Abstract
Proteomic study on membrane-integrated proteins in endoplasmic reticulum (ER) fractions was performed. In this study, we examined the effects of heat stress on Jurkat cells. The ER fractions were highly purified by differential centrifugation with sodium carbonate washing and acetone methanol precipitations. The ER membrane proteins were separated by one dimensional electrophoresis (1-DE), and some of the protein bands changed their abundance by heat stress, 12 of the 14 bands containing 40 and 60 ribosomal proteins whose expression level were decreased, on the contrary, 2 of the 14 bands containing ubiquitin and eukaryotic translation initiation factor 3 were increased. Heat treatment of human Jurkat cells led to an increase in the phosphorylation of PERK and eIF2α within 30 min of exposure. This was followed by an increase in the expression of the GRP78. Protein ubiquitination and subsequent degradation by the proteasome are important mechanisms regulating cell cycle, growth and differentiation, the result showed that heat stress enhanced ubiquitination modification of the microsomal proteins. The data of this study strongly suggest that heat treatment led to a significant reduction in protein expression and activated UPR, concomitant with protein hyperubiqutination in ER.
Keywords: Endoplasmic reticulium, Heat stress, SDS-PAGE, LC–MS/MS
Introduction
The endoplasmic reticulum (ER) is the central organelle where secretory and membrane proteins are synthesized. A number of cellular stress conditions lead to the accumulation of unfolded or misfolded proteins in the ER lumen, thereby representing a fundamental threat to cell viability. Failure to maintain proteostasis, underlies the pathology of many diseases including diabetes and cancer (Balch et al. 2008). The folding of secretory proteins is especially vulnerable to such failure, since cells must balance the activity of ribosomes in the cytoplasm with the efficiency of protein folding within the lumen of the ER (Ron and Walter 2007).
Most alive cells have an essential highly conserved, and exquisitely regulated cellular response to sudden heat exposure. The effects of heat stress on cellular function have been reported that (Park et al. 2005) reported on the effects of heat stress on cellular functions causing severe cellular injury, including impairment of DNA synthesis, transcription, mRNA processing especially splicing, translation, protein denaturation and aggregation, as well as cell death at high temperatures within a short time. Immediately after exposure to high temperature stress-related proteins are expressed as stress defense strategy of the cell. Expression of heat shock proteins (HSPs), is supposed to be involved in signal transduction transcriptional regulation and cell cycle control during heat stress (Sarkars et al. 2013). Heat-stress has a major impact on immune responses by modulating survival, proliferation, and endurance of lymphocytes. Lymphocyte persistence in turn is determined by the equilibrium between death and survival-promoting factors that regulate death receptor signaling in these cells (Kelly et al. 2007).
There are two branches of ER quality control, one is ER-associated degradation (ERAD) pathway, the ERAD is important for eviction of misfolded proteins from the ER to the cytoplasm and clearance by the ubiquitin/proteasome pathway. The other is unfolded protein response (UPR), which allows the cells to adapt to ER stress, plays a major role in maintaining ER function when the protein production exceeds the folding capacity of the ER. The α-subunit of eukaryotic initiation factor 2 (eIF2α) is part of the multimeric eIF2 complex that is involved in the initiation of cap-dependent protein translation (Wek et al. 2006). Phosphorylation of eIF2α is induced by various forms of cell stress, resulting in changes to the proteome of the cell with two diametrically opposed consequences, adaptation to stress or initiation of programmed cell death. An important mechanism for targeted degradation of regulatory proteins involves covalent linkage of ubiquitin. Protein ubiquitination is a reversible post-translational modification in which the ubiquitin peptide is covalently linked to lysine residues in target proteins (Hershko and Ciechanover 1998). A major function of ubiquitination is to target misfolded proteins for degradation via the proteasome.
Proteomics is the approach of choice to find novel gene products, expression data, and to validate genome annotations. In the case of organellar proteomics, the use of cell fractions reduces the complexity of the samples, and proteins present in smaller amounts and specific to the organelles are revealed. The proteome of organelles comprise a focused set of proteins that fulfills discrete but varied cellular functions (Taylor et al. 2003). In this way, fractionation techniques make it possible to discover potentially important gene products that are expressed at low levels, or are masked by highly expressed gene products (Ferella et al. 2008). However, very few detailed proteomic studies have been carried out on ER isolated from human Jurkat T leukemia cells. We previously reported that a cytosolic phosphoprotein of stathmin, which mediates extracellular signals in Jurkat cells, is phosphorylated by cdk1 during heat stress (Nakamura et al. 2006), and we reported the nuclear protein profiling of Jurkat cells by heat stress (Yuan et al. 2007).
In this study, we used Jurkat cells to examine the effect of heat stress on ER protein expression and signaling transduction. We evaluated a technology of 1-DE combined with liquid chromatography tandem mass spectrometry (LC/MS–MS) and a specimen of ER fraction which was isolated from Jurkat cells. We found that heat treatment led to a significant reduction in protein expression and activated UPR, concomitant with protein hyperubiqutination.
