Abstract
Cisplatin is one of the most potent and effective chemotherapeutic agents. However, its antineoplastic use is limited due to its cumulative nephrotoxic side effects. Therefore, the present study was undertaken to examine the nephroprotective potential of dietary bee honey and royal jelly against subchronic cisplatin toxicity in rats. Male Wistar rats were randomly divided into controls, cisplatin-treated, bee honey-pretreated cisplatin-treated and royal jelly-pretreated cisplatin-treated groups. Bee honey and royal jelly were given orally at doses of 20 and 100 mg/kg, respectively. Subchronic toxicity was induced by cisplatin (1 mg/kg bw, ip), twice weekly for 10 weeks. Cisplatin treated animals revealed a significant increase in serum level of renal injury products (urea, creatinine and uric acid). Histopathologically, cisplatin produced pronounced tubulointerstitial injuries, upregulated the fibrogenic factors, α-smooth muscle actin (α-SMA) and transforming growth factor β1(TGF-β1), and downregulated the cell proliferation marker, bromodeoxyuridine (Brdu). Dietary bee honey and royal jelly normalized the elevated serum renal injury product biomarkers, improved the histopathologic changes, reduced the expression of α-SMA and TGF-β1 and increased the expression of Brdu. Therefore, it could be concluded that bee honey, and royal jelly could be used as dietary preventive natural products against subchronic cisplatin-induced renal injury.
Keywords: Cisplatin, Nephrotoxicity, Honey, Royal jelly, Rat
Introduction
Cisplatin, cis-diamminedichloroplatinum, is an effective synthetic broad-spectrum antineoplastic agent (Shen et al. 2012). It has been reported to show an activity against a broad spectrum of many solid tumors, including cervical, ovarian, testicular, bladder and lung cancers as well as solid tumors resistant to other drug regimens (Kart et al. 2010; Yousef et al. 2009). The main mechanism of cisplatin cytotoxic effect is believed to result from its interaction with DNA, through the formation of covalent adducts between certain DNA bases and the platinum compound leading to cytotoxic lesions in tumors and other rapidly dividing cells (Yang et al. 2006). However, the clinical usefulness of cisplatin has been limited due to its cumulative nephrotoxic side effects (Taguchi et al. 2005). The mechanism for renal cell injury has been the focus of intense investigation over many years. Recent studies suggest that inflammation, oxidative stress injury, and apoptosis probably explained part of this injury (Jiang and Dong 2008). Furthermore, cisplatin treatment is usually associated with vomiting, nausea, hair loss, ototoxicity, neurotoxicity and cardiotoxicity (Jiang and Dong 2008; Pai and Nahata 2000).
Bee honey (BH), formed from nectar by honeybees (Apis millifera), is one of these natural products that has been recently subjected to increasing awareness. It has been used as a traditional medicine since the ancient times and this was documented in a Sumerian tablet and in an Egyptian papyrus (Bell 2007). It has many beneficial therapeutic effects such as antibacterial, anti-inflammatory, hepatoprotective, antioxidant, and antihypertensive effects (Erejuwa et al. 2010; Kassim et al. 2010). Moreover, some studies have reported promising anticancer properties of crude honey against many tumor cell lines in vitro and in vivo (Jaganathan and Mandal 2009; Swellam et al. 2003). BH is composed of at least 181 components and is supersaturated with sugars. Some components to be mentioned are flavonoids and phenolics (caffeic acid, chrysin, quercetin, kaempferol), ascorbic acid, carotenoid-like substances, organic acids, amino acids, proteins and certain enzymes such as glucose oxidase, catalase (Ariefdjohan et al. 2008; Jaganathan and Mandal 2009).
Another natural product that has received particular interest is royal jelly (RJ). RJ is a hypopharyngeal and mandibular glands’ secretion of worker honeybees (Apis mellifera) and considered as an exclusive food of the queen honeybee larva. Chemically, RJ comprises water, free amino acids, proteins, sugars, fatty acids, mineral salts, and vitamins (Nakajima et al. 2009). So far, RJ possesses antioxidant, antitumor, antibacterial, hypoglycemic, anti-inflammatory antihypercholesterolemic, and immunomodulatory activities (Kamakura et al. 2006; Nagai et al. 2006). Furthermore, it had a protective effect against paracetamol induced liver damage in mice (Kanbur et al. 2009). In addition, it counteracted cisplatin-induced testicular damage in rats (Silici et al. 2009).
Consequently, the aim of the present study was to investigate the chemoprotective effect of dietary bee honey and royal jelly against subchronic cisplatin-induced nephrotoxicity in rats.
