Abstract
Alternatives to the use of fetal bovine serum (FBS) have been investigated to ensure xeno-free growth condition. In this study we evaluated the efficacy of human platelet lysate (PL) as a substitute of FBS for the in vitro culture of some human cell lines. PL was obtained by pools of pathogen inactivated human donor platelet (PLT) concentrates. Human leukemia cell lines (KG-1, K562, JURKAT, HL-60) and epithelial tumor cell lines (HeLa and MCF-7) were cultured with either FBS or PL. Changes in cell proliferation, viability, morphology, surface markers and cell cycle were evaluated for each cell line. Functional characteristics were analysed by drug sensitivity test and cytotoxicity assay. Our results demonstrated that PL can support growth and expansion of all cell lines, although the cells cultured in presence of PL experienced a less massive proliferation compared to those grown with FBS. We found a comparable percentage of viable specific marker-expressing cells in both conditions, confirming lineage fidelity in all cultures. Functionality assays showed that cells in both FBS- and PL-supported cultures maintained their normal responsiveness to adriamycin and NK cell-mediated lysis. Our findings indicate that PL is a feasible serum substitute for supporting growth and propagation of haematopoietic and epithelial cell lines with many advantages from a perspective of process standardization, ethicality and product safety.
Keywords: Platelet lysate, Human cell lines, Fetal bovine serum, Xeno-free conditions
Introduction
In vitro culture of mammalian cells is one of the most important process in research and drug development. Expansion of human cells in vitro requires specific culture conditions and culture media providing growth factors, proteins and enzymes for cell metabolism, growth and proliferation. Fetal bovine serum (FBS) is the most widely used source of nutrients and growth factors for cell culture (Honn et al. 1975). However, there are several ethical, scientific and safety problems with the use of FBS for in vitro cell culture. The use of FBS raises animal welfare concerns regarding the harvest procedure for its production. Moreover, there is an high variability from batch-to batch that can lead to inconsistent cell culture performance and may interfere with the reproducibility of experiments (Jochems et al. 2002). Most importantly, FBS is an animal-derived component which raises severe concerns for any in vitro or ex vivo technique aimed at cell growth and propagation, due to the risk of xenogenic immune reactions against bovine antigens and transmission of animal pathogens such as viruses and prions (Gstraunthaler 2003; Sundin et al. 2007). To date, various human-derived supplements have been tested as an alternative to FBS, including growth factors, autologous and allogeneic serum, human plasma and platelet derivatives, such as platelet lysate (PL) and released factors (Ferro et al. 2012; Gstraunthaler 2003; Hankey et al. 2001; Kocaoemer et al. 2007; Lin et al. 2005; Morimoto et al. 2011; Stute et al. 2004). Platelets (PLTs) are known to play many important roles in haemostasis, tissue repair and wound healing, through their adhesive and secretory properties (Barsotti et al. 2013).
Additionally, PLTs are a very rich source of cytokines, chemokines and growth factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), basic fibroblast growth factor (FGF-b), transforming growth factor-β (TGF-β), hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), and vascular endothelial growth factor (VEGF), as well as enzymes and attachment factors (fibronectin and vitronectin) (Reed 2004; Schallmoser and Strunk 2013). PLTs can be lysed to release growth factors by a simple freeze–thaw procedure. Recently, our group developed a protocol for the production of a standardized and safe PL for clinical-grade expansion of bone marrow mesenchymal stem cells (MSCs) (Iudicone et al. 2014).
Moreover, several reports have already demonstrated successful use of human PL as a replacement for FBS in cell culture. In particular, many studies have been conducted to develop standardized protocols, in compliance with good manufacturing practices (GMP), to promote growth and proliferation of MSC without altering their phenotypic and functional characteristics (Avanzini et al. 2009; Bernardo et al. 2007; Bieback et al. 2009; Castiglia et al. 2014; Cholewa et al. 2011; Doucet et al. 2005; Fekete et al. 2012; Hemeda et al. 2014; Schallmoser et al. 2007; Trojahn Kolle et al. 2013; Warnke et al. 2013).
Furthermore, various human cell populations, including mesenchymal and epithelial cells (from cord blood, adipose tissue, bone marrow and peripheral blood), monocytes, endothelial cells, keratinocytes, tumor cells, chondrocytes, hepatocytes and fibroblasts have been used to test the growth-stimulatory effects of platelet derived growth factors in vitro (Baik et al. 2014; Barsotti et al. 2013; Bernardi et al. 2013; Hofbauer et al. 2014). Indeed, in 2003, Johansson et al. demonstrated that PL can fully replace FBS as a culture supplement both for suspension as well as anchorage-dependent cells (Johansson et al. 2003). Additionally, experiments on renal epithelial cells and human leukemia cell lines showed that the growth promoting effect of 5 % PL is almost identical compared to that of 10 % FBS (Rauch et al. 2011). Recently, Schallmoser and Strunk’s results confirmed that human PL is highly efficient for the culture of human mesenchymal progenitors and endothelial colony-forming progenitors even when used at 5 % concentration (Schallmoser and Strunk 2013).Therefore, PL is also expected to be used as a FBS substitute in human cell lines expansion. In the present study, PL and FBS were compared in terms of their abilities to support the normal rate of growth and expansion of established haematopoietic and epithelial cancer cell lines for research purposes.
