Abstract
Mycoplasmas are the most important contaminants of cell cultures throughout the world. They are considered as a major problem in biological studies and biopharmaceutical economic issues. In this study, our aim was to find the best standard technique as a rapid method with high sensitivity, specificity and accuracy for the detection of mycoplasma contamination in the cell lines of the National Cell Bank of Iran. Thirty cell lines suspected to mycoplasma contamination were evaluated by five different techniques including microbial culture, indirect DNA DAPI staining, enzymatic mycoalert® assay, conventional PCR and real-time PCR. Five mycoplasma-contaminated cell lines were assigned as positive controls and five mycoplasma-free cell lines as negative controls. The enzymatic method was performed using the mycoalert® mycoplasma detection kit. Real-time PCR technique was conducted by PromoKine diagnostic kits. In the conventional PCR method, mycoplasma genus-specific primers were designed to analyze the sequences based on a fixed and common region on 16S ribosomal RNA with PCR product size of 425 bp. Mycoplasma contamination was observed in 60, 56.66, 53.33, 46.66 and 33.33 % of 30 different cell cultures by real-time PCR, PCR, enzymatic mycoalert®, indirect DNA DAPI staining and microbial culture methods, respectively. The analysis of the results of the different methods showed that the real-time PCR assay was superior the other methods with the sensitivity, specificity, accuracy, predictive value of positive and negative results of 100 %. These values were 94.44, 100, 96.77, 100 and 92.85 % for the conventional PCR method, respectively. Therefore, this study showed that real-time PCR and PCR assays based on the common sequences in the 16S ribosomal RNA are reliable methods with high sensitivity, specificity and accuracy for detection of mycoplasma contamination in cell cultures and other biological products.
Keywords: Cell culture, Real-time PCR, DAPI staining, PCR, Mycoplasma contamination
Introduction
Since the first report of the presence of mycoplasma in cell cultures (mid-1950s), an integration of reported data including new species of mycoplasmas, their living situations, properties and reactions with cells in cell cultures have been investigated (Robinson et al. 1956). Mycoplasmas are of the most important contaminants of cell cultures worldwide. This contamination significantly affects the quality of biological products, biopharmaceutical and biotechnological studies (Armstrong et al. 2010; Baronti et al. 2013; Uphoff and Drexler 2014). Mycoplasmas are the smallest living organisms with a diameter of about 300–800 nm. They are assumed to be evolved through genomic reduction and reversible evolution from Lactobacillus, Streptococcus and Clostridium (Rottem and Barile 1993; Rottem et al. 2012). Mostly, mycoplasma genome is circular double-stranded DNA although some species with linear double-stranded DNA genome have been described. Phylogeny studies using 16S ribosomal RNA sequences analysis have shown that mycoplasmas are mainly related to Clostridium innocuum and Clostridium ramosum (Razin et al. 1998; Dandekar et al. 2002). More than 200 Mollicutes species classified in eight genera have been described. Most of them show saprophytic or commensal behavior in human, plants, animals, insects, etc. The presence of mycoplasmas in cell cultures, with or without discernable changes in color and turbidity of culture medium, influences cell growth, morphology, biochemical and immunological characteristics, genetic, metabolic and cellular physiology (Razin and Herrmann 2002). The lack of cell wall makes them more resistant to the common antibiotics such as penicillin and streptomycin. Mycoplasmas infect the cell cultures so easily and may not be visible and detectable by ordinary optical microscopic tools. Their flexibility, small-scale and polymorphism allows mycoplasmas to easily cross through anti-bacterial filters with a diameter of 0.45–0.22 µm (Folmsbee et al. 2010; Waites et al. 2013). Studies indicate that up to 87 % of cell lines in different cell banks are infected with mycoplasmas. From more than 200 species of known mollicutes, 20 of them have been isolated from infected cell cultures. It has been shown that at least eight species of mycoplasmas including M. arginini, M. fermentans, M. orale, M. hyorhinis, M. hominis, M. salivarium, M. pirum and Acholeplasma laidlawii are responsible for more than 95 % of cell lines contaminations (Nikfarjam and Farzaneh 2012; Barile and Rottem 1993). According to the published reports, mycoplasma contamination of cell cultures is commonly caused by only one strain. The infection with only one species ranges from 15 to 35 % worldwide, although, high infecting rates of 65–80 % have been reported. In general, between 7 and 62 % of cell cultures are contaminated with two or more mycoplasma species. Nevertheless, variations in the prevalence of mycoplasma contamination in cell cultures are observed in different parts of the world. Animal mycoplasma species such as M. arginini, M. hyorhinis and Acholeplasma laidlawii are the most important contaminating species in cell cultures. Using animal sera is the main source for such infection. Moreover, improper operation of laboratory staff such as pipetting through the mouth is the most important cause of contamination with human mycoplasma species including M. orale, M. fermentans, M. pirum, M. salivarium and M. hominis that comprise more than half of mycoplasma infections in cell cultures. It has been shown that M.orale is the most important contaminant species from human source which infects 20 to 40 % of cell cultures. Furthermore, infection percentage of M. hyorhinis from 10 to 40 %, M. arginini from 20 to 30 %, M. fermentans from 10 to 20 %, M. hominis from 10 to 20 % and A. laidlawii from 5 to 20 % have been announced by different researchers (Lincoln and Gabridge 1998; Drexler et al. 2002; Nikfarjam and Farzaneh 2012). Various techniques have been developed for the detection and identification of mycoplasma contaminations in cell cultures. These techniques include direct microbial culture and indirect (non-culture based) methods. The reliability, sensitivity, specificity, accuracy and cost-effectiveness are considered as determinant factors for the selection of the technique of choice. In this study, direct microbial culture (as a gold standard), enzymatic mycoalert® mycoplasma detection kit, indirect DNA DAPI staining, real-time PCR and PCR methods were utilized for the detection of mycoplasma contamination in the cell collections of the National Cell Bank of Iran. The sensitivity, specificity, accuracy, and predictive value of positive and negative results of these methods were compared in order to find the best method (McGarrity et al. 1983; Razin 1994; Harasawa et al. 2005; Volokhov et al. 2011; Uphoff and Drexler 2014).
Materials and methods
Cell cultures
Thirty different animal and human cell lines available in National Cell Bank of Iran were randomly selected and evaluated for mycoplasma contamination by microbial culture (as a gold standard), indirect DNA fluorochrome staining (DAPI, Roche, Cat No: 10236276001, Mannheim, Germany), enzymatic (mycoalert®, Lonza, Cat No: LT07-318, Switzerland), real-time PCR (PCR Mycoplasma Test Kit I/RT; Variant B, PromoKine, Cat No: PK-CA91-3025B, Germany) and conventional PCR methods. Ten different cell lines were examined and confirmed as positive (n = 5) and negative (n = 5) controls (Table 1). These cell lines were incubated at 37 °C in 88 % humidified and 5 % CO2 atmosphere. The culture medium for each cell line was prepared according to the recommended instructions and supplemented by 10–20 % fetal bovine serum (Sigma, Deisenhofen, Germany) and growth factors as described previously (Molla Kazemiha et al. 2011, 2014). In order to detect the mycoplasma contamination after harvesting, each cell line was cultured in an antibiotics-free medium for a week (at least 4–5 days) without exchanging the medium (Phelan 2006, 2007). All the cells were examined for the quality control of microbial cultures and to rule out the contamination with other microorganisms. Accordingly, Nutrient Broth (Sigma-Aldrich®, FLUKA, USA), Sabouraud Dextrose Broth (BD-Difco, Franklin Lakes, NJ, USA), Thioglycolate Broth (BD-BBL™, USA), Brain Heart Infusion Broth (BD-BBL™, USA), Trypticase Soy Broth (BD-BBL™, USA), Yeast Malt Broth (Sigma-Aldrich®, USA), Blood Agar (Sigma-Aldrich®, FLUKA, USA), Nutrient Agar (BD-Difco™, USA), MacConkey agar (Sigma-Aldrich®, FLUKA, USA), Sabouraud Dextrose Agar (BD-RODAC™, USA) and Brain Heart Infusion agar (BD-BBL™, USA) were used for investigation of microbial contaminants and quality control (Hay and Ikonomi 2005).
Table 1.