Materials and methods
Chemicals
Cisplatin (Cisplatine® vial, 1 mg/ml) in clinical formulation was purchased from MERCK (Lyon, France). Bee honey (BH) was purchased from a local market, and produced by Isis Co. (El Salam City, Cairo, Egypt) while royal jelly (RJ) soft capsules were purchased from Pharco Pharmaceuticals Co. (Alexandria, Egypt). Anti-α-SMA, anti-BrdU, and anti-TGFβ antibodies were purchased from Dako Chem Mate (Kyoto, Japan). All other chemicals used in the experiment were of analytical grade.
Animals
The experiment was performed in accordance with the Guidelines for Animal Experimentation issued by National Institute of Health and approved by local Ethics and Review Committee at the Faculty of Veterinary Medicine (Suez Canal University, Ismailia, Egypt) (the approval no 20153). All efforts were made to minimize suffering for the experimental animals used in this study. Forty-eight male Wistar Albino rats, weighing 150–200 g, were purchased from The Egyptian Organization for Biological Products and Vaccines (Gize, Egypt). Rats were kept in ventilated room under controlled laboratory conditions of normal light–dark cycle (12:12 light–dark) and temperature (25 ± 2 °C). The rats were kept for 1 week before experimentation to adapt to laboratory conditions. The rats had free access to commercial balanced diet and water throughout the experimental period.
Experimental design
The animals were randomly divided into six groups, each including eight rats. The 1st control group was given saline by intragastric intubation. The 2nd and 3rd groups were orally given BH (20 mg/kg bw) and RJ (100 mg/kg bw), respectively. The doses of these products were chosen according to Galal et al. (2012) and El-Nekeety et al. (2007), respectively. The 4th group was injected with cisplatin (1 mg/kg of bw, ip), twice weekly for 10 weeks. This dose of cisplatin was selected according to a previous study that demonstrated subchronic nephrotoxicity in rats (Marcussen 1990). The 5th and 6th groups were administered BH and RJ at the same regimen used for the 2nd and 3rd groups 1 h before intraperitoneal cisplatin administration at the same doses used for the 4th group.
Serum collection and tissue preparation
At the end of experiment, blood samples were collected via retro-orbital bleeding under light ether anaesthesia. Blood samples were collected, left to clot at room temperature, and then centrifuged at 3000 rpm for 15 min. Sera were then, separated and stored at −20 °C as aliquots for further biochemical analysis. After blood collection, rats were then euthanized using ether anaesthesia followed by cervical decapitation. Kidney was rapidly excised from each rat, and fixed in 10 % neutral buffered formalin until use for histopathologic and immunohistochemical investigations.
Serum biochemical analysis
Sera were used for estimation of renal products (Urea, creatine and uric acid) using commercial kits purchased from Biodiagnostics Co. (Cairo, Egypt) and were performed following the manufacturer’s protocol. Creatinine was determined according to Larsen (1972), urea according to Coulombe and Favreau (1963) and uric acid according to Whitehead et al. (1991).
Histopathologic examination
Kidneys were embedded into paraffin blocks, and serial sections at different levels were prepared and stained with hematoxylin and eosin (H&E). The slides were then evaluated histologically.
Immunohistochemistry (IHC)
Five-micron (5 μm) paraffin embedded sections were deparaffinized in xylene and rehydrated with gradient alcohols. Antigen retrieval was performed by placing sections in citrate buffer (pH 6.0) and a decloaker pressure cooker for 15 min at 120 °C per 18 psi. Following cool-down, potential nonspecific binding sites were blocked with 5 % normal goat or rabbit serum in phosphate-buffered saline (PBS). The sections were then incubated with anti-α-SMA (1:200), anti-BrdU (1:200), or anti-TGF-β-1 (1:100). After three 5-min washes in PBS, the sections were incubated with specific biotin-conjugated secondary antibody (Vector Laboratories, Burlingame, CA, USA). A Vector-ABC streptavidin-peroxidase kit with a benzidine substrate was used for color development. Counter-staining was done with diluted hematoxylin. Sections that were not incubated with primary antibody served as negative control. Images were collected using an Olympus vanox microscope (Tokyo, Japan) and the stained cells were extracted by using double thresholding methods or color thresholding using Image J program (http://rsb.info.nih.gov/ij/) to extract the brown color for quantitation (Russ 1995).
Statistical analysis
All data were expressed as mean ± S.E.M. and statistically analyzed using SPSS 16.0 for Windows (SPSS Inc, Chicago, IL, USA). Statistical significance of differences among different study groups was evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons test as a post hoc test. A P value of 0.05 or less was taken as a criterion for a statistically significant difference.
