Abstract
The use of adipose-derived stem cells is wide-spread in both basic biology and regenerative medicine, due to the abundance of adipose tissue and the multipotent differentiation potential of the cells. However, the methods used to isolate and culture cells vary greatly between different research groups. Identification of medium formulations which provide rapid cell expansion while maintaining cell phenotype would have clear advantages. We compared growth and differentiation potential along the adipogenic lineage in human ADSCs in nine different media. We further assessed induced and spontaneous differentiation along the adipogenic, chondrogenic and osteogenic lineage in three different media. There was significant variation in the rate of growth between different media. All media supported ADSC phenotype and adipogenic differentiation, although there was variation between the different media. Differentiation along the adipogenic, chondrogenic and osteogenic lineages in the three media was confirmed, with some upregulation of specific genes observed when cells were left to spontaneously differentiate. Our study shows a direct comparison of human ADSCs grown in different media, both reported in the literature and commercially available. It indicates that rapid proliferation occurs most often in media which contain 10 % foetal bovine serum and that differentiation along different lineages can be induced but also occurs spontaneously once cells become confluent. These data provide a tool for other researchers to facilitate the choice of medium formulation most appropriate for different applications.
Keywords: Adipose-derived stem cells, Cell therapy, Stem cells, Tissue regeneration
Introduction
Adipose-derived stem cells (ADSCs) harvested from human adipose tissue provide an abundant potential source of multipotent adult stem cells (Rodbell 1964; Zuk et al. 2001, 2002; Du et al. 2005; Katz et al. 2005; Yoshimura et al. 2006; Zhu et al. 2008; Bunnell et al. 2008; Tapp et al. 2009; Du et al. 2010; Maumus et al. 2011; Aoyagi et al. 2011; Zhou 2011; Sachs et al. 2012). These can be harvested by minimally invasive procedures with a potential for diverse applications, such as studies in stem cell biology as well as clinical applications in regenerative medicine. The ease of availability and large cell numbers upon harvest provides an alternative source of multipotent stem cells to bone marrow stem cells, which are painful to harvest and provide low cell numbers (Zuk et al. 2001; Dominici et al. 2006; Zhu et al. 2008).
Methods for isolation and culture of ADSCs vary, and different protocols and media may affect the rate of proliferation and phenotype of the ADSCs and their multipotent differentiation potential (Tapp et al. 2009). Generally, minced adipose tissue or lipoaspirate is treated by enzyme digestion followed by centrifugation resulting in the separation of the stromal vascular fraction (SVF) from the mature adipocytes. The SVF is a heterogenous population of cells that includes adipose and haematopoietic stem cells as well as endothelial cells, erythrocytes, fibroblasts, lymphocytes, monocytes, macrophages and pericytes. The adherent cell population is then expanded, which comprises primarily of ADSCs. However, the cells remain poorly defined. In 2006 Dominici et al. (Dominici et al. 2006) issued a statement defining criteria for identification of multipotent mesenchymal stem cells. Key characterisation of these stem cells includes the ability to adhere to plastic, the ability to express several common cell surface antigens and multipotent differentiation potential. Characterisation of ADSCs often fails to distinguish them from fibroblasts. The expression of cell surface markers combined with the ability to differentiate along multiple cell lineage pathways in a reproducible manner are two of the properties defined by Bourin et al. in 2013 to provide initial guidance regarding the positive identification of adipose tissue-derived cells.
Isolation of cells from adipose tissue from mice was pioneered by Rodbell et al. in the 1960s (Rodbell 1964). Zuk et al. (2001) described the isolation and culture of stem cells harvested from human lipoaspirate and Bunnell et al. (2008) later described the isolation of ADSCs from human adipose tissue. Subsequently many authors have described the isolation and culture of ADSCs from various anatomical sites and their differentiation into several mesodermal lineages. The protocols used by different researchers vary greatly. For use in cell therapy, rapid expansion to generate sufficient cell numbers as well as preservation of cellular phenotype are key.
In this study, we have compared the effect of different medium formulations on ADSC culture, which to our knowledge has not been reported previously. We tested a medium based on the method described by Bunnell et al. (2008) as well as eight other media, which were a mixture of media formulations reported in the literature and commercially available products. We subsequently assessed proliferation as well as multipotent differentiation potential with the aim to identify the media which provided fast cell expansion, while retaining ADSC characteristics and multipotent differentiation capacity, with a particular focus on suitability for applications in regenerative medicine.