List of selected cell lines for checking of mycoplasma contamination in the present study
| No. | NCBI code | Cell lines | Properties | Culture medium |
|---|---|---|---|---|
| 1 | NCBI C511 | PEER | Mouse acute T cell lymphoblastic leukemia | RPMI 1640 + 10 % FBS |
| 2 | NCBI C532 | CT-26 | Mouse colon carcinoma | |
| 3 | NCBI C639 | RAW 264.7 | Mouse monocyte macrophage | |
| 4 | NCBI C552 | YAC1 | Mouse lymphoma | |
| 5 | NCBI C624 | NCCIT | Human teratocarcinoma cell line from anterior mediastinum | |
| 6 | NCBI C615 | MKN-45 | Human gastric adenocarcinoma | |
| 7 | NCBI C628 | MCF-HGH | Human mammary gland carcinoma | |
| 8 | NCBI C110 | B95.8 (negative control) | Marmoset-EBV transformed lymphocytes | |
| 9 | NCBI C142 | NSO (negative control) | Mouse myeloma | |
| 10 | NCBI C212 | Nalm6 (negative control) | Pre B cell leukemia | |
| 11 | NCBI C515 | NB4 (positive control) | Human acute promyelocytic leukemia | |
| 12 | NCBI C453 | Saos2 (positive control) | Human osteogenic sarcoma | |
| 13 | NCBI C593 | F25 | Cat erythroleukemia | DMEM + 10 % FBS |
| 14 | NCBI C566 | SKLC6 | Human lung carcinoma | |
| 15 | NCBI C143 | COS7 (positive control) | Monkey kidney SV40 transformed | |
| 16 | NCBI C114 | EL4 (positive control) | Mouse T cell lymphoma | |
| 17 | NCBI C617 | OLN-93 | Rat oligodendroglia | |
| 18 | NCBI C622 | BHY | Human oral squamous carcinoma | |
| 19 | NCBI C635 | HEK-Blue-Null-1 | Human engineered embryonic kidney | |
| 20 | NCBI C137 | A549 (negative control) | Human-lung-carcinoma | Ham’s F12 + DMEM (1:1) + 2 mM glutamine + 10 % FBS |
| 21 | NCBI C111 | CHO (negative control) | Chinese hamster ovary | |
| 22 | NCBI C620 | C28/I2 | Juvenile costal chondrocytes | |
| 23 | NCBI C523 | RK13 | Rabbit kidney cell line | EMEM (EBSS) + 2 mM glutamine +1 % NEAA + 10 % FBS |
| 24 | NCBI C608 | BFA | Bovine aorta endothelium foetal | Ham’s F12 + 2 mM glutamine + 20 % FBS |
| 25 | NCBI C610 | GH3/B6 | Rat pituitary tumor | Ham’s F12 + 15 % Horse serum + 2.5 % bovine calf serum |
| 26 | NCBI C602 | TF-1 | Human erythroleukemia | RPMI-1640, add the following components to the base medium: 2 ng/ml recombinant human GM-CSF + 10 % FBS |
| 27 | NCBI C570 | HCT-116 | Human colon carcinoma | McCoy’s 5a medium modified + 10 % FBS, Catalog No: 30-2007 (GibCo-BRL Grand Island, NY 14072 USA) |
| 28 | NCBI C609 | MCF10A | Human non-tumorigenic breast epithelial | The base medium is MEBM, which is supplied as part of the MEGM Bullet Kit available from Clonetics Corporation, Catalog No. CC-3150 (Lonza Group Ltd CH-4002 Basel Switzerland) |
| 29 | NCBI C606 | NB2-11 | RAT lymphoma | Fischer’s medium + 10 % FBS + 10 % Horse serum (gelding) + 0.075 % Na bicarbonate + 0.05 mM 2-mercaptoethanol (2ME) + 2 mM glutamine |
| 30 | NCBI C595 | Caov-4 | Human ovary adenocarcinoma | Leibovitz’s L-15 medium with 2 mM glutamine, 80 %; FBS, 20 % |
| 31 | NCBI C638 | SW872 | Human connective tissue-fibrosarcoma | Leibovitz’s L-15 medium, Catalog No. 30-2008 + 10 % FBS [American Type Culture Collection (ATCC) Manassas, VA 20110 USA] |
| 32 | NCBI C590 | CHO/dhfr− | Chinese hamster ovary (dihydrofolate reductase) | DMEM with 4 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate and supplemented with 0.1 mM hypoxanthine, 0.016 mM thymidine,0.002 mM Methotrexate (Amethopterin) + 10 % FBS (GibCo-BRL Grand Island, NY 14072 USA) |
| 33 | NCBI C555 | MG63 (positive control) | Human osteosarcoma | DMEM + 0.1 Mm NEAA + 1.0 mM SP + 10 % heat-inactivated FBS |
| 34 | NCBI C537 | STO | Mouse SIM fetal fibroblast (HAT sensitive, ouabain resistant) | DMEM + 2 mM glutamine +10 % FBS |
| 35 | NCBI C579 | P3U1 | Mouse myeloma | DMEM + 2 mM glutamine +10 % FBS |
| 36 | NCBI C625 | D-17 | Osteosarcoma metastatic to the lung in a dog (Canis familiaris) | Eagle’s minimum essential medium, Catalog No. 30-2003 + 10 % FBS [American Type Culture Collection (ATCC) Manassas, VA 20110 USA] |
| 37 | NCBI C623 | Densovirus free C6/36 Aedes albopictus | Densovirus free C6/36 Aedes albopictus (mosquito, Asian tiger) | |
| 38 | NCBI C627 | CF41.Mg | Mammary tumor from Dog (Canis familiaris) | Dulbecco’s modified Eagle’s medium, Catalog No. 30-2002 + 10 % FBS [American Type Culture Collection (ATCC) Manassas, VA 20110 USA] |
| 39 | NCBI C611 | SH-SY5Y | Human neuroblastoma | Eagle’s minimum essential medium, Catalog No. 30-2003, and F12 medium + 10 % FBS [American Type Culture Collection (ATCC) Manassas, VA 20110 USA] |
| 40 | NCBI C614 | BE(2)-M17 | Human Caucasian neuroblastoma |
NEAA non-essential amino acids, SP sodium pyruvate
Detection of mycoplasma contamination in cell lines by microbial culture
For microbial culture detection, the contaminated cells in their specific cell culture mediums (1 ml) were added to PPLO broth medium (BD Difco™, Cat No: 255420, USA) supplemented with horse serum (Gibco®, Paisley, United Kingdom, Cat No: 16050122, New Zealand), Yeast Extract Agar (Sigma-Aldrich®, Cat No: 01497 FLUKA, USA), d-glucose (Dextrose, Gibco®, Cat No: 15023-021, USA), l-arginine (Sigma-Aldrich®, Cat No: A3909, USA) and incubated for 72 h. Afterwards, monotonous turbidity was obtained by vigorous mixing of the PPLO medium and centrifuged for 10 min. The precipitate (100 μl) was transferred to a solid PPLO agar (BD Difco™, Cat No: 241210, USA) culture plate which was carefully sealed to prevent contamination and evaporation during incubation at 37 °C for 4–6 weeks. The non-typical colonies or egg formation of mycoplasma colonies were visualized by light microscopy every 3 days (Barile and McGarrity 1983; Rottem et al. 2012).
Detection of mycoplasma contamination by indirect DNA DAPI staining
Fluorescence DNA staining with DAPI (4′,6-diamidine-2′-phenyl indole dihydro chloride, Roche, Cat No: 10236276001, Mannheim, Germany) was performed based on the rapid uptake of the dye by cells and also by binding selectively to minor grooves of cell and mycoplasmal DNA. Therefore, DNA–DAPI complex allows efficient staining with very high specificity for the nuclei detection. The maximum absorption and emission of DAPI is reported at wavelengths of 340 and 488 nm, respectively. Mycoplasma bodies can be seen as polymorphic particles with bright blue fluorescence on a dark background cytoplasm. The DAPI staining protocols and work process for adherent cells are slightly different with floating cells. The fluorescent DAPI dye (Roche) was dissolved in water to make a 1 mg/ml stock. The working solution was freshly prepared by diluting the DAPI stock into 1 µg/ml with methanol. Adherent cells suspected to mycoplasma contamination with 70–90 % confluency in their monolayer culture were harvested with a cell scraper. The number of 5 × 104–1 × 105 cells/ml were seeded on cover slip slides (Thermo Scientific, USA) and incubated at 37 °C for several hours. The cells were rinsed by DAPI-methanol working solution after having discarded their supernatant and were incubated at 37 °C for 15 min. The cells were subsequently washed with pure methanol. Afterwards, adherent cells were placed on a microscope slide and mounted with buffered glycerol or phosphate buffered saline (PBS) followed by observation of mycoplasma bodies using a drop of immersion oil on it. Conversely, there is no need to use cover slip and scraper for DAPI staining protocols of floating cells (Schaper and Converse 1985; Andrade and Arismendi 2013). Due to the limitations related to the direct analysis of cell lines by DAPI staining, mycoplasma-free Vero cell line (NCBI C101) was employed as an indicator as well as negative control. Supernatant of cell cultures suspected to have mycoplasma contamination was added to a mycoplasma-free Vero cell line in order to increase the sensitivity and specificity of the assay.
Mycoalert® mycoplasma detection kit for mycoplasma contamination in cell lines
The enzymatic mycoalert® (Lonza, Cat No: LT07-318, Switzerland) as a biochemical test (bioluminescent reaction) indicates the amount of mycoplasma enzymes activities. A rapid screening method has been provided by these enzymes for sensitive detection of mycoplasma contamination in cell cultures (Pitt et al. 2012). In the present study, mycoplasma detection was conducted by measuring the acetate kinase or carbamate kinase activity as described previously (Mariotti et al. 2008; Cheong et al. 2011; Molla Kazemiha et al. 2014).
Mycoplasma detection using conventional PCR method
Extraction of DNA from suspicious samples was performed for PCR with specific universal primers. The cells (1 × 105) were harvested at the final logarithmic growth phase. Centrifugation at 12,000g for 1 min and subsequently re-suspension of the cells in STE buffer (NaCl 10 mM, Tris–HCl 20 mM and EDTA 1 mM, pH 8.0) was conducted (Tang et al. 2000). Afterwards, the cell suspension was incubated with Sodium Dodecyl Sulfate (SDS, 1 % w/v) and proteinase K (40 μg/ml, Promega, Madison, WI, USA) for 2 h at 37 °C. The same volume of phenol–chloroform–isoamyl alcohol (1:24:25) solution was added to an equal volume of the suspended cells to precipitate the proteins. Sodium acetate (3 M, pH 5.2) and ethanol (96 %) with 1:25 ratio was added to precipitate the total nucleic acid. The cell pellet was rinsed with 70 % ethanol and dried for 30 min at 25 °C. Finally, the suspension of precipitated DNA was made in RNA–DNA free sterile deionized water and maintained at −20 °C (Molla Kazemiha et al. 2011, 2014).