Materials and methods
Human platelet lysate (PL) preparation
PLs were prepared using PLT pathogen inactivated pools as previously described (Iudicone et al. 2014). Briefly, whole blood was collected from voluntary donors selected following current criteria for blood donation. Buffy-coats (BCs) were obtained by centrifugation of whole blood donations according to the procedures validated in the routine separation of blood components for transfusion therapy. Six BC-PLT units were pooled within 24 h from collection and, after inactivation by the Intercept technology (Intercept Blood System for Platelets, Cerus Corporation, Concord, CA, USA), were resuspended in InterSol solution (additive solution, AS; Fenwall Inc., Lake Zurich, IL, USA) with a 20–30 % residual human plasma in a volume of approximately 300 mL and a PLT content ranging from 2.5 to 3.5 × 1011. Two pools of six pathogen inactivated PLT (PI-PLT) units were connected each other under sterile conditions to obtain a pool of 12 PI-PLT units, with a PLT count greater than or equal to 1 × 109 per mL. The final pooled-PLT product was frozen at −80 °C and thawed at 37 °C for three consecutive cycles to lyse PLTs, then centrifuged to remove PLT membranes and finally aliquoted and cryopreserved at −80 °C until use. Sterility tests were performed prior and after the entire procedure of PL production to exclude aerobic, anaerobic and fungal contamination (BACTEC 9240, Beckton Dickinson, San Josè, CA, USA). Several lots of PL were produced according to the above mentioned protocol and three lots named PL16, PL17, PL18 were specifically tested for their capacity to support established tumor cell lines of different tissue origin.
PL content of PLT derived growth factors
The concentration of PLT derived soluble growth factors, i.e. PDGF-AB, VEGF, FGF-b and TGF-β was evaluated in each PL lot by using commercially available immunoenzymatic kits, (Quantikine Human PDGF-AB, Quantikine Human VEGF, Quantikine Human FGF-b, Quantikine Human TGF-β 1, R&D Systems, Minneapolis, MN, USA) following manufacturer’s instructions.
Cell line growth
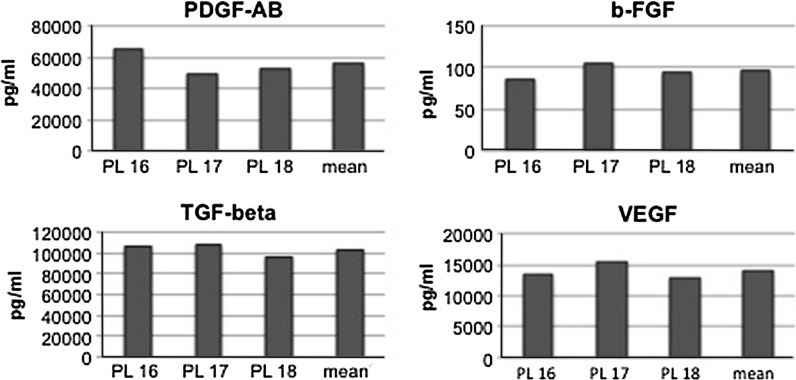
The following human cell lines were purchased from American Type Culture Collection (Manassas, VA, USA): HL-60 (acute promyelocytic leukemia), KG-1a (acute myelogenous leukemia), Jurkat (T cell leukemia), K562 (chronic myelogeneous leukaemia), MCF-7 (breast adenocarcinoma), HeLa (cervix adenocarcinoma). KG-1a and HL-60 cells were seeded at 500.000 cell per ml in Iscove’s Modified Dulbecco’s Medium (IMDM; Lonza, Walkersville, MD, USA) supplemented with 20 % FBS (Euroclone, Pero, MI, Italy) or 20 % PL. Jurkat and K562 cells were seeded at 500.000 cell per ml in RPMI 1640 medium (Lonza, Basel, Switzerland) supplemented with 10 % FBS or 10 % PL and 2 mM l-glutamine (Euroclone). Culture medium was replaced every 3 or 4 days. HeLa and MCF-7 cells were cultured in adherence at 10.000 cell per cm2 in Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Lonza) additioned with 10 % FBS or 10 % PL. Medium was renewed twice a week and when the cells reached confluence, usually within 1 week, they were detached by Trypsin–EDTA treatment (Euroclone) for counting and then re-seeded at appropriate concentration for next propagation steps. Cell enumeration at each passage was performed by flow cytometry analysis to identify lineage specific cells using the following monoclonal antibodies (Mo-Ab): CD235a FITC for K562 (Dako Denmark, Glostrup, Denmark), CD33 PE for HL60 (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany), CD3 FITC for Jurkat, CD34 PE and CD45 FITC for KG-1a, CD146 PE for HeLa, CD29 PE for MCF-7 cells (Becton-Dickinson, San José, CA, USA). Briefly, a known volume of each cell line culture was incubated 15′ in the dark with the appropriate Mo-Ab and 7-AAD (7-amino-actinomycin D) (Becton–Dickinson) to exclude dead cells. At the end of incubation, pre-defined amounts of fluorescent beads were added to the stained cells and beads were counted along with cells by using a FacsCalibur (Becton–Dickinson) to determine the absolute cell number per μl. Cells were gated on the basis of their physical features, forward and side scatter signals, and fluorescent signals. Two counts were performed for each sample and the average value of replicates was accepted as the correct absolute cell number. Doubling -time was calculated at every passage and the expansion rate was determined by dividing the total number of cells counted by the number of cells initially seeded. The three PL lots, PL-16, PL-17 and PL-18, were tested in parallel with FBS to evaluate the ability of each PL preparation to sustain the growth of the cell lines. To this aim, each cell line underwent six passages and the experiments were replicated three times. Prior to their use in growth experiments, each PL lot, including those tested in the present study, were subjected to a bone marrow-derived MSC-based “potency test” to verify that MSC confluence occurred within 15 days at the initial culture passage (P0) and that a minimum threefold expansion was documented at any further culture passage (P1, P2, P3 etc.). Similarly, the concentration of more biologically relevant growth factors were assessed as described above, to assure that each usable PL lot had concentrations not lower than the 70 % of the mean concentrations observed in 20 consecutive PL previous lots (see also Fig. 1).
