Abstract
Aspidospermine is an indole alkaloid with biological properties associated with combating parasites included in the genera Plasmodium, Leishmania and Trypanossoma. The present study evaluated the cytotoxicity (resazurin test), genotoxicity (comet assay) and mechanism of action (gene expression analysis via qRT-PCR) of this alkaloid in human HepG2 cells. The results demonstrated that treatment with aspidospermine was both cytotoxic (starting at 75 μM) and genotoxic (starting at 50 μM). There was no significant modulation of the expression of the following genes: GSTP1 and GPX1 (xenobiotic metabolism); CAT (oxidative stress); TP53 and CCNA2 (cell cycle); HSPA5, ERN1, EIF2AK3 and TRAF2 (endoplasmic reticulum stress); CASP8, CASP9, CASP3, CASP7, BCL-2, BCL-XL BAX and BAX (apoptosis); and PCBP4, ERCC4, OGG1, RAD21 and MLH1 (DNA repair). At a concentration of 50 μM (non-cytotoxic, but genotoxic), there was a significant increase in the expression of CYP1A1 (xenobiotic metabolism) and APC (cell cycle), and at a concentration of 100 μM, a significant increase in the expression of CYP1A1 (xenobiotic metabolism), GADD153 (endoplasmic reticulum stress) and SOD (oxidative stress) was detected, with repression of the expression of GR (xenobiotic metabolism and oxidative stress). The results of treatment with aspidospermine at a 100 μM concentration (the dose indicated in the literature to achieve 89 % reduction of the growth of L. amazonensis) suggest that increased oxidative stress and an unfolded protein response (UPR) occurred in HepG2 cells. For the therapeutic use of aspidospermine (antiparasitic), chemical alteration of the molecule to achieve a lower cytotoxicity/genotoxicity in host cells is recommended.
Keywords: Aspidospermine, Cytotoxicity, Genotoxicity, Gene expression, HepG2
Introduction
Species of the genus Aspidosperma are known for their medicinal effects. Low toxicity and absence of contraindications of infusions of Aspidosperma shells have contributed to their increasing use (Weniger et al. 2001; Ferreira et al. 2004). Several examples of its medicinal effects can be distinguished: A. ramiflorum is used in the treatment of leishmaniasis (Ferreira et al. 2004) and A. nitidum is used for treating uterus and ovary inflammation, diabetes, cancer, fever and rheumatism (Weniger et al. 2001). The family Apocynaceae, which includes the genus Aspidosperma, is characterized by the frequent presence of alkaloids with extensive structural diversity (Allen and Holmstedt 1980).
A new medical use for aspidospermine (Fig. 1), an indole alkaloid isolated from plants belonging to the genus Aspidosperma, has come to the attention of researchers. Aspidospermine exhibits activity in combating flagellate protozoa of the genus Trypanossoma and Leishmania, which, when they parasitize humans, cause a variety of diseases, including Chagas disease (T. cruzi), sleeping sickness (T. brucei gambiense and T. b. rhodesiense) and various forms of Leishmania (L. brasiliensis and L. amazonensis) (Galarreta et al. 2008).
Fig. 1.
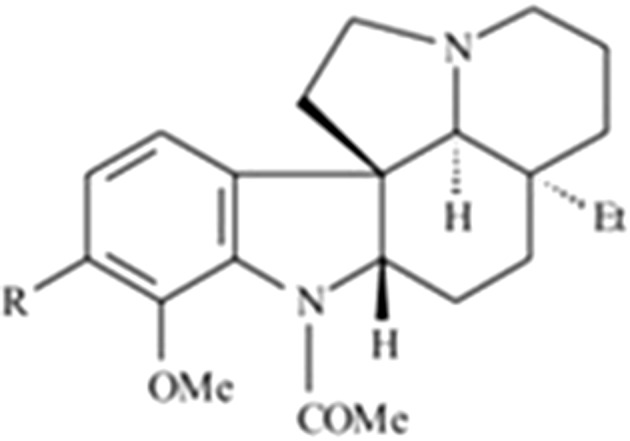
Chemical structure of aspidospermine (Pereira et al. 2007)
A common feature of these parasites is their metabolic dependence on tripanothione reductase (TryR), an NADPH-dependent flavoenzyme that helps fight oxidative stress by maintaining adequate levels of reduced tripanothione (Fairlamb and Cerami 1992). In humans, a similar activity is carried out by the enzyme glutathione reductase. Galarreta et al. (2008) assessed 23 heterocyclic compounds whose structure was compatible with known inhibitory molecules of TryR and found that treatment with 100 μM aspidospermine during the promastigote stage resulted in 89 % inhibition of L. amazonensis.
Furthermore, Mitaine-Offer et al. (2002) found that, among 12 indole alkaloids isolated from Aspidosperma, 7 showed high antiplasmodial activity, and 4 showed moderate activity against this parasite. The cytotoxicity of these alkaloids in the mouse fibroblast cell line NIH 3T3 was also evaluated. Aspidospermine showed the highest selectivity index (highest activity against the parasite and lower cytotoxicity against host cell) among the evaluated compounds.
Alkaloids have been used as main active component in various medicines due to their prominent pharmacological potential, including antimicrobial, antitumor and antiplasmodial activities (Kingston et al. 1978; Frederick et al. 1999). However, many alkaloids have also been recognized for presenting genotoxic effects (Wang and Peng 1996; Mei et al. 2004, 2005; Ansah et al. 2005).
