Abstract
This study aimed to investigate the effects of osteoprotegerin (OPG), a decoy receptor for receptor activator for nuclear factor κB ligand (RANKL), during the various stages of osteoclast differentiation, and additionally investigate its effects on osteoclast adhesion and activity. RAW264.7 murine monocytic cells were incubated with macrophage colony-stimulating factor and RANKL for 1, 3, 5, or 7 days, followed by an additional 24-h incubation in the presence or absence of OPG (80 ng/mL). We examined osteoclast differentiation and adhesion capacity using the tartrate-resistant acid phosphatase (TRAP) assay and immunofluorescence microscopy, and additionally examined cell growth in real time using the xCELLigence system. Furthermore, the expression levels of TRAP, RANK, integrin β3, matrix metalloproteinase 9, cathepsin K, carbonic anhydrase II, and vesicular-type H+-ATPase A1 were examined using western blotting. OPG exposure on day 1 enhanced the osteoclast growth curve as well as adhesion, and increased RANK and integrin β3 expression. In contrast, exposure to OPG at later time points (days 3–7) inhibited osteoclast differentiation, adhesion structure formation, and protease expression. In conclusion, the biological effects of OPG exposure at the various stages of osteoclast differentiation were varied, and included the enhanced adhesion and survival of preosteoclasts, the block of differentiation from the early to the terminal stages of osteoclastogenesis, and suppression of mature osteoclast activation following OPG exposure during the terminal differentiation stage, suggesting that the effects of OPG exposure differ based on the stage of differentiation.
Keywords: Osteoclast, Osteoprotegerin, Differentiation, Adhesion, Activation
Introduction
Osteoclasts, which are terminally differentiated cells derived from hematopoietic progenitors in the monocyte/macrophage lineage, play an essential role in bone homeostasis by carrying out the controlled bone resorption essential for bone remodeling (Udagawa et al. 1990). Osteoclastogenesis is a multistep process that involves progenitor survival, differentiation to mononuclear preosteoclasts, fusion to form multinucleate mature osteoclasts, and activation of mature osteoclasts to produce cells capable of bone resorption (Xing et al. 2012). In vitro, exposure to the growth factors macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor-κB ligand (RANKL), both of which are produced by bone stromal cells of the osteoblast lineage, is sufficient to prompt osteoclast precursor cell differentiation to multinucleate, tartrate-resistant acid phosphatase (TRAP)-positive, polarized cells, and is also sufficient for osteoclast maintenance (Boyle et al. 2003). Engagement of the RANKL receptor RANK on osteoclast precursors and mature osteoclasts results in signaling events that mediate osteoclast survival, differentiation, and activation (Chen et al. 2012; Kang et al. 2003). OPG, similar to RANK, is also a member of the TNF receptor superfamily and functions as a decoy receptor for RANKL, with a high affinity similar to that of RANKL for RANK. This decoy receptor has been reported block the differentiation of osteoclasts, resulting in the inhibition of bone resorption (Baud’huin et al. 2007; Theoleyre et al. 2004). OPG-knockout mice show severe osteoporosis due to an increase in the number of normal osteoclasts (Bucay et al. 1998). The ratio of RANKL to OPG plays an important role in regulating the delicate balance between bone formation and bone resorption.
When attached to bone, osteoclasts polarize and form a sealing zone, which is a ring-shaped belt composed of actin-rich structures termed podosomes. Bone resorption occurs in the resorption lacuna, in the area between the bone and the osteoclast defined by the sealing zone. Within the sealing zone, a unique membrane structure termed the “ruffled border” is formed, where osteoclasts generate an acidic microenvironment (pH 3.0 or less) by secreting protons. This process results in mineral (hydroxyapatite) dissolution, exposing the organic matrix components (e.g., type 1 collagen) of bone with a concomitant release of acidic hydrolases (e.g., cathepsin K and TRAP). Cathepsin K is essential for type I collagen degradation, while the role of TRAP is not completely clear (Deborah and Novack 2011; Saftig et al. 2000; Zaidi et al. 2001). Carbonic anhydrase type II (CA II) and vacuolar-type H+-ATPases (V-ATPases) participate in the extracellular acidification of the resorption lacunae (Chen et al. 2012). When the osteoclast polarizes to initiate resorption, CA II catalyzes the conversion of carbon dioxide and water to bicarbonate, with a byproduct of this reaction being protons (Lindsey et al. 1990). The V-ATPase molecules located in the outer fusion zone of the ruffled border pump the protons out into the resorption lacunae. If this enzyme function is compromised, mineral dissolution does not occur (Kornak et al. 2000). Matrix metallopeptidase-9 (MMP-9) appears to be essential for osteoclast migration and precursor recruitment into bone (Okada et al. 1995; Yu et al. 2003). Although MMP-9-knockout mice show defects in bone development, intraosseous angiogenesis and fracture repair rather than osteoclast bone resorption appear to be compromised in these animals (Colnot et al. 2003).
