Abstract
In this study we evaluated the biological activity of alcoholic and aqueous extracts from the fruit of Berberis vulgaris. The total antioxidant capacity of Berberis was characterized by FRAP, DPPH, Folin–Ciocalteu while the anthocyanins content was measured by pH differential method. Cell viability and apoptotic property were determined by MTT and DNA fragmentation assays, respectively. Alcoholic extract of Berberis was richer in antioxidants and anthocyanins compared to aqueous extract. Although both extracts significantly inhibited proliferation of breast cancer cells (MCF-7); these changes were not observed in normal human breast epithelial cells (MCF10-A). The alcoholic extract was more effective in inducing apoptosis as detected by DNA fragmentation in treated cancer cells. Our results suggest that Berberis has potent antioxidant properties and cytotoxic effects that can induce apoptosis. Therefore, Berberis can potentially be exploited for the development of therapeutics to fight against human breast cancer.
Keywords: Breast cancer, Berberis vulgaris, Cytotoxicity, Antioxidant, Apoptosis
Introduction
Breast cancer is the most common malignancy in women both in the developed and the developing countries (Wen et al. 2014). Its common treatments are surgery, radiation and chemo-hormone therapies. However, most of the patients suffering from the disease ultimately develop drug resistance and experience intense side effects. So, it is required to apply effective therapies without side effects. In this context, the use of natural components such as herbs may find a new safe way for breast cancer treatment (Wen et al. 2013; Shafi Sofi et al. 2013; Fathi Najafi et al. 2007).
Reactive Oxygen Species (ROS), i.e. free radicals are one of the major causes for the conversion of normal cell to cancerous cells (Rajkumar et al. 2011). Herbs protect human body against ROS and reduce oxidative damage to DNA in cancer initiation or promotion. Antioxidants of herbs may mediate these biological effects by directly reacting with ROS, free radical scavenging or catalytic metals chelating (Kaliora et al. 2006; Koncic et al. 2010). Furthermore, medicinal plants with antioxidant potential inhibit proliferation of different cancer cells via apoptosis induction (Samarghandian and Shabestari 2013; Bathaie et al. 2013; Hoshyar et al. 2012, 2013). Apoptosis or programmed cell death is an ordered cellular suicide process that occurs in both physiological and pathological conditions. Understanding apoptosis in cancer is very important not only in its pathogenesis but also leaves clues of cancer treatment (Samarghandian and Shabestari 2013; Wong 2011).
Berberis vulgaris L. from Berberidaceae family are grown in Europe and Asia, specially in Iran. The medicinal properties for all parts of this plant have been reported including antimicrobial, antiemetic, antioxidant and anticancer activities (Wen et al. 2013; Končić et al. 2010; Abd El-wahab et al. 2013). The aim of the present study was to examine and compare the biological in vitro activities (antioxidant, anticancer and apoptotic properties) of alcoholic and aqueous extracts of Berberis fruits in human breast cancer cells, MCF-7 in comparison with the normal human epithelial cells (MCF10-A).
Materials and methods
Chemicals and cell culture
Human breast cancer (MCF-7) and normal breast epithelial (MCF10-A) cell lines were provided from Iranian Biological Resource Center (IBRC, Teheran, Iran). RPMI-1640 and DMEM: Ham’s F-12 media, fetal bovine serum (FBS), phosphate buffered saline (PBS), trypsin/EDTA solution, cell lysis buffer and 3-(4,5-dimetylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethylsulfoxid (DMSO) were purchased from Gibco BRL (Grand Island, NY, USA) and Sigma (St. Louis, MO, USA), respectively.
Preparation of extracts of Berberis vulgaris
The Berberis fruits were collected in the South Khorasan province of Iran, dried at room temperature while keeping away from direct sunlight and then powdered. To prepare aqueous extract, Berberis fruit powder was added to boiling water and brewed for 45 min. For preparation of alcoholic extract, Berberis fruit powder was added to ethanol 70 %, and incubated overnight in the shaker at room temperature. Then extracts were filtered by Whatman No. 1. The alcoholic extract was obtained by using vacuum rotary evaporator. Finally both alcohol and water extracts were completely dried using freeze drying method.