Fig. 1.
Analysis of growth factor concentration by ELISA in PL preparations. Growth factor concentrations in PL 16, 17 and 18 were compared to the average concentrations (mean) observed in 20 consecutive previous PL lots
Cell cycle analysis
Cells obtained from each cell line culture were harvested and washed twice with PBS with 2 mmM EDTA (Sigma-Aldrich; St. Louis, MO, USA). Samples were incubated for 30 min at room temperature with a hypotonic DNA staining Solution containing 50 μg/ml Propidium Iodide (Becton–Dickinson), 6.25 μg/ml RNase A (Qiagen Inc., Valencia CA, USA) and 12.5 μl/ml Nonidet P40 (Sigma-Aldrich; St. Louis, MO, USA). Samples were run on a FacsCalibur (Becton–Dickinson) and analysed with ModFit LT 4.0 (Verity Software House, Inc., Topsham, ME, USA).
Chemosensitivity test
To measure the sensitivity to adriamycin, Jurkat, KG-1a and HL-60 cells were seeded in duplicate in culture media with or without adriamycin (Menarini, Florence, Italy) (concentration range 0.5–0.0025 μg/ml). Cells were counted 72 h after seeding by flow cytometry analysis and expressed as absolute count as described in the previous section.
Cytotoxic assay
The lytic activity of IL-2 activated CD56+ effector cells against K562 target cells, cultured with FBS or PL17 or PL18, was evaluated by carboxy fluorescein diacetate succinimidyl ester [CSFE ( Life Technology, Carlsbad, CA, USA )] based cytotoxic assay. Peripheral blood mononuclear cells (PBMC), obtained from healthy donors after written informed consent, were separated by density gradient Lympholyte (Cedarlain Laboratories, Ontario, Canada), centrifuged and cultured at 2 × 106 cells per ml in RPMI (Lonza) supplemented with 10 % FBS, l-Glutamine, antibiotics and IL-2 1000 U/mL (Miltenyi Biotech GmbH). On day 7, the lymphocyte subsets (CD3, CD4, CD8, CD45, CD16, CD56, CD19) were evaluated by Immunomonitoring Kit (Becton–Dickinson) and the CD56+ cells, both CD3+CD56+ and CD3−CD56+, were used as effector cells in the cytotoxic assay. 10 × 106 K562 target cells were labeled with 2 μM CFSE 10′ at 37 °C in the dark. Quench staining was performed on ice for 5′ by adding 5 volumes of ice-cold phosphate buffered saline (PBS) supplemented with 5 % human albumin (HA) (Grifols, Sant Cugat del Valles, BCN, Spain). Cells were then washed three times with cold PBS supplemented with 5 % HA and re-suspended at appropriate concentration in serum free medium (X-vivo 10, Lonza). CD56+ effector cells were added to viable labeled target cells at the following effector/target (E/T) ratios 20:1, 10:1, 5:1, 2, 5:1 and co-cultured for 90′. 7-AAD was then added to mark target dead cells and viable cells were evaluated by flow cytometry. The percentage of specific lysis was calculated as % of viable targets according to the following formula:
Statistical analysis
Data obtained by parallel growth experiments using FBS and PL16, PL17 and PL18 were compared during culture passages in terms of doubling time, fold expansions and sensitivity to adriamycin by a paired two-tailed Student’s t test. A p < 0.05 was considered significant.
Results and discussion
Characterization of platelet lysate
Several lots of PL were produced following a previously described GMP-compliant procedure (Iudicone et al. 2014). In our protocol PL lots were obtained by pooling 12 PLT units following a transfusional-based procedure, including pathogen inactivation by Intercept technology and three cycles of freezing and thawing, followed by membrane removal. This PL preparation can be considered a safe and well-standardized human supplement, that can be used as FBS substitute to support MSCs clinical-scale cultures without affecting their antigenic and functional properties. Moreover, we adopted the ability of PL to sustain MSC growth as a “potency test” to better characterize, beside the PL growth factor content, the quality and the potential of our PL preparations. The efficacy of PL to support in vitro cell proliferation relies on the content of growth factors, such as FGF-b, VEG-F, PDGF-AB and TGF-β, released from platelets during PL preparation process. Actually, most of these factors, released in vivo upon PLT activation, are known to play a critical role in the wound healing process; therefore the properties of PL in promoting in vitro cell growth is likely related to the presence of growth factors released from PLTs after freeze and thaw cycles. Three lots were specifically tested for their capacity to support several established tumor cell lines. These lots, named PL-16, PL-17 and PL-18, were evaluated for the content of growth factors (FGF-b, VEG-F, PDGF-AB, TGF-β) as product quality indicators, and the value of each factor was compared with the average values previously detected for 20 PL preparations (Fig. 1). As depicted in Fig. 1, the concentration of FGF-b, VEG-F, PDGF-AB and TGF-β was over 70 % of the average values (106 pg/ml ± 15 for FGF-b, 13.416 pg/ml ± 2.526 for VEGF-C, 55.547 pg/ml ± 7.673 for PDGF-AB and 94.338 pg/ml ± 13.105 for TGF-β) and no relevant variations were observed among the three distinct lots. Also the MSCs-based “potency test” (see section “Materials and methods”) revealed that each PL tested was able to support MSC growth within the acceptability limits that have been stated for product use in growth experiments.