Materials and methods
Cell isolation and culture
Adipose tissue from consented human abdominal tissue (REC: 06/Q1907/81) was harvested and ADSCs were isolated using the method described by Zuk et al. (2001) and Bunnell et al. (2008). Briefly, fibro-fatty tissue was excised and washed three times in Hanks Balanced Salt Solution (HBSS) (GIBCO, Paisley, UK) with 5 % penicillin/streptomycin (Invitrogen, Paisley, UK) and minced to approximately 0.3 cm × 0.3 cm pieces. Following digestion for 45 min at 37 °C in 0.075 % collagenase type I (Gibco, UK) the tissue pieces were passed through a 100 µm cell strainer (Fisher Scientific, Loughborough, UK) and centrifuged at 2,000 rpm for 5 min to obtain the SVF. The pellet was disrupted, mixed and centrifuged again to complete the separation of the stromal cells from the mature adipocytes. Following re-suspension and further filtering, isolated cells were seeded into 25 cm2 culture flasks (cells isolated from 1 to 2 cm cube of fatty tissue per T25 flask) and maintained in proliferation medium (Table 1) at 37 °C in 5 % CO2. We did not include a red blood cell lysis step as suggested by some groups but excluded the cells by medium exchange after 24 h. For proliferation studies, cells were maintained in proliferation medium and passaged every 4–6 days to maintain a subconfluent density. Proliferation in each medium was documented visually by light microscopy and quantified by cumulative population doublings (CPD). The following formula was used to calculate the number of population doublings (n):
Table 1.
List of proliferation media
| Medium | Composition | Author/source |
|---|---|---|
| 1 | DMEM, 10 % FBS | Modified from Bunnell et al. (2008) |
| 2 | Low glucose DMEM, 10 % FBS | Zhou et al. (2011) |
| 3 | M199, 10 % FBS, 5 ng/ml heparin, 2 ng/ml acidic FGF | Yoshimura et al. (2006) |
| 4 | DMEM:F12 1:1, FBS 10 % | Katz et al. (2005) |
| 5 | Advanced DMEM:F12, 2 % FBS | Invitrogen |
| 6 | IMDM:HAM’s F12 1:1, 10 % FBS | Frerich et al. (2012) |
| 7 | Low glucose DMEM:MCDB201 60:40, 2 % FBS, 10 ng/ml EGF, 10 ng/ml PDGF, 5 µg/ml insulin, 5 µg/ml transferrin, 200 U/ml LIF | Du et al. (2010) |
| 8 | Pre-adipocyte growth medium (500 ml pre-adipocyte basal medium-2 (PBM-2), with additives as per manufacturer’s instructions: 10 % FBS, 50 μg/ml l-glutamine, 37 ng/ml GA-1000). | Lonza, Basel, Switzerland |
| 9 | MesenPRO basal medium (500 ml), 10 ml growth supplement with 2 mM l-glutamine. | Aoyagi et al. (2011) Invitrogen |
Population doubling numbers were added at each passage to obtain the cumulative populations over time.
For differentiation studies subconfluent cultures were transferred to media containing differentiation factors (Table 2). To investigate spontaneous differentiation, cells were maintained in proliferation medium without further passaging for 14 days. All experiments were performed with cells isolated from three different tissue donors (n = 3).
Table 2.
Adipogenic differentiation media
| Medium | |
|---|---|
| 1, 2, 4, 5, 6, 7 | Proliferation medium plus 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, 0.5 μM dexamethasone |
| 3 | Proliferation medium 1 plus 0.5 mM isobutylmethylxanthine, 50 μM indomethacin, 0.5 μM dexamethasone |
| 8 | Lonza pre-adipocyte growth medium (100 ml) plus remaining supplements supplied in the PGM-2 SingleQuots kit: 0.4 ml indomethacin, 0.2 ml 3-isobutyl-1-methylxanthine, 0.2 ml dexamethasone, 2 ml insulin |
| 9 | Invitrogen StemPro adipogenesis differentiation kit |
Adipogenic differentiation
Adipogenic differentiation was induced by addition of differentiation factors (Table 2) for 14 days. To verify adipogenic differentiation intracellular triglyceride lipid droplets were visualised by Oil Red O staining (Sigma, Poole, Dorset, UK) (Ramirez-Zacarias et al. 1992).
Chondrogenic differentiation
The method of Zuk et al. (2001) was followed for chondrogenic differentiation. Briefly, 10 µl of concentrated cell suspension (1 × 106) was plated into the centre of the well in six well plates and allowed to attach at 37 °C for 2 h. Chondrogenic medium (DMEM, 1 % foetal bovine serum (FBS), insulin 6.25 µg/ml (Sigma, UK) TGF-ß1 from human platelets 10 ng/ml (Sigma, UK) ascorbate-2-phosphate 50 nM (Sigma, UK), 1 % antibiotic/antimycotic (Gibco, UK) was then gently overlaid and the cultures were maintained in chondrogenic medium for 14 days. Alcian blue (Sigma, UK) was used to stain for sulphated proteoglycans present in cartilaginous matrices. The cells were fixed in 4 % paraformaldehyde (Sigma, UK) for 15 min, rinsed with PBS (Sigma, UK) and then stained with a 1 % solution of Alcian blue in 0.1 N HCl (Sigma, UK) for 30 min. Finally, cells were rinsed for 5 min with 0.1 N HCl to remove excess stain.