Materials and methods
Reagents
N,N,N′,N′-tetramethylethylenediamine (TEMED), Coomassie brilliant blue R-250 (CBB R-250) were purchased from Nacalai Tesque (Kyoto, Japan). Dithiothreitol (DTT) and ammonium bicarbonate were purchased from Sigma (St. Louis, MO, USA). SuperSep™ PAGE 12.5 % gels were purchased from Wako (Osaka, Japan). Anti-RPL19 monoclonal antibody and PDI monoclonal antibody was from Abnova (Taipei, Taiwan), eIF2a monoclonal antibody, p-eIF2a monoclonal antibody,PERK monoclonal antibody and p-PERK monoclonal antibody were from Abcam (CA,UAS).
Cell culture and heat stress
Jurkat cells were bought from Takara Clontech (Otsu, Shiga, Japan). Jurkat cells were grown in a culture medium, Medium A [RPMI-1640 (Nissui, Tokyo, Japan) containing 0.005 % gentamycin, 2 mM l-glutamine and 10 % fetal bovine serum (Bio-West, Loire Valley, France) (Fujimoto et al. 1998)]. The cells (2 × 108) were collected by centrifugation at 500×g for 5 min. The pellet was washed and suspended in medium B (10 mM HEPES, RPMI-1640 containing 0.005 % gentamycin, 2 mM l-glutamine). The cells were separated into two dishes (2 × 106 mL) and incubated in medium B at 37 °C for 1 h, and heat stress was applied by incubating one dish of cells in a water bath at 45 °C for 30 min. Another dish of cells was incubated at 37 °C for 30 min as the control.
Preparation of ER fraction
The ER fraction of Jurkat cells was prepared as described by Peng et al. (2008) with some modifications (Chung et al. 2008). Briefly, after heat stress, cells (1 × 108) were harvested, and washed three times with ice-cold PBS, then pelleted at 1000×g, at 4 °C. The supernatant was discarded, and the cell pellet was resuspended in 5 mL of homogenization medium (0.25 M sucrose, 5 mM Tris–HCl pH 7.4, 0.5 mM Phenylmethanesulfonyl fluoride(PMSF)(Sigma), 10 μg/mL aprotinin (Sigma) and 10 μg/mL leupeptin)(Sigma). First, the suspension was incubated on ice for 5 min and homogenized in a Potter-Elvehiem homogenizer, second, the homogenate was centrifuged at 15,000g for 15 min at 4 °C, supernatants were decanted and again centrifuged at the same condition. Then the supernatant was centrifuged at 132,000g for 60 min at 4 °C in S52ST-0155 swing rotor, and the supernatant was discarded. The pellet was resuspended in 1 mL 0.1 M sodium carbonate containing 0.5 mM PMSF, 10 μg/mL aprotinin and 10 μg/mL leupeptin, stored on ice for 60 min to remove peripherally associated membrane proteins, and centrifuged at 132,000g for 60 min using S52ST-0155 swing rotor. The supernatant was discarded, the pellet (ER) was resuspended with 150 μL of Milli-Q water, aliquoted and stored at −80 °C until use.
Delipidation and protein precipitation by acetone:methanol (8:1)
Pretreatment of microsomes for SDS-PAGE as described by Rebecca and Michael (1999), simply, 700 μL of ice-cold acetone:methanol (8:1 v/v) mixture was added to 50 μL of ER fraction and incubated at 4 °C for 90 min. The precipitate was pelleted by centrifugation (2800g for 15 min at 4 °C), washed sequentially with 50 μL of acetone followed by methanol, and then air-dried. The air-dried ER fraction was resolved in SDS sample buffer (0.0625 M Tris–HCl PH 6.8, 10 % glycerol, 2.3 % SDS, 5 % 2-mercaptoethanol, 0.0025 % CBB R-250, except 2-mercaptoethanol and CBB R-250. The protein concentration of the ER fraction was determined by Lowry’s method.
SDS-PAGE
For SDS-PAGE, 20 μg ER fraction protein of each sample were mixed with 2-mercaptoethanol (final concentration 5 %) and CBB R-250 (final concentration 0.0025 %), and applied to a 12.5 % SuperSep™ PAGE gel (Wako, Osaka, Japan) with molecular weight marker. The electrophoresis was carried out at a constant current of 15 mA. The gel was fixed and stained with 30 % methanol and 10 % acetic acid containing 0.05 % CBB R-250, followed by destaining with 30 % methanol and 10 % acetic acid, and destaining with 7 % acetic acid.
Protein bands intensity level analysis
The positions of the protein bands on the gel were recorded using ProExpress 2-D Proteomic Imaging System (Perkin- Elmer,Waltham, MA, USA). Expression levels of the proteins were quantified by analyzing the intensity of each band with Progenesis PG240 software (Perkin-Elmer). We picked up the protein bands whose expression level significantly increased or decreased by more than 1.5-fold (p < 0.05) compared with that of control cell in all experiments. The intensity of each band was statistically analyzed by the Student’s t test. We performed SDS-PAGE for image analysis at five times.