Growth and proliferation
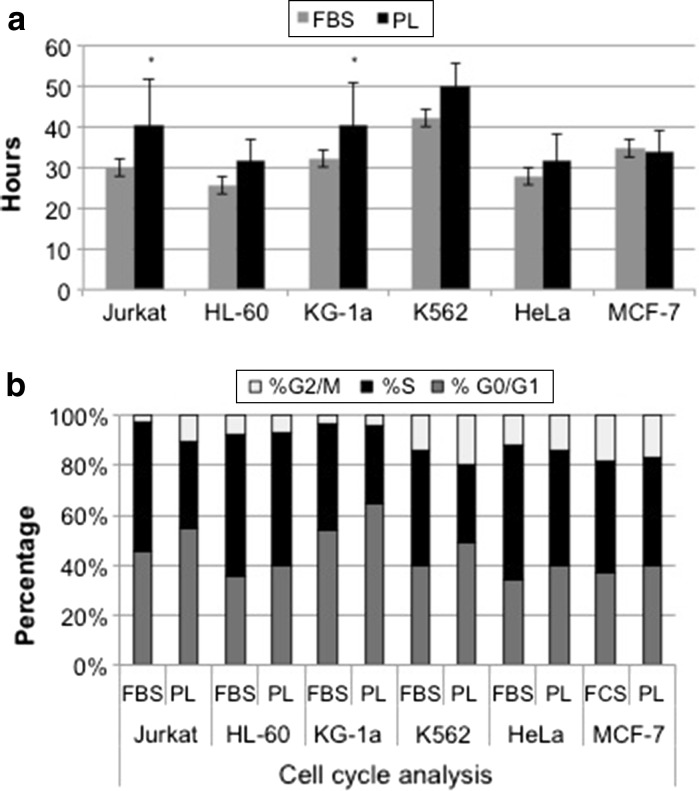
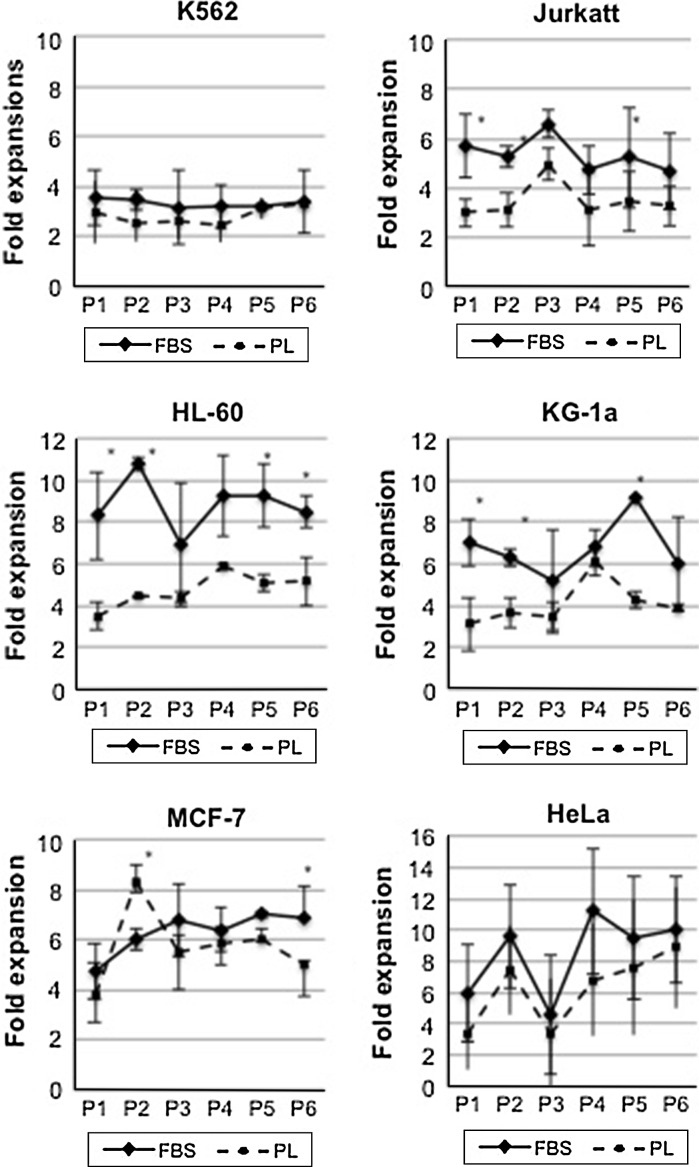
The experiments on human cell lines in vitro allow for reproducing in laboratory the behaviour of human cancer cells in vivo. Hence, these cells are often used to assess the ability of drugs, immunity or biological response modifiers to induce growth arrest, phenotypic and/or transcriptional changes with or without differentiation, epigenetic regulations or expression of new functional properties. In this field, the addition of xenogeneic material to the cultures may alter in some way the biologic response of these effectors due to the xenogeneic activation or the cell over stimulation (Balk et al. 1981).The growth promoting effect of PL was evaluated with haematopoietic and epithelial cell lines. Figure 2a shows the results in terms of doubling time average obtained from each PL lot compared to a single lot of commercial FBS. For each PL lot, three replicates of all individual cell line were evaluated, for a total of nine experiments, while three replicates of each cell line were performed for FBS. The results showed that FBS gave a faster replication rate for haematopoietic cell lines (Fig. 2a), with an average of doubling time 14 % higher then LP cultures, although the difference was significantly higher only for Jurkat and KG-1a (p < 0.05), as assessed using Student’s test. This observation was also associated with greater percentage of cells in the S-phase of the cell cycle, as depicted in Fig. 2b. An increase in G0/G1 phase of analysed cell lines, ranging from 4.6 to 11.1 %, was actually observed in the presence of PL. However, even if these differences in G0/G1 phase were consistently observed during cultures, they did not reach statistical significance. The proliferation effect of PL on these cell lines was also evaluated as fold expansion comparing cell growth during passage 1–6 (Fig. 3). The results confirmed that PL supports growth and expansion of all cell lines, although the cells cultured in presence of PL experienced a less massive proliferation compared to those grown on FBS. In particular, for MCF-7 and HL-60 the difference persisted and was statistically significant also at passage 6 (p < 0.05), resulting, respectively, in a fivefold instead of sevenfold expansion and in a sixfold instead of eightfold expansion.