Osteogenic differentiation
ADSCs were cultured in StemPro Osteogenesis differentiation kit medium (Gibco, UK) for at least 14 days. The cells were observed for alkaline phosphatase activity by staining using a leukocyte Alkaline Phosphatase kit (Sigma, UK) according to the manufacturer’s instructions and for calcium deposits using Alizarin red stain (Fluka, Gillingham, Dorset, UK). Briefly the cells were washed with HBSS, fixed in 4 % PFA and washed in deionised water then stained in Alizarin red solution for 2–3 min. The dye was aspirated and cells washed in distilled water.
Flow cytometry
Antibodies used to assess ADSC phenotype were against CD29, CD90, CD105 and CD166 (BD Biosciences, Oxford, UK). Cells were harvested at P3, fixed in 4 % PFA for 10 min and washed/stored in 0.5 % BSA (Roche Diagnostics, Burgess Hill, West Sussex UK) in PBS. Cells were then stained with the antibody according to the manufacturer’s recommendations and approximately 5 × 105 cells were re-suspended in 0.5 ml PBS for analysis on the Accuri C6 flow cytometer (BD Biosciences, UK).
qRT-PCR
RNA was isolated and analysed by quantitative real-time PCR (qRT-PCR) according to the MIQE Guidelines (Bustin et al. 2009). RNA was extracted using Qiagen RNeasy Mini Kit without DNase treatment (Qiagen, Manchester, UK). RNA concentration was quantified using Nanodrop spectrophotometry (Molecular Devices, UK) and DNA contamination using Qubit fluorometer (Invitrogen, UK). Reverse transcription was performed using Bio-Rad iScript Reverse Transcription kit (Bio-Rad, Hemel Hempstead, Hertfordshire, UK) and quantitative PCR using Bio-Rad Sso Advanced Universal SYBR Green Supermix (Bio-Rad, UK).
Cycling parameters were: 95 °C—30 s, (95 °C—5 s, 60 °C—30 s) × 40 cycles, followed by melt curve acquisition as per the manufacturer’s handbook (Bio-Rad, UK). Data was analysed using Bio-Rad CFX manager (Bio-Rad, UK) and Microsoft Excel (Microsoft, Redmond, WA, USA). Results were validated as follows: specificity was assessed by melt curve analysis and no reverse transcriptase control samples; Cq of non-template control was 0 or greater than 35; GAPDH primers were designed using Primer-Blast (NCBI) and primer efficiencies were assessed by serial dilution of primers (NM_002046.3 Forward: TCTTTTGCGTCGCCAGCCGAG Reverse: TGACCAGGCGCCCAATACGAC). Primers for GLUT4 (SLC2A4) (adipogenesis), RUNX2 (osteogenesis) and SOX9 (chondrogenesis) were pre-validated primer sets (Bio-Rad, UK). Biological (n = 3) and technical replicates (n = 3) were incorporated into the experimental design. One way ANOVA all-pairwise comparison was performed using SigmaStat (Systat, San Jose, CA, USA).