Despite the proven effect of aspidospermine against parasites, no study has previously been conducted to deeply investigate its effect on human cells. Thus, the objective of the present study was to evaluate the cytotoxic and genotoxic effects and the mechanism of action (via gene expression analysis) of aspidospermine in a cell culture system using human hepatoma HepG2 cells, a metabolizing cell line. One type of analysis that is relevant to the study of a natural product is the evaluation of gene expression using real-time PCR. Applying this methodology, various cellular pathways were investigated in this study, including the metabolism of xenobiotics (CYP1A1 and GSTP1), oxidative stress (SOD1, GPX1, CAT and GR), cell cycle control (TP53, APC and CCNA2), endoplasmic reticulum stress (HSPA5, ERN1, EIF2AK3, TRAF2 and GADD153), apoptosis (CASP8, CASP9, CASP3, CASP7, BCL-2, BCL-XL, BAX, BAK and PCBP4) and DNA repair (ERCC4, OGG1, RAD21 and MLH1). The study of such pathways aids in the determination of the processes that are activated or inactivated in the cell due to treatment with the referred compound.
Materials and methods
Aspidospermine and chemical agents induce cellular damage
Root ethanolic extract of A. polyneuron was submitted to acid–base treatment for alkaloid extraction (de Barros et al. 2011). The product was submitted to column chromatography on silica gel eluted by solvents in increasing gradient of polarity (hexane, acetone, methyl acetate and methanol) resulting in 469 fractions (250 mL). The APR-108–110 (APA-8) was eluted in hexane:acetone 30 %. This fraction was submitted to the purification with preparative thin layer chromatography plates of silica gel using dichloromethane methanol (9:1) yielding needle crystals. Through spectroscopy methods (NMR,1H/13C, CG/MS, IV) the crystals were identified. Aspidospermine (mm = 354,49 g) was dissolved in Dimethyl sulfoxide (DMSO) and diluted in Dulbecco’s Modified Eagle’s medium (DMEM) (Life Technologies, Gaithersburg, MD, USA). The DMSO concentration did not exceed 1 % of the total quantity of the medium in the culture.
After evaluation of the cytotoxicity test results, the concentrations of aspidospermine for use in the comet and qRT-PCR assays were determined. As a positive control for the resazurin and comet assays, methyl methanesulfonate (MMS) (Sigma-Aldrich, St. Louis, MO, USA) was employed at a concentration of 300 μM in the culture medium, representing a known cytotoxic concentration (positive control).
Cell line
The human hepatoma HepG2 cell line was chosen since it represents a metabolizing cell line with the capacity of altering the original structure of the referred compound and thus modifying its biological properties. HepG2 cells were acquired from the cell bank of the Federal University of Rio de Janeiro (UFRJ) and were cultured in 25 cm2 flasks containing DMEM supplemented with 10 % fetal bovine serum (Gibco, Grand Island, NY, USA) in a 37 °C incubator with 5 % CO2. Under these conditions, the cell cycle of this lineage takes approximately 24 h.
Cytotoxicity assay
The resazurin (7-hydroxy-10-oxide-phenoxazin-10-ium-3-one) cytotoxicity test was based on the method employed by McMillian et al. (2002) and was performed using 24-well plates. The following treatments were included: medium (without cells), negative control (1 % DMSO, the same concentration used in other treatments), positive control (300 μM MMS) and aspidospermine at 5, 25, 50, 75 and 100 μM. The applied aspidospermine concentrations were chosen according to the assessment of cytotoxicity conducted by Mitaine-Offer et al. (2002) based on [3H]-hypoxanthine incorporation in NIH 3T3 fibroblast cells. In this case, the IC50 of aspidospermine was 53.2 ± 0.8 μM after 24 h of incubation. Each treatment was repeated three times in each plate, and each well was seeded with 2 × 104 cells.
After 24 h of treatment, the supernatant was discarded, and resazurin was added at a final concentration of 60 μM per well. The cells were incubated at 37 °C for 3 h. Then, the fluorescence was read at an excitation wavelength of 530–560 nm and emission wavelength of 580–600 nm. The obtained absorbance data were used to generate a regression curve for determination of the IC50. This experiment was replicated 3 times.
Single-cell gel electrophoresis (SCGE), or comet assay, and cellular viability test
The comet assay was conducted as described by Tice et al. (2000). Approximately 5 × 105 cells from the experimental cultures were plated per flask, and after stabilization for 24 h, the following experimental treatments were realized: positive control (MMS, 300 µM), negative control (1 % DMSO, the same concentration used in the other treatments), two non-cytotoxic concentrations of aspidospermine (5 and 50 µM) and a cytotoxic concentration of aspidospermine (100 µM) chosen previously based on the results of the resazurin cytotoxicity test. Three repetitions were performed.
After 3 h of treatment, the cells were washed with PBS, trypsinized (Gibco, Grand Island, NY, USA), centrifuged for 5 min (64.4 g) and then resuspended in culture medium. A mixture of 20 μL of the resuspended cells and 120 μL of low-melting-point agarose (LMP, 0.5 %) was applied to pre-gelatinized slides with normal melting point agarose (1.5 %). Next, the slides were submerged in an alkaline lysis solution for 1 h, followed by incubation for 40 min in electrophoresis buffer and then electrophoresis for 20 min. After this step, the slides were neutralized for 5 min 3 times and then submerged in absolute alcohol for 5 min for fixation. Staining of the slides was performed with ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) (200 μg/mL).