Evaluation of antioxidant activity of Berberis vulgaris
Ferric Reducing Antioxidant Power (FRAP) assay
The total antioxidant capacity of Berberis extracts was determined by FRAP assay (Benzie and Strains 1996). The results were expressed in M Fe(II)/g dry weight of plant extracts (DW).
Folin–Ciocalteu assay
The total phenolic contents of herbal extracts were measured using the Folin–Ciocalteu method (Rakitzis 1975). The data were expressed as milligram Gallic acid equivalents (GAE)/g dried weight of plant extracts (DW).
DPPH radical-scavenging activity
Effect of the herb extracts on 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical was measured, based on previous study (Brand-Williams et al. 1995). The percentage of DPPH free radical scavenges ability of Berberis is reported as (%).
Determination of Total monomeric anthocyanins (TMA)
The TMA have been estimated by a pH differential method (Giusti and Wrolstad 2005) using UV–Vis spectrophotometer. Results were expressed as mg of cyanidin-3-glucoside equivalents per liter of Berberis extracts.
Evaluation of anticancer effects of Berberis vulgaris on cells
Cell culture and cell viability assay
The MCF-7 and MCF-10A cells were cultured in RPMI-1640 and DMEM: Ham’s F-12 media, respectively. Cultures supplemented with 10 % FBS serum, 100 units/mL penicillin and 100 mg/mL streptomycin. Both cell lines were grown at 37 °C in humidified atmosphere containing 5 % CO2. The cells were seeded in 96-well plates (5 × 103 cells/well) and allowed to attach overnight. Before treatment, the cells were grown to 90 % confluency and starved by incubation in a basal medium for 24 h to synchronize cells to the resting stage. Then the synchronized cells were treated with different concentrations of Berberis (0–6 mg/ml) at various time intervals (0–72 h).The cytotoxic effect of Berberis extracts against MCF-7 and MCF-10A cells was evaluated by MTT assay. For analysis of the cytotoxic efficiency, the IC50 value of Berberis was calculated using the dose- and time-dependent curves by linear interpolation (Hoshyar et al. 2013).
DNA fragmentation assay
Biochemically, apoptosis is characterized by the activation of a nuclear endonuclease that cleaves DNA into multimers of base pairs and can be visualized as an oligosomal ladder by standard agarose gel electrophoresis. In brief, MCF-7 cells were treated with alcoholic extract of Berberis (1.5 and 3 mg/ml) for 48 h. DNA samples were isolated after cell lysis according to previous studies (Aghbali et al. 2013; Samarghandian and Shabestari 2013). The samples were analyzed by electrophoresis on 1.5 % agarose gel in TAE buffer containing 0.5 µg/ml ethidium bromide. Fragmented DNA of treated cells was compared with untreated cells as control, by UV illuminator.
Statistical analysis
All experiments were accomplished in triplicate and the data were expressed as mean ± standard error of the mean (SEM). Results were analyzed using one-way ANOVA followed by Tukey’s post hoc test using SPSS version 16 software. Differences at p < 0.05 were considered to be significant.
Results
Total antioxidant activity (TAA)
The FRAP values of adequate concentrations of both aqueous and alcoholic Berberis extracts (2.5 g/l) indicated that the reducing power of the alcoholic extract was higher compared to aqueous extract (Table 1).
Table 1.