Results
Proliferation
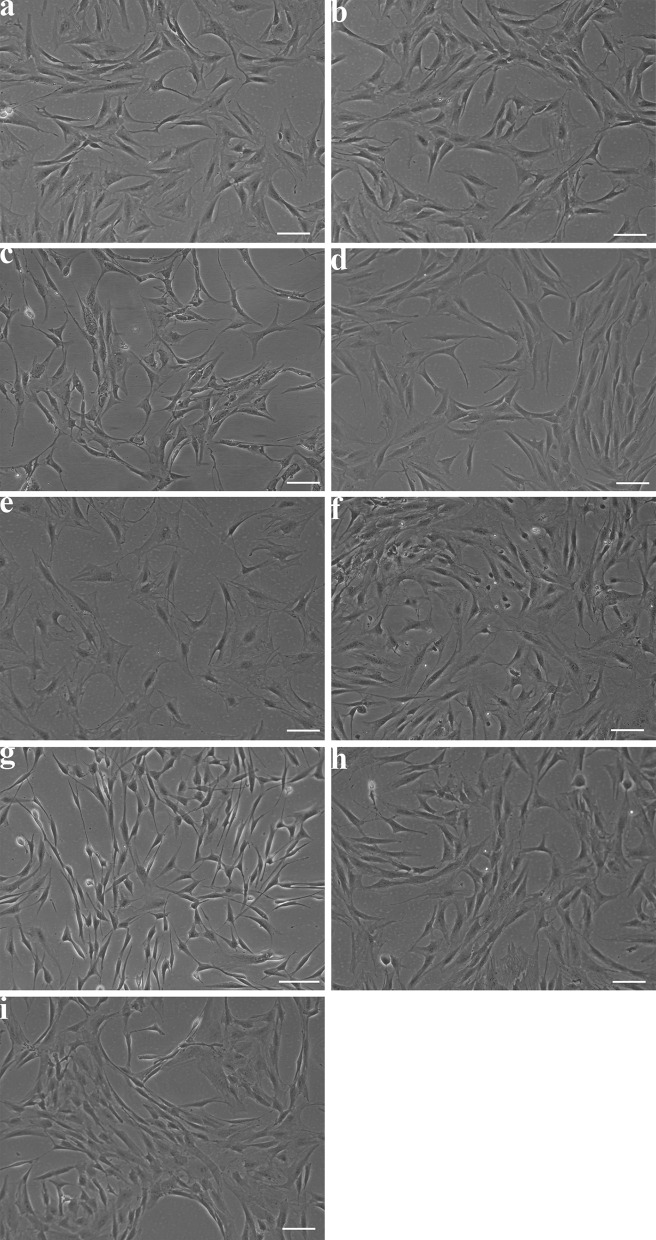
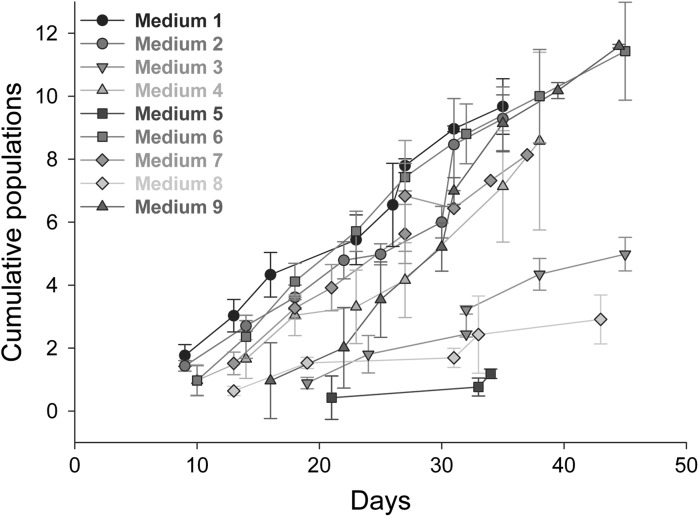
Proliferation in the different media (Table 1) was assessed visually (Fig. 1) and by cumulative population doubling (Fig. 2). Visual assessment showed that the cells proliferated in all media and displayed a homogenous fibroblastic phenotype, except in medium seven, where cells displayed a more elongated phenotype (Fig. 1g). Quantification of population doubling showed the most rapid growth in media one, two, four, six and seven. Cell growth was slower in medium nine while being very slow in media three, five and eight.
Fig. 1.
Light microscopy shows ADSC phenotype in different medium formulations. Representative images of ADSCs show differences in morphology in different medium formulations, scale bar 50 µm. a Medium one, b medium two, c medium three, d medium four, e medium five, f medium six, g medium seven, h medium 8, i medium 9
Fig. 2.
Cumulative population doubling shows growth rates in the different medium formulations. Cumulative population doubling indicates differences in growth rates in different medium formulations. Variation between different biological replicates exists, indicated by the variation in the cell numbers present at the time of passage. n = 3, error bars SD
Confirmation of stem cell phenotype by flow cytometry
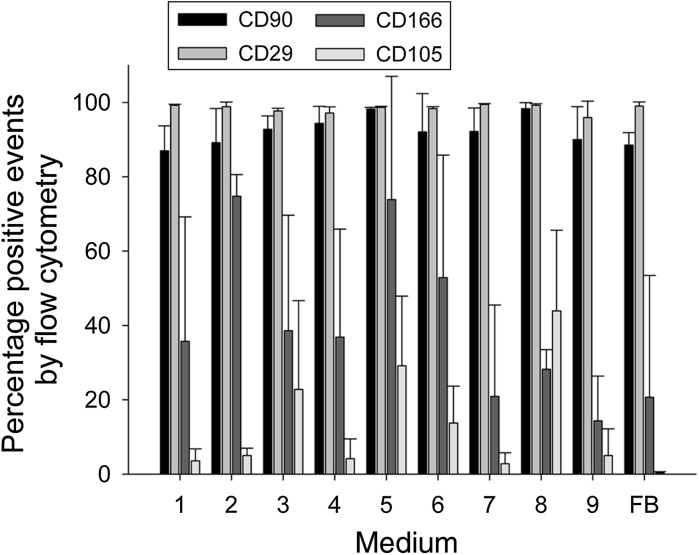
Flow cytometry analysis showed that the average percentage expression of CD29 and CD90 by cells cultured in all proliferation media was 90–100 % (Fig. 3). CD166 and CD105 were also present in all media, however the average percentage expression was lower and more variable. Expression of all these surface marker proteins has been used to characterise ADSCs (Bourin et al. 2013). However, we also observed that these markers are expressed by fibroblasts, although CD105 only at very low levels.