Results
Serum biochemical analysis
The effects of subchronic cisplatin administration as well as the preventive effects of bee honey and RJ on serum biochemical analyses are shown in Table 1. Significant increases (P ≤ 0.05) in serum renal product biomarkers (urea, creatinine, and uric acid) were recorded in cisplatin intoxicated rats as compared to the untreated control group (about 315, 213, 323 %, respectively). Pre-treatment with bee honey and RJ at doses of 20 and 100 mg/Kg BW, respectively, (1 h prior to cisplatin administration) reversed the changes in the studied serum parameters. The results indicate that BH and RJ effectively reduced cisplatin-induced nephrotoxicity. Bee honey administration at a dose of 20 mg/kg significantly (P ≤ 0.05) reduced the serum renal products: urea, creatinine and uric acid (about 47, 62 and 53 %, respectively) compared to the cisplatin-intoxicated non-treated group. Similarly, RJ pre-administration at a dose of 100 mg/kg BW significantly (P ≤ 0.05) reduced urea, creatinine and uric acid (about 34, 57 and 50 %, respectively) compared to the cisplatin-intoxicated non-treated group.
Table 1.
Serum biochemical parameters in control and different treated groups
| Experimental groups | Parameters (mg/dL) | ||
|---|---|---|---|
| Urea | Creatinine | Uric acid | |
| Control | 25.02 ± 0.98 | 0.43 ± 0.03 | 23.39 ± 1.07 |
| BH | 23.78 ± 0.85 | 0.41 ± 0.02 | 23.11 ± 1.17 |
| RJ | 23.22 ± 0.93 | 0.40 ± 0.02 | 22.69 ± 1.24 |
| Cisplatin | 78.82 ± 2.56* | 0.92 ± 0.06* | 75.63 ± 4.92* |
| Cisplatin-BH | 37.16 ± 1.36** | 0.58 ± 0.02** | 40.51 ± 2.40** |
| Cisplatin-RJ | 27.50 ± 0.66** | 0.53 ± 0.03** | 38.35 ± 1.84** |
Data are presented as mean ± SE (n = 8)
BH bee honey, RJ royal jelly
* Significantly different from normal non-treated control group (P ≤ 0.05)
** Significantly different from ciplatin-intoxicated group (P ≤ 0.05)
Histopathological findings
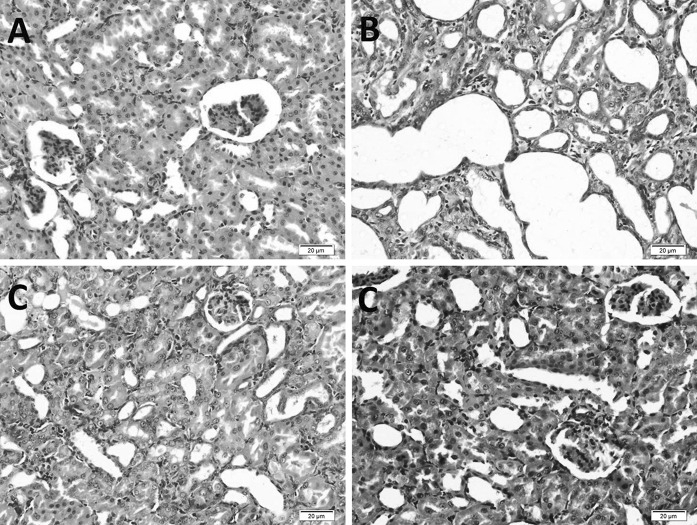
Microscopically, 60 % of the renal tubules of rats in cisplatin-intoxicated group suffered from varying degrees of necrosis and degeneration, especially those located at cortico-medullary junction. Tubules were moderately ectatic, tortuous, and lined with attenuated epithelium. Multifocally, tubular epithelial cells showed increased cytoplasmic basophilia with large vesicular nuclei and prominent nucleoli. Occasionally, some lining epithelial cells had hypereosinophilic cytoplasm and pyknotic nuclei. Some tubules were filled with proteinous cast. Multifocally, interstitium was moderately expanded with fibrous connective tissue and some inflammatory cells, mostly macrophages, lymphocytes, and plasma cells (Fig. 1a, b).
Fig. 1.
Histopathologic section stained with H&E, ×100, a control untreated kidney showing normal renal architecture. b Markedly dilated tubules in rats treated with ciplatin only and inflammatory cells along with fibrosis expanded in the interstitial tissue. c Minimal histopathologic changes are seen in honey treated rats. d Some tubules are moderately ectatic, RJ group
Bee honey group, about 5 % of the renal tubules showed a milder degree of the previously mentioned changes with few numbers of inflammatory cells in the interstitial tissue. No fibrosis was detected (Fig. 1c). On the other hand, approximately 10 % of the renal tubules of the rats treated with RJ showed histopathologic changes similar to those of cisplatin ones (Fig. 1d).