Fig. 2.
a Doubling times observed in the different cell lines grown in the presence of FBS or PL 16, 17 and 18. Results are presented as the means ± standard deviations (SD) obtained from triplicates observed with FBS or PL 16, 17 and 18 for 6 passages. Thus, for PL cultures the means and SD of nine observations are shown. *Statistically significant differences between the two values (Student’s t test, p < 0.05). b Representative evaluation of cell cycle analysis of various cell lines carried out in the presence of FBS and PL 17. The average values obtained in a triplicate analysis are presented for both FBS and PL 17
Fig. 3.
Expansion rates expressed as fold expansions of the different cell lines grown in the presence of FBS or PL 16, 17 and 18. Results are presented as the means ± standard deviations (SD) obtained from triplicates observed with FBS or PL 16, 17 and 18. Thus, in the case of PL the means and SD of nine observations are shown. *Statistically significant differences between the two values (Student’s t test, p < 0.05)
Phenotype and morphology
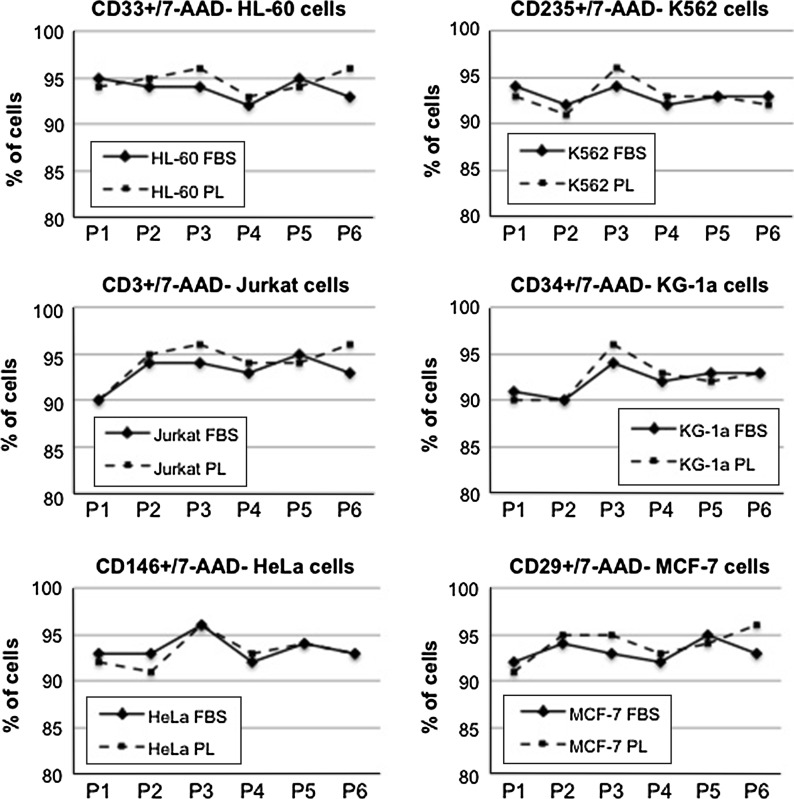
To analyse the lineage stability of cells cultured with PL, we performed phenotypic analysis using a constitutive marker for each cell line. In particular, we used CD235a for K562, CD33 for HL60, CD3 for Jurkat, CD34 and CD45 for KG-1a, CD146 for HeLa and CD29 for MCF-7. As depicted in Fig. 4, we found a comparable percentage of viable specific marker-expressing cells for each cell line cultured with either FBS or PL, which confirmed the persistence of lineage fidelity in all cultures at all passages (Fig. 4).
Fig. 4.
A representative example of phenotypic analysis of various cell lines carried out in the presence of FBS and PL17. The average values obtained in a triplicate analysis are presented for both FBS and PL 17
Figure 5 shows the morphologic aspect of cell lines during culture in presence of FBS or PL (PL17). As observed by microscopic examination, both K562 and Jurkatt (lymphoblasts) and KG1-a and HL-60 (myeloblasts) showed a typical spherical shape and were grown in suspension without attaching to the plate surface in both culture conditions (LP or FBS). Also for HeLa and MCF-7 (ephitelial cells) cells, which showed a typical polygonal shape and were grown attached to the plate, no differences were revealed between FBS or PL driven cultures.
Fig. 5.