Fig. 3.
Flow cytometry analysis confirms ADSC phenotype. Flow cytometry for markers CD29, CD90, CD105 and CD166 shows the presence of ADSCs in all medium formulations, although CD105 and CD166 display significantly more variation. Fibroblasts (FB) also stain positively for CD29, CD90 and CD166 but only contain very low levels of CD105-positive events. n = 3, error bars SD
Adipogenic proliferation
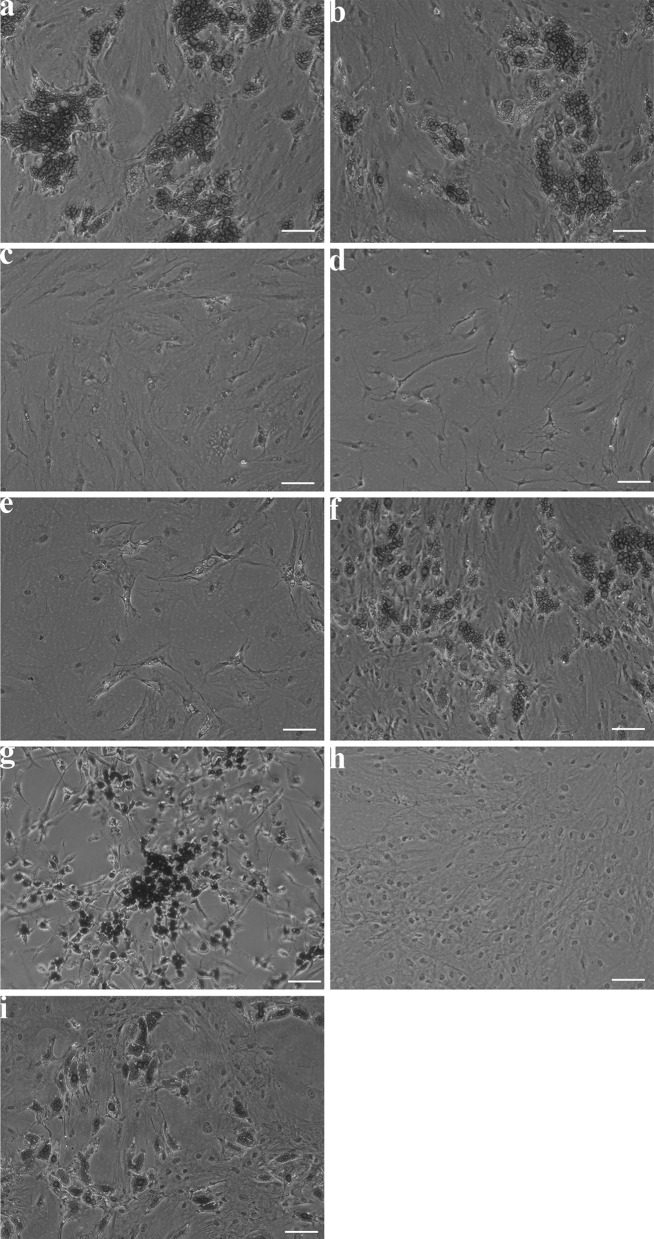
To assess differentiation along the adipogenic lineages, differentiation factors were added to culture media (Table 2). Assessment of adipogenic differentiation by Oil Red O stain showed the greatest accumulation of lipid-containing cells in media one, two, six, seven and nine after differentiation for 14 days (Fig. 4 a, b, f, g, i). Due to the unusual morphology of cells grown in medium seven, this was excluded from further studies. The media chosen for further analysis were media one, two and six.
Fig. 4.
Oil red O staining shows adipogenic differentiation in different medium formulations. Representative images of ADSCs in differentiation medium formulations (Table 2) show differences in lipid droplet content, scale bar 50 µm. a Medium one, b medium two, c medium three, d medium four, e medium five, f medium six, g medium seven, h medium eight
Induced and spontaneous multipotent differentiation in different media
In order to further assess the potential to differentiate along the classic ADSC lineages (adipogenic, osteogenic and chondrogenic), upregulation of genes characteristic for these lineages was assessed after differentiation was induced.