Immunohistochemical study
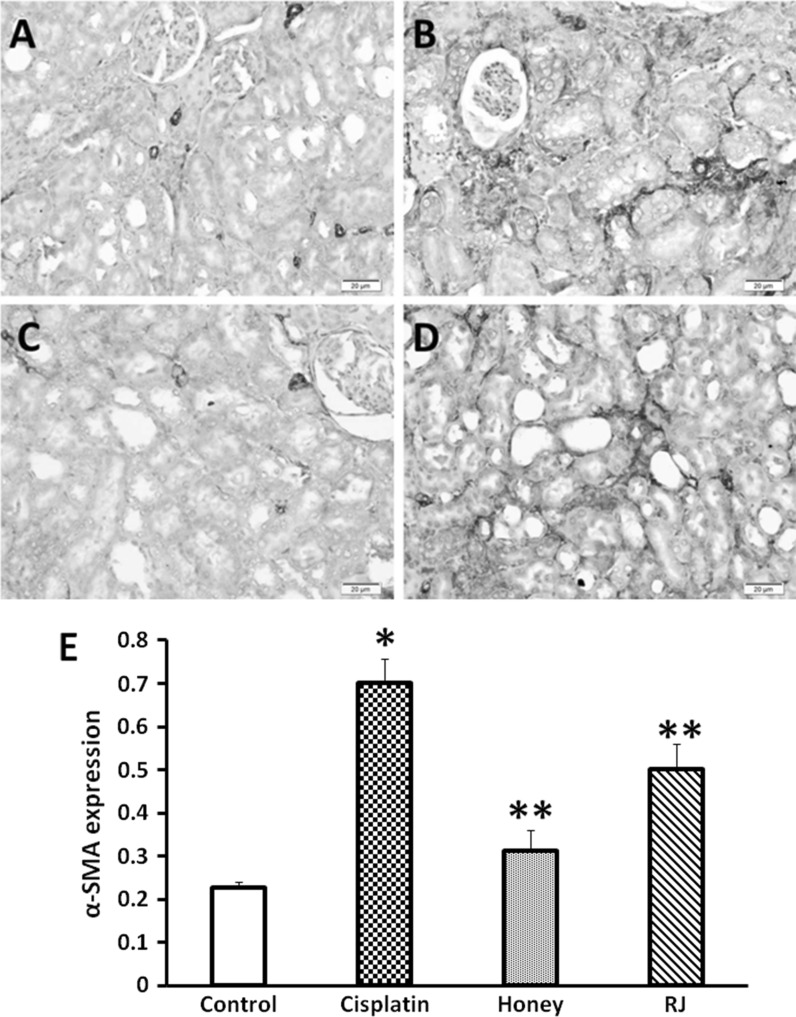
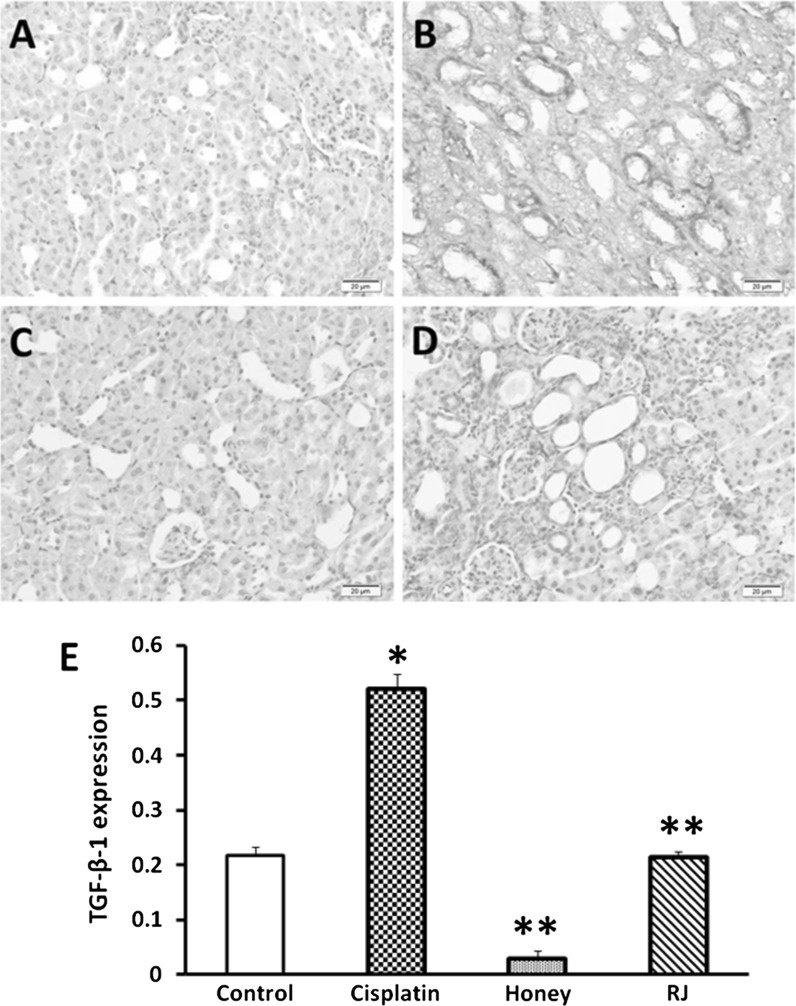
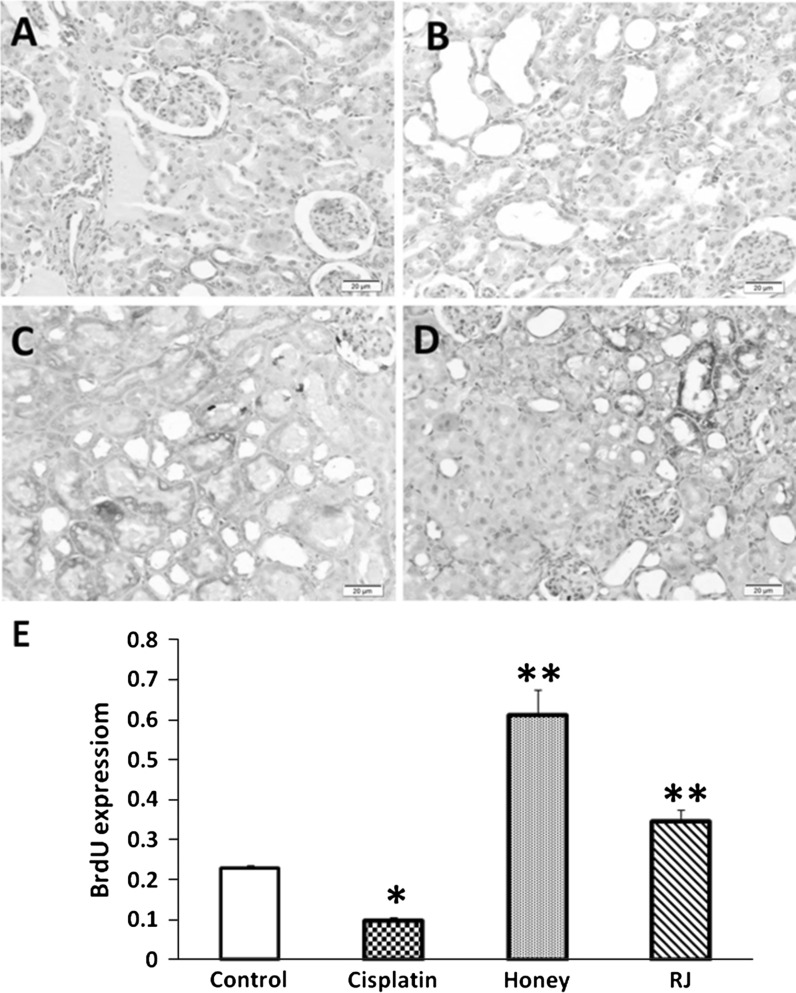
Immunohistochemical staining of α-SMA sections from the cisplatin-intoxicated group revealed a moderate immunoreactivity in the interstitial tissue around the dilated tubules (Fig. 2b). As shown in Fig. 2c and d, treatment with honey and RJ significantly (P < 0.05) reduced the expression α-SMA. TGF-β-1- positive cells were noticed in many renal tubules of kidneys treated with cisplatin only. However, the number of these cells were significantly (P < 0.05) reduced with BH and RJ treatment (Fig. 3). In contrary, the immunostaining of BrdU was increased in the tubular epithelial cells of BH and RJ pre-treated groups than in the cisplatin group (Fig. 4).
Fig. 2.
Immunohistochemical staining of α-SMA, ×100. a Control untreated kidney showing normal expression of SMA within the wall of interstitial arterioles. b Cisplatin treated rat showing a moderate reaction in the interstitial tissue. c Honey treated rats expressing no reactivity. d RJ treated rats expressing minimal reaction. e Data represent the expression level of α-SMA in cisplatin, honey, and RJ groups: *significant difference from normal control group at P < 0.05, **significant difference from cisplatin group at P < 0.05
Fig. 3.
Immunohistochemical staining of TGF-β-1, ×100. a Control untreated kidney showing minimal expression of TGF within the renal tubular epithelial cells. b Cisplatin treated rat showing many positive epithelial cells in the renal tubules. c Honey treated rats revealing low reactivity. d RJ treated rats showing reduced reaction. e Data represent the expression level of TGF-β-1 in cisplatin, honey, and RJ groups: *significant difference from normal control group at P < 0.05, **significant difference from cisplatin group at P < 0.05
Fig. 4.
