Abstract
Differentiation of embryonic stem (ES) cells is a heterogeneous process which is influenced by different parameters, including growth and differentiation factors. The aim of the present study was to investigate the effect of bone morphogenetic protein-4 (BMP4) signaling on differentiation of mouse ES cells to endodermal lineages. For this purpose, differentiation of the ES cells was induced by embryoid body (EB) formation through hanging drop method. During the suspension stage, EBs were treated with BMP4 in a medium containing either fetal bovine serum (FBS) or knockout serum replacement (KoSR). After plating, EBs showed differentiation to a heterogeneous population of specialized cell types. Two weeks after plating, all the experimental groups expressed three germ layer markers and some primitive and definitive endoderm-specific genes. Quantitative real-time PCR analysis showed higher expression levels of Sox17, Pdx1, Cdx2 and Villin mRNAs in the KoSR plus BMP4 condition and higher Gata4 and Afp expression levels in the FBS plus BMP4 condition. Formation of visceral endoderm and derivatives of definitive endoderm was detected in the BMP4 treated EBs. In conclusion, we demonstrated that both BMP4 signaling and serum composition have significant roles in differentiation of mouse ES cells towards endodermal lineages.
Keywords: Mouse ES cell, BMP4, Differentiation, Primitive endoderm, Definitive endoderm
Introduction
Embryonic stem (ES) cells are derived from the inner cell mass of blastocyst-stage embryos. They can be propagated in an undifferentiated state in vitro. However, when allowed to be differentiated in suspension or in hanging drops, they aggregate to form embryoid bodies (EBs). Spontaneous differentiation of EBs in vitro mimics many key aspects of early mouse embryonic development, including differentiation of ectodermal, mesodermal and endodermal lineages and cavitation (Martin et al. 1977; Coucouvanis and Martin 1995). Differentiation of EBs is a heterogeneous process. Nevertheless, desired lineages can be enriched using various strategies (Liu et al. 2006), including application of growth and differentiation factors and extracellular matrix (ECM) proteins. Although several studies have addressed these topics, more fundamental studies are required in order to explore the precise role of different factors and culture conditions in ES cell differentiation.
Bone morphogenetic protein-4 (BMP4) is a member of transforming growth factor-β (TGF-β) superfamily. BMPs are involved in most of the morphogenetic processes during development (Hogan 1996). BMP signaling has critical roles in endodermal morphogenesis and ectodermal patterning (Davis et al. 2004). Treatment of ES cells with BMP4 in a serum-free chemically defined medium triggers a process similar to primitive streak formation (Wiles and Johansson 1999). BMP4 signaling has a pivotal role in mesodermal and epidermal differentiation of ES cells (Aberdam et al. 2007; Kennedy et al. 2007; Hansson et al. 2009). Moreover, BMP4 has been identified as a inducer for visceral and definitive endoderm (Conley et al. 2007; Gouon-Evans et al. 2006; Zorn and Wells 2009; Mathew et al. 2012; Sudheer et al. 2012). In several studies, the effect of BMP4 on ES cell differentiation to endodermal lineages has been investigated in combination with the other factors. Moreover, some investigators have claimed that BMP4 alone can induce differentiation of human and mouse ES cells to trophoblastic lineages (Amita et al. 2013; Hayashi et al. 2010; Xu et al. 2002). In the present study, we investigated the role of BMP4 signaling in differentiation of mouse ES cells to primitive and definitive endoderm.
Materials and methods
Mouse ES cells culture and differentiation
The mouse ES cell line Royan B1 (Royan Stem Cell Bank, Royan Institute, RSCB0001) was used in the present study. The ES cells were cultured on top of a feeder layer of mitomycin C-treated mouse embryonic fibroblasts (MEF) and in the presence of leukemia inhibitory factor (LIF, Chemicon, ES-GRO, Boronia, Victoria, Australia), as described previously (Taha et al. 2007). To initiate differentiation, the ES cells were dissociated from the MEF feeder layer, and embryoid bodies (EBs) were generated using hanging drop method (Taha et al. 2007). Differentiation medium consisted of 0.1 mM β-mercaptoethanol (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany), 1 mM l-glutamine, 1 % nonessential amino acid stock and 1 % penicillin–streptomycin in Knockout™ Dulbecco’s Modified Eagle’s Medium, supplemented with 15 % fetal bovine serum (FBS, ES qualified) (all materials from Gibco, Grand Island, NY, USA). Medium of the suspension stage contained 15 % FBS or 15 % Knockout™ serum replacement (KoSR, Gibco) and 10 ng/ml BMP4 (Sigma). Untreated EBs were included as the control group.