Several studies have indicated that OPG affects the expression of key molecules involved in osteoclast differentiation and function, mainly at the gene level (Chen et al. 2012; Fu et al. 2013b, Wittrant et al. 2002). However, little is known about the effects of OPG exposure during the various stages of osteoclastogenesis. To investigate this area further, we treated osteoclast cultures derived from RAW264.7 murine monocytic cells with OPG at days 1, 3, 5, or 7, and examined the effects on osteoclast differentiation, the formation of adhesion structures, and osteoclast activity.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), Eagle’s minimum essential medium alpha modification (α-MEM), and fetal bovine serum (FBS) were purchased from Gibco Laboratories (Grand Island, NY, USA). The tartrate-resistant acid phosphatase (TRAP) kit 387-A, rhodamine phalloidin, 4′,6-diamidino-2-phenylindole (DAPI), l-glutamine, penicillin, streptomycin and rabbit anti-vinculin polyclonal antibody were obtained from Sigma Chemical Co. (St. Louis, MO, USA). M-CSF, RANKL, and OPG were obtained from Peprotech Inc. (Rocky Hill, NJ, USA). Rabbit anti-cathepsin K, anti-CA II, anti-MMP-9 polyclonal antibodies and anti-integrin β3 monoclonal antibody were purchased from Abcam (Cambridge, UK). Rabbit anti-RANK, anti-V-ATPase A1, and anti-β-actin polyclonal antibodies were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG were obtained from Cell Signaling Technology Inc. (Danvers, MA, USA). A bicinchoninic acid (BCA) protein assay kit was provided by Beyotime Institute of Biotechnology (Jiangsu, China). Radio-immunoprecipitation assay (RIPA) lysis buffer was from Solarbio Inc. (Beijing, China). Protease inhibitor cocktail was from Applygen Technology Inc. (Beijing, China). Enhanced chemiluminescence (ECL) detection kit was from ECL Millipore Ltd. (Burlington, MA, USA).
Cell culture
The mouse monocyte cell line RAW264.7 was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM supplemented with 10 % FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM l-glutamine at 37 °C in a 5 % CO2 atmosphere (Thermo HERAcell 150i, Waltham, MA, USA). To generate osteoclasts, RAW264.7 cells were suspended in α-MEM containing 10 % FBS and were allowed to differentiate for 1, 3, 5 or 7 days in the presence of 30 ng/mL RANKL and 25 ng/mL M-CSF, at the end of which they were incubated in the presence or absence of OPG (80 ng/mL) for another 24 h. The medium was changed every other day during the culture period.
TRAP staining
TRAP staining was performed as per the manufacturer’s protocol. Multinucleated TRAP-positive cells containing three or more nuclei were identified as osteoclast-like cells. To determine the number and area of the osteoclast-like cells, mean values were calculated for 10 visual fields from at least three wells using an inverted phase contrast microscope (LEICA DMI 3000B, Wetzlar, Germany) and the ImageJ 1.46 (NIH, Bethesda, MD, USA).
Study of growth curves using the xCELLigence system
To study the dynamic effects of OPG on osteoclastogenesis, OPG-treated or untreated osteoclast cultures were monitored using the xCELLigence system (Roche 2.0, Mannheim, Germany) composed of four main parts: the real-time cell analyzer (RTCA) computer with integrated software, the RTCA analyzer, the RTCA single plate (SP) station and a 16-well or 96-well E-plate. The special E-plate is a micro-electronic plate with gold microelectrode arrays integrated to glass substrates at the bottom of each well, to measure cellular status in real time. The impedance between the electrodes in an individual well depends on electrode geometry, ionic concentration and whether the cells are attached to the electrodes. Impedance change can occur either due to the number of cells attached to the electrodes or due to dimensional change of attached cells on the electrodes. Proliferation of cells or cell spreading results in enhanced electrical impedance. The impedance value of each well was automatically monitored by the xCELLigence system every 15 min and expressed as a Cell Index value (CI). To compare the effects of osteoprotegerin (OPG) on cells at different stages of osteoclastogenesis, the Normalized Cell Index (NCI) was used. The NCI was calculated as the CI at a given time point (CIti) divided by the CI at the normalization time point (CInml_time) as follows: NCI = CIti/CInml_time, with the normalization time point being the last time point before OPG addition. Only the curve of the NCI after treatment was calculated and chosen for analysis.