In-gel trypsin digestion
The protein bands of interest were cut out from the gels with sterile single-use scalpels. The gel pieces were washed once with Milli-Q water (100 μL), and CBB R-250 dye was removed by three rinses in 60 % methanol, 50 mM ammonium bicarbonate, and 5 mM DTT for 15 min and twice in 50 % ACN, 50 mM ammonium bicarbonate and 5 mM DTT for 10 min. The gel pieces were dehydrated twice in 100 % ACN for 30 min, and then reswollen with an in-gel digestion reagent containing 10 mg/mL sequencing grade trypsin (Promega, Madison, WI, USA) overnight at 30 °C. The tryptic peptides were extracted from the gel pieces and lyophilized in a lyophilization machine for 18 h. Then they were eluted with 0.1 % formic acid into a microfuge tube for LC–MS/MS analysis.
Amino acid sequencing by LC–MS/MS
The lyophilized peptide was dissolved in a solution of 0.1 % formic acid and then centrifuged at 15,000 g for 5 min, after which 20 μL of the supernatant was fixed in 100 μL of polypropylene for the LC–MS/MS analysis. LC–MS/MS is liquid chromatography tandem mass spectrometry. Samples were analyzed by using an Agilent 1100 LC/MSD Trap XCT system (Agilent Technologies. Inc. Palo Alto, CA, USA). The 1100 LC/MSD Trap XCT has a standard resolution of <0.6 m/z (FWHM) at a scan rate of 26,000 m/z per second. In the high-resolution mode (<0.4 compared to <0.45 on the earlier model), the scan speed is 8,100. The Agilent 1100 capillary pump was operated under the following conditions: Solvent A: 0.1 % formic acid; Solvent B: CH3CN in 0.1 % formic acid. Column flow: 0.3 mL/min, primary flow: 300 μL/min. Gradient: 5 min 2 % B, 60 min 60 % B. Stop time: 60 min. Results were analyzed with a Spectrum Mill MS Proteomics Workbench, which matched observed peptide masses and product ion masses with the theoretical values for all proteins in the SWISSPROT data-base. UniProtKB/Swiss-Prot is a manually annotated, non-redundant protein sequence database. It combines information extracted from scientific literature and biocurator-evaluated computational analysis. The aim of UniProtKB/Swiss-Prot is to provide all known relevant information about a particular protein. Annotation is regularly reviewed to keep up with current scientific findings. The manual annotation of an entry involves detailed analysis of the protein sequence and of the scientific literature. The criteria for positive identification of proteins were set as follows: filter by protein score >8, percentage scored peak intensity.
Western blotting
ER fraction (20 μg of total proteins) were subjected to SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes (Nomura et al. 1993) at 90 mA for 78 min using blotting buffer (40 mM glycine, 48 mM Tris Base, 20 % methanol, 1.3 mM SDS), blocked with 5 % milk containing TBS buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl) and then incubated with primary antibodies specific for or RPL19 (mouse monoclonal, 1:500 dilution in 5 % milk/TBS), p-eIF2α (rabbit monoclonal, 1:500 dilution in 5 % milk/TBS), p-PERK (rabbit polyclonal, 1:200 dilution in 5 % milk/TBS), PERK (rabbit polyclonal, 1:500 dilution in 5 % milk/TBS), ubiquitin (mouse monoclonal, 1:250 dilution in 5 % milk/TBS), and followed by reactions with horseradish peroxidase-linked secondary antibodies (West Grove, PA, USA) for 1 h, and developed with the ECL-kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) or ECL plus Western Blotting Detection System (GE healthcare) or ImmunoStar Long Detection (Wako, Japan).
RT-PCR
The PERK primers were forward, 5′-ATCCCCCCATGGAACGACCTG-3′ and reverse, 5′-ACCCGCCAGGGACAAAAATG-3′; the eIF2α primers were forward, 5′-GAGTGTGTGGTTGTCATTAGGGTGG-3′ and reverse, 5′-GACAGCCTGTGGGGGTCAAGCGCC-3′; the RPL19 primers were forward, 5′-GAGGCTCGCCTCTAGTGTCCTCCGC-3′ and reverse, 5′-GAGCAGCCGGCGCAAAATCCTCATT-3′. The β-actin primers used for loading control were forward, 5′-CGACAGGATGCAGAAGGAG-3′ and reverse, 5′-ACATCTGCTGGAAGGTGGA-3′. PCR was performed in 50 μl of PCR buffer containing 1.5 mM MgCl2, 80 mM dNTP, 0.4 mM of each primer and 1.25 U of TaqDNA polymerase and 5 μl of cDNA reaction mixture. The amplification was performed using the following steps: reverse transcription at 37 °C for 1 h, predenaturation at 94 °C for 1 min, cycles of PCR (denaturation at 94 °C for 40 s, annealing at 60 °C for 1 min, and extension at 72 °C for 1 min and 10 s), and extension at 72 °C for 3 min. RT-PCR products were separated by electrophoresis on 2 % w/v agarose-TAE (40 mM Tris and 1 mM EDTA) gels containing ethidium bromide, visualized with UV light and photographed using an AE-6916 instant gel camera.
Statistical analysis
Protein levels were quantified by analyzing the intensity of each band with ProExpress 2-D Proteomic Imaging System. The differences in protein level between control and heat-stressed Jurkat cells were analyzed by the Student’s t test. P < 0.05 was considered to be significant.