Analysis of gene expression by RT-PCR and quantitative real-time PCR
Total RNA was extracted using High Pure RNA Isolation Kit (Roche Applied Science, Mannheim, Germany). 1 µg of total RNA was reverse transcribed to cDNA using cDNA Synthesis Kit (Thermo Scientific, Karlsruhe, Germany). PCR was performed using specific primers described in Table 1.
Table 1.
Primers used for RT-PCR and qPCR
| Genes | Forward primers | Reverse primers | Size (bp) | Accession number |
|---|---|---|---|---|
| Tubb5 | 5′-GGAACATAGCCGTAAACTGC-3′ | 5′-TCACTGTGCCTGAACTTACC-3 | 317 | NM-011655 |
| Pax6 | 5′-TGCCCTTCCATCTTTGCTTG-3′ | 5′-TCTGCCCGTTCAACATCCTTAG-3′ | 178 | NM_001244200 |
| Brachyury | 5′-ATGCCAAAGAAAGAAACGAC-3′ | 5′-AGAGGCTGTAGAACATGATT-3′ | 835 | NM_009309 |
| Afp | 5′-TCGTATTCCAACAGGAGG-3′ | 5′-AGGCTTTTGCTTCACCAG-3′ | 174 | NM_007423 |
| Gsc | 5′-GCTGGCCAGGAAGGTGCACC-3′ | 5′-CGGCGAGGCTTTTGAGGACGT-3′ | 148 | NM_010351 |
| Gata4 | 5′-TCTCACTATGGGCACAGCAG-3′ | 5′-GCGATGTCTGAGTGACAGGA-3′ | 133 | NM_013633 |
| Sox17 | 5′-GGCACAGCAGAACCCAGAT-3′ | 5′-TTGTAGTTGGGGTGGTCCTG-3′ | 150 | NM_011441 |
| Pdx1 | 5′-GAAATCCACCAAAGCTCACGC-3′ | 5′-ATTCCTTCTCCAGCTCCAGCA-3′ | 128 | NM_008814 |
| Cdx2 | 5′-TAGGAAGCCAAGTGAAAACCAG-3′ | 5′-CTTGGCTCTGCGGTTCTGA-3′ | 191 | NM_007673 |
| Villin | 5′-CTTCTTCGATGGTGACTGCTAT-3′ | 5′-AAGTCTCGCTCTCGTTGCCT-3′ | 203 | NM_009509 |
| Plf | 5′- CCAGGCTCACACACTATTCA-3′ | 5′- CTGTGGCTTTGGAGATGATTAT-3′ | 138 | NM_031191 |
Quantitative assessment of gene expression by real-time PCR (qPCR) was performed using RealQ PCR Master (Ampliqon A/S, Odense, Denmark) with Green dye on a Rotor-Gene™ 6000 (Corbett Research, Qiagen, Hilden, Germany) real-time analyzer. Comparative quantification were performed using REST 2009 (Relative Expression Software Tool, Qiagen) based on Pair Wise Fixed Reallocation Randomization Test® (Pfaffl et al. 2002). At least, three biologic replicates of each group were included in the qPCR experiments, and β-tubulin 5 (Tubb5) and eukaryotic elongation factor 2 (Eef2) were used as the housekeeping genes for normalization of the quantitative data.
Transmission and scanning electron microscopy (TEM and SEM)
For TEM study, mechanically dissected areas were fixed using 2.5 % glutaraldehyde, post-fixed using 1 % osmium tetroxide (OsO4), dehydrated in graded degrees of ethanol and embedded in Araldite 6005 (Sigma). Semithin sections were stained with toluidine-blue for light microscopy. Ultrathin (50–70 nm) sections were double-stained with uranyl acetate and lead citrate and were used for ultrastructural evaluation by a transmission electron microscope (EM900, Zeiss, Oberkochen, Germany).
