Abstract
Cisplatin (cDDP) is one of the most widely used anticancer-drugs in both therapy and research. However, cDDP-resistance is the greatest obstacle for the successful treatment of cancer patients. In the present study, the possible joint anticancer effect of bee venom (BV), as a natural toxin, and cDDP towards human glioblastoma A1235 cells was evaluated. Treatment with BV alone in concentrations of 2.5–30 μg/ml displayed dose-dependent cytotoxicity towards A1235 cells, as evaluated with different cytotoxicity assays (MTT, Cristal violet and Trypan blue exclusion assay), with an IC50 value of 22.57 μg/ml based on the MTT results. Furthermore, BV treatment induced necrosis, which was confirmed by typical morphological features and fast staining with ethidium-bromide dye. Pre-treatment with BV induced cell sensitization to cDDP, indicating that BV could improve the killing effect of selected cells when combined with cDDP. The isobologram method used to determine the extent of synergism in combining two agents to examine their possible therapeutic effect showed that combined treatment induced an additive and/or synergistic effect towards selected cells depending on the concentration of both. Hence, a greater anticancer effect could be triggered if BV was used in the course of chemotherapy. The obtained results indicate that joint treatment with BV could be useful from the point of minimizing the cDDP concentration during chemotherapy, thus reducing and/or postponing the development of drug resistance. Our data, in accordance with previously reported results, suggests that BV could be used in the development of a new strategy for cancer treatment.
Keywords: Biotoxins, Bee venom, Cisplatin, Combination therapy, Cytotoxicity, Human glioblastoma cells
Introduction
A major problem in standard chemotherapy for treating cancer patients is the development of drug resistance during the course of the treatment. Cisplatin (cDDP) is one of the most effective anticancer drugs for the treatment of a variety of solid tumours, although treatment itself can often lead to development of cDDP resistance as well as numerous undesirable side effects such as severe kidney problems, allergic reactions, decreased immunity to infections, gastrointestinal disorders, haemorrhages, and hearing loss; especially in younger patients (Stewart 2007; Macciò and Madeddu 2013; Stathopoulos 2013; Dasari and Bernard Tchounwou 2014).
The effectiveness of cDDP seems to be due to its unique properties. It enters cells via multiple pathways and forms different DNA-platinum adducts while initiating a cellular self-defence system by activating or silencing a variety of different genes, resulting in epigenetic and/or genetic alternations (Shen et al. 2012). cDDP is a DNA damaging agent, because its cytotoxic effect is based upon the formation of platinum–DNA adducts and cross-links leading to cell cycle arrest which allows the cell to repair the damage. If repair fails, apoptosis is induced by the activation of different pathways (Fink and Howell 2000; Fuertes et al. 2003). cDDP can also induce non-DNA damage due to formation of reactive oxygen species (ROS) (Brozović et al. 2010). The therapeutic result of cDDP-based chemotherapy can be compromised by the development of cDDP resistance which can be a consequence of several molecular mechanisms that may occur in the same cell population such as decreased accumulation and increased detoxification of cDDP, more effective removal of platinum–DNA adducts, greater capacity to replicate past adducts, inhibition of apoptosis and/or cell adhesion-mediated cDDP resistance (Stewart 2007; Brozović et al. 2010).
In order to overcome cDDP resistance and reduce normal cell toxicity, combination therapies of cDDP with other drugs, especially natural toxins have been highly considered. In the search for new cancer agents, biotoxins have recently attracted attention owing to their novel mode of actions and decreased possibility of resistance development (Gaspar et al. 2013; Gajski et al. 2014; Liu et al. 2014). Bee venom (BV) has been used in oriental medicine as a source of drugs to cure different disorders (Son et al. 2007; Gajski and Garaj-Vrhovac 2011; Oršolić 2012; Liu et al. 2014; Premratanachai and Chanchao 2014). There are several reports in the literature about the beneficial properties of BV, such as radioprotective (Varanda and Tavares 1998), antimutagenic (Varanda et al. 1999), antinociceptive (Chen and Lariviere 2010), antibacterial (Boukraâ and Sulaiman 2009), antiviral (Kouros 2013) and anticancer effects (Son et al. 2007; Oršolić 2012). Several mechanisms of BV cytotoxicity on different types of cancer cells are known, like cell cycle alterations, effects on proliferation and growth inhibition, as well as induction of cell death either by apoptosis or necrosis both in vivo and in vitro (Son et al. 2007; Gajski et al. 2011; Oršolić 2012; Gajski and Garaj-Vrhovac 2013).