Immunofluorescence
Cells were grown on coverslips and treated as described above. Following a 24-h treatment with OPG or control, the coverslips were washed, fixed with 4 % paraformaldehyde, permeabilized using 0.5 % Triton X-100, and blocked in 5 % BSA. The cells were then incubated with an anti-vinculin antibody in 2 % BSA–PBS overnight at 4 °C, followed by staining with an anti-rabbit IgG (Alexa Fluor 488 Conjugate) for 1 h. Rhodamine phalloidin (1:40 diluted in PBS) was used to detect F-actin, while nuclei were visualized following staining with 1 μg/mL DAPI. Imaging was carried out using a fluorescence microscope (LEICA DMI 3000B).
Western blotting analysis
After treatment, cells were washed twice with cold PBS, and total protein extraction was done on ice using the RIPA buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 1 % NP-40; 0.1 % SDS) containing protease inhibitor cocktail. After sonication and centrifugation, the protein concentration in the cell extracts was determined using the BCA protein assay kit. Lysate aliquots were diluted with 6× sodium dodecyl sulfate (SDS) sample buffer and boiled for 10 min. Equivalent amounts of protein were separated on 10 % SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After blocking with TBS containing 0.05 % Tween 20 and 5 % nonfat dry milk, the membranes were then probed with primary and peroxidase-coupled secondary antibodies. Band intensities were determined using standard scanning densitometry with normalization to β-actin.
Statistical analysis
All experimental values were expressed as the mean ± standard deviation (SD) of values from three independent experiments. Statistical differences between groups were evaluated by Student’s t test using SPSS v.17.0 software. Differences were considered significant when p ≤ 0.05.
Results
OPG exposure at different points has differential effects on osteoclastogenesis
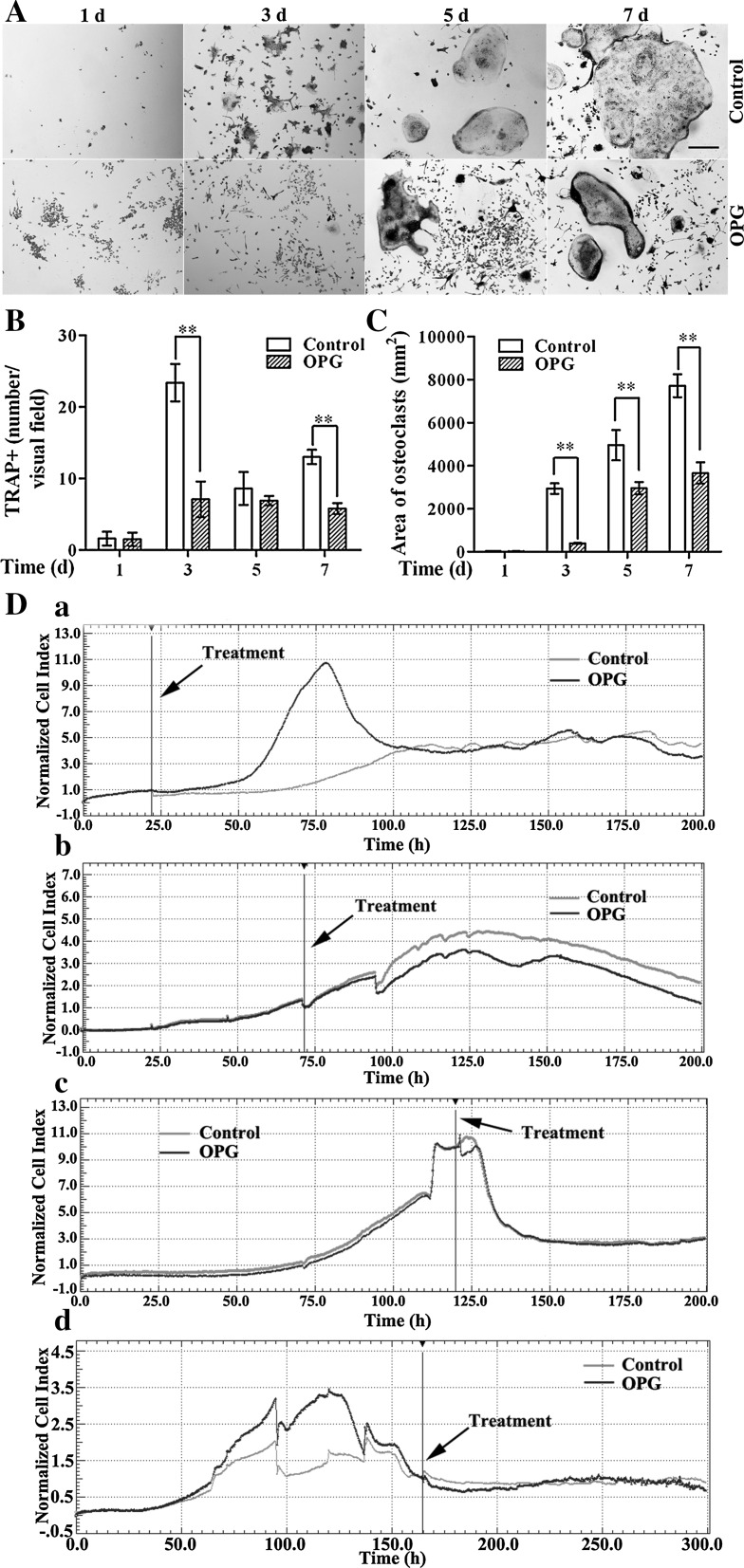
To determine the effects of OPG exposure at different time points during osteoclastogenesis, RAW264.7 cells were incubated with M-CSF and RANKL to initiate osteoclast differentiation, and treated with or without OPG at different points (days 1, 3, 5 or 7) for another 24 h. The increase in the number of osteoclasts [identified as multinucleate (three or more nuclei) TRAP-positive cells stained rose red] was maximal after 4 days of culture (Fig. 1A, B), while osteoclast area increased as the incubation progressed (Fig. 1A, C). The 24-h treatment with OPG had little effect on cells that had undergone differentiation for only 1 day, but inhibited osteoclastogenesis when the cells had been allowed to differentiate for longer periods (3, 5 or 7 days) prior to OPG exposure (Fig. 1A–C). OPG treatment at day 3 or later significantly decreased the osteoclast number at certain points (by 69.66 ± 10.60 and 55.38 ± 5.85 % compared to the corresponding control group values at days 3 and 7, respectively; Fig. 1B), and decreased osteoclast area at all points (by 86.73 ± 0.77, 40.40 ± 5.73 and 52.59 ± 6.42 % compared to the corresponding control group values at days 3, 5, and 7, respectively; Fig. 1C).