Simultaneously to the comet assay, 20 μL of the cell suspension was mixed with 20 μL of trypan blue (0.2 %) for cell viability analysis. Only the treatments resulting in an index >80 % were considered.
The nucleoid were finally displayed under a fluorescent microscope (excitation filter of 420–490 and 520 nm barrier filter, magnified 400×). For each treatment, 300 nucleoids were analyzed with CometScore software, v. 1.5 (TriTek, Sumerduck, VA, USA). The parameter evaluated was the total size of the comet (comet total length).
Analysis of gene expression via qRT-PCR
To assess the mechanism of action of aspidospermine in HepG2 cells, the effect of the alkaloid on the expression of key genes involved in xenobiotic metabolism, oxidative stress, cell cycle regulation, endoplasmic reticulum stress, apoptosis and DNA repair (Table 1) was tested using the qRT-PCR technique. To obtain RNA, 106 cells were seeded in 25 cm2 culture flasks. After stabilization for 24 h, the following treatments were performed for 6 h: negative control, 50 µM aspidospermine (non-cytotoxic concentration) and 100 µM aspidospermine (cytotoxic concentration). These concentrations were chosen since they present differences regarding their cytotoxicity and were closely related to the effective concentration against L. amazonensis found by Galarreta et al. (2008). The experiment was replicated 3 times.
Table 1.
Primers used in the present study
| Sequence | |
|---|---|
| CYP1A1 | F: TCA TCC CTA TTC TTC GCT ACC R: CAG GAG ATA GCA GTT GTG AC |
| GSTP | F: CAA TAC CAT CCT GCG TCA CC′ R: GGA GAT GTA TTT GCA GCG GA |
| SOD1 | F: CTAGCGAGTTATGGCGAC′ R: GAATGTTTATTGGGCGATC′ |
| GPX 1 | F: CAACCAGTTTGGGCATCAG′ R: CGATGTCAATGGTCTGGAAG′ |
| CAT | F: CATCGCCACATGAATGGATA′ R: CCAACTGGGATGAGAGGGTA′ |
| GR | F: GAAAAAGTTTACCGCTCCAC′ R: TAAACGCCTTTGACGTTGGTA′ |
| TP53 | F: 5′ TCACACCATCCACTACAACT 3′ R: 5′ GACAGGCACAAACACGCAC 3′ |
| APC | F: 5′ AAAGCGCCATGATATTGCACGGTC 3′ R: 5′ TGTTTGCTGTGCTCACGTTTCCAG 3′ |
| CCNA1 | F: 5′ GACCCTGCATTTGGCTGTG 3′ R: 5′ ACAAACTCTGCTACTTCTGG 3′ |
| HSPA5 | F: 5′ ACGGACGTCAAGTTTGATCC 3′ R: 5′ TTGGTAGACGCAGACAGTGG 3′ |
| ERN1 | F: 5′ ACGGACGTCAAGTTTGATCC 3′ R: 5′ TTGGTAGACGCAGACAGTGG 3′ |
| EIF2 AK3 | F: 5′ CCTCACCATTTGCCTAAGGA 3′ R: 5′ GGGGGACTTTCCTTCTTCTG 3′ |
| TRAF 2 | F: 5′ AGTTTTGTGGTGCTGGCTCT 3′ R: 5′ ACTCAGCCCCCGTAAGATTT 3′ |
| GADD153 | F: 5′ CCCTCACTCTCCAGATTCCA 3′ R: 5′ CTGGGGAATGACCACTCTGT 5′ |
| CASP8 | F: 5′ GCAAAAGCACGGGAGAAAGT 3′ R: 5′ TGCATCCAAGTGTGTTCCATT3′ |
| CASP9 | F: 5′ GCTCTTCCTTTGTTCATCTCC 3′ R: 5′ GTTTTCTAGGGTTGGCTTCG 3′ |
| CASP3 | F: 5′ GTGCTACAATGCCCCTGGAT 3′ R: 5′ GCCCATTCATTTATTGCTTTC C 3′ |
| CASP7 | F: 5′ TCACCATGCGATCCATCAAGACCA 3′ R: 5′ TTTGTCTGTTCCGTTTCGAACGCC 3′ |
| BCL-2 | F: 5′ CCTCGTCCAAGAATGCAAAGCACA 3′ R: 5′ ATCTCCCGGTTATCGTACCCTGTT 3′ |
| BCL-XL | F: 5′ TGGGCTCACTCTTCAGTCGGAAAT 3′ R: 5′ ATGTAGTGGTTCTCCTGGTGGCAA3′ |
| BAX | F: 5′ TTTCTGACGGCAACTTCAACTGGG 3′ R: 5′ TGTCCAGCCCATGATGGTTCTGAT 3′ |
| BAK | F: 5′ CAAGAT TGCCACCAGCCTGTT TGA 3′ R: 5′ ATGCAGTGATGCAGCATGAAGTCG 3′ |
| PCBP4 | F: 5′ CTGATGCACGGGAAGGAAGT R: 5′ CCCGGATTCGCTTTACAGTCT |
| ERCC4 | F: 5′ AATTCCAAGGTGTGCGACTG R: 5′ CGATGTTGTTGTTGGAGGAAC |
| OGG1 | F: 5′ AATTCCAAGGTGTGCGACTG R: 5′ CGATGTTGTTGTTGGAGGAAC |
| RAD21 | F: 5′ CAATGCCAACCATGACTGAT R: 5′ CGGTGTAAGACAGCGTGTAAA |
| MLH1 | F: 5′ CTTGTACCCCCCGGAGAAG R: 5′ TGCAACATCTCCCGGAGAAC |
| GAPDH | F: 5′ GAAGGTGAAGGTCGGAGTC 3′ R: 5′ GGAAGATGGTGATGGGATTT 3′ |
RNA was extracted using the LS-TRIzol (Life Technologies, Gaithersburg, MD, USA) reagent according to manufacturer‘s instructions. RNA integrity was confirmed through 1 % agarose gel electrophoresis, and the concentration and purity of the RNA were verified based on the A260/A280 absorbance ratios obtained using a spectrophotometer.
