-cell-mediated xenogeneic responses. Transplantation. 2008;85:1841–1848. doi: 10.1097/TP.0b013e3181734793. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Yamano T, Watanabe S, Hasegawa H, Suzuki T, Abe R, Tahara H, Nitta T, Ishimaru N, Sprent J, Kishimoto H. Ex vivo-expanded DCs induce donor-specific central and peripheral tolerance and prolong the acceptance of donor skin grafts. Blood. 2011;117:2640–2648. doi: 10.1182/blood-2010-07-293860. [DOI] [PubMed] [Google Scholar]
- Yi S, Ji M, Wu J, Ma X, Phillips P, Hawthorne WJ, O’Connell PJ. Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin-10 in humanized mice. Diabetes. 2012;61:1180–1191. doi: 10.2337/db11-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]