For SEM study, samples were fixed using 4 % glutaraldehyde and post-fixed with 1 % OsO4. After dehydration with the graded series of ethanol, the samples were freeze dried, coated in a sputter-coater with a layer of gold and observed under a scanning electron microscope (SEM360, Cambridge, UK).
Results
Morphological analysis
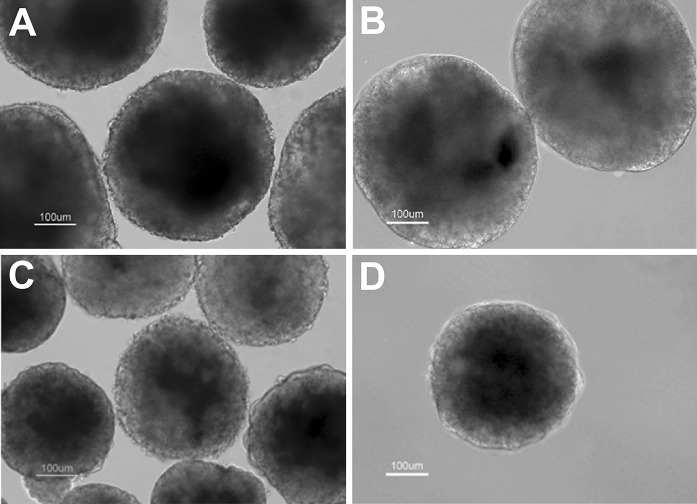
Differentiation of mouse ES cells was initiated by formation of EBs using hanging drop method. At the end of suspension stage, the EBs which were cultured in the FBS + BMP4 condition showed the maximum size, while the EBs cultured in KoSR condition without BMP4 treatment demonstrated the minimum size (Fig. 1a–d).
Fig. 1.
Seven-day old EBs of FBS (a), FBS + BMP4 (b), KoSR (c) and KoSR + BMP4 (d) groups
Differentiation of ES cells yielded a heterogenous cell population. Within the first week after plating, numerous cells with different morphologies drifted out of the EBs. Some tubule-like structures were also detectable in the outgrowths of BMP4 treated EBs. These structures were mechanically dissected for TEM analysis.
Gene expression analysis by RT-PCR and qPCR
Two-week differentiated EBs in all the experimental groups expressed Paired box gene 6 (Pax6) and Brachyury genes which determine the formation of neuroectoderm and mesoderm layers, respectively. Alpha fetoprotein (Afp) and Goosecoid (Gsc) genes were also expressed in the differentiated EBs. Afp is a hepatocyte-specific marker, but it is also expressed in the extraembryonic visceral endoderm (Dziadek and Adamson 1978). Gsc expression shows generation of a bi-potent mesendodermal population which can give rise to both mesoderm and definitive endoderm (Tada et al. 2005). Differentiated EBs expressed GATA binding protein 4 (Gata4) and SRY-box containing gene 17 (Sox17) transcription factors. Gata4 is expressed in the primitive endoderm and its derivatives, visceral and parietal endoderm. It is also expressed in the definitive endoderm and in the mesoderm of early mouse embryo (Kuo et al. 1997; Molkentin et al. 1997). Sox17 is expressed in both visceral and definitive endoderms (Kanai-Azuma et al. 2002). Pancreatic and duodenal homeobox 1 (Pdx1), Caudal-type homeobox transcription factor 2 (Cdx2) and Villin genes were also expressed in 2-week differentiated EBs. The expression of Pdx1 determines the pancreatic buds and portions of stomach and duodenum (Offield et al. 1996). Cdx2 is expressed in the endoderm of entire postgastric epithelium (Beck et al. 1995; Silberg et al. 2000), and Villin is found in many absorptive epithelia. In mouse embryo, Villin is first expressed in the primitive endoderm and then in the visceral endoderm. During development of definitive endoderm and in adults, Villin is expressed in the intestinal epithelium (Maunoury et al. 1992, 1988). Plf gene, which is exclusively transcribed in the trophoblastic giant cells (Lee et al. 1988), was also expressed in all the experimental groups (Fig. 2).
Fig. 2.
The expression of genes specific to pluripotency, germ layer specification, endodermal differentiation and trophoblastic giant cell formation in the EBs, 2 weeks after plating
Octamer-binding transcription factor 4 (Oct4/Pou5f1) gene was not expressed in the differentiated EBs. Nanog mRNA was weakly expressed in the EBs which were differentiated in the absence of BMP4 (Fig. 2). Undifferentiated mouse ES cells expressed the pluripotency markers but not the other genes related to endodermal specification (Fig. 2).