Immunohistochemical staining of BrdU, ×100. a Control untreated kidney showing minimal expression of BrdU within the renal tubular epithelial cells. b Few epithelia cells showing a weak reaction in the cispaltin group. c The honey group has many positive epithelial cells. d In the RJ treated group epithelial cells reacting to BrdU are seen at moderate numbers of renal tubules. e Data represent the expression level of BrdU in cisplatin, honey, and RJ groups: *significant difference from normal control group at P < 0.05, **significant difference from cisplatin group at P < 0.05
Discussion
In the last few years, many consumers and researchers have shown a renewed interest in using natural products for treatment of human and animal diseases (Abdel-Daim 2014; Abdel-Daim et al. 2013; Eldahshan and Abdel-Daim 2014). These materials are very cheap, readily available, and devoid of many side effects of synthetic chemicals (Abdel-Daim et al. 2014b; Al-Sayed and Abdel-Daim 2014).
In this study, we aimed to fulfill the hypothesis that bee honey and RJ could block cisplatin-induced renal damage. As a primary organ for drug filtration, concentration, and excretion, renal tissues (especially proximal tubule cells) are selectively damaged by cisplatin during cancer therapy. The kidney selectively accumulates cisplatin to a higher degree than other organs, probably via mediated transport (Ali and Al Moundhri 2006). This is because the kidney is the major route for its excretion. Cisplatin concentration in proximal tubular epithelial cells, especially S3 segment, is about five times its serum concentration (Kuhlmann et al. 1997). The in vivo mechanisms of cisplatin nephrotoxicity are complex and involve oxidative stress, apoptosis, inflammation, and fibrogenesis (Lieberthal et al. 1996). Reactive oxygen species (ROS) directly act on cell components, including lipids, proteins, and DNA, and destroy their structure (Abdel-Daim et al. 2014a; Madkour and Abdel-Daim 2013).
Renal injuries caused by subchronic cisplatin administration in the present study may be attributed to the production of fibrogenic factors and inhibition of cell proliferation. Subchronic cisplatin intoxication increased serum renal product injury markers; urea, creatinine and uric acid (Table 1). In addition, it induced fibrosis, interstitial inflammation, and tubular necrosis (Fig. 1). Moreover, Fibrogenic factors such as TGF-β1 and α-SMA were overexpressed (Figs. 2, 3). Furthermore, the expression of Brdu, a cell proliferation biomarker, was downregulated (Fig. 4).
Acute and subchronic cisplatin administration led to renal degeneration and increased serum levels of urea, creatinine, uric acid, BUN and lipid peroxidation in rats and mice (Hassan et al. 2013; Kawai et al. 2009). In the current study, the pre-administration of BH and RJ (20 and 100 mg/kg respectively) reduced the serum renal injury biomarkers. These results are consistent with the earlier study reporting that, BH prevented renal injury induced by CCl4 intoxication in rats (El Denshary et al. 2012). In addition, RJ modulated renal injury in rats treated with cisplatin (Silici et al. 2011).
The pathologic events of cisplatin-induced renal damage can be tentatively divided into three main events, which at times may overlap: initial cytotoxic, inflammatory and fibroproliferative events (Taguchi et al. 2005). Our results showed that BH and RJ groups were devoid of the main histologic changes observed in cisplatin group such as fibrosis, interstitial inflammation, and tubular necrosis. Development of irreversible tubule-interstitial fibrosis is a relatively late change found in the kidneys of cisplatin-treated experimental animals. Fibrogenic factors such as TGF-β-1, HSP47, and TNF have the potential to mediate both human and experimental fibrotic diseases. They are released by the activated and phenotypically altered resident cells, especially macrophages. In turn, these fibrogenic factors stimulate the transcription of genes encoding extra cellular matrix proteins. Studies have convincingly demonstrated that blocking TGF-β-1 results in the suppression of collagen production and subsequent modulation of fibrotic processes (Razzaque et al. 1998; Yamate et al. 2004). An increased expression of TGF-β-1 has been detected in tubular epithelial cells and interstitial cells in the kidneys of cisplatin treated rats by in situ hybridization (Basile 2001). We have concluded that, BH and RJ significantly reduced the expression of TGF-β-1.
The alpha-smooth muscle (α-SMA) actin isoform is expressed normally by vascular smooth muscle cells and by stromal fibroblastic cells. Interstitial overexpression of α-SMA which indicates myofibroblast activation is correlated with the degree of interstitial fibrosis (Boukhalfa et al. 1996). To confirm that honey and RJ decreases the interstitial fibrosis, we evaluated the expression of α-SMA. Immnohistochemically, BH and RJ treatment significantly reduced the α-SMA expression. Also we measured the expression of BrdU, which is a thymidine analog that is incorporated in the DNA of dividing cells during the S-phase of the cell cycle. As such, BrdU is used for birth dating and monitoring cell proliferation (Taupin 2007). Interestingly, our results indicated that BrdU expression was significantly higher in the BH and RJ groups in comparison to cisplatin-intoxicated group. This is consistent with the results of Yamate et al. (2005) who reported that BrdU-positive cell number was significantly gradually decreased from day 6 after injection of rats with cisplatin (Yamate et al. 2005). Moreover, this result is a good indicator for BH and RJ which stimulate epithelial cells to proliferate for replacing lost cells.