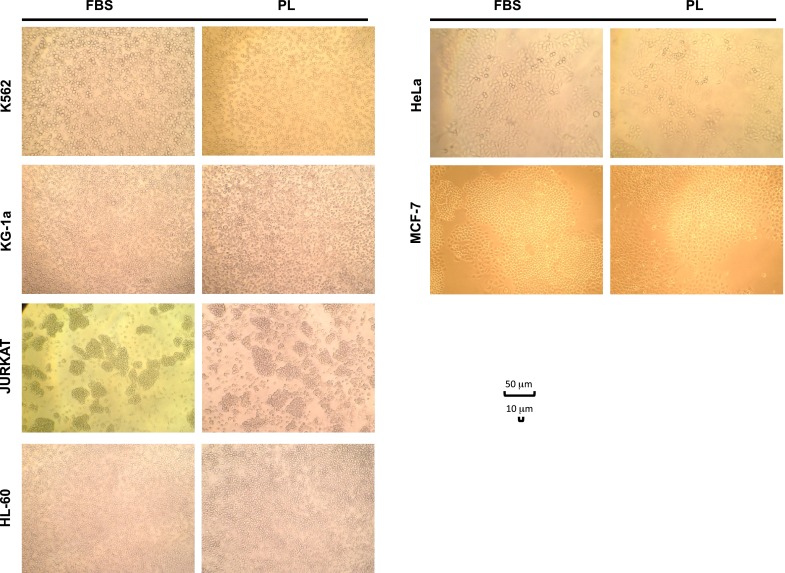
Microscope examination of various cell lines cultured in the presence of FBS or PL 17, showing similar cell characteristics at morphologic analysis (×100 magnification)
Functional assays
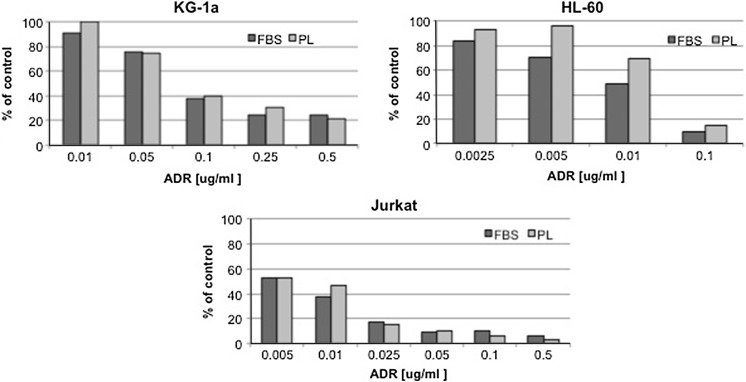
In order to evaluate the functional characteristics and responsiveness of tumor cell lines grown with either PL or FBS, we performed a more in depth analysis with either drug sensitivity test and cytotoxicity assay in vitro. Using KG-1a, HL-60 and Jurkat haematopoietic cell lines only, we tested their sensitivity to adriamycin exposure, using a drug dose range from 0.0025 to 0.5 μg/ml. Figure 6 shows that adriamycin maintains a dose-related effect on growth of each cell line in both FBS- and PL-supported cultures in a representative experiment using PL17. In the case of FBS cultures, a greater sensitivity to low doses of adriamycin was observed and this might correlate with the increased replication rate of these cells in presence of FBS, as indicated by the increased S-phase in their cultures. In fact, it seems that the presence of FBS rendered these cells more sensitive to the drug, likely altering the real capacity of these effectors to induce growth arrest and cell death. Moreover, regarding this aspect, it has been reported that primary leukemic cells showed an S-phase ranging from 20 to 45 %, which is the same range that we found in PL cultures, which may more likely resemble the kinetic behaviour of these primary cells (Ishiyama et al. 1994). However, a proper dose–response curve was also observed for cells cultured in PL, indicating a preserved cell sensitivity to the drug, with differences that did not reach statistical significance at any dose-point, except for HL-60 at 0.005 and 0.01 μg/ml (p < 0.05).
Fig. 6.
A representative example of cell exposure to adriamycin of KG-1a, HL-60 and Jurkat haematopoietic cell lines carried out in the presence of FBS and PL 17. The average values obtained in triplicate assays are presented for both FBS and PL 17 in a dose range from 0.0025 to 0.5 μg/ml
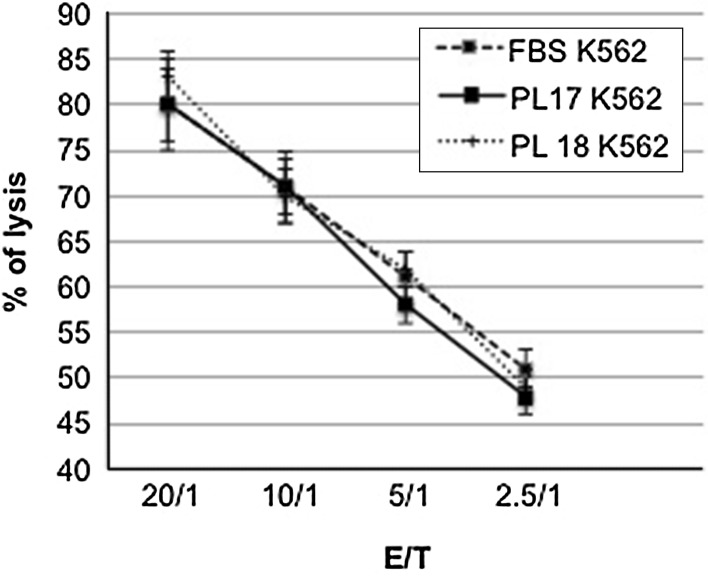
Finally, as K562 is a cell line susceptible to lysis by NK effectors, we tested the ability of IL-2 activated NK cells to lyse K562 target cells cultured with FBS or PL to verify whether the presence of PL in culture medium could influence sensitivity of K562 to NK killing. Three distinct NK cell populations were generated by culturing PBMC from three healthy donors in presence of high dose IL-2 and their lytic potential was tested against K562 cultivated, respectively, with FBS or PL (lots PL17 and PL18). NK effector cells were co-cultured with FBS-K562 or LP-K562 target cells for 90 min at an E/T cell ratio ranging between 20/1 and 2.5/1. All the three NK cells showed an high lytic activity against both FBS or LP driven K562 targets with a percentage of specific lysis between 80 and 20 % according to the E/T ratios. These findings confirmed that PL-supported K562 cells retained sensitivity to NK cell-mediated lysis with a proper dose–response curve comparable, at any E/T ratios, to the curve obtained with FBS-cultured K562 cells (Fig. 7).