cDNA synthesis was performed using 200 units of M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA), 1 μg of total RNA and oligonucleotides with an oligo(dT) primer according to the protocol provided by the manufacturer of the enzyme.
Real-time PCR assays were carried out in a PTC 200 DNA Engine Cycler (a thermal cycler) using the Chromo 4 detection system (MJ Research BIO-RAD, Richmond, CA, USA). The amplified products were detected with a fluorescence measurement system using SYBR Green dye (Invitrogen). The thermal cycling conditions for PCR included an initial step at 95 °C for 5 min; 40 cycles at 95 °C for 20 s, 60 °C for 30 s and 72 °C for 20 s; 1 cycle at 95 °C for 10 s; and, finally, 1 cycle at 40 °C for 1 min. A melting curve analysis was performed at the end of each reaction at temperatures between 50 and 90 °C.
The obtained data were normalized to the endogenous glyceraldehyde phosphate dehydrogenase (GAPDH) gene, which was amplified in each set of PCR experiments. The primers used (n = 28) are listed in Table 1.
Statistical analysis
The data from the resazurin cytotoxicity assay and comet assay were subjected to variance analysis (ANOVA), followed by a Dunnett Test (p < 0.05).
For the qRT-PCR gene expression assays, analysis of the relative values of gene expression and standardization using the reference gene GAPDH were conducted via the Pffafl method (Pfaffl et al. 2002).
To decrease the likelihood of false positive or false negative results, the analyses were restricted to the statistically significant gene expression data that showed at least a twofold increase or decrease from the control.
Results
Treatment with 75 or 100 μM aspidospermine induces cytotoxicity
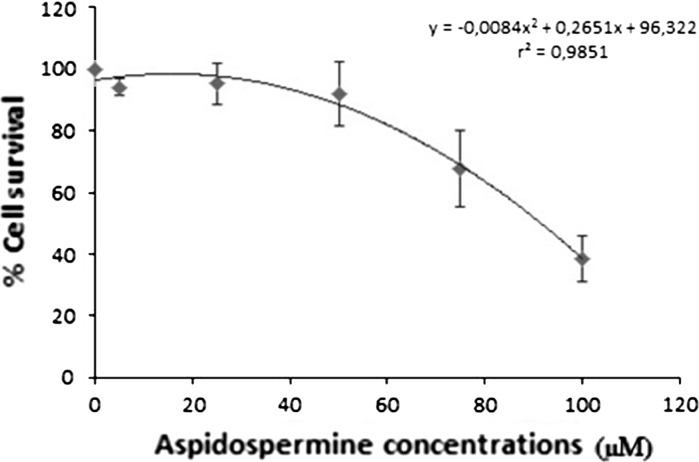
When the values obtained from the resazurin cytotoxicity test were examined, it was possible to observe a dose-dependent reduction of cell survival (Fig. 2; Table 2). After 24 h of treatment, the reduction of cell survival detected in HepG2 cells was significant for aspidospermine concentrations of 75 μM (67.88 %) and 100 μM (38.55 %) when compared to the control.
Fig. 2.
Cell survival curve obtained from the resazurin cytotoxicity test in HepG2 cells treated with aspidospermine at different concentrations for 24 h. The dots represent the average survival ± standard deviation values obtained in three independent experiments
Table 2.
Average percentage of cell survival rates ± SD
| Treatment | Mean ± SD |
|---|---|
| Negative control | 100 ± 0.00 |
| Positive control | 20.30 ± 2.85 |
| Aspidospermine 5 μM | 93.96 ± 6.66 |
| Aspidospermine 25 μM | 95.58 ± 10.37 |
| Aspidospermine 50 μM | 92.02 ± 12.31 |
| Aspidospermine 75 μM | 68.02 ± 7.54*** |
| Aspidospermine 100 μM | 39.04 ± 22.34*** |
Calculated from the resazurin assay after 24 h of treatment
Statistical significance: *** p < 0.001 relative to the control
The straight polynomial equation (r2 = 0.98) obtained through regression analysis showed that the IC50 for a 24-h treatment with aspidospermine in HepG2 cells was 92.46 μM.