BV is a mixture of different active components with beneficial properties and the two major ones are melittin (MEL) and phospholipase A2 (PLA2) (Son et al. 2007; Garaj-Vrhovac and Gajski 2009; Oršolić 2012; Gajski and Garaj-Vrhovac 2013). MEL is a small lytic peptide that exerts its activity through interactions with the plasma membrane and the enzyme system of the cells (Dempsey 1990; Raghuraman and Chattopadhyay 2007). PLA2 is an enzyme that catalys the hydrolysis of the sn-2 fatty acyl-ester bond of membrane glycero-3-phospholipids to generate free fatty acids and lysophospholipids suggested for anticancer therapies (Six and Dennis 2000; Putz et al. 2006; Oršolić 2012).
Previously, we reported that MEL is cytotoxic towards different types of tumour cells including human glioblastoma A1235 cells (Gajski et al. 2011). Therefore, the current study aims to investigate the possible combined anticancer activity of BV and cDDP towards A1235 cells in vitro. To the best of our knowledge, this is the first report on the combined effect of BV and cDDP on A1235 cells. Cytotoxicity and effects on proliferation of BV alone was evaluated using three distinct assays (MTT, Cristal violet and Trypan blue exclusion assay). As sublethal concentrations of BV can stimulate cell growth, in the frame of the present study we used higher BV concentrations lower than those which induce the death of the majority of treated cells according to our previous data (Gajski et al. 2011). Moreover, we also examined morphological changes and the type of cell death induced by BV and investigated the combined treatment of cDDP and BV on A1235 cells in order to see if this combined treatment had additive and/or synergistic effects that could be applied in cancer treatment later on.
Materials and methods
Cell cultures and treatment
In the presents study, human glioblastoma A1235 cells were used. The cells were given by Dr. Branko Brdar (described in Brdar and Matulić 1988). Cells were kept as a monolayer culture in Dulbecco’s modified Eagle’s medium, DMEM (GIBCO, Gaithersburg, MD, USA) with 10 % foetal bovine serum (GIBCO) and antibiotics (penicillin and streptomycin; Sigma, St Louis, MO, USA) in a humidified atmosphere at 37 °C with 5 % CO2. Cells were seeded and after overnight incubation, they were treated with BV (Sigma). BV was dissolved in sterile redistilled water and further on dissolved in growth medium to a broad range of concentrations. At a certain time point after the treatment, cells were collected for further experiments. Each experiment was repeated at least two times.
Cytotoxicity and proliferation assays
MTT assay
Cytotoxicity of BV alone was evaluated using modified colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma) assay (Mickisch et al. 1990). The same test was also used to evaluate cell response to cDDP (Sigma) treatment as well as to joint treatment with BV and cDDP. Approximately 5 × 103 cells were plated in each well of a 96-well culture plate. On the following day, the medium was aspirated and replaced with fresh growth medium in which BV diluted in medium was added at different concentrations. The cells were treated with different concentrations of BV for 72 h at 37 °C in triplicate. For the joint treatment, cells were first pre-treated with different concentrations of BV for 1 h and then different concentrations of cDDP were added to cell cultures. Afterwards, the medium was aspirated and 20 μg of MTT dye/0.04 ml medium was added to each well. After 4 h of incubation at 37 °C, formazan crystals were dissolved in dimethyl sulfoxide (DMSO; 0.17 ml/well; Kemika, Zagreb, Croatia) and the plates were mechanically agitated for 5 min. Absorbance of each well was measured on a plate reader (Awareness Technology Inc., Palm City, FL, USA) at 545 nm.
Cristal violet assay
The proliferation of A1235 cells following exposure to BV was determined using a modified Crystal violet assay (Kueng et al. 1989). Crystal violet (Sigma) stains cells (alive or dead) that adhere to the bottom of a well. Cells were plated and treated as described above. Afterwards, cells were stained for 15 min with 50 μl of a solution containing 0.5 % Crystal violet in 20 % methanol (Kemika). The staining solution was then removed and wells were rinsed twice with water and dried at room temperature. To solubilize the stained cells, 100 μl per well of a solution of 0.1 M citrate sodium pH 4.2 and 50 % methanol were added and plates were kept for 30 min at room temperature. The absorbance of each well was measured on a plate reader (Awareness Technology Inc.) at 545 nm.
Trypan blue exclusion assay
Cytotoxicity of the BV alone towards A1235 cells was also studied using Trypan blue exclusion technique (Strober 2001). Cells were plated and treated as described above. Afterwards, the cells were stained with Trypan blue dye (0.4 %; BDH Chemicals, Poole, UK) and analysed under a light microscope (Olympus CX41, Tokyo, Japan). The viable (uncoloured) and dead (blue) cells were counted.