Based on the qPCR analysis, the expression levels of Afp, Gata4, Sox17, Pdx1, Cdx2 and Villin mRNAs in the FBS + BMP4 group were 2.767, 2.642, 1.65, 4.57, 2 and 2.66 fold higher than in the FBS group and in the KoSR + BMP4 group were 2.16, 2.87, 3.96, 4.25, 3.7 and 4.5 fold higher than in the KoSR group, respectively (Fig. 3).
Fig. 3.
Quantitative real-time PCR analysis of gene expression in the differentiated EBs, 2 weeks after plating. Tubb5 and Eef2 were used as the internal controls. *Significant differences were indicated by P value (Pair Wise Fixed Reallocation Randomization Test® performed by REST 2009 software)
The expression levels of Sox17, Pdx1 and Cdx2 mRNAs in the KoSR + BMP4 group were 3.49, 2 and 5.63 fold higher than in the FBS + BMP4 group, respectively. Gata4 expression in the KoSR + BMP4 group was about one-third of that in the FBS + BMP4 group. The expression levels of Afp and Villin mRNAs were not significantly different between the KoSR + BMP4 and FBS + BMP4 groups (Fig. 3).
Fine structural study
Primitive and visceral endoderm specification
In the absence of BMP4, the superficial layer of EBs in both the FBS and KoSR conditions was specified as a monolayer of cells with flat morphology (Fig. 4a, b). However, in the EBs treated with 10 ng/ml BMP4, a layer of cuboidal cells was formed on the surface of EBs (Fig. 4c, d).
Fig. 4.
Semithin sections of the ES-cell derived EBs after toluidine blue staining. a–d 2-week differentiated EBs of the FBS, FBS + BMP4, KoSR and KoSR + BMP4 groups, respectively
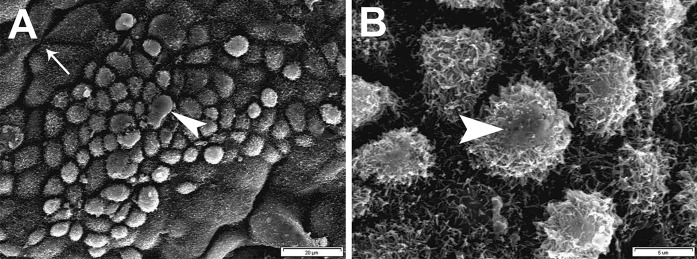
At day 21 after plating, SEM analysis revealed a layer of microvilliated cells on the surface of BMP4 treated EBs. Some cells seemed to be flat, while the others appeared dome-shaped and in some bulges of their apical membrane, microvilli decreased and a secretory appearance was observable. Moreover, in some areas, a ductal arrangement of the cells was detected (Fig. 5).
Fig. 5.
Scanning electron micrograph of the differentiated EBs in the KoSR + BMP4 group. a, b Microvilliated cells which cover the surface of 3-week differentiated EBs. Some cells seemed to be flat, while the others appeared dome-shaped and in some bulges of their apical membrane, a secretory appearance was observed (arrowheads). Moreover, in some areas, a duct-like arrangement of the cells was detected (arrow)
Definitive endoderm specification
Two weeks after plating, gut-like structures were developed within the EBs’ outgrowth of the KoSR + BMP4 group. Based on TEM analysis, these tubular structures were composed of a lining epithelium, sub-epithelial connective tissue and smooth muscle cells. Epithelial cells were interconnected with apical tight junctions, some desmosome-like junctions and developed interdigitations. Some secretory cells and some cells with pinocytic vesicles were also observed in the epithelium (Fig. 6).
Fig. 6.
TEM micrographs of the differentiated EBs in the KoSR + BMP4 group. a–d Cross section of one gut-like structure. Ep epithelium, GC golgi complex, j junctional complexes, Lum lumen, PV pinocytotic vesicles, SC secretory cells, CT sub-epithelial connective tissue, SM smooth muscle layer, TJ tight junction
Discussion
EB formation by hanging drop method
In the current study, we investigated the role of BMP4 in differentiation of mouse ES cells to endodermal lineages. For this aim, ES cells were differentiated through hanging drop method which results in uniform-sized EBs. EB formation mimics many key aspects of embryo development, including pre-gastrulation and early gastrulation stages (Martin et al. 1977; Coucouvanis and Martin 1995; Yamamoto et al. 2007). EB culture can be used as a useful model to study the interplay of different germ layers and their influence on differentiation of various cell types (Pekkanen-Mattila et al. 2010).