Fig. 1.
OPG differently affects osteoclastogenesis when added at different stages of culture. RAW264.7 cells were differentiated for 1, 3, 5 or 7 days in the presence of RANKL and M-CSF, followed by an additional 24-h incubation with or without 80 ng/mL OPG. A Cells were stained for TRAP and observed by inverted phase contrast microscopy (magnification ×100, scale bar 100 μm). B The number of TRAP-positive multinucleated cells (TRAP+) containing three or more nuclei were counted (magnification ×100). C The osteoclast area was measured in square pixels using ImageJ software (magnification ×100). The results are expressed as the mean ± SD. **p < 0.01 and *p < 0.05 versus control. D Dynamic monitoring of osteoclast responses to OPG using the xCELLigence system. OPG (80 ng/mL) was added when cells were incubated with cytokines for 1 (a), 3 (b), 5 (c) and 7 (d) days. Cell index (CI) values were normalized to those at the time point of OPG addition. The black lines indicate the point of OPG addition. Representative data are averaged from three wells. All experiments were repeated at least three times
To better understand the effects of OPG on osteoclastogenesis, we monitored dynamic changes in cell growth following OPG exposure using impedance technology. For cells incubated for 1 day, after treatment with OPG, real time monitoring revealed a sustained increase in the NCI above that in the control cultures during the first 10 to 55 h, followed by a decrease, with NCI reaching the control level within approximately 20 h after the peak increase (Fig. 1D, panel a). For cells differentiated for 3 days prior to OPG exposure, it took approximately 20 h after OPG treatment to see a difference between the two groups, but subsequently the OPG-treated cells showed a decrease in the NCI that was sustained till the end of the culture, though the trends in changes in the NCI with time were similar in both groups (Fig. 1D, panel b). For cells incubated for 5 days, OPG treatment resulted in an immediate short-lived decline in the NCI, which returned to the control levels 8 h following the treatment (Fig. 1D, panel c). For cells incubated for 7 days, OPG triggered a small decrease in the NCI immediately after the treatment that the curve of the OPG-treated group was lower than the curve of the control group, with the NCI trace reaching the control level after 50 h (Fig. 1D, panel d).