Treatment with 50 or 100 μM aspidospermine induces genotoxicity
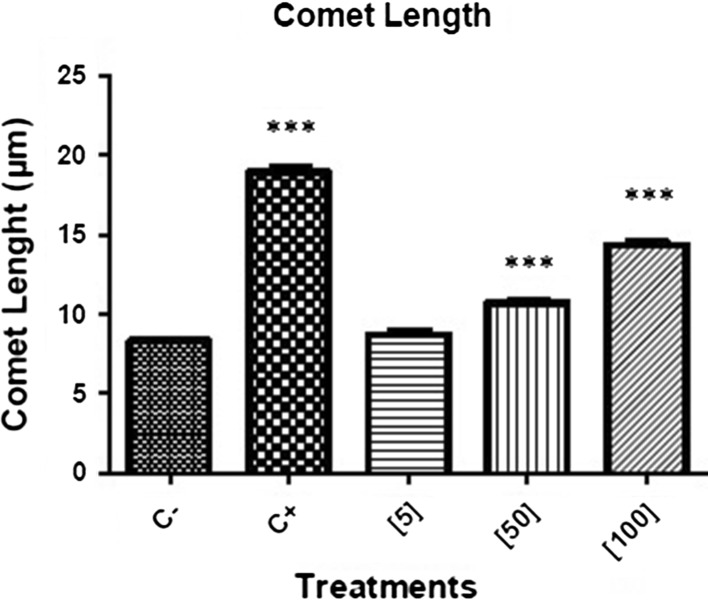
The results obtained for the comet length parameter in the comet assay showed that the genotoxicity associated with aspidospermine treatment for 3 h was dose dependent (Fig. 3; Table 3). There were significant differences between the negative control (8.3 μm) and the treatment with aspidospermine at the concentrations of 50 μM (10.7 μm) and 100 μM (14.3 μm). The concentration of 5 μM (8.7 μm) was not genotoxic to HepG2 cells. Cell viability remained above 80 % in all treatments.
Fig. 3.
Genotoxicity values (comet length) obtained from comet assay after a 3 h treatment of HepG2 cells with 5 [5], 50 [50] and 100 μM [100] aspidospermine. Statistical significance: *** = p < 0.001 relative to the negative control without treatment (C−). Positive control (C +) was performed with 300 μM MMS
Table 3.
Mean ± standard deviation of Comet length and increase (%) of this parameter in the aspidospermine treatments compared to the negative control
| Comet length (μm) ± SD | Increase (%) related to negative control | |
|---|---|---|
| Negative control | 8.320 ± 1.492 | 0 |
| Positive control | 18.914 ± 6.336*** | 127.3 |
| Aspidospermine 5 μM | 8.711 ± 5.10S | 4.7 |
| Aspidospermine 50 μM | 10.710 ± 2.637*** | 28.7 |
| Aspidospermine 100 μM | 14.348 ± 2.991*** | 72.5 |
Values obtained from the comet assay and evaluated by software CometScore vl.5
Statistical significance: *** p < 0.001 relative to the control
Treatment with 50 μM aspidospermine causes increased expression of CYP1A1 and APC
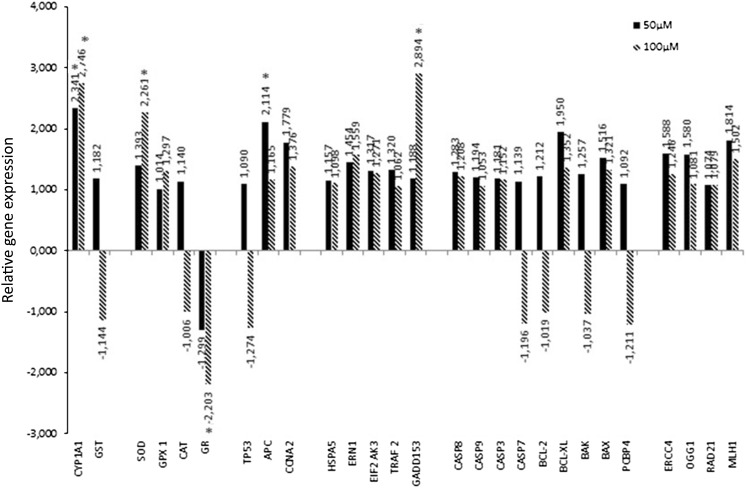
To assess the mechanism of aspidospermine action in human cells, the effect of aspidospermine on the expression of key genes involved in various types of process of the cellular metabolism, was analyzed via qRT-PCR after 6 h of treatment (Fig. 4).
Fig. 4.
Relative gene expression obtained via qRT-PCR for genes assessed after treatment of HepG2 cells for 6 h with 50 or 100 µM aspidospermine. *Significant modulation of expression
For the non-cytotoxic, but genotoxic concentration of 50 μM aspidospermine, increased CYP1A1, but not GSTP1 expression was observed. In relation to the oxidative stress genes, 50 μM aspidospermine showed a tendency to inhibit the expression of GR and to increase the expression of SOD, but these results were not significant. With regard to the examined cell cycle control genes, there was a significant increase of APC expression detected.
There was no significant modulation of GSTP1, SOD, GPX1, CAT, GR, TP53, CCNA1, HSPA5, ERN1, EIF2AK3, TRAF2, GADD153, CASP8, CASP9, CASP3, CASP7, BCL-2, BCL-XL, BAX, BAK, PCBP4, ERCC4, OGG1, RAD21 or MLH1 under the 50 μM aspidospermine treatment.