Positive and negative controls for PCR technique
The DNA of different bacterial and mollicutes strains were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and the National Collection of Type Cultures (NCTC, Salisbury, UK). Mycoplasma genitalium (NCTC 10195), Mycoplasma salivarium (NCTC 10113), Ureaplasma urealiticum (NCTC 10177T), Mycoplasma pneumonia (NCTC 10119), Acholeplasma laidlawii (ATCC 23206), Mycoplasma fermentans (ATCC 19989), Mycoplasma hyorhinis (ATCC 17981), Mycoplasma hominis (ATCC 23114), Mycoplasma orale (ATCC 23714), Mycoplasma arginini (ATCC 23828) and Mycoplasma pirum (NCTC 11702) were utilized as positive controls for genus specific PCR. In addition, genomic DNA from gram positive and negative bacteria like Staphylococcus saprophyticus (ATCC 15305), Proteus vulgaris (ATCC 29905), Bacillus cereus (ATCC 14579) and Klebsiella oxytoca (ATCC 8724) were considered as negative controls to confirm the non-cross reactivity of mycoplasma universal primers.
Oligonucleotides and specific PCR for mycoplasma detection in cell lines
In addition to 11 species-specific primer pairs, a universal primer pair was designed based on 16S rRNA mollicutes to detect the mycoplasma contamination of cell lines with PCR method which is indicated in Table 2 (Molla Kazemiha et al. 2009, 2011, 2014). Master mix of PCR was prepared by 2.5 μl PCR 10 × Buffer, 1 μl Taq DNA polymerase enzyme (1 U), 1 μl dNTPs (50 μM), 1 μl magnesium chloride (1.5 mmol), 3 μl forward and reverse primers (15 pmol), 12.5 μl distilled water and 1 μl mycoplasma genomic DNA (0.1 μg/μl) or cell DNA (1 μg/μl). The temperature of the mixture was elevated to 94 °C and kept for 3 min. Afterwards, 32 cycles of amplification including 94 °C for 60 s, 60 °C for 30 s, 72 °C for 1 min were applied to the mixture. The temperatures for species-specific and universal primers were set at 60 and 55 °C, respectively. The PCR products were separated by agarose gel (1 % w/v) electrophoresis and visualized on a UV transilluminator.
Table 2.
The sequences of oligonucleotide primers used for detection of mycoplasmas
| Mycoplasma species | Primer sequence | Amplicon size (bp) | GC % | TM |
|---|---|---|---|---|
| Universal primer | S: GTG GGG AGG AAA YAG GAT TAG A AS: GGC ATG ATG ATT TGA CGT CRT |
425 | 45–50 45–48 |
53–54.8 50.5–52.4 |
| M. arginini | S: TGA TCA TTA GTC GGT GGA GAG TTC AS: TAT CTC TAG AGT CCT CGA CAT GAC TC |
326 | 55.7 58 |
46 46 |
| M. orale | S: TGA TCA TTA GTC GGT GGA AAA CTA AS: TAT CTC TAG AGT CCT CGA CAT GAC TC |
325 | 52.3 58 |
38 46 |
| M. hyorhinis | S: CGA TGA TCA TTA GTT GGT GGA ATA AAT AS: AGG CAG TAT CTC TAG AGT CCT TAA CTT A |
334 | 53.7 57 |
33 39 |
| M. fermentans | S: TGA TCA TTA GCT GAT GGG GAA CT AS: TCT CTT AGA GTC CTC AAC TAA ATG |
324 | 53.5 52.3 |
43 38 |
| M. genitalium | S: ATA GAT ACT AGC TGT CGG AGC GAT AS: CCA ATT TAC ATT AGC AGT CTC GTT AA |
335 | 55.7 53.2 |
46 35 |
| A. laidlawii | S: GAT GAG AAC TAA GTG TTG GCC ATA A AS: CGC TAG AGT CCC CAA CTT AAT GA |
300 | 54.4 55.3 |
40 48 |
| M. hominis | S: ATC ATT AGT CGG TGG AGA ATC A AS: GCA GTA TCT CTA CTA GAG TCC TCA ACT TAAT |
301 | 55.1 59.1 |
41 39 |
| M. pirum | S: TGG ATG TTA GAT GTC GGG GTA AA AS: GTT GGC AGT ATC GCT AGA CAA A |
324 | 53.5 56.7 |
43 41 |
| M. pneumoniae | S: GAT ACT AGC TGT CGG GGC GAT AS: AAT TTG CAT TAG TAG CAG TCT CGC TAG |
329 | 56.3 56.7 |
57 41 |
| M. salivarium | S: GAT CAT TAG TCG GCA GAG AAC TCG AS: TAT CTC TAG AGT CCT CGA CAT GAC TC |
324 | 57.4 58 |
50 46 |
| U. urealyticum | S: CAT CAT TAA ATG TCG GCT CGA A AS: CGG TAG CAG TAT CGC TAG AAA AGC |
323 | 51.1 57.4 |
41 50 |
TM melting temperature
Real-time PCR for the detection of mycoplasma contamination in cell lines
The real-time PCR master mix was prepared by adding internal amplification (inhibition) control, hot start Taq DNA polymerase, primers, and probes (PCR Mycoplasma Test Kit I/RT; Variant B, PromoKine, Cat No: PK-CA91-3025B, Heidelberg, Germany). This kit can detect as low as 15 fg of mycoplasma DNA which corresponds to approximately 10–15 mycoplasmas per sample volume. According to the manufacturer’s instruction, PCR reactions at the total volume of 26.0 μl consisted of 8.0 μl DNA-free water, 14.0 μl of primers and probes, 1.0 μl inhibition control DNA, 1.0 μl DNA polymerase, and 2.0 μl sample (as a template DNA). Thermal cycle setting for StepOne Plus™ real-time PCR System was as follows: initial polymerase activation at 95 °C for 2 min and 45 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 60 °C for 45 s. Mycoplasma contaminations were analyzed by the assessment of the increase in fluorescence signal during the real-time PCR test in FAM channel. The inhibition internal control amplification was indicated in VIC channel (Espy et al. 2006; Volokhov et al. 2011).
Statistical evaluation
The data analysis was done using IBM SPSS statistical software version 22. P values less than 0.05 (P value <0.05) were considered statistically significant. Descriptive statistics (i.e. mean, standard deviation, SD of the mean, confidence interval 95 %, frequencies and percentages) was used to summarize the quantitative variables. Data were analyzed by t test for comparing two tests and Friedman Test for comparing five tests. Sensitivity, specificity, accuracy and predictive value of positive and negative results were calculated using microbial culture as the gold standard (Galen and Gambino 1975; Hopert et al. 1993; Mariotti et al. 2008). Sensitivity: true positives/(true positives + false negatives); Specificity: true negatives/(true negatives + false positives); Predictive value of a positive result and of a negative result: true positives/(true positives + false positives) and true negatives/(true negatives + false negatives), respectively; Accuracy: (true positives + true negatives)/total number of cases.
Results
In this study, 30 different human and animal cell lines were randomly selected and assessed for mycoplasma contamination using microbial culture as a reference standard, indirect DNA DAPI staining, enzymatic mycoalert®, PCR and real-time PCR methods. Meanwhile, five cell lines were considered and confirmed as a positive control and five cell lines as a negative control (Table 1). Furthermore, Vero cell line infected with mycoplasma and Vero cell line free of mycoplasma infection (NCBI C101) were evaluated by these methods and considered as positive and negative controls, respectively (Fig. 1a–d). Three different strains including M. hyorhinis, M. arginini and M. fermentans were detected in the positive control (Vero cell line infected with mycoplasma) by species-specific PCR primers (Fig. 2). Microbial culture, DAPI staining, enzymatic mycoalert®, PCR and real-time PCR tests showed 33.33 % (10 cases), 46.66 % (14 cases), 53.33 % (16 cases), 56.66 % (17 cases) and 60 % (18 cases) infection among the 30 studied cell lines, respectively. Indeed, microbial culture (Gold Standard), DAPI staining, enzymatic mycoalert®, PCR and real-time PCR tests indicated the number of false negative 8, 7, 2, 1 and 0, respectively. There were no false positive cases observed except in the indirect DNA DAPI staining with three cases (Table 3). Comparison of cell lines with enzymatic mycoalert® and microbial culture methods showed that 22 cell culture samples (13 negative cases and 9 positive cases) perfectly match together (concordance) and 8 cell cultures (8 false negative cases) were non-compliant (discordance). By contrast, indirect DNA DAPI staining with microbial culture revealed the concordance in 22 cell cultures (14 negative cases and 8 positive cases) and discordance in 8 cell cultures (5 false negative cases and 3 false positive cases). Conversely, PCR results and standard microbial culture exhibited concordance in 23 cell culture samples (13 negative cases and 10 positive cases) and discordance in 7 cell lines (7 false negative cases).