Classification of proteins
Identified proteins were classified according to their subcellular localization and biological function using Swiss-Prot.
Results
Heat Stress-induced differences in the protein level of the ER fraction from human Jurkat cells
ER fraction from Jurkat cells which were incubated at 37 and 45 °C for 30 min were separated by 1-DE and visualized by CBB R-250 staining. More than 50 protein bands in 5 pairs of gels were compared by means of ProExpress 1 D Proteomic Imaging System. As shown in Fig. 1, fourteen of which were found to have changed, among which twelve had decreased after heat stress, while the other two had increased by at least 1.5-fold.
Fig. 1.
SDS-PAGE images of untreated and heat-treated Jurkat cells. Proteins (20 μg) were separated by SDS-PAGE using 12.5 % SuperSepTM PAGE gels stained with CBB-R250. The size of the molecular weight marker is indicated in kDa towards the left side of gel (M). Bands 1–12 decreased, while bands 13–14 increased after heat stress
Identification of ER proteins by LC–MS/MS
The identification of these 14 protein bands was accomplished by measuring tryptic peptide masses using the microcapillary ion trap Agilent 1100 LC–MS/MS Trap XCT system in the positive ion mode and carrying out a database search with Spectra Mill III, as summarized in Table 1, 14 protein bands contain 36 proteins. The peak that is labeled with a lozenge was unique to RPL19 (Fig. 2a). The primary data interpretation showed that 5 distinct peptides could match the RPL19 sequence well with coverage of 15 % (30 of 196 amino acids) (data not shown). We found protein bands that were decreased by heat stress; these proteins included 60S, 40S ribosomal proteins, THO complex subunit 4, and eukaryotic initiation factor 4A-I (see Table 1). While in 2 bands were increased by the heat stress, those bands were ubiquitin, eukaryotic translation initiation factor 3 subunit 10, clathrin heavy chain 1, protein diaphanous homolog 1, as well as ubiquitin, spectrin beta chain, brain 1, and talin-1 in the second band (see Table 1).
Table 1.
Identification of proteins differentially expressed by heat stress
| Band no. (Mr) |
Density ratio Control/heat shocked |
Protein name | Accession no | pI | Mr | Distinct peptides | Score |
|---|---|---|---|---|---|---|---|
| 1(16800) | 0.64 | 60S ribosomal protein L28 | P46779 | 12 | 15748 | 3 | 36.84 |
| 40S ribosomal protein S18 | P62269 | 11 | 17719 | 3 | 35.49 | ||
| 60S ribosomal protein L32 | P62910 | 11.3 | 15860 | 2 | 26.58 | ||
| 60S ribosomal protein L26 | P61254 | 10.6 | 17258 | 4 | 44.28 | ||
| 2(18000) | 0.39 | 60S ribosomal protein L23a | P62750 | 10.4 | 17695 | 2 | 24.23 |
| 3(17700) | 0.56 | 60S ribosomal protein L21 | P46778 | 10.5 | 18565 | 2 | 27.55 |
| 4(18000) | 0.59 | 60S ribosomal protein L29 | P47914 | 11.7 | 17752 | 4 | 58.39 |
| 5(20700) | 0.6 | 40S ribosomal protein S5 | P46782 | 9.7 | 22877 | 2 | 23.29 |
| 6(22000) | 0.6 | 60S ribosomal protein L18 | Q07020 | 11.7 | 21635 | 3 | 30.72 |
| 7(23900) | 0.58 | 60S ribosomal protein L15 | P61313 | 11.6 | 24146 | 2 | 24.96 |
| 8(25800) | 0.45 | 60S ribosomal protein L19 | P84098 | 11.5 | 23466 | 5 | 25.31 |
| 9(31400) | 0.49 | 40S ribosomal protein S2 | P15880 | 10.3 | 31325 | 3 | 47.04 |
| 40S ribosomal protein S3a | P61247 | 9.8 | 29945 | 3 | 36.59 | ||
| THO complex subunit 4 | Q86V81 | 11.2 | 26888 | 2 | 31.59 | ||
| 40S ribosomal protein S6 | P62753 | 10.9 | 28681 | 2 | 29.14 | ||
| Histone H1.5 | P16401 | 10.9 | 22580 | 2 | 27.7 | ||
| NADH-cytochrome b5 reductase | P00387 | 7.2 | 34236 | 2 | 21.99 | ||
| 10(32600) | 0.49 | Heterogeneous nuclear ribonucleoprotein A1 | P09651 | 9.3 | 38846 | 5 | 70.60 |
| Emerin | P50402 | 5.3 | 28994 | 3 | 41.36 | ||
| 60S ribosomal protein L5 | P46777 | 9.7 | 34363 | 3 | 37.3 | ||
| 40S ribosomal protein S3a | P61247 | 9.8 | 29945 | 3 | 33.95 | ||
| 40S ribosomal protein S6 | P62753 | 10.9 | 28681 | 2 | 25.56 | ||
| 11(34500) | 0.5 | 60S ribosomal protein L6 | Q02878 | 10.6 | 32728 | 7 | 102.07 |
| 60S ribosomal protein L4 | P36578 | 11.1 | 47698 | 3 | 32.72 | ||
| 60S acidic ribosomal protein P0 | P05388 | 5.7 | 34274 | 2 | 23 | ||
| Heme oxygenase 2 | P30519 | 5.3 | 360333 | 2 | 22.18 | ||
| 12(47500) | 0.51 | 60S ribosomal protein L4 | P36578 | 11.1 | 47698 | 12 | 173.98 |
| Eukaryotic initiation factor 4A-I | P60842 | 5.3 | 46154 | 4 | 65.32 | ||
| Nuclease sensitive element-binding protein 1 | P67809 | 9.9 | 35924 | 3 | 32.66 | ||
| 13(217000) | 1.59 | Ubiquitin | P62988 | 6.6 | 8565 | 6 | 92.29 |
| Eukaryotic translation initiation factor 3 subunit 10 | Q14152 | 6.4 | 166570 | 4 | 43.16 | ||
| Clathrin heavy chain 1 | Q00610 | 5.5 | 191616 | 2 | 22.62 | ||
| Protein diaphanous homolog 1 | O60610 | 5.3 | 138979 | 2 | 21.88 | ||
| 14(280000) | 1.87 | Ubiquitin | P62988 | 6.6 | 8565 | 5 | 80.75 |
| Spectrin beta chain, brain 1 | Q01082 | 5.4 | 274611 | 3 | 46.38 | ||
| Talin-1 | Q9Y490 | 5.8 | 269769 | 3 | 36.3 |
Fig. 2.