OPG exposure at different points differentially regulates the formation of podosomes and adhesion structures
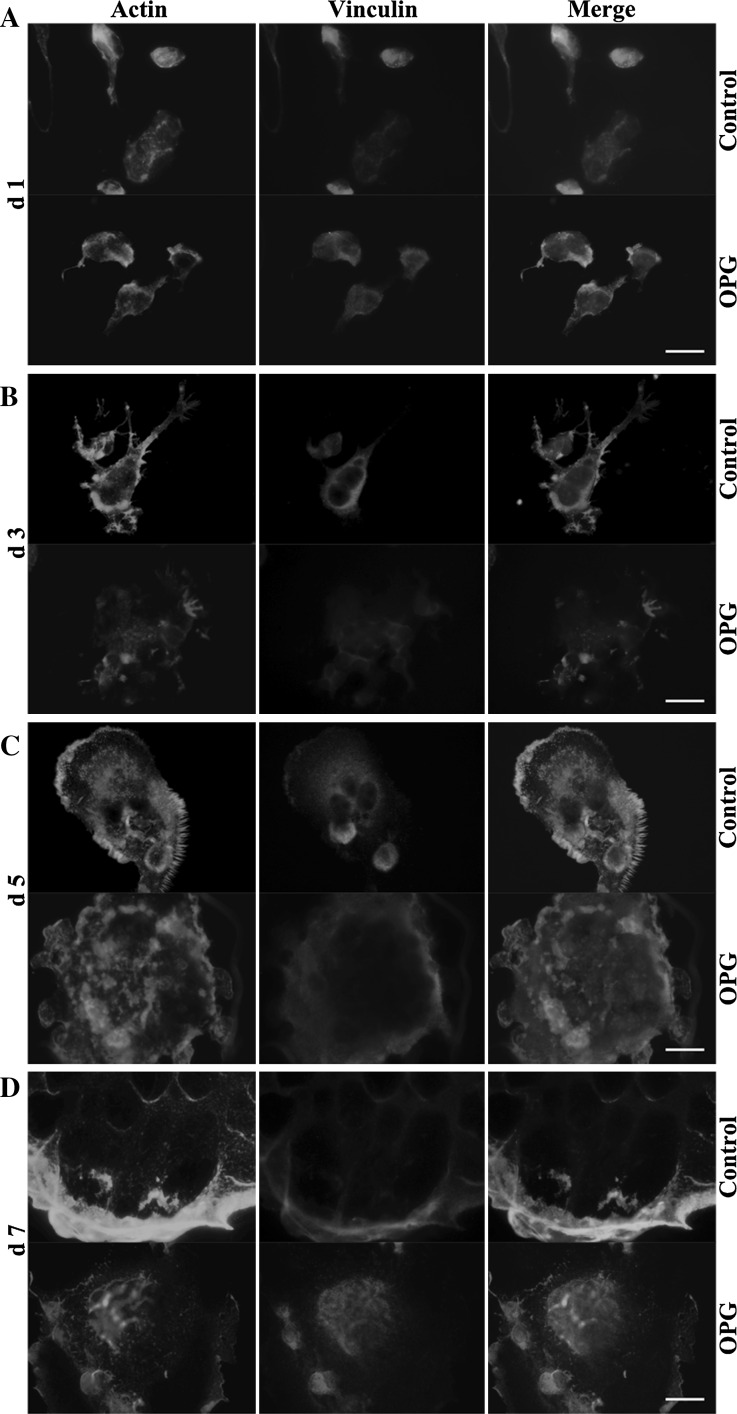
Immunofluorescence staining revealed osteoclast differentiation triggered by treatment with RANKL and M-CSF for 2 days yielded preosteoclasts with a uniform distribution of F-actin and vinculin (Fig. 2A, upper panel). When the incubation was allowed to proceed for 4 days, the resulting osteoclasts showed a characteristic podosome belt, revealed by both the F-actin and vinculin staining patterns (Fig. 2B, upper panel). When the differentiation was allowed to proceed further (up to day 5 or 7 before OPG addition), the peripheral circular zone grew larger and thicker, with the nuclei being pushed closer to the margin (Fig. 2C, D, upper panels). OPG treatment disrupted the F-actin clustering and abrogated the ring formation, triggered a change in distribution of vinculin from the periphery to the central region, and disrupted the colocalization of actin and vinculin in the periphery (Fig. 2B–D, lower panels). Moreover, osteoclasts treated at the 3- or 7-day time points were more sensitive to OPG treatment than cells treated at the 5-day time point. Interestingly, in contrast to the effects of OPG treatment at later points during osteoclastogenesis, OPG exposure early during the process (on day 1) increased the immunostaining for F-actin and vinculin at the cell periphery (Fig. 2A, lower panel).