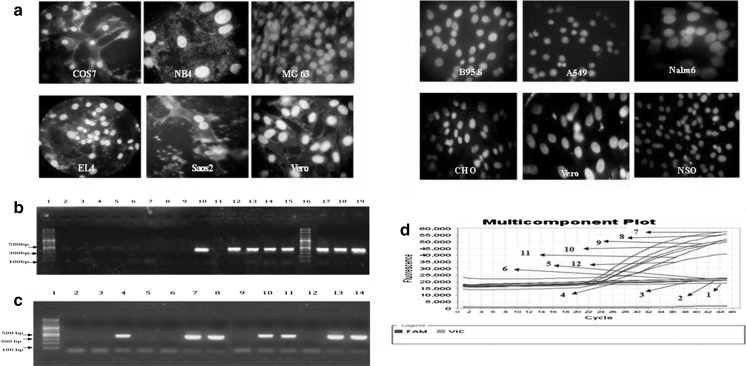
Fig. 1.
a Indirect DNA DAPI staining of COS7, NB4, MG63, EL4, Saos2 and Vero cell lines infected with mycoplasma in the left figure (positive controls) and B95.8, A549, Nalm6, CHO, Vero and NSO cell lines free of mycoplasma contamination in the right figure (negative controls). b PCR gel electrophoresis results for detection of mycoplasma contamination in five positive control cell lines, five negative control cell lines and Vero cell line of positive and negative control and other cell lines. Lane 1 DNA Size marker (100 bp DNA Ladder, Roche XIV), lane 2 negative control (DNA-free water), lane 3 A549 (negative control), lane 4 B95.8 (negative control), lane 5 NSO (negative control), lane 6 CHO (negative control), lane 7 Nalm6 (negative control), lane 8 Vero (negative control), lane 9 Densovirus free C6/36 (negative), lane 10 Vero (positive control), lane 11 Vero (negative control), lane 12 MG63 (positive control), lane 13 EL4 (positive control), lane 14 NB4 (positive control), lane 15 Saos2 (positive control), lane 16 DNA Size marker (100 bp DNA Ladder, Roche XIV), lane 17 COS7 (positive control), lane 18 NCCIT (positive), lane 19 Vero (positive control). c PCR gel electrophoresis results for detection of mycoplasma contamination in Vero (positive control), NSO (negative control) and other cell lines. Lane 1 DNA Size marker (100 bp DNA Ladder, Roche XIV), lane 2 NSO (negative control), lane 3 NB2-11 (negative), lane 4 BFA (positive), lane 5 F25 (negative), lane 6 YAC1 (negative), lane 7 OLN-93 (positive), lane 8 MCF-HGH (positive), lane 9 TF1 (negative), lane 10 C28/I2 (positive), lane 11 SH-SY5Y (positive), lane 12 SKLC6 (negative), lane 13 D17 (positive), lane 14 Vero (positive control). d Analysis real-time PCR results of positive and negative control cell lines based on the linear amplification plot and reporter dyes (FAM Target Probe and VIC Internal Control) and Quencher (none). 1-negative control (DNA-free water), 2-NSO (negative control), 3-B95.8 (negative control), 4-A549 (negative control), 5-CHO (negative control), 6-Nalm6 (negative control), 7-MG63 (positive control), 8-COS7 (positive control), 9-NB4 (positive control), 10-EL4 (positive control), 11-Saos2 (positive control), 12-Positive control kit
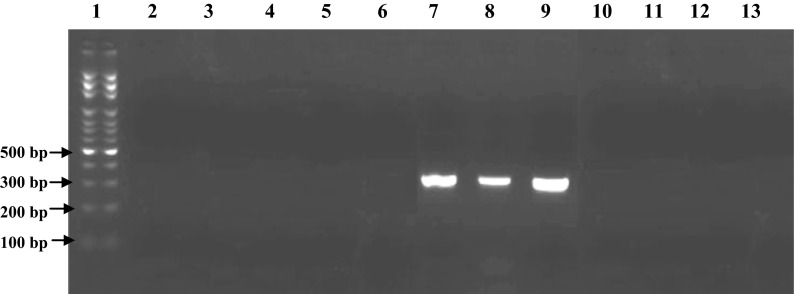
Fig. 2.
PCR gel electrophoresis results for detection of mycoplasma contamination with three different mycoplasma species in Vero cell line as positive control with mycoplasma species-specific primers. Lane 1 DNA Size marker (100 bp DNA Ladder, Roche XIV), lane 2 M. hominis-specific primer (negative), lane 3 M. pirum-specific primer (negative), lane 4 M. pneumoniae-specific primer (negative), lane 5 A. laidlawii-specific primer (negative), lane 6 M. salivarium-specific primer (negative), lane 7 M. hyorhinis-specific primer (positive with Amplicone size 334 bp), lane 8 M. arginini-specific primer (positive with Amplicone size 326 bp), lane 9 M. fermentans-specific primer (positive with Amplicone size 324 bp), lane 10 M. orale-specific primer (negative), lane 11 M. genitalium-specific primer (negative), lane 12 U. urealyticum-specific primer (negative), lane 13 DNA-free water (negative control)
Table 3.
The evaluation results of 30 cell lines for the detection of mycoplasma contamination by five different methods (microbial culture, DAPI staining, enzymatic mycoalert® ,method, molecular PCR and real-time PCR assays)
| No. | NCBI code | Cell lines | Test results | ||||
|---|---|---|---|---|---|---|---|
| Microbial culture | Mycoalert® | DAPI staining | PCR | Real time PCR | |||
| 1 | NCBI C635 | HEK-Blue-Null-1 | Negative | Negative | Negative | Negative | Negative |
| 2 | NCBI C579 | P3U1 | Negative | Negative | Positive (false-positive) | Negative | Negative |
| 3 | NCBI C606 | NB2-11 | Negative | Negative | Positive (false-positive) | Negative | Negative |
| 4 | NCBI C623 | Densovirus free C6/36 Aedes albopictus | Negative | Negative | Negative | Negative | Negative |
| 5 | NCBI C608 | BFA | Positive | Positive | Positive | Positive | Positive |
| 6 | NCBI C639 | RAW 264.7 | Negative (false-negative) | Positive | Positive | Positive | Positive |
| 7 | NCBI C638 | SW872 | Positive | Positive | Positive | Positive | Positive |
| 8 | NCBI C624 | NCCIT | Positive | Positive | Positive | Positive | Positive |
| 9 | NCBI C625 | D-17 | Negative (false-negative) | Positive | Positive | Positive | Positive |
| 10 | NCBI C615 | MKN-45 | Positive | Positive | Positive | Positive | Positive |
| 11 | NCBI C570 | HCT-116 | Negative | Negative | Negative | Negative | Negative |
| 12 | NCBI C609 | MCF10A | Negative | Negative | Negative | Negative | Negative |
| 13 | NCBI C627 | CF41.Mg | Negative (false-negative) | Positive (weak positive) | Negative (false-negative) | Negative (false-negative) | Positive |
| 14 | NCBI C532 | CT-26 | Negative (false-negative) | Positive (weak positive) | Positive | Positive | Positive |
| 15 | NCBI C566 | SKLC6 | Negative | Negative | Negative | Negative | Negative |
| 16 | NCBI C622 | BHY | Positive | Positive | Positive | Positive | Positive |
| 17 | NCBI C595 | Caov-4 | Positive | Positive | Negative (false-negative) | Positive | Positive |
| 18 | NCBI C537 | STO | Negative (false-negative) | Positive | Negative (false-negative) | Positive | Positive |
| 19 | NCBI C511 | PEER | Positive | Negative (false-negative) | positive | Positive | Positive |
| 20 | NCBI C610 | GH3/B6 | Positive | positive | positive | Positive | Positive |
| 21 | NCBI C593 | F25 | Negative | Negative | Positive (false-positive) | Negative | Negative |
| 22 | NCBI C590 | CHO/dhfr− | Negative | Negative | Negative | Negative | Negative |
| 23 | NCBI C552 | YAC1 | Negative | Negative | Negative | Negative | Negative |
| 24 | NCBI C523 | RK13 | Negative | Negative | Negative | Negative | Negative |
| 25 | NCBI C602 | TF-1 | Negative | Negative | Negative | Negative | Negative |
| 26 | NCBI C611 | SH-SY5Y | Negative (false-negative) | Negative (false-negative) | Negative (false-negative) | Positive | Positive |
| 27 | NCBI C614 | BE(2)-M17 | Negative (false-negative) | Positive | Negative (false-negative) | Positive | Positive |
| 28 | NCBI C617 | OLN-93 | Negative (false-negative) | Positive | Negative (false-negative) | Positive | Positive |
| 29 | NCBI C628 | MCF-HGH | Positive | Positive | positive | Positive | Positive |
| 30 | NCBI C620 | C28/I2 | Positive | Positive | Negative (false-negative) | Positive | Positive |
Moreover, real-time PCR results were compared to the reference microbial culture method and demonstrated concordance in 22 cell culture samples (12 negative cases and 10 positive cases) and discordance in 8 cell cultures (8 false negative cases).