Treatment with 100 μM aspidospermine causes an increase in the expression of CYP1A1, GADD153 and SOD and inhibition of the expression of GR
For the cytotoxic and genotoxic concentration of 100 μM aspidospermine, among the group of biotransformation genes, an increase in the expression of the CYP1A1, but not GSTP1 was observed, which was the same pattern observed under the 50 μM concentration (Fig. 4).
Regarding the examined oxidative stress genes, the pattern observed at the 50 μM concentration was confirmed under the 100 μM concentration. Aspidospermine treatment significantly inhibited GR expression and caused an increase in SOD1 expression.
The expression of the APC gene, which was upregulated by treatment with 50 μM aspidospermine, showed no significant change when the concentration of aspidospermine was increased to 100 μM. Among the genes in the endoplasmic reticulum (ER) stress group, an increase in the expression of the pro-apoptotic gene GADD153 was noted. There was no change in the expression of DNA repair and cell cycle control genes detected in HepG2 cells.
For the 100 μM concentration of aspidospermine, there was no change in the gene expression of GSTP1, GPX1, CAT, TP53, APC, CCNA1, HSPA5, ERN1, EIF2AK3, TRAF2, CASP8, CASP9, CASP3, CASP7, BCL-2, BCL-XL, BAX, BAK, PCBP4, ERCC4, OGG1, RAD21 and MLH1.
Discussion
Studies have suggested that the alkaloid aspidospermine is effective in the treatment of parasites of the Leishmania, Trypanossoma and Plasmodium genera, which cause numerous diseases. However, reports discussing the effect of aspidospermine on host cells are scarce. One study that addressed this topic was performed by Mitaine-Offer et al. (2002). In this study, the cytotoxicity of aspidospermine in mouse fibroblasts (NIH3T3 cell line) was assessed by the [3H]-hypoxanthine incorporation method (Mitaine-Offer et al. 2002). IC50 values observed were 53.2 and 46.2 μM for 24 and 72 h periods of incubation with aspidospermine, respectively. In the present study, the IC50 found for a 24 h incubation period with aspidospermine was 92.46 μM, indicating that a higher concentration of aspidospermine was necessary to induce cytotoxicity in 50 % of HepG2 cells when compared to fibroblasts.
HepG2 cells are widely used in toxicological studies because they exhibit the ability to metabolize xenobiotics and generate experimental conditions that are closer to those that occur in vivo. When testing the cytotoxicity of the alkaloids warifteine and milonine, Melo et al. (2003) found similar IC50 values in hepatocytes and fibroblasts and demonstrated that the toxic effects of both compounds are independent of the cytochrome P450 metabolism system.
In the present study, analysis of the expression of important genes involved in xenobiotic metabolism revealed a dose-dependent increase in CYP1A1 gene expression under treatment with 50 and 100 μM concentrations of aspidospermine, indicating that the aspidospermine can be metabolized by HepG2 cells. In contrast, the levels of the GSTP1 gene transcript were not modulated significantly. It is possible that the intermediary metabolite generated by phase I metabolism of aspidospermine is a substrate of a phase II enzyme other than GSTP1. Thus, the difference between the HepG2 and NIH3T3 IC50s could be attributed to the formation of less toxic metabolites of aspidospermine by the biotransformation process of HepG2 cells. This phenomenon has also been observed for other compounds (Li et al. 2012).
The results of the gene expression analysis for genes involved in apoptosis were consistent with the pattern observed in the cytotoxicity assay. Non-cytotoxic treatment, with 50 μM aspidospermine, resulted in no significant increase in the expression of any of the evaluated genes, whereas cytotoxic treatment, with 100 μM aspidospermine, resulted in increased expression of GADD153. This suggests that the changes in cellular metabolism that occur when the concentration of aspidospermine is increased to 100 μM can be harmful to normal protein folding, resulting in the formation of immature, unfolded proteins and/or poorly folded proteins. In this study, the cells were apparently unable to restore the correct protein folding pattern because there was no increase in the expression of the genes responsible for repression of the UPR located upstream in the ER stress pathway (EIF2AK3, ERN1 and HSPA5), which can cause the targeting of apoptosis via GADD153.
Regarding the group of genes involved in the oxidative stress response, a trend of increased expression of SOD1 in response to treatment with aspidospermine was observed at the 50 μM concentration, and an additional significant increase was observed at the 100 μM concentration. This may be an indication that treatment with aspidospermine triggers an increase in superoxide anions that are then converted to H2O2 by the enzyme superoxide dismutase 1, which is overexpressed. On the other hand, there was no change in gene expression detected for CAT or GPX1, which are the enzymes responsible for the neutralization of H2O2 to H2O.
The parasites of the Leishmania and Trypanossoma genera exhibit metabolic dependence on trypanothione reductase (TryR), which helps to combat oxidative stress (Fairlamb and Cerami 1992). In humans, an analogue activity is carried out by the enzyme glutathione reductase (GR). In a recent study, Galarreta et al. (2008) observed significant inhibition (89 %) of the growth in L. amazonensis during the promastigote stage when treated with 100 μM aspidospermine. Additionally, the 100 μM aspidospermine treatment did not have a direct effect on the enzyme activity of glutathione reductase (analogue to TryR in humans) in HepG2 cells.