Identification of RPL19 by tandem mass spectrometry. a LC–MS spectra of tryptic digests of RPL19; precursor ion m/z is 972.11. b LC–MS/MS spectrum of a precursor ion with m/z 972.11 marked by a lozenge in a. The tandem mass spectrum is identified as the partial tryptic peptide VWLDPNETNEIANANSR from RPL19 processed with a spectrum MILL workbench. The spectrum of the precursor shows a doubly charged peptide ion
Confirmation of differential expression of RPL19 by Western blotting and RT-PCR
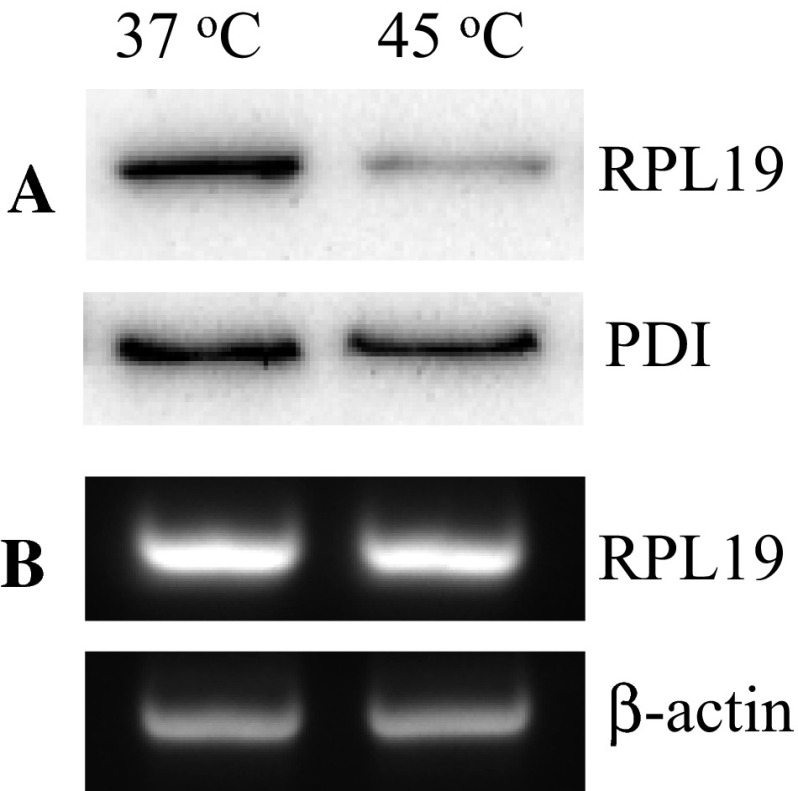
To verify the results of the proteomic analysis, western blot analysis was conducted. As shown in Fig. 3a, heat induced a significant decrease of 60S ribosomal protein L19 (RPL19) protein expression in Jurkat cells compared to control cells which were incubated at 37 °C and 45 °C, respectively, for 30 min, was consistent with the results obtained by SDS-PAGE (Fig. 2, band 8), and there was no significant change in the expression of RPL19 mRNA between control and heat-treated cells (Fig. 3b).
Fig. 3.
Western blotting analysis and RT-PCR for RPL19 in Jurkat cells. a Shows patterns of the 23 kDa band of RPL19 with anti-RPL19 antibodies specific for the RPL19 from amino acid 1 to amino acid 101, which decreased at 45 °C compared to control, PDI acted as the loading control in our experiments. b Shows RPL19 mRNA levels were analysed by semi-quantittive RT-PCR, with β-actin as loading controls. Data are representative of three independent experiments
Heat stress induces GRP78 expression
To elucidate the underlying mechanisms by which heat stress induces apoptosis (Nakamura et al. 2006), we performed a comprehensive proteomic analysis to identify molecules mediating the process. After matching with 1-DE maps, 14 protein bands were selected for further investigation by LC–MS/MS. Unfortunately, among them, GRP78 was not identified (Fig. 1). To determine whether heat stress induces GRP78 protein expression, we investigated the expression pattern of GRP78 in Jurkat cells. Western blot analysis demonstrated marked accumulation of GRP78 in ER fraction after heat stress as compared with that in the control (Fig. 4).