Limit of detection (LOD) of mycoalert® test has been indicated to be 50 cfu/ml for A. laidlawii, mycoplasma hyorhinis and mycoplasma orale. In this study, serial dilutions (1/2–1/4096) were prepared from Vero cell line (NCBI C101) infected with mycoplasma, the kit positive control and also mycoplasma hyorhinis strain. LOD of 1/256 dilution was observed for the three samples (Table 4). To ensure that the cell lines were not contaminated by other positive and negative gram bacteria samples taken from the cell line cultures were transferred to various microbial culture media. No bacterial growth was detected for these cultures (data not shown), which confirmed the specificity of mycoalert® results. In the PCR experiments, the universal pair primers could amplify a 425 bp product from the DNA of all mollicutes strains. This was confirmed by the sequencing of randomly selected PCR products obtained from different amplification experiments. The results of the sequencing showed the consistency of the PCR product sequence to the reference mollicutes 16S rRNA conserved sequence at Gene Bank data base (Fig. 3b). Furthermore, there was no cross-reaction with rat DNA, human DNA, mouse DNA as well as prokaryotes and bacteria DNA such as Staphylococcus saprophyticus, Proteus vulgaris, Bacillus cereus and Klebsiella oxytoca (data not shown). In order to examine the sensitivity of the universal primers, different serial dilutions of the DNA extracted from Mycoplasma hyorhinis (30 ng) were prepared. The LOD of the amplified product showed the dilution of 10−3 (30 pg of DNA) (Fig. 3a). Whereas, the sensitivity (LOD) of PromoKine’s PCR Mycoplasma Test Kit I/RT assay with specific primers and probes (Promokine diagnostic kits) was up to 10−4 (3 pg) by using serial dilutions of template DNA from M. hyorhinis culture. These results showed the high sensitivity, specificity and accuracy of the real-time PCR method in comparison with conventional PCR and the other direct and indirect mycoplasma detection methods (Fig. 4a, b).
Table 4.
LOD (limit of detection) evaluation of Vero cell line infected with mycoplasma (positive control cell line)
| Vero cell line mycoplasma positive | Reading A | Reading B | B/A | Test results |
|---|---|---|---|---|
| Undiluted positive control Vero cell line | 9/514 | 267/860 | 28/154 | Positive > 1 |
| Dilution 1/2 | 13/673 | 132/044 | 9/657 | Positive > 1 |
| Dilution 1/4 | 17/407 | 95/361 | 5/478 | Positive > 1 |
| Dilution 1/8 | 11/005 | 74//011 | 6/725 | Positive > 1 |
| Dilution 1/16 | 7/262 | 48/519 | 6/681 | Positive > 1 |
| Dilution 1/32 | 5/025 | 15/477 | 3/080 | Positive > 1 |
| Dilution 1/64 | 5/555 | 14/522 | 2/614 | Positive > 1 |
| Dilution 1/128 | 3/660 | 7/194 | 1/956 | Positive > 1 |
| Dilution 1/256 | 3/119 | 4/375 | 1/402 | Positive > 1 |
| Dilution 1/512 | 3/390 | 2/685 | 0/792 | Negative < 1 |
| Dilution 1/1024 | 4/686 | 2/121 | 0/452 | Negative < 1 |
| Dilution 1/2048 | 2/279 | 0/931 | 0/408 | Negative < 1 |
| Dilution 1/4096 | 1/892 | 0/682 | 0/360 | Negative < 1 |
| Control positive kit | 6/051 | 216/435 | 35/768 | Positive > 1 |
| Control negative kit | 3/075 | 0/459 | 0/149 | Negative < 1 |
| Vero cell line mycoplasma free (negative) | 2/235 | 0/585 | 0/261 | Negative < 1 |
Different serial dilutions in the range of 1/2 to 1/4,096 were prepared
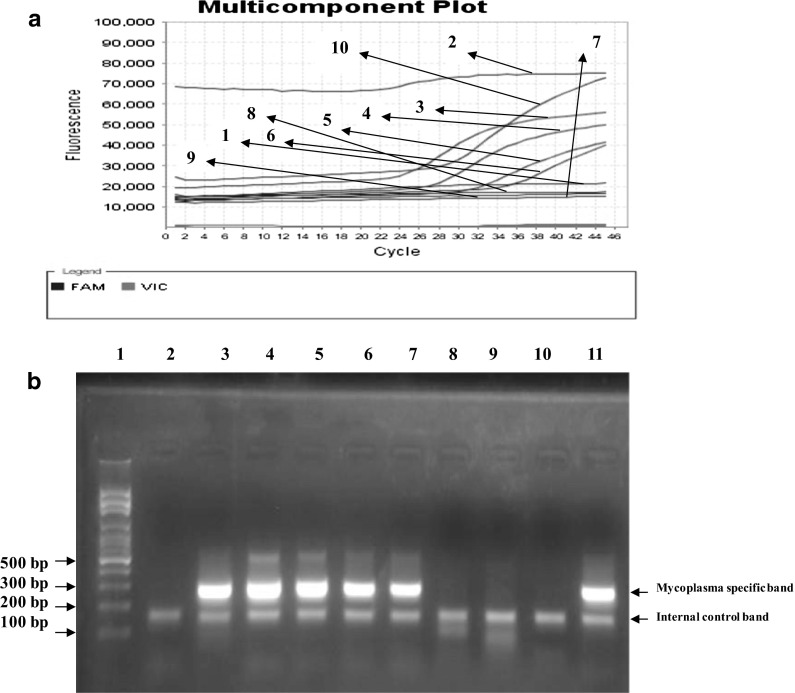
Fig. 3.
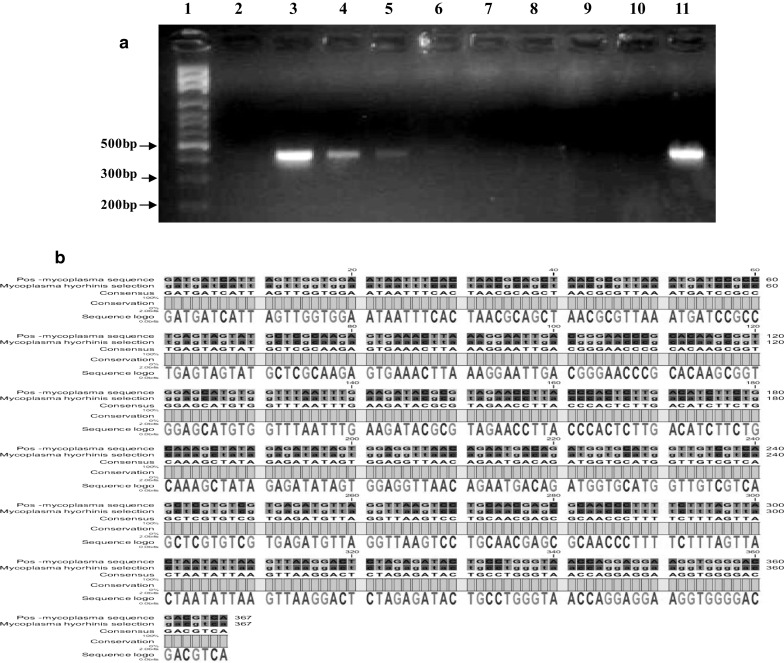
a Determination of the limit of detection for the PCR assay. Gel electrophoresis results of optimized PCR assay performed with universal primers on different serial dilutions of DNA obtained from a culture of M. hyorhinis. Lane 1 DNA Size marker (100 bp DNA Ladder, Roche XIV), lane 2 DNA-free water (negative control), lane 3 3 ng (positive), lane 4 300 pg (positive), lane 5 30 pg (positive), lane 6 3 pg (negative), lane 7 300 fg (negative), lane 8 30 fg (negative), lane 9 3 fg (negative), lane 10 300 atg (negative), lane 11 Undiluted-DNA strain of M. hyorhinis as a positive control (30 ng). b The alignment of the sequence of PCR product obtained by the amplification of M. hyorhinis 16S rRNA with the corresponding reference sequence at the NCBI Gene Bank data base. This comparison confirmed that the universal primers used in the study specifically amplified the desired fragment of a conserved sequence in all mycoplsma species
Fig. 4.
a The results of real-time PCR assay using PromoKine’s PCR Mycoplasma Test Kit I/RT performed with specific primers and probes (Promokine diagnostic kit) on different serial dilutions of DNA obtained from a culture of M. hyorhinis Amplification curve 1 negative control (DNA-free water), 2 Undiluted-DNA strain of M. hyorhinis (30 ng, positive), 3 3 ng (positive), 4 300 pg (positive), 5 30 pg (positive), 6 3 pg (positive), 7 300 fg (negative), 8 30 fg (negative), 9 3 fg (negative), 10 positive control kit. b Gel electrophoresis results of the real-time PCR assay products. Lane 1 DNA Size marker (100 bp DNA Ladder, Roche XIV), Lane 2 negative control (DNA-free water), lane 3 Undiluted-DNA strain of M. hyorhinis (30 ng, positive), lane 4 3 ng (positive), lane 5 300 pg (positive), lane 6 30 pg (positive), lane 7 3 pg (positive), lane 8 300 fg (negative), lane 9 30 fg (negative), lane 10 3 fg (negative), lane 11. The kit positive control. FAM Fluorescein amidite (6-carboxyfluoresceine), VIC a proprietary fluorescent dye produced by Applied Biosystems. Detection of target amplification was performed in FAM channel whereas monitoring of inhibition control amplification in VIC channel.