Embryonic stem (ES) cell-derived EBs were treated with 10 ng/ml BMP4 in a medium containing either FBS or KoSR. KoSR is a commercially available synthetic serum replacement which is completely devoid of any undefined growth and differentiation factors (Goldsborough et al. 1998). Supplementation of the culture media with this serum substitute induces more directed differentiation of the ES cells and also may prevent negative interactions between BMP4 and FBS constituents.
Gene expression analysis
Differentiation of ES cells results in a heterogenous cell population. Therefore, the comparison between the control and treatment groups is based on the quantitative gene or protein expression analyses. Here we showed the expression of several endodermal genes in differentiated EBs of all the experimental groups. However, based on qPCR analysis, treatment of the EBs with 10 ng/ml BMP4 increased the expression of endodermal markers including Afp, Gata4, Sox17, Pdx1, Cdx2 and Villin. BMP4 treatment in KoSR condition upregulated the overall expression of endodermal genes that may reflect better development of visceral (Sox17 and Villin), definitive (Sox17), foregut (Pdx1) and hindgut (Villin and Cdx2) endoderms under this condition. However, some immunofluorescence co-stainings can be used to exactly determine the formation of definitive endoderm (SOX17/FOXA2) and extraembryonic lineages (SOX17/SOX7). Meanwhile, it should be noted that differentiated cells of all the experimental groups expressed Plf gene which is exclusively transcribed in the trophoblastic giant cells (Lee et al. 1988). This finding indicates that some proportion of the ES cells may be differentiated toward a trophoblastic phenotype.
Primitive endoderm specification
Following aggregation of ES cells, the outer layer of EBs is specified as extraembryonic primitive endoderm (Doetschman et al. 1985; Hamazaki et al. 2004). In the present study, BMP4 treatment of the EBs induced formation of a superficial layer of visceral endoderm with microvilliated cells. Some cells also showed a secretory characteristic which is in agreement with the secretory role of visceral endoderm. As known, visceral endoderm synthesizes and secretes some proteins such as transferrin and apolipoproteins (Gardner 1983). Our results are consistent with a previous study showing that BMP signaling is both capable of promoting and required for the differentiation of visceral endoderm (Coucouvanis and Martin 1999). Moreover, Artus et al. (2012) showed the role of BMP4 in visceral endoderm specification.
Definitive endoderm specification
So far, several investigators have demonstrated the roles of BMPs in the differentiation of mouse and human ES cells to definitive endoderm. As reported previously, exposure of definitive endoderm to BMP and FGF ligands induces generation of hepatocyte-like cells (Mfopou et al. 2013). Treatment of the endodermal progenitors with BMP4, bFGF and activin A also results in the development of mature hepatocytes (Gouon-Evans et al. 2006). A combination of BMP4 and activin A induces the differentiation of human ES cells first to definitive endoderm and then to pancreatic cells (Teo et al. 2012). In contrast to these studies, some investigators have demonstrated that BMPs negatively regulates the formation of endodermal progenitors (Poulain et al. 2006; Sumi et al. 2008). Sumi et al. (2008) showed that BMP4 treatment does not induce the differentiation of human ES cells to mesendoderm/mesoderm progenitors. Moreover, Amita et al. (2013) demonstrated that trophoblast is the predominant cell type which is derived from human ES cells in response to 10 ng/ml BMP4. Zhang et al. (2008) showed that long-term treatment of human ES cells with BMP4 results in trophoblast and extraembryonic endoderm specification, while short-term treatment can induce differentiation of early mesoderm.