Fig. 4.
Heat stress induced GRP78 activation. Cells were treated at 45 °C for 30 min. Western blot analysis showed that heat significantly upregulated GRP78 protein expression at 45 °C for 30 min compared with control cells, with PDI as loading controls. Data are representative of three independent experiments
Heat stress preferentially induces PERK-eIF2α pathway
We investigated the activation of UPR pathways induced by heat stress. Under ER stress, ER stress sensor PERK leads to activation of eIF2α, a key step in attenuation of protein translation (Kondo et al. 2005). However, ER stress-induced phosphorylation of eIF2α can lead to selective translation of specific mRNAs encoding functions in modulating amino acid metabolism, anti-oxidative stress response, and apoptosis (Wek et al. 2006). To evaluate activation of the UPR pathways under heat stress, ER proteins and RNA were extracted from control and heat-stressed Jurkat cells. Western blot analysis and RT-PCR were performed. As shown in Fig. 5a, exposure to heat which was performed by a water bath at 45 °C for 30 min, clearly induced PERK and eIF2α phosphorylation compared to that in the control cells. Heat stress did not induce any significant change in the PERK and eIF2α mRNA levels (Fig. 5b). These results indicate that PERK- eIF2α level pathway is a potential target in response to heat stress.
Fig. 5.
Heat stress induces activation of PERK and eIF2-α in Jurkat cells. a Western blotting with anti-p-PERK and anti-p-eIF2-α in heat-treated Jurkat cells shows the activation of PERK and eIF2-α. b PERK (top) and eIF2-α (middle) mRNA levels were analyzed, PDI is loading control by semi-quantitative RT-PCR, with β-actin as loading controls. Data are representative of three independent experiments
Effects of heat stress on the level of cellular ubiquitination proteins in the ER fraction from Jurkat cells
Kelly et al. (2007) reported that stimulation in ubiquitin modification was observed in CHO cells exposed to heat-stress (42 °C, 30 min), and increased ubiquitin modification in lysates from heat-shocked cells is likely due to unfolding or misfolding of a subset of especially thermally cytosolic and nuclear proteins (Malzer et al. 2010). In this study we wanted to investigate whether heat stress could enhance ubiquitination modification of the microsomal proteins in Jurkat cells or not. The total level of ubiquitinated microsomal proteins was determined by Western blotting with anti-ubiquitin antibody. As shown in Fig. 6, heat stress enhanced ubiquitination modification of the microsomal proteins from human Jurkat cells. The increased ubiquitination modification might result from the enhancement of ubiquination and the inhibition of deubiquitination.
Fig. 6.
Ubiquitination assay in Jurkat cells. To detect the effect of heat stress on the ubiquitination modification, Jurkat cells were exposed to hyperthermia (45 °C for 30 min). Using the Western blotting by ImmunoStar Long Detection systems with an anti-ubiquin antibody, increased ubiquitin was detected in heat-treated cells as indicated by the parenthesis. Data are representative of three independent experiments
Discussion
In previous studies we performed nuclear protein profiling of Jurkat cells during heat stress generating some interesting data (Yuan et al. 2007; Nakamura et al. 2006) leading directly to the question on the effects of heat stress on ER. Thus we continued these studies using proteomic analysis of heat stressed Jurkat cells.
The ER is a highly dynamic organelle that plays a central role in lipid and protein biosynthesis. The ER has multiple cellular functions in the synthesis of integral membrane proteins, proper folding and oligomerization of proteins. The glucose regulated proteins (GRPs) are traditionally regarded as ER proteins with chaperone and calcium binding properties. The GPRs are constitutively expressed at basal levels in all organs, and as stress-induced ER chaperones, they are major players in protein folding, assembly and degradation. The most abundant ER chaperone is GRP78/Bip, which is responsible for maintaining the permeability barrier of the ER during protein translocation, guiding protein folding and assembly, and targeting misfolded proteins for degradation (Zhu and Lee 2014). Our results have demonstrated that heat stress induced up-regulation of the ER stress marker GRP78 (Fig. 4) after heat shock, indicating, the increased expression of GRP78 in Jurkat cells is that to relieve the ER stress.