The total antioxidant activity, total phenolics and total anthocyanins of both Berberis fruits extracts
| Antioxidant contents | Aqueous extract | Alcoholic extract |
|---|---|---|
| TAA1 (M Fe(II)/g DW) | 397.68 ± 7.11 | 542.93 ± 6.32 |
| TPC2 (GAE/g DW) | 184.1 ± 5.30 | 291.22 ± 2.52 |
| TAC3 (mg cy-3-glu/l) | 602 ± 6.8 | 785 ± 9.2 |
Data are mean ± standard error of the mean (SEM; n = 3)
1Total antioxidant activity expressed in M Fe(II) per gram dry weight (DW) of plant extracts
2Total phenol content expressed in milligrams of Gallic acid equivalents (GAE) per gram dried weight (DW) of plant extracts
3Total monomeric anthocyamins expressed as mg cy-3-glu per liter of plant extracts
Total phenolics contents (TPC)
The results of total phenolic values of Berberis extracts are presented in Table 1. The amounts of total phenolic content of equal concentration of aqueous and alcoholic extracts of Berberis (2.5 g/l) were 184.1 ± 5.30 and 291.22 ± 2.52 GAE/g DW, respectively.
Total anthocyanins contents (TAC)
Total monomeric anthocyanins of Berberis extracts are shown in Table 1. The data illustrate that TMA of ethanolic extract was higher than that of water extract of this herb.
Radical scavenging activity
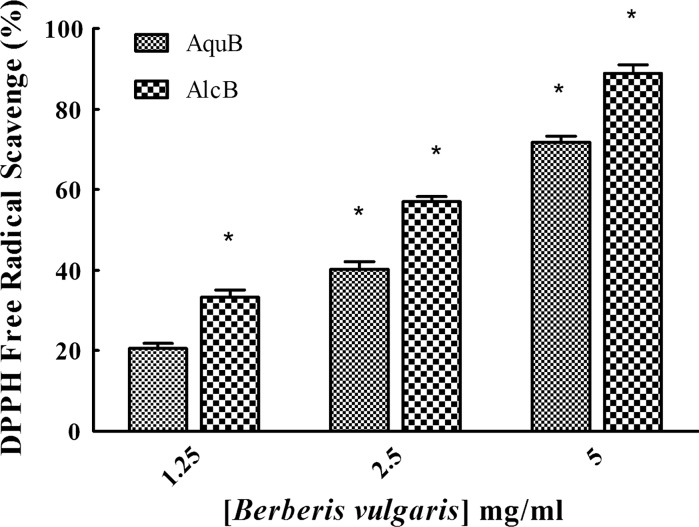
The results of DPPH assay showed (Fig. 1) that various concentrations (1.25, 2.5 and 5 mg/ml) of alcoholic extract of Berberis exhibited greater free radical scavenging activity (33.17, 56.97 and 88.9 %, respectively) than that of its aqueous extract with 20.55, 43.02 and 74.71 %. All results were compared with ascorbic acid as a control.
Fig. 1.
Percentage of DPPH radical quenching activity of various concentrations of alcoholic and aqueous extracts of Berberis compared to lowest concentration of water extract (1.25 mg/ml, as a reference). Data are expressed as mean ± SEM (n = 3) and the histograms marked with asterisk are significantly different at P < 0.05
Cytotoxic activity
The cytotoxic effects of various concentrations of alcoholic and aqueous extracts of Berberis on MCF-7 and MCF-10A cells for 24, 48 and 72 h were determined by MTT assay. As shown in Fig. 2 the viability of treated cancer cells was significantly reduced in a time- and dose-dependent manner compared to untreated cancer cells. Also the IC50 values (minimum concentration of extract to reduce cell viability to 50 %) of alcoholic and aqueous extracts of Berberis after incubation for 24, 48, and 72 h are reported in Table 2. The inhibitory effect of the alcoholic extract on cell proliferation was significantly superior to that of aqueous extract. Parallel treatment of the normal cells with this herb demonstrated a much less inhibitory effect on the viability of MCF-10A cells (Fig. 3).
Fig. 2.
Cytotoxicity of various concentrations of aqueous (Aqu) and alcoholic (Alc) extracts of Berberis (0–6 mg/ml) against MCF-7 cells after incubation for 24, 48 and 72 h (a–c). All tests were performed in triplicate and results are reported as the mean ± SEM. *P < 0.05, compared with untreated cells
Table 2.