Fig. 7.
Evaluation of NK cell-mediated cell lysis of K562 cells grown in the presence of FBS or PL17 and PL18 at variable effector/target ratios ranging from 20/1 to 2.5/1. Each data point is presented as the mean ± standard deviation (SD) of three different K562 cell lysis obtained culturing FBS- or PL-supported cells with three different NK cell populations obtained from three different healthy donors
Conclusion
The efforts to find a substitute to replace FBS has become a relevant goal in the field of cell and tissue culture since the use of FBS may present several disadvantages, both for the ethical concerns about its collection and production, and for the requirement of safe, defined and animal-free culture conditions for experimental cultures and cell therapy use (Bernardo et al. 2007; Gstraunthaler 2003; Jochems et al. 2002; Sundin et al. 2007). Moreover, the replacement of FBS, which can often display high batch-to-batch variability, would lead to a better quality and reproducibility of experimental data (Balls and Morton 2010; Brunner et al. 2010). For these reasons, many human alternatives to animal serum have been tested in the last decades, including growth factors, human serum and plasma, tissue extract and PL. (Ferro et al. 2012; Gstraunthaler 2003; Hankey et al. 2001; Kocaoemer et al. 2007; Lin et al. 2005; Morimoto et al. 2011; Stute et al. 2004). PL can be obtained from outdated donor PLTs and contains a wide series of growth factors. The stimulatory effect of PL for in vitro cell propagation has been already proven by several reports, that, for the most part, have been conducted to find a human FBS replacement for clinical-grade expansion of MSCs. Nevertheless, the development of cellular therapeutics requires the use of standardized products for cell culture with the mandatory avoidance of animal-derived components and the compliance with GMP (Fekete et al. 2012). Thus, in view of the rapidly increasing applications of MSCs, as previously showed, we have set up a clinical grade protocol to produce a standardized and safe PL for clinical-grade cell expansion (Iudicone et al. 2014). In the present study, we analyzed the ability of PL to support the normal rate of growth and expansion of established haematopoietic and epithelial cancer cell lines in comparison with FBS. We tested three different lots of PL for the content of growth factors and the ability to sustain MSCs growth. LP was added to culture medium at the same concentration as used for the competitor FBS. The growth promoting effect of PL was evaluated in terms of doubling time and fold expansion during passage 1–6. The results confirmed that PL supports growth and expansion of all cell lines, although the cells cultured in presence of PL experienced a less massive proliferation compared to those grown on FBS. However, we found no differences in terms of cell morphology, phenotype and sensitivity to adriamycin and to NK cell-mediated lysis. In conclusion, we have demonstrated that human PL (now trademarked as Mesengen and produced by Futura Re-Life, Rome, Italy) is able to support growth and propagation of human cell lines without altering their morphologic, phenotypic and functional characteristics. PL can be produced at relatively low cost, without ethical issues and safety concerns, and can offer substantial advantages for various applications in the field of cell and tissue culture, both from a perspective of performance, process standardization, ethicality and product safety.
Acknowledgments
This work was supported in part by a grant of Futura Stem Cells sa.
Contributor Information
R. Fazzina, Email: raffaella.fazzina@inscientiafides.com
P. Iudicone, Email: piudicone@scamilloforlanini.rm.it
A. Mariotti, Email: andrea.mariotti@rm.unicatt.it
D. Fioravanti, Email: dfioravanti@scamilloforlanini.rm.it
A. Procoli, Email: annabella.procoli@gmail.com
E. Cicchetti, Email: elisabetta.cicchetti1988@gmail.com
G. Scambia, Email: giovanni.scambia@rm.unicatt.it
G. Bonanno, Email: giuseppina.bonanno@rm.unicatt.it
L. Pierelli, Phone: (0039) 06 58703288-3500, Email: lpierelli@me.com
References
- Avanzini MA, Bernardo ME, Cometa AM, Perotti C, Zaffaroni N, Novara F, Visai L, Moretta A, Del Fante C, Villa R, Ball LM, Fibbe WE, Maccario R, Locatelli F (2009) Generation of mesenchymal stromal cells in the presence of platelet lysate: a phenotypic and functional comparison of umbilical cord blood- and bone marrow-derived progenitors. Haematologica 94:1649–1660 [DOI] [PMC free article] [PubMed]
- Baik SY, Lim YA, Kang SJ, Ahn SH, Lee WG, Kim CH. Effects of platelet lysate preparations on the proliferation of HaCaT cells. Ann Lab Med. 2014;34:43–50. doi: 10.3343/alm.2014.34.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk SD, Levine SP, Young LL, LaFleur MM, Raymond NM. Mitogenic factors present in serum but not in plasma. Proc Natl Acad Sci USA. 1981;78:5656–5660. doi: 10.1073/pnas.78.9.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balls M, Morton D. FRAME and the three Rs: yesterday, today and tomorrow. Altern Lab Anim. 2010;38:269–274. doi: 10.1177/026119291003800402. [DOI] [PubMed] [Google Scholar]
- Barsotti MC, Losi P, Briganti E, Sanguinetti E, Magera A, Al Kayal T, Feriani R, Di Stefano R, Soldani G (2013) Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS One 8:e84753 [DOI] [PMC free article] [PubMed]
- Bernardi M, Albiero E, Alghisi A, Chieregato K, Lievore C, Madeo D, Rodeghiero F, Astori G (2013) Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow-derived mesenchymal stromal cells. Cytotherapy 15:920–929 [DOI] [PubMed]