Heat Stress-induced different protein expression of the ER fraction from Jurkat cells, fourteen protein bands were found to have changed, among which twelve had decreased, while the other two had increased by at least 1.5-fold. This is because shutdown of translation is a common response of cells to a severe form of stress. The ER has a dynamic capacity to accommodate increases in the demand for protein folding. However, extracellular stimuli and changes in intracellular homeostasis cause protein misfolding in the ER. The ER uses its protein folding status as a signal to orchestrate downstream adaptive or apoptotic responses. The unfolded protein response (UPR) is a cellular adaptive response that evolved to restore protein-folding homeostasis by reducing protein synthesis through phosphorylation of eIF2α (Han et al. 2013). The UPR pathway, which is conserved from yeast to humans, is triggered by activation of three sensors that are localized in the ER membrane. Progress in the field has provided insight into the regulatory mechanisms and signalling crosstalk of the three branches of the UPR (Hetz 2012). In response to accumulation of unfolded proteins, PERK is activated by auto-phosphorylation and phosphorylates eIF2α. This phosphorylation potently inhibits the initiation of protein translation. in fact, under extreme ER stress conditions protein synthesis can be brought to a complete halt (Goldfinger et al. 2011). The signal transduction cascade resulting in stress-induced shutdown of translation is initiated by activation of eIF2α kinase. Phosphorylation of eIF2α leads to impairment of the initiation process of protein synthesis (Paschen 2003). When cells are challenged with ER stress, phosphorylated eIF2α is increased which mediates both a transient decrease in global translation and the translational upregulation of selected stress-induced mRNAs (Saito et al. 2011). Heat stress clearly induced PERK and eIF2α phosphorylation compared to that in the control cells (Fig. 5a). Furthermore, among the decreased proteins, most of them were ribosomal proteins. Ribosomal proteins are involved in protein production. These results may indicate the suppression of protein biosynthesis in ER during heat stress.
Proteins are one of the major building blocks of cells, their production and degradation must be precisely controlled and regulated. Ubiquitination is one of the ways the cell uses to mark and label proteins for degradation. In an effort to maintain protein homeostasis, cells must not only actively control the production but also the degradation of proteins. Attachment of mono- or poly-ubiquitin to the protein of interest may trigger a diverse set of events, which could affect the location, interaction, and most importantly, the timing and extent of protein degradation inside the cell (Varshavsky 2012; Xu and Jaffrey 2011). The post-translational modification of cellular proteins by ubiquitination is a dynamic and reversible process that is orchestrated and precisely controlled via several ubiquitinating and deubiquitinating enzymes. Ubiquitin proteasome system (UPS) function depends on the availability of free ubiquitin. Intracellular level of free ubiquitin is determined by synchronous activities of ubiquitination and deubiquitination processes (Neutzner and Neutzner 2012). UPS determines the timing and extent of protein turnover in cells, and it is one of the most strictly controlled cellular mechanisms.
Protein maturation in the cell can be an inefficient process. For secretory cargo that matures in the ER, a quality control process selects terminally misfolded substrates for degradation by cytosolic proteasomes through the ERAD pathway. This process requires dislocation of the defective protein to the cytoplasm for ubiquitination and subsequent proteasomal degradation. The degradation of aberrant secretory cargo prevents potential blocks within the secretory pathway, creates antigens and provides a mechanism for destroying toxic substrates (Tamura et al. 2008). The ERAD is important for eviction of misfolded proteins from the ER to the cytoplasm and clearance by the ubiquitin/proteasome pathway. Finley et al. (1984) have proposed a direct role for ubiquitin in regulating the heat shock response. Both postulated that heat shock transcription factors are normally ubiquitinated and inactivated or rapidly degraded, and both envision that competition between heat-denatured proteins and transcription factors for ubiquitin would spare or activate heat shock transcription factors. Although once thought to be restricted to cytosolic and nuclear protein, degradation by the ubiquitin/proteasome system also plays a major role in regulating the level of proteins synthesized within the ER (Park et al. 2005). Two protein bands containing ubiquitin and eukaryotic translation initiation factor 3 were increased (Fig. 3, band 13 and 14), showing that heat treated Jurkat cells may accumulate more misfolded and/or aggregated proteins, these proteins need to be degraded via the ubiquitin-proteosome pathway and permit the cells to survive at 45 °C.
In conclusion, the present study showed that the heat shocked Jurkat cells underwent ER stress. We demonstrated that the UPR was triggered after heat stress and PERK, eIF2α kinases were integral to cellular stress pathways induced by heat, and may be central to the efficacy of heat that target the ubiquitin/proteasome pathway.
Acknowledgments
MPI preparation was supported by Prof. Bill Jordan (Center for Biodiscovery and School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand).
Conflict of interest
The authors declared that they have no competing interests.