IC50 (mg/ml) values for both extracts of Berberis against MCF-7 cells after incubations for 24, 48 and 72 h
| Time (h) | 24 h | 48 h | 72 h |
|---|---|---|---|
| Alcoholic extract | 4 ± 0.05 | 2 ± 0.03 | 1 ± 0.07 |
| Aqueous extract | 3 ± 0.08 | 1.5 ± 0.04 | 0.5 ± 0.02 |
Results are mean ± SEM of three replications
Induction of apoptotic DNA fragmentation by Berberis extracts
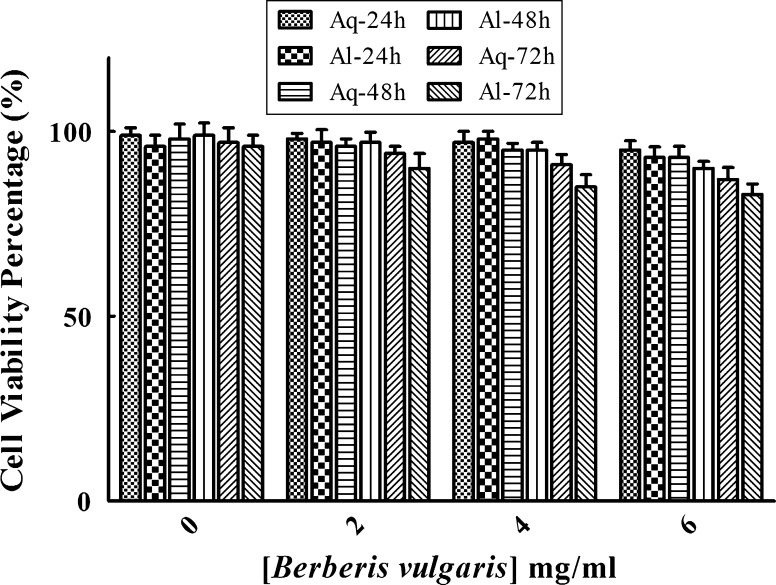
Fig. 3.
Effect of Berberis on the viability of human epithelial MCF-10A cells. Cells were treated with different concentrations of aqueous (Aq) and alcoholic (Al) extracts of this herb (0–6 mg/ml) for 24, 48, and 72 h. Results are reported as the mean ± SEM
DNA fragmentation is a qualitative hallmark of apoptosis. To show Berberis-induced apoptosis in MCF-7 cells, DNA of untreated cells and cells treated with effective doses of the alcoholic extract (1.5 and 3 mg/ml) for 48 h was analyzed by agarose gel 1.5 % electrophoresis. DNA of treated cells was fragmented whereas control cells did not show typical DNA fragmentation (Fig. 4).
Fig. 4.

DNA fragmentation after 48 h treatment with alcoholic extracts of Berberis (C: 1.5 mg/ml and D: 3 mg/ml) compared to untreated cells (B) and marker (A) values
Discussion
The mortality due to breast cancer is increasing in many countries (Taghavi et al. 2012). Cancer cells reproduce uncontrollably; loose sensitivity to antigrowth signals, get resistant to apoptosis and are enabled to replicative immortality (Hanahan and Weinberg 2011).
There are many evidences that commercially available anticancer drugs are derived from medicinal plants (Kitagishi et al. 2012; Zong et al. 2012). The antitumor effects of herbs are associated with modulation of cell-cycle and induction of apoptosis (Pandey and Rizvi 2009; Kuno et al. 2012). It has been also illustrated that herb extracts possess antioxidant properties which neutralize production of free radicals, prevent damage of DNA in cancer cells and reduced side effects of the most common types of cancer treatment (Charoensin 2014). Berberis vulgaris is one of these medicinal plants that have various biological properties including anti-proliferative, anti-migratory and antioxidant activities (Mahata et al. 2011; Wen et al. 2013). Previous studies mentioned that the phytochemical constituents of Berberis such as antioxidants and anthocyanins increased its bioactivity and was affected by different extraction procedures or the type of solvents used (Abd El-wahab et al. 2013; Annegowda et al. 2011).