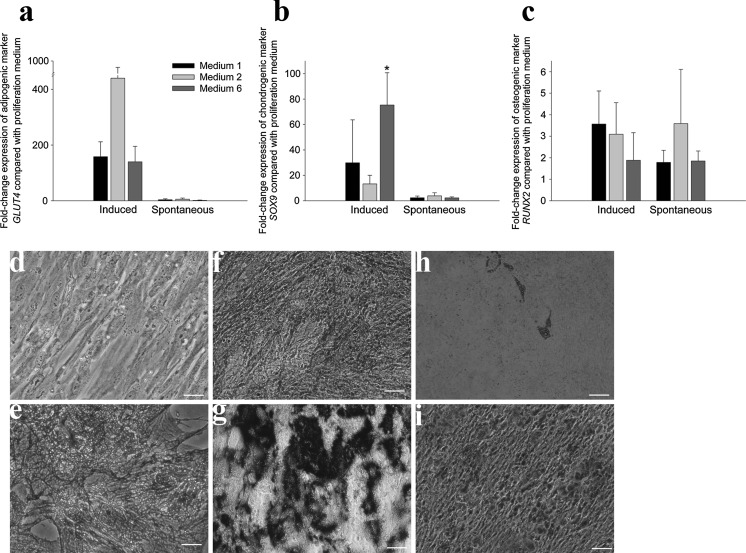
ADSCs cultured in media one, two and six with addition of adipogenic differentiation factors showed upregulation of GLUT4, a glucose transporter and indicator of adipogenic differentiation when compared to cells in proliferation medium, although this was not statistically significant (Fig. 5a). In medium one, GLUT4 expression was upregulated 158-fold (±54), in medium two 440-fold (±534) and in medium six 140-fold (±56). There was significant variation between biological replicates, leading to large standard deviations. Successful adipogenic differentiation was confirmed, however, by Oil Red O staining (Fig. 4). To assess spontaneous differentiation, cells were left in proliferation medium without further passaging. An upregulation of GLUT4 between 1 and fivefold was observed, although this was not statistically significant due to the large variation between biological replicates.
Fig. 5.
Gene expression and induced differentiation confirm multipotency. Fold-change gene expression in cells induced to differentiate and cells left to spontaneously differentiate upon confluency relative to undifferentiated cells as measured by qRT-PCR for a adipogenic marker GLUT4, b chondrogenic marker Sox9 and c osteogenic marker RUNX2. n = 3, p < 0.05, error bars SD. Representative images of Alcian blue staining to confirm chondrogenic differentiation in d undifferentiated and e induced cells. Representative images of alkaline phosphatase staining in f undifferentiated and g induced cells and Alizarin Red staining in h undifferentiated and i induced cells to confirm osteogenic differentiation
Differentiation in chondrogenic medium showed upregulation of SOX9, a marker of chondrogenesis (Fig. 5b) by 30-fold (±34) in medium one, by 13-fold (±7) in medium 2 and by 76-fold (±25) in medium 6, which was statistically significant only for the change observed in medium six. Successful chondrogenic differentiation was confirmed by Alcian blue staining (Fig. 5d, e). Cells left in proliferation medium showed an upregulation of SOX9 between 2 and fourfold, which was not statistically significant.
Differentiation with osteogenic factors resulted in upregulation of RUNX2, a marker of osteogenesis, (Fig. 5c) by fourfold (±1.5) in medium one, by threefold (±1.5) in medium 2 and by twofold (±1.3) in medium six, although these were not statistically significant. Successful osteogenic differentiation was confirmed by alkaline phosphatase (Fig. 5f, g) and alizarin red staining (Fig. 5h, i). Cells left in proliferation medium also displayed spontaneous differentiation as shown by upregulation of RUNX2, although this was also not statistically significant.