As a result of this study, we can conclude that BH and RJ are promising protective natural agents for cisplatin-induced subchronic nephrotoxicity through inhibiting fibrogenic factors production. The present study reveals that, cisplatin exposure resulted in varying degree of alterations of serum biochemical parameters, histopathological and immunohistochemical nephrotoxic lesions in rats. BH and RJ pre-exposure provided near complete protection in terms of serum biochemical changes, renal histopathology and immunohistochemistry of fibrogenic biomarkers.
Conclusions
Chemotherapy diminishes the normal homeostasis of the body, a fact which is particularly applicable for cisplatin treatment. Cisplatin-induced nephrotoxicity has been considered as the main complication during the normal clinical regimens of treatment.
The present study reveals that, cisplatin exposure causes a varying degree of alterations of serum biochemical, histopathological and immunohistochemical parameters in rats. Pretreatment of BH and RJ shows protective effects on cisplatin-induced renal damage.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abdelazim Ibrahim, Mabrouk Abd Eldaim and Mohamed Abdel-Daim have contributed equally in this research work.
References
- Abdel-Daim MM. Pharmacodynamic interaction of Spirulina platensis with erythromycin in Egyptian Baladi bucks (Capra hircus) Small Rumin Res. 2014;120:234–241. doi: 10.1016/j.smallrumres.2014.05.013. [DOI] [Google Scholar]
- Abdel-Daim MM, Abuzead SM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol. 2014;92:679–685. doi: 10.1139/cjpp-2014-0144. [DOI] [PubMed] [Google Scholar]
- Abdel-Daim MM, Ghazy EW, Fayez M. Synergistic protective role of mirazid (Commiphora molmol) and ascorbic acid against tilmicosin-induced cardiotoxicity in mice. Can J Physiol Pharmacol. 2014 doi: 10.1139/cjpp-2014-0336. [DOI] [PubMed] [Google Scholar]
- Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44:1173–1183. doi: 10.1016/j.fct.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Al-Sayed E, Abdel-Daim MM. Protective role of cupressuflavone from Cupressus macrocarpa against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Planta Med. 2014;80:1665–1671. doi: 10.1055/s-0034-1383211. [DOI] [PubMed] [Google Scholar]
- Ariefdjohan MW, Martin BR, Lachcik PJ, Weaver CM. Acute and chronic effects of honey and its carbohydrate constituents on calcium absorption in rats. J Agric Food Chem. 2008;56:2649–2654. doi: 10.1021/jf073357w. [DOI] [PubMed] [Google Scholar]
- Basile DP. Transforming growth factor-beta as a target for treatment in diabetic nephropathy. Am J Kidney Dis. 2001;38:887–892. doi: 10.1053/ajkd.2001.27721. [DOI] [PubMed] [Google Scholar]
- Bell SG. The therapeutic use of honey. Neonatal netw NN. 2007;26:247–251. doi: 10.1891/0730-0832.26.4.247. [DOI] [PubMed] [Google Scholar]
- Boukhalfa G, Desmouliere A, Rondeau E, Gabbiani G, Sraer JD. Relationship between alpha-smooth muscle actin expression and fibrotic changes in human kidney. Exp Nephrol. 1996;4:241–247. [PubMed] [Google Scholar]
- Coulombe JJ, Favreau L. A new simple semimicro method for colorimetric determination of urea. Clin Chem. 1963;9:102–108. [PubMed] [Google Scholar]
- El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Exp Toxicol Pathol. 2012;64:753–760. doi: 10.1016/j.etp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Eldahshan OA, Abdel-Daim MM. Phytochemical study, cytotoxic, analgesic, antipyretic and anti-inflammatory activities of Strychnos nux-vomica. Cytotechnology. 2014 doi: 10.1007/s10616-014-9723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon. 2007;50:256–269. doi: 10.1016/j.toxicon.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Erejuwa OO, Gurtu S, Sulaiman SA, Ab Wahab MS, Sirajudeen KN, Salleh MS (2010) Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int J Vitam Nutr Res 80:74–82. doi:10.1024/0300-9831/a000008 [DOI] [PubMed]
- Galal RM, Zaki HF, Seif El-Nasr MM, Agha AM (2012) Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch Iran Med 15:674–680. doi:0121511/AIM.006 [PubMed]
- Hassan HA, Edrees GM, El-Gamel EM, El-Sayed EA (2013) Amelioration of cisplatin-induced nephrotoxicity by grape seed extract and fish oil is mediated by lowering oxidative stress and DNA damage. Cytotechnology. 2013 Jun 13. [Epub ahead of print]. doi:10.1007/s10616-013-9589-8 [DOI] [PMC free article] [PubMed]