The results of the present study demonstrated a trend of inhibition of GR gene expression in response to the 50 µM aspidospermine treatment that became significant when the concentration was increased to 100 µM. Thus, aspidospermine may not have a direct effect on the catalytic activity of glutathione reductase but does cause inhibition of the gene expression in HepG2 cells. As a result, the GSH/GSSG ratio may decrease, and the antioxidant capacity of the cells related to glutathione could be reduced. An excess of H2O2 in the cell can cause serious damage to proteins, lipids and DNA (Wakamatsu et al. 2008). Using comet assays, it was possible to observe that the genotoxicity caused by a 3-h treatment with aspidospermine was dose dependent and was significant starting at the 50 μM concentration, which may be a result of a buildup of H2O2 in the cells.
Despite detection of DNA damage beginning at a concentration of 50 µM, only the highest concentration tested in this study (100 μM) was also cytotoxic, suggesting that at the 50 μM concentration, DNA repair mechanisms remained active, and the number of viable cells was consequently unaltered. However, none of the repair genes assessed via qRT-PCR showed significant modulation under the 50 or 100 μM aspidospermine concentration. It is possible that the examined repair genes are involved in a more immediate response, and consequently, no changes in their expression were observed after the 6-h treatment with aspidospermine. For example, Cortes et al. (2011) observed modulation of the expression of repair genes after 2 h of treatment with glucose oxidase, an enzyme that induces oxidative stress in breast cell lines.
Still, treatment with 50 μM aspidospermine triggered an increase in the expression of APC, a tumor suppressor, reinforcing the idea that the cell cycle is paused to allow repair of genotoxic damage under these conditions.
Drugs that are genotoxic can be modified to achieve greater therapeutic activity and lower toxicity. For example, several active components of vegetable origin, some of which exhibit toxic properties, have been used as precursors of important substances incorporated into therapy, including catarantine and vindoline, isolated from Catharanthus roseus; camptothecin, isolated from Camptotheca acuminata; podophyllotoxin, isolated from rhizomes of Podophyllum peltatum and P. hexandru; scopolamine isolated from Datura ssp.; stigmasterol, isolated from Glycine max; and diosgenin, isolated from Dioscores ssp. (Simões et al. 2002).
By virtue of its cytotoxic nature, aspidospermine can also be investigated as a potential drug for anticancer treatment, similar to other alkaloids with the same properties. An example of such a compound is the cytotoxic alkaloid camptothecin (CPT), which has inhibitory effect on the enzyme topoisomerase I (Efferth et al. 2007). While CPT showed excellent antitumor activity in preliminary clinical trials, it also presented a low solubility and led to numerous adverse reactions. Due to these disadvantages, a wide variety of compounds have been synthesized based on the structure of CPT, two of which (topotecan and irinotecan) have been approved and are currently being used in chemotherapy for cancer treatment (Samuelsson 1992; Ulukan and Swaan 2002; Takimoto and Calvo 2008).
Conclusion
The cytotoxic and genotoxic effects triggered in HepG2 cells treated with aspidospermine, which is useful for combating parasites included in the genera Trypanossoma, Leishmania and Plasmodium, were observed to be dose dependent. The results obtained in this study suggest that aspidospermine is biotransformed by cells into a metabolite with properties that are less toxic than the initial molecule and that treatment with this compound causes modulation of oxidative stress-related enzymes, which may be the cause of the observed genotoxic damage. These findings suggest that treatment with 50 µM aspidospermine results in cell cycle arrest to allow repair of the damage and that treatment with 100 µM aspidospermine induces apoptosis via a persistent UPR response. Based on the effects reported here, it is recommended that the original chemical structure of aspidospermine be altered to decrease its cytotoxicity and genotoxicity in host cells, making it more viable for clinical use. Additionally, because of its potential cytotoxicity, aspidospermine can be evaluated for use as a potential anticancer treatment.