Fig. 2.
OPG exposure at different time points differentially regulates the formation of podosomes and adhesion structures. RAW264.7 cells were seeded on coverslips and cultured for 1 (A), 3 (B), 5 (C), or 7 (D) days, as described in the “Methods” section. Cells were treated with 80 ng/mL OPG for 24 h (lower panels) or left untreated (upper panels) and then were fixed and stained to detect F-actin (red), vinculin (green), and nuclei (blue), and samples were imaged using a fluorescent microscope (magnification ×1000, scale bar = 10 μm). (Color figure online)
OPG exposure at different time points differentially regulates the expression of osteoclast enzymes and markers
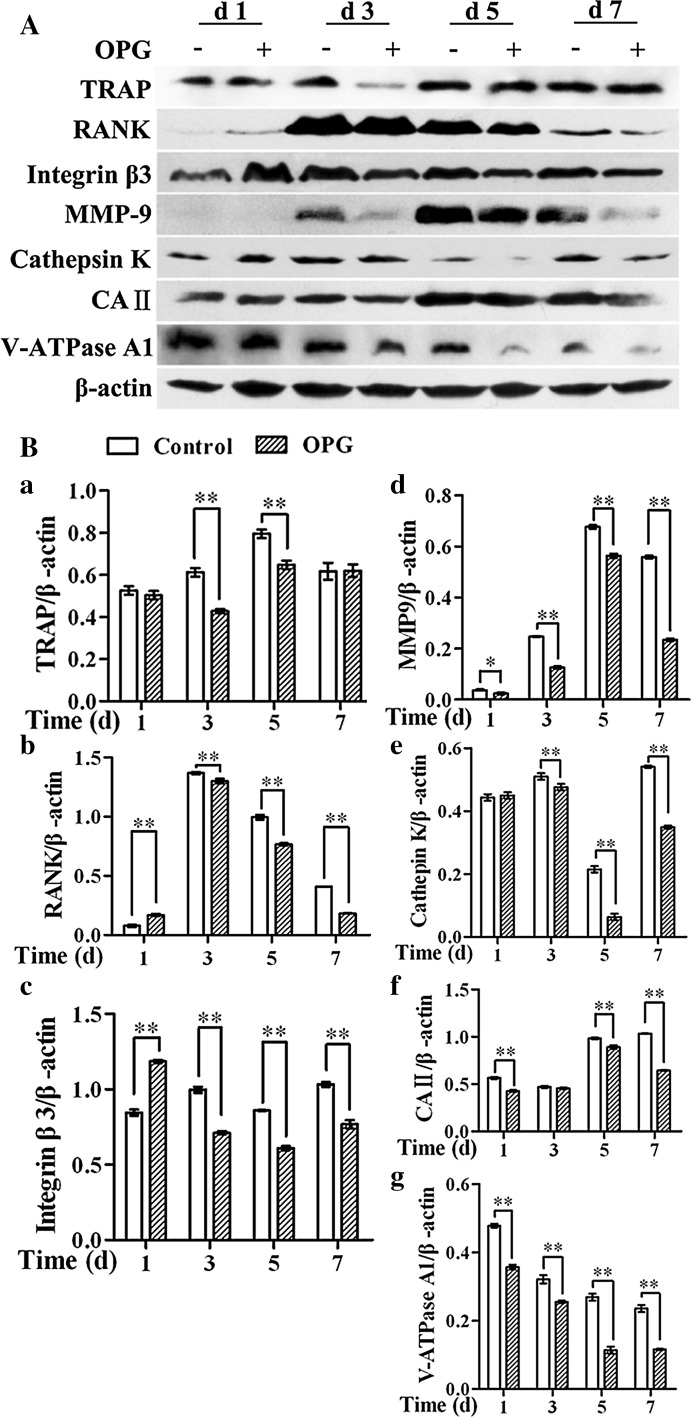
Cell lysates of RAW264.7 cells treated with OPG at different stages of osteoclastogenesis (days 1, 3, 5, or 7) were subjected to western blotting to analyze the expression of various markers of osteoclast maturation and function. OPG significantly down-regulated TRAP expression when cells at a more advanced stage of differentiation (day 3 or 5) were exposed to OPG (Fig. 3B, panel a). Additionally, the expression of RANK and integrin β3, which play a role in osteoclast adhesion, was also down-regulated when cells at later stages of differentiation (days 3–7) were exposed to OPG. In contrast to these findings, the expression of RANK and integrin β3 was increased when preosteoclasts differentiated for 1 day were exposed to OPG, while that of TRAP was unchanged (Fig. 3B, panels a–c). Additionally, the expression of enzymes involved in osteocyte-mediated bone resorption (MMP-9 and cathepsin K) was significantly down-regulated when cells at later stages of differentiation (days 3–7) were exposed to OPG (Fig. 3B, panels d, e). Similarly, the expression of enzymes involved in osteocyte vesicular transport (CA II and V-ATPase A1) was also down-regulated in cells at all stages of differentiation (days 1–7) following OPG exposure, with the exception of CA II in the group where cells differentiated for 3 days were treated with OPG (Fig. 3B, panels f, g).
Fig. 3.
OPG exposure at different points differentially regulates the expression of osteoclast enzymes and markers. A Cell lysates were immunoblotted with the indicated antibodies and analyzed by western blotting. B The band intensities were quantified by densitometry, and β-actin was used as a loading control. The results are expressed as the mean ± SD. **p < 0.01 and *p < 0.05 versus control
Discussion