- Jaganathan SK, Mandal M. Antiproliferative effects of honey and of its polyphenols: a review. J Biomed Biotechnol. 2009;2009:830616. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Dong Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol Exp Ther. 2008;327:300–307. doi: 10.1124/jpet.108.139162. [DOI] [PubMed] [Google Scholar]
- Kamakura M, Moriyama T, Sakaki T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J Pharm Pharmacol. 2006;58:1683–1689. doi: 10.1211/jpp.58.12.0017. [DOI] [PubMed] [Google Scholar]
- Kanbur M, Eraslan G, Beyaz L, Silici S, Liman BC, Altinordulu S, Atasever A. The effects of royal jelly on liver damage induced by paracetamol in mice. Exp Toxicol Pathol. 2009;61:123–132. doi: 10.1016/j.etp.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol. 2010;62:45–52. doi: 10.1016/j.etp.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr Res. 2010;30:650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Satoh T, Hibi D, Ohno Y, Kohda Y, Miura K, Gemba M. The effect of antioxidant on development of fibrosis by cisplatin in rats. J Pharmacol Sci. 2009;111:433–439. doi: 10.1254/jphs.09185FP. [DOI] [PubMed] [Google Scholar]
- Kuhlmann MK, Burkhardt G, Kohler H. Insights into potential cellular mechanisms of cisplatin nephrotoxicity and their clinical application. Nephrol Dial Transpl. 1997;12:2478–2480. doi: 10.1093/ndt/12.12.2478. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay in the presence of protein with LKB 8600 reaction rate analyser. Clin Chim Acta. 1972;38:475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs. necrosis. Am J Physiol. 1996;270:F700–F708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- Madkour FF, Abdel-Daim MM. Hepatoprotective and antioxidant activity of Dunaliella salina in paracetamol-induced acute toxicity in rats. Indian J Pharm Sci. 2013;75:642–648. [PMC free article] [PubMed] [Google Scholar]
- Marcussen N. Atubular glomeruli in cisplatin-induced chronic interstitial nephropathy. Exp Stereol Investig APMIS. 1990;98:1087–1097. doi: 10.1111/j.1699-0463.1990.tb05039.x. [DOI] [PubMed] [Google Scholar]
- Nagai T, Inoue R, Suzuki N, Nagashima T. Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food. 2006;9:363–367. doi: 10.1089/jmf.2006.9.363. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Tsuruma K, Shimazawa M, Mishima S, Hara H. Comparison of bee products based on assays of antioxidant capacities. BMC Complement Altern Med. 2009;9:4. doi: 10.1186/1472-6882-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Kumatori A, Harada T, Taguchi T. Coexpression of collagens and collagen-binding heat shock protein 47 in human diabetic nephropathy and IgA nephropathy. Nephron. 1998;80:434–443. doi: 10.1159/000045217. [DOI] [PubMed] [Google Scholar]
- Russ C. The image processing handbook. 2. Boca Raton, FL, USA: CRC Press; 1995. [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silici S, Ekmekcioglu O, Eraslan G, Demirtas A. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology. 2009;74:545–551. doi: 10.1016/j.urology.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Silici S, Ekmekcioglu O, Kanbur M, Deniz K. The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J Urol. 2011;29:127–132. doi: 10.1007/s00345-010-0543-5. [DOI] [PubMed] [Google Scholar]
- Swellam T, Miyanaga N, Onozawa M, Hattori K, Kawai K, Shimazui T, Akaza H. Antineoplastic activity of honey in an experimental bladder cancer implantation model: in vivo and in vitro studies. Int J Urol. 2003;10:213–219. doi: 10.1046/j.0919-8172.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–121. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Bevan EA, Miano L, Leonardi A. Defects in diagnostic kits for determination of urate in serum. Clin Chem. 1991;37:879–881. [PubMed] [Google Scholar]
- Yamate J, Machida Y, Ide M, Kuwamura M, Sawamoto O, LaMarre J. Effects of lipopolysaccharide on the appearance of macrophage populations and fibrogenesis in cisplatin-induced rat renal injury. Exp Toxicol Pathol. 2004;56:13–24. doi: 10.1016/j.etp.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Yamate J, Machida Y, Ide M, Kuwamura M, Kotani T, Sawamoto O, LaMarre J. Cisplatin-induced renal interstitial fibrosis in neonatal rats, developing as solitary nephron unit lesions. Toxicol Pathol. 2005;33:207–217. doi: 10.1080/01926230490523978. [DOI] [PubMed] [Google Scholar]
- Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res. 2006;12:5817–5825. doi: 10.1158/1078-0432.CCR-06-1037. [DOI] [PubMed] [Google Scholar]
- Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]