Discussion
In this study we isolated and cultured ADSCs in seven proliferation media as described by various authors (Katz et al. 2005; Yoshimura et al. 2006; Bunnell et al. 2008; Du et al. 2010; Zhou 2011; Aoyagi et al. 2011; Frerich et al. 2012) and two other proprietary media. Rate of proliferation is an important factor for clinical application, where autologous cells are required for reconstructive surgery. This may include growing the cells within a scaffold prior to being implanted into the patient. Therefore, one of our main criteria in this study was proliferation rate. Overall we found proliferation rates were faster in media which contained 10 % FBS and slower in media with lower concentrations of FBS but there were exceptions: medium seven described by Du et al. (2010) contained only 2 % FBS but also included growth factors and supported vigorous and fast proliferation. Medium three, described by Yoshimura et al. (2006) contained 10 % FBS but we found proliferation to be slower. We also tested MesenPro RS medium which contains 2 % serum which supported proliferation and adipogenic differentiation but proliferation was considerably slower. The use of FBS as a supplement for growing cultured cells raises regulatory issues for clinical application, thus media which do not need FBS should be further investigated. Others have investigated serum- and xenobiotic-free medium systems or those which substitute FBS with human serum (Shih et al. 2011; Chase et al. 2012; Yang et al. 2012; Patrikoski et al. 2013; Al Saqi et al. 2014). For example, Patrikoski et al. (2013) have reported the culture of ADSCs in xeno- and serum-free media as well as in medium supplemented with human serum. Their findings included a reduction in adhesion of the cells to tissue culture plastic in xeno- and serum-free media without applying a carboxyl coating but cell proliferation in further passages was good and population doubling faster than in media containing human serum. More recently, Al-Saqi et al. (2014) have compared ADSC culture in a commercially available xenobiotic-free medium formulation to culture in DMEM with 10 % FBS and found the former to support more rapid cell proliferation and differentiation. Further work on establishing robust xenobiotic-free medium formulations in the development of protocols for clinical application for human patients should include comparison of several such formulations to produce sufficient data to obtain health authority approval for use of such systems.
Identification of the ADSC cell type was confirmed by flow cytometry. Cells cultured in all media expressed markers of ADSCs, such as CD29 and CD90, which were universally expressed, as well as CD105 and CD166, which were more variable between different media and expressed in lower percentages. A recent statement by Bourin et al. (2013) suggested CD13 as an alternative or supplement to CD105 as the expression level is often higher and more stable. These cell surface markers have been shown to identify ADSCs (Rodbell 1964; Zuk et al. 2001; Maumus et al. 2011; Zhou et al. 2011; Patrikoski et al. 2013) but do not distinguish them from other cell types such as fibroblasts, which was confirmed in our study. It has been shown that CD105 is not expressed by freshly isolated ADSCs but can first be observed after culture for approximately 1 week and continues to be expressed in cultures up to 20 weeks (Katz et al. 2005). Our results support these findings by showing higher levels of CD105 in the slower growing cultures.
In our study cells grown in all nine proliferation media were also cultured in adipogenic medium to confirm differentiation along the adipogenic lineage. Following addition of adipogenic factors staining with Oil Red O confirmed the presence of mature adipocytes in all cultures, although there was variation in the amount of lipid-containing cells between the media.
We identified three media to investigate further, selected on the criteria of rapid proliferation, confirmation of ADSC phenotype and differentiation along the adipogenic lineage. Although some of the slower proliferating cultures expressed higher levels of CD105, rapid proliferation was our main criteria for suitability for clinical application. We therefore selected media one, two and six for further investigation and showed that upon treatment with appropriate differentiation factors, the cells could successfully differentiate along the adipogenic, chondrogenic and osteogenic lineages. qRT-PCR indicated upregulation of relevant genes, although a significant amount of biological variation was observed.
We also investigated the potential for ADSCs to spontaneously differentiate towards the adipogenic, chondrogenic and osteogenic lineages if maintained in proliferation medium without further passaging. The ability for spontaneous differentiation could have clinical implications which may be beneficial or damaging and may be affected by the location in the body where the cells are implanted. Aoyagi et al. (2011) found that ADSCs seeded in a fibrinogen scaffold spontaneously accumulate lipid droplets and lower concentrations of fibrinogen appear to stimulate earlier adipogenesis. However, Zhu et al. (2008) and Abdallah et al. (2005) recorded no spontaneous differentiation in cell culture. Both studies were performed on subconfluent cells which were continuously passaged and did not include cells maintained without passaging. Continually passaged cells have been reported by others to maintain their phenotype throughout many passages (Zuk et al. 2001; Yoshimura et al. 2006; Bunnell et al. 2008; Zhu et al. 2008) In our study, we observed some upregulation of genes indicative of differentiation in cells maintained in proliferation medium for 14 days without further passaging, although this was not statistically significant.
Such biological variation in studies using primary human sources has also been observed by others, who have commented that age and health status of the donor may affect the behaviour, phenotype and lifespan of the cultured cells (Sachs et al. 2012). Maumus et al. (2011) have considered how changes in adipose tissue microenvironment may participate in ADSC commitment and contribute to excessive adipose tissue development in obesity. Various studies have also shown that ADSC yield can vary depending on donor site (Du et al. 2005; Katz et al. 2005; Bunnell et al. 2008; Maumus et al. 2011; Frerich et al. 2012). In our own studies using primary human tissue from other sources, such as skin, we have previously reported variation between tissues from different donors (Jubin et al. 2011; Lenihan et al. 2014), especially when analysed at the level of gene expression, and this may also explain the large amount of biological variation observed in the qRT-PCR results in this study. However, histochemical staining for adipose, osteogenic and chondrogenic differentiation did confirm that this process was successful, if not easily quantifiable in our culture model.