Morphological changes and cell death evaluation
To determine the morphological changes and type of cell death, light and fluorescence microscopy were used, respectively. Approximately, 5 × 103 cells/well of A1235 cells were plated in tissue culture plates. Afterwards, cells were incubated with different concentrations of BV for 1 h. Cells were also treated with cDDP (20 µM) for 24 h as a positive control for apoptotic cell death. The type of cell death was assessed by differential staining with acridine-orange (AO; Serva, Heidelberg, Germany) and ethidium-bromide (EtBr; Serva). Live cells with a functional membrane display uniform green staining of the nucleus and dead cells were characterized with the uniform red staining of the nucleus. Briefly, adherent and floating cells were collected by centrifugation and resuspended to a small volume of culture medium, after which in 10 µl of cell suspension 2 µl of AO (15 µg/ml in phosphate saline buffer (PBS)) and 2 µl of EtBr (50 µg/ml in PBS) were added. Samples were examined under an epifluorescence microscope (Axiovert 35, Zeiss, Jena, Germany). Fluorescence was detected through the BP 450–490, FT 510, LP 520 filter and images were taken by camera (Pixera Pro150ES, San Jose, CA, USA).
Statistics
To determine the extent of synergism in combining two agents for yielding a possible therapeutic effect, the isobologram analysis method was used. The synergy index (q) was calculated using the formula q = E(A + B)/(EA + EB − EA × EB) (Jin 1980). This method was used to reveal a possible additive and/or synergistic effect of BV and cDDP. E(A + B) represents the inhibition rate of combination group, while EA and EB represent the individual group. The value of q, which ranges from 0.85 to 1.15, indicates that the combination gives a simple addictive effect, while q values from 1.15 to 2.0 indicate a synergistic effect. As it was >2.0, this means that there is a significant synergistic effect between the two agents. Experimental results were statistically analysed using Student’s t test. p < 0.05 was considered significant.
Results
The cytotoxic and antiproliferative effect of BV alone was investigated on A1235 cells using three different assays (MTT, Cristal violet and Trypan blue exclusion assays). BV displayed cytotoxicity and antiproliferative effect towards A1235 cells, and the observed effects were dose-dependent. The IC50 value was (i.e. the concentration that reduces viability of treated cells to 50 %) 22.56 μg/ml based on the MTT results (Fig. 1).
Fig. 1.
Cytotoxic and antiproliferative effects of bee venom (BV) on human glioblastoma A1235 cells. Cells were plated in 96-well tissue culture plates and treated after 24 h with different concentrations of BV for 72 h. Cell viability was evaluated with the MTT assay, Crystal violet (CV) assay and Trypan blue (TB) exclusion assay. *Statistically significant compared to corresponding control (p < 0.05)
Morphological changes were induced rapidly after the addition of BV and were characterized with round and granulated cells, cell shrinkage, and separation from neighbouring cells and eventual detachment from the culture plates (Fig. 2). Moreover, BV induced a necrotic type of cell death compared to treatment with cDDP where apoptosis was observed (Fig. 2). The detection of cells dying by necrosis is determined by the fact that compared to apoptosis, the cell membrane becomes permeable very early, but the nucleus disintegrates only later on. Hence, pyknotic and fragmented nuclei are characteristic for apoptosis, but round and intact nuclei point to necrosis. It is important to note that cells in the late stages of apoptosis are also membrane permeable due to secondary necrosis.
Fig. 2.

Effects of bee venom (BV) on the morphology and type of cell death of human glioblastoma A1235 cells. Cells were treated with different concentrations of BV for 1 h and viewed and photographed under light and epifluorescent microscope. a For the morphology determination pictures were taken at 400× magnification. Bar = 20 µm. b For determination of apoptotic and/or necrotic cell death pictures were taken at 1000× magnification. Bar = 10 µm. Cisplatin (cDDP, 20 μM for 24 h) was used as a positive control of apoptotic cell death
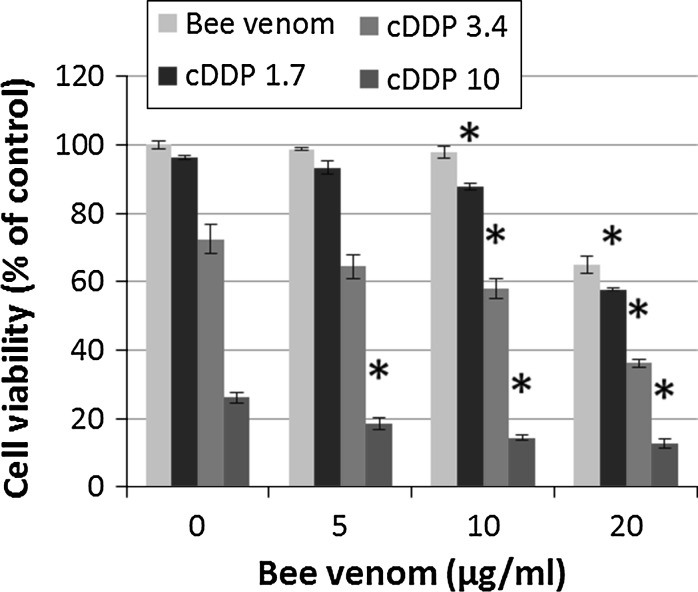
For the evaluation of the combined treatment with BV and cDDP, A1235 cells were pre-treated with different concentrations of BV for 1 h, after which cDDP was added. After pre-treatment with BV, cell sensitization to cDDP was noticed for higher doses of BV and cDDP. The isobologram method revealed that the effect of BV and cDDP given in combination on A1235 cells was synergistic (q ranges from 1.15 to 2.0) for a broad range of BV and cDDP combinations, although a significant synergistic effect was observed only for the lowest cDDP concentration and 10 μg/ml BV, while some combinations of BV and cDDP only gave an additive effect, regardless of the concentration of both (Fig. 3).