In the current study, we demonstrated the role of BMP4 signaling in differentiation of mouse ES cells to some definitive endodermal genes-expressing cells. Gsc expression shows generation of a bi-potent mesendodermal population (Tada et al. 2005). The expression of Sox17, Gata4, Afp, Pdx1, Cdx2 and Villin points towards development of definitive endoderm derivatives. Afp is expressed in hepatoblasts during liver development (Dziadek and Adamson 1978). Pdx1 expression determines the pancreatic buds and portions of stomach and duodenum (Offield et al. 1996). Cdx2 is expressed in the endoderm of entire postgastric epithelium (Beck et al. 1995; Silberg et al. 2000), and Villin is found in the absorptive intestinal epithelium (Maunoury et al. 1992, 1988). Based on the qPCR analysis, BMP4 signaling was more effective for the expression of definitive endoderm-specific genes in the KoSR condition.
As revealed by TEM analysis, 2-week differentiated EBs of the KoSR condition showed formation of well-developed tubular structures with the ultrastructural characteristics of gut. The presence of some secretory cells and some cells with pinocytic vesicles in the epithelium can support the functionality of these gut-like structures. As previously described, BMPs have an early role in development and patterning of the endodermal anterior intestinal portal structure (Faure et al. 2002). BMPs are involved in the normal epithelial differentiation and homeostasis of the gut (Howe et al. 2001) and in formation of stomach gland (Narita et al. 2000). BMP4 is also important for regulation of smooth muscle differentiation and for determining the proper thickness of mesodermal layers in the different regions of gut (Roberts et al. 1998; Smith et al. 2000).
In conclusion, both BMP4 signaling and serum supplementation had significant roles in differentiation of mouse ES cells. BMP4 treatment of the ES cell-derived EBs induced formation of both primitive and definitive endoderms. It is clear that understanding these pathways has a significant value for directing the ES cells differentiation toward a specific cell type and enrichment of desired cells for potential clinical applications. Also, generation of gut-like structures is another important finding in this regard.
Acknowledgments
This study was supported by a research grant from National Institute of Genetic Engineering and Biotechnology (434). We would like to thank Mrs. Ilnaz Sadeghi Sadr and Mr. Shahram Pour Beiranvand for their technical advice.
References
- Aberdam D, Gambaro K, Rostagno P, Aberdam E, de la Forest Divonne S, Rouleau M. Key role of p63 in BMP-4-induced epidermal commitment of embryonic stem cells. Cell Cycle. 2007;6:291–294. doi: 10.4161/cc.6.3.3800. [DOI] [PubMed] [Google Scholar]
- Amita M, Adachi K, Alexenko AP, Sinha S, Schust DJ, Schulz LC, Roberts RM, Ezashi T. Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci USA. 2013;110:E1212–E1221. doi: 10.1073/pnas.1303094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artus J, Douvaras P, Piliszek A, Isern J, Baron MH, Hadjantonakis AK. BMP4 signaling directs primitive endoderm-derived XEN cells to an extraembryonic visceral endoderm identity. Dev Biol. 2012;361:245–262. doi: 10.1016/j.ydbio.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck F, Erler T, Russell A, James R. Expression of Cdx-2 in the mouse embryo and placenta: possible role in patterning of the extra-embryonic membranes. Dev Dyn. 1995;204:219–227. doi: 10.1002/aja.1002040302. [DOI] [PubMed] [Google Scholar]
- Conley BJ, Ellis S, Gulluyan L, Mollard R. BMPs regulate differentiation of a putative visceral endoderm layer within human embryonic stem-cell-derived embryoid bodies. Biochem Cell Biol. 2007;85:121–132. doi: 10.1139/o06-145. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Dziadek M, Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J Embryol Exp Morphol. 1978;43:289–313. [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- Goldsborough MD, Tilkins ML, Price PJ, Lobo-Alfonso J, Morrison J, Stevens ME, Meneses J, Pederson R, Koller B, Latour A. Serum-free culture of murine embryonic stem (ES) cells. Focus. 1998;20:8–12. [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Oka M, Yamanaka S, Terada N. Aggregation of embryonic stem cells induces Nanog repression and primitive endoderm differentiation. J Cell Sci. 2004;117:5681–5686. doi: 10.1242/jcs.01489. [DOI] [PubMed] [Google Scholar]
- Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, Klein T, Maddox-Hyttel P, Serup P. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol. 2009;330:286–304. doi: 10.1016/j.ydbio.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Furue MK, Tanaka S, Hirose M, Wakisaka N, Danno H, Ohnuma K, Oeda S, Aihara Y, Shiota K, Ogura A, Ishiura S, Asashima M. BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell Dev Biol Anim. 2010;46:416–430. doi: 10.1007/s11626-009-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, Vogelstein B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Talamantes F, Wilder E, Linzer DI, Nathans D. Trophoblastic giant cells of the mouse placenta as the site of proliferin synthesis. Endocrinology. 1988;122:1761–1768. doi: 10.1210/endo-122-5-1761. [DOI] [PubMed] [Google Scholar]
- Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Martin GR, Wiley LM, Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977;61:230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Mathew S, Jaramillo M, Zhang X, Zhang LA, Soto-Gutierrez A, Banerjee I. Analysis of alternative signaling pathways of endoderm induction of human embryonic stem cells identifies context specific differences. BMC Syst Biol. 2012;6:154. doi: 10.1186/1752-0509-6-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunoury R, Robine S, Pringault E, Huet C, Guenet JL, Gaillard JA, Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988;7:3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunoury R, Robine S, Pringault E, Leonard N, Gaillard JA, Louvard D. Developmental regulation of villin gene expression in the epithelial cell lineages of mouse digestive and urogenital tracts. Development. 1992;115:717–728. doi: 10.1242/dev.115.3.717. [DOI] [PubMed] [Google Scholar]
- Mfopou JK, Geeraerts M, Dejene R, Van Langenhoven S, Aberkane A, Van Grunsven LA, Bouwens L. Efficient definitive endoderm induction from mouse embryonic stem cell adherent cultures: a rapid screening model for differentiation studies. Stem cell Res. 2013;12:166–177. doi: 10.1016/j.scr.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Narita T, Saitoh K, Kameda T, Kuroiwa A, Mizutani M, Koike C, Iba H, Yasugi S. BMPs are necessary for stomach gland formation in the chicken embryo: a study using virally induced BMP-2 and Noggin expression. Development. 2000;127:981–988. doi: 10.1242/dev.127.5.981. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Pekkanen-Mattila M, Pelto-Huikko M, Kujala V, Suuronen R, Skottman H, Aalto-Setala K, Kerkela E. Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem Cell Biol. 2010;133:595–606. doi: 10.1007/s00418-010-0689-7. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36 [DOI] [PMC free article] [PubMed]
- Poulain M, Furthauer M, Thisse B, Thisse C, Lepage T. Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling. Development. 2006;133:2189–2200. doi: 10.1242/dev.02387. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Smith DM, Goff DJ, Tabin CJ. Epithelial–mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–971. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- Smith DM, Nielsen C, Tabin CJ, Roberts DJ. Roles of BMP signaling and Nkx2.5 in patterning at the chick midgut–foregut boundary. Development. 2000;127:3671–3681. doi: 10.1242/dev.127.17.3671. [DOI] [PubMed] [Google Scholar]
- Sudheer S, Bhushan R, Fauler B, Lehrach H, Adjaye J. FGF inhibition directs BMP4-mediated differentiation of human embryonic stem cells to syncytiotrophoblast. Stem Cells Dev. 2012;21:2987–3000. doi: 10.1089/scd.2012.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, activin/nodal and BMP signaling. Development. 2008;135:2969–2979. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Taha MF, Valojerdi MR, Mowla SJ. Effect of bone morphogenetic protein-4 (BMP-4) on cardiomyocyte differentiation from mouse embryonic stem cell. Int J Cardiol. 2007;120:92–101. doi: 10.1016/j.ijcard.2006.08.118. [DOI] [PubMed] [Google Scholar]
- Teo AK, Ali Y, Wong KY, Chipperfield H, Sadasivam A, Poobalan Y, Tan EK, Wang ST, Abraham S, Tsuneyoshi N, Stanton LW, Dunn NR. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30:631–642. doi: 10.1002/stem.1022. [DOI] [PubMed] [Google Scholar]
- Wiles MV, Johansson BM. Embryonic stem cell development in a chemically defined medium. Exp Cell Res. 1999;247:241–248. doi: 10.1006/excr.1998.4353. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Tase N, Okuno T, Kondo Y, Akiba S, Shimozawa N, Terao K. Monitoring of gene expression in differentiation of embryoid bodies from cynomolgus monkey embryonic stem cells in the presence of bisphenol A. J Toxicol Sci. 2007;32:301–310. doi: 10.2131/jts.32.301. [DOI] [PubMed] [Google Scholar]
- Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]