Conclusion
Our study has shown that medium selection can cause considerable variation in the rate of proliferation and phenotype of cells and their ability to differentiate into mature adipocytes. Addition of FBS appears to enhance proliferation but low FBS with separate growth factors as in medium seven will also result in fast and vigorous growth. Of the three media we selected for further testing, all three supported rapid proliferation of cells which had multilineage differentiation potential.
These three media all contained 10 % heat inactivated FBS but varied in their basal medium: medium one contained DMEM with Glutamax, medium two contained low glucose DMEM without Glutamax and medium six was a 50:50 mixture of IMDM with Glutamax: Ham’s F12. We have demonstrated the isolation and proliferation of ADSCs in the three selected media by characterisation and multilineage differentiation into mature adipocytes, osteocytes and chondrocytes. Our study confirms significant variation between media used by different groups and indicates which media provide rapid proliferation, which is key if cells are to be used in clinical application.
Acknowledgments
The work was funded by charitable donations to the Blond McIndoe Research Foundation.
Conflict of interest
No conflicts of interest exist.
References
- Abdallah BM, Haack-Sorensen M, Burns JS, Elsnab B, Jakob F, Hokland P, Kassem M. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326:527–538. doi: 10.1016/j.bbrc.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Al Saqi SH, Saliem M, Asikainen S, Quezada HC, Ekblad A, Hovatta O, Le Blanc K, Jonasson AF, Gotherstrom C. Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy. 2014;16:915–926. doi: 10.1016/j.jcyt.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Kuroda M, Asada S, Bujo H, Tanaka S, Konno S, Tanio M, Ishii I, Aso M, Saito Y. Fibrin glue increases the cell survival and the transduced gene product secretion of the ceiling culture-derived adipocytes transplanted in mice. Exp Mol Med. 2011;43:161–167. doi: 10.3858/emm.2011.43.3.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chase LG, Yang S, Zachar V, Yang Z, Lakshmipathy U, Bradford J, Boucher SE, Vemuri MC. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl Med. 2012;1:750–758. doi: 10.5966/sctm.2012-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Roh DS, Funderburgh ML, Mann MM, Marra KG, Rubin JP, Li X, Funderburgh JL. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–2689. [PMC free article] [PubMed] [Google Scholar]
- Frerich B, Winter K, Scheller K, Braumann UD. Comparison of different fabrication techniques for human adipose tissue engineering in severe combined immunodeficient mice. Artif Organs. 2012;36:227–237. doi: 10.1111/j.1525-1594.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- Jubin K, Martin Y, Lawrence-Watt DJ, Sharpe JR. A fully autologous co-culture system utilising non-irradiated autologous fibroblasts to support the expansion of human keratinocytes for clinical use. Cytotechnology. 2011;63:655–662. doi: 10.1007/s10616-011-9382-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- Lenihan C, Rogers C, Metcalfe AD, Martin YH. The effect of isolation and culture methods on epithelial stem cell populations and their progeny-toward an improved cell expansion protocol for clinical application. Cytotherapy. 2014;16:1750–1759. doi: 10.1016/j.jcyt.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Maumus M, Peyrafitte JA, D’Angelo R, Fournier-Wirth C, Bouloumie A, Casteilla L, Sengenes C, Bourin P. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes. 2011;35:1141–1153. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, Mannerstrom B, Miettinen S. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4:27. doi: 10.1186/scrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Sachs PC, Francis MP, Zhao M, Brumelle J, Rao RR, Elmore LW, Holt SE. Defining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sites. Cell Tissue Res. 2012;349:505–515. doi: 10.1007/s00441-012-1423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DT, Chen JC, Chen WY, Kuo YP, Su CY, Burnouf T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51:770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- Tapp H, Hanley EN, Jr, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med . 2009;234:1–9. doi: 10.3181/0805-MR-170. [DOI] [PubMed] [Google Scholar]
- Yang S, Pilgaard L, Chase LG, Boucher S, Vemuri MC, Fink T, Zachar V. Defined xenogeneic-free and hypoxic environment provides superior conditions for long-term expansion of human adipose-derived stem cells. Tissue Eng Part C. Methods. 2012;18:593–602. doi: 10.1089/ten.tec.2011.0592. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yan Z, Zhang H, LuW, Liu S, Huang X, Luo H, Jin Y (2011). Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A 17:2981–2997 [DOI] [PubMed]
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]