Fig. 3.
Effect of combined treatment with bee venom (BV) and cisplatin (cDDP) on human glioblastoma A1235 cells. Cells were plated in 96-well tissue culture plates and treated after 24 h with different concentrations of BV for 1 h after which cDDP was added. Cell viability was evaluated with the MTT assay after 72 h of treatment. *Statistically significant compared to corresponding cDDP treatment (p < 0.05)
Discussion
Cancer is one of the most important public health problems today. Its high morbidity and mortality highlights the need for more effective treatment options. Several therapies have been included in cancer treatment today such as radiation therapy, surgery, chemotherapy, immunotherapy and hormonal therapy (Stewart and Kleihues 2003; Gotay 2010), with chemotherapy as the predominant option. Although successful, a large number of patients often develop a resistance to chemotherapeutics acquired by tumours, which greatly reduces the efficacy of cancer treatment. Additionally, chemotherapy can induce serious side effects (Stewart 2007; Dasari and Bernard Tchounwou 2014). All of this has led to the development of new strategies to achieve more successful cancer treatments. Therefore, new anticancer drugs developed from natural sources may increase the effectiveness of conventional chemotherapeutic drugs. Biotoxins have emerged as rich sources of potential compounds with the ability to target and kill cancer cells (Liu et al. 2014).
One biotoxin that tops the list of the many natural compounds that have been newly introduced as anticancer agents is BV isolated from Apis mellifera (Deorukhkar et al. 2007; Son et al. 2007; Garaj-Vrhovac and Gajski 2009; Oršolić 2012; Gajski and Garaj-Vrhovac 2013). BV is a complex mixture of a variety of active compounds and its toxic effects could be mostly attributed to its small peptide MEL, enzyme PLA2, as well as other peptide components such as apamin, mast cell degranulating (MCD) peptide and/or tertiapin. These components have known cytotoxic effects towards different cells (Son et al. 2007; Oršolić 2012; Gajski and Garaj-Vrhovac 2013) and are likely to be responsible for the effects observed in our study as well. In our previous study, peptides MEL, apamin, MCD peptide and tertiapin were identified as components of BV and we determined the concentration of MEL and its mass fraction in a BV sample, estimating it at 0.19 (Gajski et al. 2014). The identified peptides are also in agreement with earlier findings concerning peptide components of BV (Son et al. 2007; Oršolić 2012).
Until now, it has been demonstrated that BV has the ability to inhibit the growth of several types of cancer cell lines and induce cytotoxic, immunomodulatory, apoptotic and necrotic effects both in vivo and in vitro (Son et al. 2007; Oršolić 2012; Gajski et al. 2014; Liu et al. 2014). Previously, we reported that BV is cytotoxic towards different types of tumour and non-tumour cells in vitro (Gajski et al. 2011) with preferential cytotoxicity against tumour cells. Besides evaluating the cytotoxic effect of BV alone, morphological changes and type of cell death induced after BV treatment, the current study also investigated the possible combined anticancer effect of BV and cDDP towards A1235 cells in vitro, in a narrower concentration range around IC50 values established previously.
Using different cytotoxicity and proliferation assays (MTT assay, Crystal violet and Trypan blue exclusion assays), we evaluated cell viability and effects on proliferation after BV treatment. Furthermore, we evaluated morphological changes and the type of cell death induced by BV using light and fluorescent microscopy, respectively. The obtained results indicate that BV displayed dose-dependent cytotoxic and antiproliferative effects towards A1235 cells based on all three assays employed. The observed cytotoxic effects of BV could be based on the amphipathic properties of MEL and its ability to disturb cell membrane bilayer integrity, either by creation of defects, disruption, or through pore formation in the lipid bilayer that eventually leads to the collapse of transmembrane electrochemical gradients (Dempsey 1990; Raghuraman and Chattopadhyay 2007; Gajski and Garaj-Vrhovac 2013). Compared to normal cells with low membrane potential, the cell membranes of tumour cells maintain a large membrane potential. As a result, MEL selectively is more likely to disrupt the tumour cell membranes than those of normal cells. This could be an important mechanism of the anticancer activity of BV (Son et al. 2007; Gajski and Garaj-Vrhovac 2013; Premratanachai and Chanchao 2014).