Statistical results
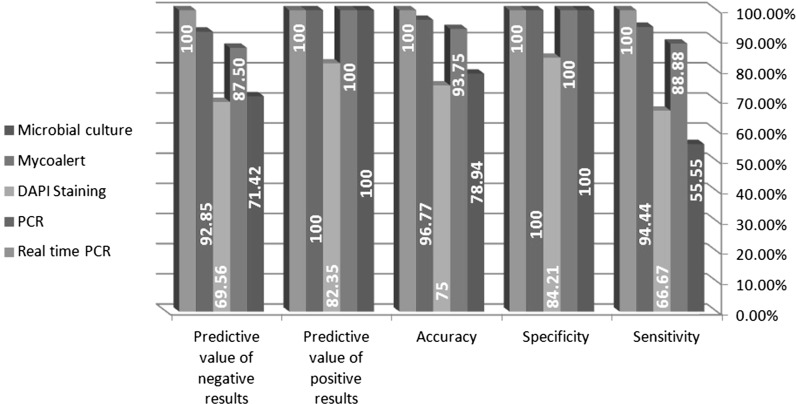
The specificity of all the methods was 100 % with the exception of the indirect DNA DAPI staining (84.21 %). The sensitivity of real-time PCR, PCR, enzymatic mycoalert®, DAPI staining, microbial culture methods was 100, 94.44, 88.88, 66.67 and 55.55 %, respectively. In addition, the highest (100 %) and lowest (75 %) accuracy was measured for real-time PCR and DAPI staining methods, respectively. The predictive value of positive results was determined as 100 % for all the methods other than DAPI staining (82.35 %), while the predictive value of negative results was 100, 92.85, 87.50, 71.42 and 69.56 % for real-time PCR, PCR, enzymatic mycoalert®, microbial culture and DAPI staining techniques, respectively (Fig. 5). Table 5 shows the performance of the methods by comparing their statistical data using standard t test analysis. The differences between DAPI staining and microbial culture methods, PCR and enzymatic mycoalert®, real-time PCR and mycoalert® and also real-time PCR and PCR methods were not statistically significant (P value >0.05). On the other hand, the comparison of enzymatic mycoalert® and microbial culture methods, PCR and microbial culture, real-time PCR and microbial culture, mycoalert® and DAPI staining, PCR and DAPI staining and also real-time PCR and DAPI staining methods showed statistically significant differences with P values <0.05. According to the ranking method using Friedman test, the importance of the variable is the highest at the lowest average ranking. The average ranking for real-time PCR, PCR, enzymatic mycoalert®, microbial culture and DAPI staining was calculated as 2.63, 2.72, 2.80, 3.30 and 3.55, respectively. Moreover, the P value in Friedman test was also significant (P value 0.000). Therefore, the similarities in the ranking of these five methods were discarded. These five methods were ranked as real-time PCR, PCR, enzymatic mycoalert®, microbial culture and DAPI staining, respectively.
Fig. 5.
Comparison of the statistical parameters related to each method. The specificity, sensitivity, accuracy, predictive value of positive and negative results for all five methods were calculated
Table 5.
Pairwise multiple comparison test procedures (t-test)
| Type of testing | t-statistic | Mean | Standard deviation (SD) | SD of the mean | P value | Confidence interval 95 % | |
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Microbial culture-Mycoalert® | 2.262 | 0.200 | 0.48423 | 0.08841 | 0.031 | 0.01918 | 0.38082 |
| Microbial culture-DAPI staining | −1.608 | −0.300 | 1.02217 | 0.18662 | 0.119 | −0.0681 | 0.081 |
| Microbial culture-PCR | 2.971 | 0.2333 | 0.43018 | 0.07854 | 0.006 | 0.072 | 0.393 |
| Microbial culture-real-time PCR | 3.247 | 0.26667 | 0.44978 | 0.08212 | 0.003 | 0.098 | 0.434 |
| Mycoalert®-DAPI staining | −2.812 | −0.500 | 0.97379 | 0.17779 | 0.009 | −0.8636 | −0.1363 |
| Mycoalert®-PCR | 0.571 | 0.03333 | 0.31984 | 0.05839 | 0.573 | −0.8610 | 0.15276 |
| Mycoalert®-real-time PCR | 1.439 | 0.06667 | 0.25371 | 0.04632 | 0.161 | −0.028 | 0.16140 |
| DAPI stainig-PCR | 3.117 | 0.53333 | 0.93710 | 0.17109 | 0.004 | 0.183 | 0.883 |
| DAPI stainig-real-time PCR | 3.319 | 0.56667 | 0.93526 | 0.17075 | 0.002 | 0.217 | 0.915 |
| PCR-real-time PCR | 1 | 0.03333 | 0.18257 | 0.03333 | 0.326 | 0.034 | 0.101 |
Discussion
Mycoplasmas are common and inevitable causes of cell cultures contamination, which is considered as a major problem in biological studies and pharmaceutical processes. Mycoplasma can affect several parameters in cell cultures including biochemical, genetic and immunological changes. Therefore, screening for mycoplasma infection of cell cultures must be performed before their use for research and pharmaceutical purposes. An ideal method for mycoplasma detection should be highly sensitive, specific, rapid, easily interpretable, and cost-effective (Dallo and Baseman 2000; Drexler et al. 2002; Young et al. 2010; Uphoff and Drexler 2013). Direct microbial culture, as the standard reference technique, is frequently applied as a sensitive and reliable method for the detection of mycoplasma in cell cultures. Slow rate of mycoplasma growth and difficulties in the interpretation of the results are considered as the disadvantages of microbial cultivation (Volokhov et al. 2011). In our study, there were false negative results for 8 cases (26.66 %) using this method. However, for more accurate quality control, the results of microbial cultures must be confirmed by an alternative method such as DAPI fluorochrome or Hoechst 33258 staining. Although, the staining procedure is relatively quick and easy to perform, it is sometimes difficult to interpret the results. This difficulty arises from the presence of contaminating bacteria or nucleic acid decomposition. In our study, 7 false negative cases (23.33 %) and 3 false positive cases (10 %) were observed (Jung et al. 2003; Uphoff and Drexler 2013). In contrast, the sensitivity, accuracy, specificity and rapidity (<20 min) are the main advantages of enzymatic test (mycoalert® kit). In comparison with molecular methods, detection of enzymatically-active viable mycoplasma germs is a highly remarkable feature of the enzymatic test, particularly for antibiotic treatment purposes. The main disadvantage of this method is false negative results due to the impaired function of the enzymes. The quality, quantity and stability of acetate kinases and carbamate kinases must be at appropriate level for the detection by a luminometer. In addition, this method is unable to discriminate different genus and species of ureaplasmas such as Ureaplasma urealyticum, Ureaplasma parvum as well as specific mycoplasma species. In this study, the false negative results for enzymatic method were observed in only two cell lines i.e., PEER (NCBI C511) and SH–SY5Y (NCBI C611). In the other 28 cases, the mycoalert® results were concordant with the real-time PCR results (Mariotti et al. 2008; Markoullis et al. 2009; Volokhov et al. 2011; Molla Kazemiha et al. 2014).
PCR-based technique has been widely used for the detection of mycoplasma infections in cell cultures. The methods which are based on nucleic acids detection are rapid, reliable, cost-effective, sequence-specific and highly sensitive for the detection of DNA and RNA in complex biological samples. However, PCR techniques which are based on the amplification of target DNA sequences are incapable to distinguish between viable and non-viable mycoplasma cells. Moreover, under special circumstances such as antibiotic treatment procedures and contamination of cell culture medium components NBS, Trypsin, PBS, etc.), the Nested PCR method is more sensitive than direct PCR while more likely to give false positive results (Sung et al. 2006; Lawrence et al. 2010; Dabrazhynetskaya et al. 2014). We developed a single-step PCR technique which was able to identify at least 30 pg DNA of M. hyorhinis species and showed no cross-reactivity with genomic DNA of other organisms including human, mouse, Saccharomyces cerevisiae, gram-positive and gram-negative bacteria (data not shown). The ability to monitor the amplification reaction and allowing quick analysis of multiple genomic targets simultaneously with high throughput capability are the advantages of the real-time PCR method. In our study, the LOD of the real-time PCR assay was the serial dilution of 10−4 (equivalent to 3 pg of M. hyorhinis DNA). This indicates the higher sensitivity of the real-time PCR technique in comparison with the other direct and indirect methods for mycoplasma detection in cell cultures. Detection of mycoplasma contamination by specific PCR of 16S ribosomal RNA genomic region in cell cultures was compared to microbial culture, DNA fluorochrome staining and DNA-RNA Hybridization by Van Kuppeveld et al. (1994). Overall, the sensitivity, specificity and accuracy of the PCR method was superior to the other assays (Van Kuppeveld et al. 1994). In another study by Tang et al. (2000), a Nested PCR assay with combination of specific primers for conserved regions of 16S and 23S ribosomal RNA genes were designed. The sensitivity of the assay was in the range of 20–180 cfu/ml. No cross-reactivity with rats DNA, humans DNA and other bacteria DNA was observed. Ishikawa et al. (2006) used three pairs of primers based on the genomic region of 23S ribosomal RNA to develop a real-time PCR assay with LOD of 100 fg genomic DNA. They confirmed the higher accuracy of the real-time PCR method for the detection of eight major species infecting cell cultures in comparison with fluorochrome DAPI staining method. In contrast, Kumar et al. (2008) concluded that simultaneous use of two cytological staining techniques (i.e., Direct Hoechst staining and Immuno Fluorescence-Antibody or IFA) can be correctly interpreted. In addition, they also showed that optimal results of Nested PCR techniques (because of frequent false positive cases) are particularly useful in screening of cell cultures. In another study by Lawrence et al. (2010) a cell line (NKm) infected with two mycoplasma strains (M. fermentans and M. hyorhinis) were analyzed by three methods including microbial culture, conventional PCR and quantitative real-time PCR. There was no growth in microbial culture method, whereas, both molecular tests were positive up to the 10−7 dilution. Besides, the sensitivity of molecular techniques (NAT tests) for the detection of mycoplasma contamination was quite similar. In SYBR green real-time PCR method there were no false positives (no cross-reactivity) detected for the bacteria that are phylogenetically and evolutionary associated with mycoplasma such as Clostridium sporogenes, Lactobacillus acidophilus and Streptococcus bovis.