In this study the antioxidant properties of alcoholic and aqueous extracts of Berberis were compared. A complete picture of total antioxidants capacity of both aqueous and ethanolic extracts of Berberis fruits determined was obtained via analysis by FRAP, DPPH and Ciocalteu assays. The results showed that the ethanol extract has a higher content in total antioxidants, total phenolices (Table 1) and free radical scavenging property (Fig. 1) than the aqueous extract. The high antioxidant capacity of Berberis fruit is primarily due to the high levels of phenolic and anthocyanin content found in this fruit (Ozgen et al. 2012). The best solvents for isolation of phenolic compounds from herbal extracts are organic solvents such as ethanol (Mohammedi and Atik 2011; Abd El-Wahab et al. 2013). Our FRAP, DPPH and Ciocalteu data confirmed these reports as indicated in Table 1 and Fig. 1.
One of the richest sources of anthocyanins is the purple-black of barberries which has strong antioxidant capacity (Ozgen et al. 2012). To investigate the difference of antioxidant activity between ethanolic and aqueous extracts, the anthocyanins amount was determined by spectrophotometry. It was found that the ethanolic extract was richer in anthocyanins compared to the aqueous extract. Previously, another study found that the ethanol extract of V. vinifera was richer in anthocyanins than the aqueous extract (Mansour et al. 2013).
The main biological property of cancer cells is uncontrolled and often rapid proliferation so arresting of tumor growth is considered as valid treatment option in different types of cancer therapies. The anti-proliferative effect of Berberis recognized in various cancers including breast, liver and colon cancers but its molecular mechanisms of action are not yet established (Kim et al. 2010; Mahata et al. 2011; Wang et al. 2009). In agreement with the last finding, our data showed that both aqueous and alcoholic extracts of Berberis significantly decreased cancer cell proliferation in a time- and dose-dependent manner (Fig. 3). The IC50 values strongly indicated that the effective doses of alcoholic extracts of Berberis were lower compared to aqueous extracts after different incubation times (0–72 h; Table 2). Therefore alcoholic extract of Berberis has inhibited growth of MCF-7 cells more effectively than aqueous extract. The cytotoxic effect of ethanolic extract may be related to its higher content of phenolic and anthocyanin components. MTT results indicated that various concentrations of Berberis had no cytotoxic effect on normal (MCF-10A) cells after incubations of 24, 48 and 72 h (Fig. 3).
Induction of apoptosis is one the most important marker of antitumor agents. Apoptosis is characterized by morphological changes for instance, cell shrinkage, membrane blebbing, chromatine condensation, DNA fragmentation and loosing organelles position in cytoplasm (Aghbali et al. 2013; Galluzzi et al. 2007). It has been shown that some plants such as saffron, curcumin and Ginger suppressed carcinogenic progress through induction of apoptosis (Bakhshi et al. 2008, 2010, Hoshyar et al. 2013; Bathaie et al. 2013). In our study, Berberis extracts have caused main morphological changes in the treated cancer cells (data are not shown). Moreover, we examined the apoptotic activity of alcoholic extraction of Berberis by DNA agarose gel electrophoresis. The isolated DNA from apoptotic cells showed a typical pattern of DNA fragments (Fig. 4).
In conclusion, our data illustrated that various cytotoxic effect of ethanol and water extracts of Berberis related to different amounts of total antioxidant, phenolics and anthocyanin in herb. The ethanol extract exerted a higher level of phenolics and anyhocyanin, seems to be more effective against proliferation of cancer cells. Besides, ethanolic extract of Berberis induced apoptosis in MCF-7 cells that could be explained by its powerful antioxidant and free radical scavenging capacities. Thus, Berberis as a selective anticancer agent against MCF-7 cells is a potent phyto-drug to be explored further for its cytotoxic property.