In A1235 cells, we also noticed a breakdown of membrane integrity which was proven by EtBr staining, which enters only those cells that have damaged membranes. Furthermore, morphological changes induced by BV occurred quickly after cell treatment. Observed cell rounding and granulation, detachment from cultured plates and fast staining with EtBr indicate cell membrane damage as the cause of cell death and the necrotic type of the cell death in the concentration range tested. There are contradictory data about the type of cell death induced by BV. BV caused apoptosis in lung carcinoma cells (Jang et al. 2003), hepatoma cells (Hu et al. 2006), leukemic cells (Moon et al. 2006), breast cancer cells (Ip et al. 2008a), cervical epidermoid carcinoma cells (Ip et al. 2008b), synovial fibroblasts (Hong et al. 2005), prostate cancer cells (Park et al. 2011), bladder cancer cells, (Ip et al. 2012) ovarian cancer cells (Jo et al. 2012) and melanoma cells (Liu et al. 2002; Tu et al. 2008). On the contrary, in fibroblast-like synoviocytes in addition to apoptotic-like cell death, necrosis was present as well (Stuhlmeier 2007), whereas in human mammary carcinoma cells (Oršolić et al. 2003), cervical carcinoma cells and Chinese hamster lung fibroblasts (Oršolić 2009) both apoptosis and necrosis were observed. Moreover, BV did not induce either sub-G1 fractions or the cleavage of caspase-9, -3, or poly-(ADP-ribose)-polymerase (PARP) in normal human lymphocytes and lymphoma HL-60 cells, indicating cell death, although not through apoptosis pathways (Lee et al. 2007).
Based on our results, the effects of BV can be related to necrosis, which in addition to apoptosis and according to a large number of experimental data is also a promising way of cancer cell death. Thus far, necrotic processes have been characterized as accidental and uncontrolled cell death, whose typical characteristics include disruption of plasma membrane and induction of inflammation. However, recent studies indicate that necrosis could also be controlled as it shared the same stimuli, signalling pathways and protective mechanisms as apoptosis (Edinger and Thompson 2004; Golstein and Kroemer 2007; Vanden Berghe et al. 2014; Wang et al. 2014). Therefore, necrosis could also be deemed as one of the possible ways of cancer cell death although the significance of necrosis in cancer therapy needs to be further clarified.
A large number of anticancer agents, which includes cDDP, are capable of inducing drug resistance. In order to interact with intracellular targets, they need to pass the membrane barrier. Once they reach the cytosol many are deactivated and transported out of the cell before interacting with intracellular targets (Stewart 2007). To prevent such resistance and reduce normal cell toxicity, new drugs and strategies for cancer treatment need to be developed. In light of this, peptides like MEL have recently attracted attention due to their specific mode of action and reduced possibility of resistance development (Schweizer 2009; Gajski and Garaj-Vrhovac 2013; Carmona-Ribeiro and de Melo Carrasco 2014).
Here we observed that pre-treatment with BV increases cytotoxicity of cDDP in A1235 cells. The isobologram analysis method was used to determine the extent of synergism/additivity after joint treatment with BV and cDDP. The synergy index (q) on A1235 cells showed that some combinations of BV and cDDP could be synergistic, although a significant synergistic effect was observed only for the lowest cDDP concentration and 10 μg/ml BV, while some combinations only gave an additive effect. Even so, not only was there a direct effect of BV on the growth of A1235 cells, but joint treatment with cDDP also potentiated BV induced cytotoxicity which could be useful from the point of minimizing the cDDP concentration during cancer treatment or reducing cDDP resistance. Some of the possibilities could be creation of defects, disruption and pore formation in the cell membrane with MEL, facilitating cDDP uptake and accumulation, consequently causing a greater cytotoxic effect.
In our previous study (Gajski et al. 2014), we investigated the combined anticancer ability of BV and cDDP towards two pairs of tumour cell lines: parental cervical carcinoma HeLa and laryngeal carcinoma HEp-2 cells, and their cDDP-resistant HeLa CK and CK2 sublines, respectively. The results showed that BV applied alone displayed dose-dependent cytotoxicity against all cell lines tested triggering a necrotic type of cell death. Combined treatment induced an additive and/or weak synergistic effect towards the tested cell lines, suggesting that BV could increase the killing effect of selected cells when combined with cDDP. On the contrary, the results of the present study showed that the BV and cDDP combination was also significantly synergistic, suggesting a tumour type-dependent response to the selected combination.