Mycoplasmal agents were also identified (Kong et al. 2007) by using a modified PCR/microarray assay based on genetic differences between mollicutes at the 16S-23S rRNA intergenic transcribed spacer (ITS). The application of nano-gold/silver enhancement technology instead of previously used fluorescent dyes significantly simplified the readout of microarray results and allowed them to avoid using expensive scanning equipment. This modification was able to expand the implementation of microarray techniques into laboratories involved in diagnostic testing of mycoplasma contamination in cell substrates and potentially in other biological and pharmaceutical products. In another study by Störmer et al. (2009), a multiplex real-time PCR was developed using LNA technology based on conserved regions of mycoplasma TUF gene (which encodes peptide chain elongation factor Tu) for the detection of mycoplasma contamination in cell culture supernatants, clinical specimens and blood products. This method showed high specificity, sensitivity of 1 copy/µl of sample and 95 % detection limit was calculated to 10 copies/µl of M. pneumonia and M. orale samples. Moreover, no false positive results due to cross-reaction with other bacterial-DNA, fungi and human DNA were observed. Recently, a novel rapid method for the detection of mycoplasma genomic DNA by using gated materials was examined (Climent et al. 2013). In this assay the DNA-capped mesoporous silica nanoparticles loaded with a dye were used to detect the common mycoplasma contaminant in contaminated cell-culture media without needing Polymerase Chain Reaction (PCR) techniques (with a detection limit of 70 copies/μl of genomic DNA). In another study by Degeling et al. (2014) a simple and sensitive assay to monitor mycoplasma contamination (mycosensor) were introduced based on degradation of the Gaussia luciferase reporter in the conditioned medium of cells. This assay proved to be more sensitive as compared to a commercially available bioluminescent assay (enzymatic mycoalert®) in detecting mycoplasma contamination in seven different cell lines. Schnee et al. (2012) have designed a DNA microarray carrying 70 oligonucleotide probes derived from the 23S rRNA gene and 86 probes from the tuf gene target regions. Following a PCR amplification and biotinylation step, hybridization on the array was shown to specifically identify 31 Mycoplasma spp., as well as 3 Acholeplasma spp. and 3 Ureaplasma spp. The main advantages of the microarray assay include ease of operation, rapidity, high information content, and affordability. The new test’s analytical sensitivity is equivalent to that of real-time PCR and allows examination of field samples without the need for culture. In another investigation by Schmitt and Pawlita (2009), a novel high-throughput Multiplex cell Contamination Test (McCT) was developed and validated which was able to detect 37 contamination markers in a single reaction. The assay was designed based on multiplex PCR with target-specific primers and subsequent hybridization of amplimers to specific oligonucleotide probes. McCT proved to be highly specific, sensitive and robust with the ability to analyze more than 1000 cell lysates per week in assessing cell line purity.
Conclusion
In this study, five different methods for mycoplasma detection in cell cultures including microbial culture as a gold standard method, indirect DNA DAPI staining, mycoalert® detection kit, conventional PCR and, real-time PCR were compared. In terms of sensitivity, specificity, accuracy, and predictive value of positive and negative results the real-time PCR assay was superior to the other methods with respect to (Fig. 5). The enzymatic mycoalert® method was the third best assay compared to the real-time PCR and PCR assay and can be used as an alternative method to conventional microbial culture and DNA fluorochrome staining methods. Therefore, real-time PCR (PCR Mycoplasma Test Kit I/RT) and conventional PCR assays based on conserved sequences in 16S ribosomal RNA were confirmed to be a reliable, highly sensitive, highly specific and accurate methods for mycoplasma detection in cell cultures and biological products. The PCR Mycoplasma Test Kit I/RT may be able to detect a range of 1 × 106–1 × 108 organisms in cell culture supernatants which guarantees a sensitive real-time PCR assay. The test responded specific to a broad range of mycoplasma species and exhibited no cross-reactivity to phylogenetically related bacteria (for example: Clostridium acetobutylicum, Lactobacillus acidophilus and Streptococcus pneumoniae) or other bacterial DNA and eukaryotic DNA. Due to limitations in terms of sensitivity, specificity and accuracy in the various diagnostic tests of mycoplasma detection (given the advantages and disadvantages of direct and indirect methods), mycoplasmologists suggest using at least 2–3 methods in parallel for the detection of mycoplasma contamination in cell cultures. In accordance with the US, EP and JP Pharmacopoeia and FDA, these tests should be performed independently and simultaneously to assess, identify and complete screening of cell cultures contamination (Volokhov et al. 2011; Nikfarjam and Farzaneh 2012; Uphoff and Drexler 2014). Thus, we recommend the combination of the real-time PCR assay with mycoalert assay and microbial culture to confirm active mycoplasma contamination.
Acknowledgments
The authors would like to express their appreciation to Dr. Ehsan Mostafavi (Head of the Department of Epidemiology, Pasteur Institute of Iran) and also would like to thank National Cell Bank of Iran at Pasteur Institute for their financial support.
Contributor Information
Mohammad Ali Shokrgozar, Phone: +98-21-66492595, Email: mashokrgozar@pasteur.ac.ir.
Reza Mahdian, Phone: +98-21-66480780, Email: mahdian@pasteur.ac.ir.
References
- Andrade NM, Arismendi NL (2013) DAPI staining and fluorescence microscopy techniques for phytoplasmas. In: Dickinson M, Hodgetts J (eds) Phytoplasma. Humana Press, Chicago, pp 115–121 [DOI] [PubMed]
- Armstrong SE, Mariano JA, Lundin DJ. The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals. 2010;38:211–213. doi: 10.1016/j.biologicals.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Barile MF, McGarrity GJ. Isolation of mycoplasmas from cell cultures by agar and broth techniques. Methods Mycoplasmol. 1983;2:159–165. doi: 10.1016/B978-0-12-583802-3.50024-0. [DOI] [Google Scholar]
- Barile MF, Rottem S (1993) Mycoplasmas in cell culture. In: Rapid diagnosis of mycoplasmas. Springer, US, pp 155–193
- Baronti C, Pastorino B, Charrel R, De Lamballerie X. Mycoplasma removal: simple curative methods for viral supernatants. J Virol Methods. 2013;187:234–237. doi: 10.1016/j.jviromet.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Cheong KA, Agrawal SR, Lee AY. Validation of nested PCR and a selective biochemical method as alternatives for mycoplasma detection. J Basic Microbiol. 2011;51:215–219. doi: 10.1002/jobm.201000066. [DOI] [PubMed] [Google Scholar]
- Climent E, Mondragón L, Martínez-Máñez R, Sancenón F, Marcos MD, Murguía JR, Amorós P, Rurack K, Pérez-Payá E. Selective, highly sensitive, and rapid detection of genomic DNA by using gated materials: mycoplasma detection. Angew Chem Int Ed. 2013;52:8938–8942. doi: 10.1002/anie.201302954. [DOI] [PubMed] [Google Scholar]
- Dabrazhynetskaya A, Furtak V, Volokhov D, Beck B, Chizhikov V. Preparation of reference stocks suitable for evaluation of alternative NAT-based mycoplasma detection methods. J Appl Microbiol. 2014;116:100–108. doi: 10.1111/jam.12352. [DOI] [PubMed] [Google Scholar]
- Dallo S, Baseman J. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000;29:301–309. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- Dandekar T, Snel B, Schmidt S, Lathe W, Suyama M, Huynen M, Bork P (2002) Comparative genome analysis of the mollicutes. In: Razin S, Herrmann R (eds) Molecular biology and pathogenicity of mycoplasmas. Springer, US, pp 255–278
- Degeling MH, Bovenberg MSS, Tannous M, Tannous BA (2014) Gaussia luciferase-based mycoplasma detection assay in mammalian cell culture. In: Badr CE (ed) Bioluminescent imaging. Humana Press, Chicago, pp 47–55 [DOI] [PubMed]
- Drexler HG, Uphoff CC, Dirks WG, MacLeod RA. Mix-ups and mycoplasma: the enemies within. Leuk Res. 2002;26:329–333. doi: 10.1016/S0145-2126(01)00136-9. [DOI] [PubMed] [Google Scholar]