Acknowledgments
The authors would like to thank personnel of research laboratory in Birjand University of Medical Sciences. This study was made possible by a grant from the Research Vice Presidency of Birjand University of Medical Sciences. We also thank Mozhde Partovifar (Shiraz University, Shiraz, Iran) for performing the antioxidant assays.
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Reyhane Hoshyar and Zahra Mahboob contributed equally to this study.
References
- Abd El-Wahab A, Ghareeb D, Sarhan E, Abu-Seri E, Demellawy M. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine: antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218–230. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghbali A, Vosough Hosseini S, Delazar A, Kalbasi Gharavi N, Zare Shahneh F, Orangi M, Bandehagh A, Baradaran B. Induction of apoptosis by grape seed extract (Vitis vinifera) in oral squamous cell carcinoma. Bosn J Basic Med Sci. 2013;13:186–191. doi: 10.17305/bjbms.2013.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegowda HV, Mordi MN, Ramanathan S, Hamdan MR, Mansor SM. Effect of extraction techniques on phenolic content, antioxidant and antimicrobial activity of Bauhinia purpurea: HPTLC determination of antioxidants. Food Anal Methods. 2011;5:226–233. doi: 10.1007/s12161-011-9228-y. [DOI] [Google Scholar]
- Bakhshi H, Smitha S, Manik S. Assessment of cytotoxic and apoptogenic activity of Crocus sativus on human cancer cell lines. Ind J Appl Life Sci. 2008;4:34–38. [Google Scholar]
- Bakhshi H, Smitha S, Rozati R, Sultan Ph, Islam T, Rathore B, Lone Z, Sharma M, Triphati J, Saxena RC. DNA fragmentation and cell cycle arrest: hallmark of apoptosis induced by Crosin from Kashmiri Saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev. 2010;11:675–679. [PubMed] [Google Scholar]
- Bathaie SZ, Hoshyar R, Miri HR, Sadeghizadeh M. Anticancer effects of crocetin in both human adenocarcinoma gastric cancer cells and rat model of gastric cancer. Biochem Cell Biol. 2013;91:397–403. doi: 10.1139/bcb-2013-0014. [DOI] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Charoensin S. Antioxidant and anticancer activities of Moringa oleifera leaves. J Med Plant Res. 2014;8:318–325. doi: 10.5897/JMPR2013.5353. [DOI] [Google Scholar]
- Fathi Najafi M, Vahedy F, Seyyedin M, Jomehzadeh HR, Bozary K. Effect of the water extracts of propolis on stimulation and inhibition of different cells. Cytotechnology. 2007;54:49–56. doi: 10.1007/s10616-007-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Kroemer Zitvogel L. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In: Wrolstad RE, Schwartz SJ, editors. Handbook of food analytical chemistry. New York: Wiley; 2005. pp. 19–31. [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hoshyar R, Bathaie SZ, Kyani A, Mousavi MF. Is there any interaction between telomeric DNA structures, G-quadruplex and I-motif, with saffron active metabolites? Nucleosides Nucleotides Nucleic Acids. 2012;31:801–812. doi: 10.1080/15257770.2012.730164. [DOI] [PubMed] [Google Scholar]
- Hoshyar R, Bathaie SZ, Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol. 2013;32:50–57. doi: 10.1089/dna.2012.1866. [DOI] [PubMed] [Google Scholar]