Other authors also noticed joint effects of BV and MEL on the cytotoxicity of cytostatic drugs in different cancer cells (Oršolić 2009; Alizadehnohi et al. 2012; Safaeinejad et al. 2013; Choi et al. 2014). Oršolić (2009) observed potentiation of BV cytotoxicity with the DNA-damaging drug bleomycin on HeLa and Chinese hamster lung fibroblast V79 cells. Alizadehnohi et al. (2012) also showed that effects of cDDP in ovarian cancerous cDDP-resistant A2780cp cells could be enhanced by the addition of a non-lethal dose of BV. Most recently, Safaeinejad et al. (2013) evaluated the synergistic cytological effects of BV and novel palladium (II) complex [Pd (bpy) (Pi-Pydtc)]NO3 on human T-cell lymphoblastic leukemia MOLT-4 cells. They also found a clear synergistic effect of BV on a Pd complex and the apoptotic pathway activated by BV in combination with Pd complex was caspase-3-dependent. Furthermore, Choi et al. (2014) showed that treatment with BV in combination with docetaxel or cDDP resulted in a strong synergistic inhibitory effect on cell growth of human lung cancer A549 and NCI-H460 cells. Their results suggest that the combination of BV with lower doses of selected chemotherapeutics caused a significantly greater inhibition of lung cancer cell growth compared with either agent alone.
Conclusions
In the present study, BV given alone displayed cytotoxic and antiproliferative effects towards glioblastoma A1235 cells. Additionally, combined treatment with BV and cDDP induced an additive and/or synergistic effect towards the tested cell line, suggesting that BV could enhance the killing effect when combined with cDDP. Hence, a greater anticancer effect could be set off if BV is used in the course of chemotherapy. Although the detailed mechanism for the joint action of BV and cDDP needs further clarification, the favourable effect of such a combination on tumour cells is apparent. Minimizing the cDDP concentration during the course of therapy with compounds that increase their cytotoxicity could be very useful for patients with regard to unwanted side effects, as well as from the point of reducing and/or postponing the development of cDDP resistance during the course of standard chemotherapy. Our data, in accordance with previously reported results, suggests that BV could be used in the development of a new strategy for cancer treatment, although more studies are needed to show the suitability and particularly safety of this combination in cancer treatment. Therefore, further research to determine the exact mechanism of action and in vivo studies on animal models are warranted.
Acknowledgments
This work was supported by the Ministry of Science, Education and Sport of the Republic of Croatia (Grant Nos. 022-0222148-2125 and 098-0982913-2748).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alizadehnohi M, Nabiuni M, Nazari Z, Safaeinejad Z, Irian S. The synergistic cytotoxic effect of cisplatin and honey bee venom on human ovarian cancer cell line A2780cp. J Venom Res. 2012;3:22–27. [PMC free article] [PubMed] [Google Scholar]
- Boukraâ L, Sulaiman SA. Rediscovering the antibiotics of the hive. Recent Pat Antiinfect Drug Discov. 2009;4:206–213. doi: 10.2174/157489109789318505. [DOI] [PubMed] [Google Scholar]
- Brdar B, Matulić M. Induction of plasminogen activator by N-methyl-N’-nitro-N-nitrosoguanidine in mer+ and mer− human tumour cell strains. Carcinogenesis. 1988;9:2191–2195. doi: 10.1093/carcin/9.12.2191. [DOI] [PubMed] [Google Scholar]
- Brozović A, Ambriović-Ristov A, Osmak M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit Rev Toxicol. 2010;40:347–359. doi: 10.3109/10408441003601836. [DOI] [PubMed] [Google Scholar]
- Carmona-Ribeiro AM, de Melo Carrasco LD. Novel formulations for antimicrobial peptides. Int J Mol Sci. 2014;15:18040–18083. doi: 10.3390/ijms151018040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lariviere WR. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol. 2010;92:151–183. doi: 10.1016/j.pneurobio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KE, Hwang CJ, Gu SM, Park MH, Kim JH, Park JH, Ahn YJ, Kim JY, Song MJ, Song HS, Han SB, Hong JT. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins (Basel) 2014;6:2210–2228. doi: 10.3390/toxins6082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey CE. The actions of melittin on membranes. Biochim Biophys Acta. 1990;1031:143–161. doi: 10.1016/0304-4157(90)90006-X. [DOI] [PubMed] [Google Scholar]
- Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs. 2007;16:1753–1773. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Fink D, Howell SB. How does cisplatin kill cells? In: Kelland LR, Farrell N, editors. Platinum-based drugs in cancer therapy 7. Totowa, New Jersey: Humana Press Inc; 2000. pp. 149–167. [Google Scholar]
- Fuertes MA, Alonso C, Perez JM. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev. 2003;103:645–662. doi: 10.1021/cr020010d. [DOI] [PubMed] [Google Scholar]
- Gajski G, Garaj-Vrhovac V. Bee venom induced cytogenetic damage and decreased cell viability in human white blood cells after treatment in vitro: a multi-biomarker approach. Environ Toxicol Pharmacol. 2011;32:201–211. doi: 10.1016/j.etap.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Gajski G, Garaj-Vrhovac V. Melittin: a lytic peptide with anticancer properties. Environ Toxicol Pharmacol. 2013;36:697–705. doi: 10.1016/j.etap.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Gajski G, Čimbora-Zovko T, Osmak M, Garaj-Vrhovac V. Bee venom and melittin are cytotoxic against different types of tumor and non-tumor cell lines in vitro. Cancer Res J. 2011;4:159–174. [Google Scholar]
- Gajski G, Čimbora-Zovko T, Rak S, Rožman M, Osmak M, Garaj-Vrhovac V. Combined antitumor effects of bee venom and cisplatin on human cervical and laryngeal carcinoma cells and their drug resistant sublines. J Appl Toxicol. 2014;34:1332–1341. doi: 10.1002/jat.2959. [DOI] [PubMed] [Google Scholar]
- Garaj-Vrhovac V, Gajski G. Evaluation of the cytogenetic status of human lymphocytes after exposure to a high concentration of bee venom in vitro. Arh Hig Rada Toksikol. 2009;60:27–34. doi: 10.2478/10004-1254-60-2009-1896. [DOI] [PubMed] [Google Scholar]
- Gaspar D, Veiga AS, Castanho MA. From antimicrobial to anticancer peptides: a review. Front Microbiol. 2013;4:294. doi: 10.3389/fmicb.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gotay CC. Cancer prevention: major initiatives and looking into the future. Expert Rev Pharmacoecon Outcomes Res. 2010;10:143–154. doi: 10.1586/erp.10.9. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Rim GS, Yang HI, Yin CS, Koh HG, Jang MH, Kim CJ, Choe BK, Chung JH. Bee venom induces apoptosis through caspase-3 activation in synovial fibroblasts of patients with rheumatoid arthritis. Toxicon. 2005;46:39–45. doi: 10.1016/j.toxicon.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Hu H, Chen D, Li Y, Zhang X. Effect of polypeptides in bee venom on growth inhibition and apoptosis induction of the human hepatoma cell line SMMC-7721 in vitro and Balb/c nude mice in-vivo. J Pharm Pharmacol. 2006;58:83–89. doi: 10.1211/jpp.58.1.0010. [DOI] [PubMed] [Google Scholar]
- Ip SW, Liao SS, Lin SY, Lin JP, Yang JS, Lin ML, Chen GW, Lu HF, Lin MW, Han SM, Chung JG. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo. 2008;22:237–245. [PubMed] [Google Scholar]
- Ip SW, Wei HC, Lin JP, Kuo HM, Liu KC, Hsu SC, Yang JS, Mei-Dueyang Chiu TH, Han SM, Chung JG. Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res. 2008;28:833–842. [PubMed] [Google Scholar]
- Ip SW, Chu YL, Yu CS, Chen PY, Ho HC, Yang JS, Huang HY, Chueh FS, Lai TY, Chung JG. Bee venom induces apoptosis through intracellular Ca(2+) -modulated intrinsic death pathway in human bladder cancer cells. Int J Urol. 2012;19:61–70. doi: 10.1111/j.1442-2042.2011.02876.x. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Lim S, Han SM, Park HJ, Shin I. Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J Pharmacol Sci. 2003;91:95–104. doi: 10.1254/jphs.91.95. [DOI] [PubMed] [Google Scholar]
- Jin ZJ. Addition in drug combination. Acta Pharmacol Sin. 1980;1:70–76. [PubMed] [Google Scholar]
- Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Kouros N. New research finds HIV can be killed with bee venom. Monash Bioeth Rev. 2013;31:4. [PubMed] [Google Scholar]
- Kueng W, Silber E, Eppenberger U. Quantification of cells cultured on 96-well plates. Anal Biochem. 1989;182:16–19. doi: 10.1016/0003-2697(89)90710-0. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kang SJ, Kim BM, Kim YJ, Woo HD, Chung HW. Cytotoxicity of honeybee (Apis mellifera) venom in normal human lymphocytes and HL-60 cells. Chem Biol Interact. 2007;169:189–197. doi: 10.1016/j.cbi.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen D, Xie L, Zhang R. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in vitro and growth of murine B16 melanomas in-vivo. J Pharm Pharmacol. 2002;54:1083–1089. doi: 10.1211/002235702320266235. [DOI] [PubMed] [Google Scholar]
- Liu CC, Yang H, Zhang LL, Zhang Q, Chen B, Wang Y. Biotoxins for cancer therapy. Asian Pac J Cancer Prev. 2014;15:4753–4758. doi: 10.7314/APJCP.2014.15.12.4753. [DOI] [PubMed] [Google Scholar]