- Espy M, Uhl J, Sloan L, Buckwalter S, Jones M, Vetter E, Yao J, Wengenack N, Rosenblatt J, Cockerill F. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmsbee M, Howard G, McAlister M. Nutritional effects of culture media on mycoplasma cell size and removal by filtration. Biologicals. 2010;38:214–217. doi: 10.1016/j.biologicals.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Galen RS, Gambino SR. Beyond normality: the predictive value and efficiency of medical diagnoses. New York: Wiley; 1975. [Google Scholar]
- Harasawa R, Mizusawa H, Fujii M, Yamamoto J, Mukai H, Uemori T, Asada K, Kato I. Rapid detection and differentiation of the major mycoplasma contaminants in cell cultures using real-time PCR with SYBR Green I and melting curve analysis. Microbiol Immunol. 2005;49:859–863. doi: 10.1111/j.1348-0421.2005.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Hay RJ, Ikonomi P. Detection of microbial and viral contaminants in cell lines. Cell Biol. 2005;1:49. doi: 10.1016/B978-012164730-8/50008-3. [DOI] [Google Scholar]
- Hopert A, Uphoff CC, Wirth M, Hauser H, Drexler HG. Specifity and sensitivity of polymerase chain reaction (PCR) in comparison with other methods for the detection of mycoplasma contamination in cell lines. J Immunol Methods. 1993;164:91–100. doi: 10.1016/0022-1759(93)90279-G. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Kozakai T, Morita H, Saida K, Oka S, Masuo Y. Rapid detection of mycoplasma contamination in cell cultures using SYBR Green-based real-time polymerase chain reaction. In Vitro Cell Dev Biol Anim. 2006;42:63–69. doi: 10.1290/0505035.1. [DOI] [PubMed] [Google Scholar]
- Jung H, Wang S-Y, Yang I-W, Hsueh D-W, Yang W-J, Wang T-H, Wang H-S. Detection and treatment of mycoplasma contamination in cultured cells. Chang Gung Med J. 2003;26:250–258. [PubMed] [Google Scholar]
- Kong H, Volokhov DV, George J, Ikonomi P, Chandler D, Anderson C, Chizhikov V. Application of cell culture enrichment for improving the sensitivity of mycoplasma detection methods based on nucleic acid amplification technology (NAT) Appl Microbiol Biotechnol. 2007;77:223–232. doi: 10.1007/s00253-007-1135-1. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ali A, Yerneni LK. Tandem use of immunofluorescent and DNA staining assays to validate nested PCR detection of mycoplasma. In Vitro Cell Dev Biol Anim. 2008;44:189–192. doi: 10.1007/s11626-008-9081-5. [DOI] [PubMed] [Google Scholar]
- Lawrence B, Bashiri H, Dehghani H. Cross comparison of rapid mycoplasma detection platforms. Biologicals. 2010;38:218–223. doi: 10.1016/j.biologicals.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Lincoln CK, Gabridge MG. Cell culture contamination: sources, consequences, prevention, and elimination. Methods Cell Biol. 1998;57:49–65. doi: 10.1016/S0091-679X(08)61571-X. [DOI] [PubMed] [Google Scholar]
- Mariotti E, Mirabelli P, Di Noto R, Fortunato G, Salvatore F. Rapid detection of mycoplasma in continuous cell lines using a selective biochemical test. Leuk Res. 2008;32:323–326. doi: 10.1016/j.leukres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Markoullis K, Bulian D, Hölzlwimmer G, Quintanilla-Martinez L, Heiliger K-J, Zitzelsberger H, Scherb H, Mysliwietz J, Uphoff CC, Drexler HG. Mycoplasma contamination of murine embryonic stem cells affects cell parameters, germline transmission and chimeric progeny. Transgenic Res. 2009;18:71–87. doi: 10.1007/s11248-008-9218-z. [DOI] [PubMed] [Google Scholar]
- McGarrity GJ, Steiner T, Vanaman V. Detection of mycoplasmal infection of cell cultures by DNA fluorochrome staining. Methods Mycoplasmol. 1983;2:183–190. doi: 10.1016/B978-0-12-583802-3.50027-6. [DOI] [Google Scholar]
- Molla Kazemiha V, Shokrgozar MA, Arabestani MR, Moghadam MS, Azari S, Maleki S, Amanzadeh A, Tehrani MJ, Shokri F. PCR-based detection and eradication of mycoplasmal infections from various mammalian cell lines: a local experience. Cytotechnology. 2009;61:117–124. doi: 10.1007/s10616-010-9252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla Kazemiha V, Azari S, Amanzadeh A, Bonakdar S, Moghadam MS, Anbouhi MH, Maleki S, Ahmadi N, Mousavi T, Shokrgozar MA. Efficiency of Plasmocin™ on various mammalian cell lines infected by mollicutes in comparison with commonly used antibiotics in cell culture: a local experience. Cytotechnology. 2011;63:609–620. doi: 10.1007/s10616-011-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla Kazemiha V, Amanzadeh A, Memarnejadian A, Azari S, Shokrgozar MA, Mahdian R, Bonakdar S (2014) Sensitivity of biochemical test in comparison with other methods for the detection of mycoplasma contamination in human and animal cell lines stored in the National Cell Bank of Iran. Cytotechnology 66:861–873 [DOI] [PMC free article] [PubMed]
- Nikfarjam L, Farzaneh P. Prevention and detection of mycoplasma contamination in cell culture. Cell J. 2012;13:203–212. [PMC free article] [PubMed] [Google Scholar]
- Phelan MC (2006) Techniques for mammalian cell tissue culture. Curr Protoc Mol Biol 74:A.3F.1–A.3F.8. [DOI] [PubMed]
- Phelan MC (2007) Basic techniques in mammalian cell tissue culture. Curr Protoc Cell Biol 36:1.1.1–1.1.18 [DOI] [PubMed]
- Pitt A, Crouch SPM, Slater KJ, Cox A (2012) Assay for detecting mycoplasma by measuring acetate kinase or carbamate kinase activity. European Patent No. EP 2264181
- Razin S. DNA probes and PCR in diagnosis of mycoplasma infections. Mol Cell Probes. 1994;8:497–511. doi: 10.1006/mcpr.1994.1071. [DOI] [PubMed] [Google Scholar]
- Razin S, Herrmann R. Molecular biology and pathogenicity of mycoplasmas. NY: Springer; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LB, Wichelhausen RH, Roizman B. Contamination of human cell cultures by pleuropneumonialike organisms. Science. 1956;124:1147–1148. doi: 10.1126/science.124.3232.1147. [DOI] [PubMed] [Google Scholar]
- Rottem S, Barile MF. Beware of mycoplasmas. Trends Biotechnol. 1993;11:143–151. doi: 10.1016/0167-7799(93)90089-R. [DOI] [PubMed] [Google Scholar]
- Rottem S, Kosower NS, Kornspan JD (2012) Contamination of tissue cultures by mycoplasmas. In: Luca Ceccherini-Nelli (ed) Biomedical Tissue Culture. InTech, pp 35–38
- Schaper U, Converse R. Detection of mycoplasmalike organisms in infected blueberry cultivars by the DAPI technique. Plant Dis. 1985;69:193–196. doi: 10.1094/PD-69-193. [DOI] [Google Scholar]
- Schmitt M, Pawlita M (2009) High-throughput detection and multiplex identification of cell contaminations. Nucleic Acids Res 37:e119 [DOI] [PMC free article] [PubMed]
- Schnee C, Schulsse S, Hotzel H, Ayling RD, Nicholas RA, Schubert E, Heller M, Ehricht R, Sachse K. A novel rapid DNA microarray assay enables identification of 37 mycoplasma species and highlights multiple mycoplasma infections. PLoS ONE. 2012;7:e33237. doi: 10.1371/journal.pone.0033237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer M, Vollmer T, Henrich B, Kleesiek K, Dreier J. Broad-range real-time PCR assay for the rapid identification of cell-line contaminants and clinically important mollicute species. Int J Med Microbiol. 2009;299:291–300. doi: 10.1016/j.ijmm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Sung H, Kang SH, Bae YJ, Hong JT, Chung YB, Lee C, Song S. PCR-based detection of mycoplasma species. J Microbiol. 2006;44:42. [PubMed] [Google Scholar]
- Tang J, Hu M, Lee S, Roblin R. A polymerase chain reaction based method for detecting Mycoplasma Acholeplasma contaminants in cell culture. J Microbiol Methods. 2000;39:121–126. doi: 10.1016/S0167-7012(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Uphoff CC, Drexler HG. Detection of mycoplasma contaminations. In: Helgason CD, editor. Basic cell culture protocols. Chicago: Humana Press; 2013. pp. 1–13. [Google Scholar]
- Uphoff CC, Drexler HG (2014) Detection of mycoplasma contamination in cell cultures. Curr Protoc Mol Biol 106:28.4:28.4.1–28.4.14 [DOI] [PubMed]
- Van Kuppeveld F, Johansson K, Galama J, Kissing J, Bölske G, Van der Logt J, Melchers W. Detection of mycoplasma contamination in cell cultures by a mycoplasma group-specific PCR. Appl Environ Microbiol. 1994;60:149–152. doi: 10.1128/aem.60.1.149-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokhov DV, Graham LJ, Brorson KA, Chizhikov VE. Mycoplasma testing of cell substrates and biologics: review of alternative non-microbiological techniques. Mol Cell Probes. 2011;25:69–77. doi: 10.1016/j.mcp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Waites KB, Xiao L, Paralanov V, Viscardi RM, Glass JI (2013) Mycoplasma and ureaplasma. In: Filippis I, McKee ML (eds) Molecular typing in bacterial infections. Humana Press, Chicago, pp 229–281
- Young L, Sung J, Stacey G, Masters JR. Detection of mycoplasma in cell cultures. Nat Protoc. 2010;5:929–934. doi: 10.1038/nprot.2010.43. [DOI] [PubMed] [Google Scholar]