Abbreviations
- ER
Endoplasmic reticulium
- RPL19
60S ribosomal protein L19
- CBB R-250
Coomassie brilliant blue R-250
- SDS
Sodium lauryl sulfate
- PAGE
Polyacrylamide gel electrophoresis
- ACN
Acetonitrile
- TBS
Tris buffered saline
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Chung M, Nakamura K, Jordan TW. The AOHUPO membrane proteomics initiative, fourth workshop 22 June 2008, Cairns, Australia. Proteomics. 2008;8:3920–3923. doi: 10.1002/pmic.200800605. [DOI] [PubMed] [Google Scholar]
- Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, Docampo R, Andersson B. Proteomics in Trypanosoma cruzi–localization of novel proteins to various organelles. Proteomics. 2008;8:2735–2749. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Ciechanover A, Varshavsky A. Thermolability of ubiquitin-activating enzyme from the mammalian cell cycle mutant ts85. Cell. 1984;37:43–55. doi: 10.1016/0092-8674(84)90299-X. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Nagasaka Y, Tanaka T, Nakamura K (1998) Analysis of heat shock-induced monophosphorylation of stathmin in human T lymphoblastic cell line JURKAT by two-dimensional gel electrophoresis. Electrophoresis 19:2515–2520 [DOI] [PubMed]
- Goldfinger M, Shmuel M, Benhamron S, Tirosh B. Protein synthesis in plasma cells is regulated by crosstalk between endoplasmic reticulum stress and mTOR signaling. Eur J Immunol. 2011;41:491–502. doi: 10.1002/eji.201040677. [DOI] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Robert Gildersleeve R, Shan JX, Yuan CL, Krokowski D, Wang SY, Hatzoglou M, Kilberg MS, Sartor MA, Kaufman RJ. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Kelly SM, Vanslyke JK, Musil LS. Regulation of ubiquitin-proteasome system-mediated degradation by cytosolic stress. Mol Bio Cell. 2007;18:4279–4291. doi: 10.1091/mbc.E07-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, Iseki K, Wanaka A, Imaizumi K. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- Malzer E, Daly ML, Moloney A, Sendall TJ, Thomas SE, Ryder E, Ryoo HD, Crowther DC, Lomas DA, Marciniak SJ. Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. J Cell Sci. 2010;123:2892–2900. doi: 10.1242/jcs.070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Zhang XL, Kuramitsu Y, Fujimoto M, Yuan XQ, Akada J, Aoshima-Okuda M, Mitani N, Itoh Y, Katoh T, Morita Y, Nagasaka Y, Yamazaki Y, Kuriki T, Sobel A. Analysis on heat stress-induced hyperphosphorylation of stathmin at serine 37 in Jurkat cells by means of two-dimensional gel electrophoresis and tandem mass spectrometry. J Chromatogr A. 2006;1106:181–189. doi: 10.1016/j.chroma.2005.12.068. [DOI] [PubMed] [Google Scholar]
- Neutzner M, Neutzner A. Enzymes of ubiquitination and deubiquitination. Essays Biochem. 2012;52:37–50. doi: 10.1042/bse0520037. [DOI] [PubMed] [Google Scholar]
- Nomura S, Kashiwagi S, Ito H, Mimura Y, Nakamura K. Degradation of fibrinogen and fibrin by plasmin and nonplasmin proteases in the chronic subdural hematoma: evaluation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot. Electrophoresis. 1993;14:1318–1321. doi: 10.1002/elps.11501401202. [DOI] [PubMed] [Google Scholar]
- Park HG, Han SI, Oh SY, Kang HS. Cellular responses to mild heat stress. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W. Shutdown of translation: lethal or protective? Unfolded protein response versus apoptosis. J Cereb Blood Flow Metab. 2003;23:773–779. doi: 10.1097/01.WCB.0000075009.47474.F9. [DOI] [PubMed] [Google Scholar]
- Peng LF, Rawson P, Mclauchlan D, Lehnert K, Snell R, Jordan TW. Proteomic analysis of microsomes from lactating bovine mammary. J Proteome Res. 2008;7:1427–1432. doi: 10.1021/pr700819b. [DOI] [PubMed] [Google Scholar]
- Rebecca M, Michael H. Protein delipidation and precipitation by tri-n-butylphosphate, acetone, and methanol treatment for isoelectric focusing and two-dimensional gel electrophoresis. Anal Biochem. 1999;273:313–315. doi: 10.1006/abio.1999.4224. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K (2011) Endoplasmic reticulum stress response mediated by the PERK-eIF2α-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem 286(6):4809–4818 [DOI] [PMC free article] [PubMed]
- Sarkars R, Mukherjee S, Roy M. Targeting heat shock proteins by phenethyl isothiocyanate results in cell-cycle arrest and apoptosis of human breast cancer cells. Nutr Cancer. 2013;65:480–493. doi: 10.1080/01635581.2013.767366. [DOI] [PubMed] [Google Scholar]
- Tamura T, Cormier JH, Hebert DN. Sweet bays of ERAD. Trends Biochem Sci. 2008;33:298–300. doi: 10.1016/j.tibs.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Taylor SW, Fahy E, Ghosh SS. Global orgallar proteomics. Trends Biotechnol. 2003;21:82–88. doi: 10.1016/S0167-7799(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system, an immense realm. Annu Rev Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST0340007. [DOI] [PubMed] [Google Scholar]
- Xu GQ, Jaffrey SR. The new landscape of protein ubiquitination. Nat Biotechnol. 2011;29:1098–1100. doi: 10.1038/nbt.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XQ, Kuramitsu Y, Furumoto H, Zhang XL, Hayashi E, Fujimoto M, Nakamura K. Nuclear protein profiling of Jurkat cells during heat stress-induced apoptosis by 2-DE and MS/MS. Electrophoresis. 2007;28:2018–2026. doi: 10.1002/elps.200600821. [DOI] [PubMed] [Google Scholar]
- Zhu G, Lee AS (2014) Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. doi:10.1002/jcp.24923 [DOI] [PMC free article] [PubMed]