- Macciò A, Madeddu C. Cisplatin: an old drug with a newfound efficacy— from mechanisms of action to cytotoxicity. Expert Opin Pharmacother. 2013;14:1839–1857. doi: 10.1517/14656566.2013.813934. [DOI] [PubMed] [Google Scholar]
- Mickisch G, Fajta S, Keilhauer G, Schlick E, Tschada R, Alken P. Chemosensitivity testing of primary human renal cell carcinoma by a tetrazolium based microculture assay (MTT) Urol Res. 1990;18:131–136. doi: 10.1007/BF00302474. [DOI] [PubMed] [Google Scholar]
- Moon DO, Park SY, Heo MS, Kim KC, Park C, Ko WS. Key regulators in bee venom-induced apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells through downregulation of ERK and Akt. Int Immunopharmacol. 2006;6:1796–1807. doi: 10.1016/j.intimp.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Oršolić N. Potentiation of bleomycin lethality in HeLa and V79 cells by bee venom. Arh Hig Rada Toksikol. 2009;60:317–326. doi: 10.2478/10004-1254-60-2009-1936. [DOI] [PubMed] [Google Scholar]
- Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012;31:173–194. doi: 10.1007/s10555-011-9339-3. [DOI] [PubMed] [Google Scholar]
- Oršolić N, Šver L, Verstovšek S, Terzić S, Bašić I. Inhibition of mammary carcinoma cell proliferation in vitro and tumor growth in vivo by bee venom. Toxicon. 2003;41:861–870. doi: 10.1016/S0041-0101(03)00045-X. [DOI] [PubMed] [Google Scholar]
- Park MH, Choi MS, Kwak DH, Oh KW, do Yoon Y, Han SB, Song HS, Song MJ, Hong JT. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate. 2011;71:801–812. doi: 10.1002/pros.21296. [DOI] [PubMed] [Google Scholar]
- Premratanachai P, Chanchao C. Review of the anticancer activities of bee products. Asian Pac J Trop Biomed. 2014;4:337–344. doi: 10.12980/APJTB.4.2014C1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz T, Ramoner R, Gander H, Rahm A, Bartsch G, Thurnher M. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate. Cancer Immunol Immunother. 2006;55:1374–1383. doi: 10.1007/s00262-006-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman H, Chattopadhyay A. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. 2007;27:189–223. doi: 10.1007/s10540-006-9030-z. [DOI] [PubMed] [Google Scholar]
- Safaeinejad Z, Nabiuni M, Nazari Z. Potentiation of a novel palladium (II) complex lethality with bee venom on the human T-cell acute lymphoblastic leukemia cell line (MOLT-4) J Venom Anim Toxins Incl Trop Dis. 2013;19:25. doi: 10.1186/1678-9199-19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer F. Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol. 2009;625:190–194. doi: 10.1016/j.ejphar.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/S1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115:246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Stathopoulos GP. Cisplatin: process and future. J BUON. 2013;18:564–569. [PubMed] [Google Scholar]
- Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Stewart BW, Kleihues P. World cancer report. 2. Lyon: IARC Press; 2003. [Google Scholar]
- Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol A. 2001;3:A3B. doi: 10.1002/0471142735.ima03bs21. [DOI] [PubMed] [Google Scholar]
- Stuhlmeier KM. Apis mellifera venom and melittin block neither NF-kappa B-p50-DNA interactions nor the activation of NF-kappa B, instead they activate the transcription of proinflammatory genes and the release of reactive oxygen intermediates. J Immunol. 2007;179:655–664. doi: 10.4049/jimmunol.179.1.655. [DOI] [PubMed] [Google Scholar]
- Tu WC, Wu CC, Hsieh HL, Chen CY, Hsu SL. Honeybee venom induces calcium-dependent but caspase-independent apoptotic cell death in human melanoma A2058 cells. Toxicon. 2008;52:318–329. doi: 10.1016/j.toxicon.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- Varanda EA, Tavares DC. Radioprotection: mechanism and radioprotective agents including honey bee venom. J Venom Anim Toxins. 1998;4:5–21. doi: 10.1590/S0104-79301998000100002. [DOI] [Google Scholar]
- Varanda EA, Monti R, Tavares DC. Inhibitory effect of propolis and bee venom on the mutagenicity of some direct- and indirect-acting mutagens. Teratog Carcinog Mutagen. 1999;19:403–413. doi: 10.1002/(SICI)1520-6866(1999)19:6<403::AID-TCM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Feng Y, Wang N, Cheung F, Tan HY, Zhong S, Li C, Kobayashi S. Chinese medicines induce cell death: the molecular and cellular mechanisms for cancer therapy. Biomed Res Int. 2014;2014:530342. doi: 10.1155/2014/530342. [DOI] [PMC free article] [PubMed] [Google Scholar]