References
- Allen JRF, Holmstedt BR. The simple β-carboline alkaloids. Phytochemistry. 1980;19:1573–1582. doi: 10.1016/S0031-9422(00)83773-5. [DOI] [Google Scholar]
- Ansah C, Khan A, Gooderham NJ. In vitro genotoxicity of the West African anti-malarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Toxicology. 2005;208:141–147. doi: 10.1016/j.tox.2004.11.026. [DOI] [PubMed] [Google Scholar]
- de Barros IBD, Daniel JFDS, Pinto JP, Rezende MI, Filho RB, Ferreira DT (2011) Phytochemical and antifungal activity of anthraquinones and root and leaf extracts of Coccoloba mollis on phytopathogens. Braz Arch Biol Technol 54:535–541
- Cortes DF, Sha W, Hower V, Blekherman G, Laubenbacher R, Akman S, Torti SV, Shulaev V, et al. Differential gene expression in normal and transformed human mammary epithelial cells in response to oxidative stress. Free Radic Biol Med. 2011;50:1565–1574. doi: 10.1016/j.freeradbiomed.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Fu Y-J, Zu Y-G, Schwarz G, Konkimalla VSB, Michael W, et al. Molecular target-guided tumor therapy with natural products derived from traditional Chinese medicine. Curr Med Chem. 2007;14:2024–2032. doi: 10.2174/092986707781368441. [DOI] [PubMed] [Google Scholar]
- Fairlamb AH, Cerami A. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- Ferreira ICP, Lonardoni MVC, Machado GMC, Leon LL, Filho LG, Pinto LHB, de Oliveira AJB, et al. Anti-leishmanial activity of alkaloidal extract from Aspidosperma ramiflorum. Mem Inst Oswaldo Cruz. 2004;99:325–327. doi: 10.1590/S0074-02762004000300015. [DOI] [PubMed] [Google Scholar]
- Frederick M, Hayette MP, Tits M, De Mol P, Angenot L, et al. In vitro activities of Strychnos alkaloids and extracts against Plasmodium falciparum. Antimicrob Agents Chemother. 1999;43:2328–2331. doi: 10.1128/aac.43.9.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta BC, Sifuentes R, Carrillo AK, Sanchez L, Amado MRI, Maruenda H, et al. The use of natural product scaffolds as leads in the search for trypanothione reductase inhibitors. Bioorganic Med Chem. 2008;16:6689–6695. doi: 10.1016/j.bmc.2008.05.074. [DOI] [PubMed] [Google Scholar]
- Kingston DGI, Gerhart BB, Ionescu F, Mangino MM, Sami SM, et al. Plant anticancer agents. V: new bisindole alkaloids from Tabernaemontana johnstonii stem bark. J Pharm Sci. 1978;67:249–251. doi: 10.1002/jps.2600670232. [DOI] [PubMed] [Google Scholar]
- Li A, Shen G, Jiao SY, Li H, Wang Q, et al. Metabolic detoxification of bakuchiol is mediated by cytochrome P450 enzymes in human liver microsomes. Beijing Da Xue Xue Bao. 2012;44:431–436. [PubMed] [Google Scholar]
- McMillian MK, Li L, Parker JB, Patel L, Zhong Z, Gunnett JW, Powers WJ, Johnson MD, et al. An improved resazurin-based cytotoxicity assay for hepatic cells. Cell Biol Toxicol. 2002;18:157–173. doi: 10.1023/A:1015559603643. [DOI] [PubMed] [Google Scholar]
- Mei N, Heflich RH, Chou MW, Chen T. Mutations induced by the carcinogenic pyrrolizidine alkaloid riddelliine in the liver cII gene of transgenic big blue rats. Chem Res Toxicol. 2004;17:814–818. doi: 10.1021/tx049955b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Guo L, Fu PP, Heflich RH, Chen T, et al. Mutagenicity of comfrey (Symphytum Officinale) in rat liver. Br J Cancer. 2005;92:873–875. doi: 10.1038/sj.bjc.6602420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo PS, De Medeiros Cavalcante HM, Barbosa-Filho JM, Diniz MFFM, Medeiros IA, Haun M, et al. Warifteine and milonine, alkaloids isolated from Cissampelos sympodialis Eichl: cytotoxicity on rat hepatocyte culture and in V79 cells. Toxicol Lett. 2003;142:143–151. doi: 10.1016/S0378-4274(03)00064-X. [DOI] [PubMed] [Google Scholar]
- Mitaine-Offer AC, Sauvain M, Valentin A, Callapa J, Mallie MZ, Zèches-Hanrot M, et al. Antiplasmodial activity of aspidosperma indole alkaloids. Phytomedicine. 2002;9:142–145. doi: 10.1078/0944-7113-00094. [DOI] [PubMed] [Google Scholar]
- Pereira MM, Jácome RL, Alcântara AFCDC, Alves RB, Raslan DS, et al. Indole alkaloids from species of the Aspidosperma (Apocynaceae) genus. Quim Nova. 2007;30:970–983. doi: 10.1590/S0100-40422007000400037. [DOI] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;e30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson G. Drugs of Natural Origin: a textbook of pharmacognosy. Stockholm: Swedish Pharmaceutical Press; 1992. [Google Scholar]
- Simões CMO, Schenkel EP, Gosmann G, Mello JCP de, Mentz LA, Petrovick PR (2002) Farmacognosia: da planta ao medicamento, 5th edn. Porto Alegre/Florianópolis: Editora da UFRGS/Editora da UFSC
- Takimoto CH, Calvo E (2008) Principles of oncologic pharmacotherapy. In: Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (eds) Cancer management: a multidisciplinary approach, 11th edn
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35(3):206–221 [DOI] [PubMed]
- Ulukan H, Swaan PW. Camptothecins: a review of their chemotherapeutic potential. Drugs. 2002;62:2039–2057. doi: 10.2165/00003495-200262140-00004. [DOI] [PubMed] [Google Scholar]
- Wakamatsu TH, Dogru M, Tsubota K. Tearful relations: oxidative stress, inflammation and eye diseases. Arq Bras Oftalmol. 2008;71:72–79. doi: 10.1590/S0004-27492008000700015. [DOI] [PubMed] [Google Scholar]
- Wang CK, Peng CH. The mutagenicities of alkaloids and N-nitrosoguvacoline from betel quid. Mutat Res—Environ Mutagen Relat Subj. 1996;360:165–171. doi: 10.1016/s0165-1161(96)90013-8. [DOI] [PubMed] [Google Scholar]
- Weniger B, Robledo S, Arango GJ, et al. Antiprotozoal activities of Colombian plants. J Ethnopharmacol. 2001;78:193–200. doi: 10.1016/S0378-8741(01)00346-4. [DOI] [PubMed] [Google Scholar]