- Bernardo ME, Avanzini MA, Perotti C, Cometa AM, Moretta A, Lenta E, Del Fante C, Novara F, de Silvestri A, Amendola G, Zuffardi O, Maccario R, Locatelli F (2007) Optimization of in vitro expansion of human multipotent mesenchymal stromal cells for cell-therapy approaches: further insights in the search for a fetal calf serum substitute. J Cell Physiol 211:121–130 [DOI] [PubMed]
- Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, Kluter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331–2341. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- Brunner D, Frank J, Appl H, Schoffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. Altex. 2010;27:53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- Castiglia S, Mareschi K, Labanca L, Lucania G, Leone M, Sanavio F, Castello L, Rustichelli D, Signorino E, Gnetti M, Bergallo M, Bordiga AM, Ferrero I, Fagioli F (2014) Inactivated human platelet lysate with psoralen: a new perspective for mesenchymal stromal cell production in Good Manufacturing Practice conditions. Cytotherapy 16:750–763 [DOI] [PMC free article] [PubMed]
- Cholewa D, Stiehl T, Schellenberg A, Bokermann G, Joussen S, Koch C, Walenda T, Pallua N, Marciniak-Czochra A, Suschek CV, Wagner W (2011) Expansion of adipose mesenchymal stromal cells is affected by human platelet lysate and plating density. Cell Transpl 20:1409–1422 [DOI] [PubMed]
- Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, Lataillade JJ. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–236. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- Fekete N, Rojewski MT, Furst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 2012;7:e43255. doi: 10.1371/journal.pone.0043255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro F, Spelat R, Beltrami AP, Cesselli D, Curcio F. Isolation and characterization of human dental pulp derived stem cells by using media containing low human serum percentage as clinical grade substitutes for bovine serum. PLoS One. 2012;7:e48945. doi: 10.1371/journal.pone.0048945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. Altex. 2003;20:275–281. [PubMed] [Google Scholar]
- Hankey DP, McCabe RE, Doherty MJ, Nolan PC, McAlinden MG, Nelson J, Wilson DJ. Enhancement of human osteoblast proliferation and phenotypic expression when cultured in human serum. Acta Orthop Scand. 2001;72:395–403. doi: 10.1080/000164701753542069. [DOI] [PubMed] [Google Scholar]
- Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014;16:170–180. doi: 10.1016/j.jcyt.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Hofbauer P, Riedl S, Witzeneder K, Hildner F, Wolbank S, Groeger M, Gabriel C, Redl H, Holnthoner W (2014) Human platelet lysate is a feasible candidate to replace fetal calf serum as medium supplement for blood vascular and lymphatic endothelial cells. Cytotherapy 16:1238–1244 [DOI] [PubMed]
- Honn KV, Singley JA, Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975;149:344–347. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- Ishiyama K, Satoh S, Igarashi Y, Kumagai H, Yahagi A, Sasaki H. Flow cytometric analysis of the cell cycle of the leukemic cell lines treated with etoposide and cytosine arabinoside. Tohoku J Exp Med. 1994;174:95–107. doi: 10.1620/tjem.174.95. [DOI] [PubMed] [Google Scholar]
- Iudicone P, Fioravanti D, BonannoG, Miceli M, Lavorino C, Totta P, Frati L, Nuti M, Pierelli L (2014) Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J Transl Med 12:28 [DOI] [PMC free article] [PubMed]
- Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002;30:219–227. doi: 10.1177/026119290203000208. [DOI] [PubMed] [Google Scholar]
- Johansson L, Klinth J, Holmqvist O, Ohlson S. Platelet lysate: a replacement for fetal bovine serum in animal cell culture? Cytotechnology. 2003;42:67–74. doi: 10.1023/B:CYTO.0000009820.72920.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaoemer A, Kern S, Kluter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells. 2007;25:1270–1278. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- Lin HT, Tarng YW, Chen YC, Kao CL, Hsu CJ, Shyr YM, Ku HH, Chiou SH (2005) Using human plasma supplemented medium to cultivate human bone marrow-derived mesenchymal stem cell and evaluation of its multiple-lineage potential. Transpl Proc 37:4504–4505 [DOI] [PubMed]
- Morimoto N, Takemoto S, Kanda N, Ayvazyan A, Taira MT, Suzuki S. The utilization of animal product-free media and autologous serum in an autologous dermal substitute culture. J Surg Res. 2011;171:339–346. doi: 10.1016/j.jss.2009.11.724. [DOI] [PubMed] [Google Scholar]
- Rauch C, Feifel E, Amann EM, Spotl HP, Schennach H, Pfaller W, Gstraunthaler G. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. Altex. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- Reed GL. Platelet secretory mechanisms. Semin Thromb Hemost. 2004;30:441–450. doi: 10.1055/s-2004-833479. [DOI] [PubMed] [Google Scholar]
- Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stademeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D (2007) Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 47:1436–1446 [DOI] [PubMed]
- Schallmoser K, Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol Biol. 2013;946:349–362. doi: 10.1007/978-1-62703-128-8_22. [DOI] [PubMed] [Google Scholar]
- Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol. 2004;32:1212–1225. doi: 10.1016/j.exphem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- Trojahn Kolle SF, Olivieri RS, Glovinski PV, Kirchhoff M, Mathiasen AB, Elberg JJ, Andersen PS, Drzewiecki KT, Fischer-Nielsen A ( 2013) Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy 15:1086–1097 [DOI] [PubMed]
- Warnke PH, Humpe A, Strunk D, Stephens S, Warnke F, Wiltfang J, Schallmoser K, Alamein M, Bourke R, Heiner P, Liu Q (2013) A clinically-feasible protocol for using human platelet lysate and mesenchymal stem cells in regenerative therapies. J Craniomaxillofac Surg 41:153–161 [DOI] [PubMed]