- Kaliora AC, Dedoussis GVZ, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kim JB, Yu JH, Ko E, Lee KW, Song AK, Park SY, Shin I, Han W, Noh DY. The alkaloid Berberine inhibits the growth of Anoikis-resistant MCF-7 and MDA-MB-231 breast cancer cell lines by inducing cell cycle arrest. Phytomedicine. 2010;17:436–740. doi: 10.1016/j.phymed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Kitagishi Y, Kobayashi M, Matsuda S. Protection against cancer with medicinal herbs via activation of tumor suppressor. J Oncol. 2012;2012:1–7. doi: 10.1155/2012/236530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncic M, Kremer D, Karlovic K, Kosalec I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem Toxicol. 2010;48:2176–2180. doi: 10.1016/j.fct.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Kuno T, Tsukamoto T, Hara A, Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem. 2012;3:156–173. doi: 10.4236/jbpc.2012.32018. [DOI] [Google Scholar]
- Mahata S, Bharti AC, Shukla S, Tyagi A, Husain SA, Das BC. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol Cancer. 2011;10:1–14. doi: 10.1186/1476-4598-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour R, Haouas N, Ben Kahla-Nakbi A, Hammami S, Mighri Z, Mhenni F, Babba H. The effect of Vitis vinifera L. leaves extract on Leishmania infantum. Iran J Pharm Res. 2013;12:349–355. [PMC free article] [PubMed] [Google Scholar]
- Mohammedi Z, Atik F. Impact of solvent extraction type on total polyphenols content and biological activity from Tamarix aphylla (L.) karst. Int J Pharma Bio Sci. 2011;2:609–615. [Google Scholar]
- Ozgen M, Saraçoglu O, NurGeçer E. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Hort Environ Biotechnol. 2012;53:447–451. doi: 10.1007/s13580-012-0711-1. [DOI] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar V, Guha G, Kumar A. Antioxidant and antineoplastic activities of Picrorhiza kurroa extracts. Food Chem Toxicol. 2011;49:363–369. doi: 10.1016/j.fct.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Rakitzis ET. Reaction of thioureas with Folin–Ciocalteu reagent. Anal Chim Acta. 1975;78:495–497. doi: 10.1016/S0003-2670(00)00176-8. [DOI] [Google Scholar]
- Samarghandian S, Shabestari MM. DNA fragmentation and apoptosis induced by safranal in human prostate cancer cell line. Indian J Urol. 2013;29:177–183. doi: 10.4103/0970-1591.117278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi Sofi M, Sateesh MK, Bashir M, Harish G, Lakshmeesha TR, Vedashree S, Vedamurthy AB. Cytotoxic and proapoptotic effects of Abrus precatorius L. on human metastatic breast cancer cell line, MDA-MB-231. Cytotechnology. 2013;65:407–417. doi: 10.1007/s10616-012-9494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi A, Fazeli Z, Vahedi M, Baghestani AR, Pourhoseingholi A, Barzegar F, Pourhoseingholi MA. Increased trend of breast cancer mortality in Iran. Asian Pac J Cancer Prev. 2012;13:367–370. doi: 10.7314/APJCP.2012.13.1.367. [DOI] [PubMed] [Google Scholar]
- Wang GY, Lv QH, Dong Q, Xu RZ, Dong QH. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J Asian Nat Prod Res. 2009;11:219–228. doi: 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- Wen CJ, Wu LX, Fu LJ, Yu J, Zhang YW, Zhang X, Zhou HH. Genomic screening for targets regulated by berberine in breast cancer cells. Asian Pac J Cancer Prev. 2013;14:6089–6094. doi: 10.7314/APJCP.2013.14.10.6089. [DOI] [PubMed] [Google Scholar]
- Wen CJ, Wu LX, Fu LJ, Shen DY, Zhang X, Zhang YW, Yu J, Zhou HH. Preferential induction of CYP1A1 over CYP1B1 in human breast cancer MCF-7 cells after exposure to berberine. Asian Pac J Cancer Prev. 2014;15:495–499. doi: 10.7314/APJCP.2014.15.1.495. [DOI] [PubMed] [Google Scholar]
- Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:1–14. doi: 10.1186/1756-9966-30-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources: a review of recent research. Carbohydr Polym. 2012;90:1395–1410. doi: 10.1016/j.carbpol.2012.07.026. [DOI] [PubMed] [Google Scholar]