Osteoclastogenesis includes several steps, including the conversion of hematopoietic progenitors to preosteoclasts, the formation of multinucleated quiescent osteoclasts, and activation to produce bone resorbing osteoclasts (Xing et al. 2012). The RANKL–RANK pathway is crucial in driving osteoclast formation from precursors and in the activation of mature osteoclasts. OPG, on the other hand, has been shown to negatively regulate RANK–RANKL interactions by binding to RANKL to inhibit bone resorption (Baud’huin et al. 2007). In the present study, we found that OPG inhibited osteoclast differentiation and adhesion structure formation, especially when the OPG exposure occurred at the point of initiation of formation of multinucleated cells. Interestingly, our study also revealed that exposure to OPG early during osteoclastogenesis enhanced the generation as well as the attachment capacity of preosteoclasts. An examination of the effects of OPG on the various functional effectors of osteoclasts revealed that OPG suppressed these to varying degrees when OPG exposure occurred after the preosteoclast stage, except in the case of CA II when the OPG treatment was carried out at the point of multinucleate cell formation (day 3). Surprisingly, but in agreement with the differential effects observed when OPG exposure occurred early during osteoclastogenesis, OPG up-regulated the expression of RANK and integrin β3 in preosteoclasts. Collectively, these findings provide the first evidence that the effects of OPG during early osteoclastogenesis differ from its effects at later points during osteocyte development.
RANK/RANKL interactions, which are essential for the survival, differentiation, and activation of osteoclasts, can be modulated by OPG, a decoy receptor for RANKL. However, the effects of OPG are complex, as it has multiple binding partners; in addition to RANKL, it also interacts with TNF-related apoptosis-inducing ligand (TRAIL) (Baud’huin et al. 2013). TRAIL induces apoptosis in numerous tumor cell lines, and OPG is able to block TRAIL-induced apoptosis (Emery et al. 1998; Neville-Webbe et al. 2004; Shipman and Croucher 2003). As osteoclasts express TRAIL, OPG inhibits osteoclast apoptosis by binding and thus inhibiting the effects of endogenously produced TRAIL in human osteoclast cultures (Chamoux et al. 2008). Thus, OPG interactions with both RANKL and TRAIL may regulate bone biology via direct effects on osteoclasts. Cell adhesion may be also modulated by OPG. Indeed, OPG stimulates the proliferation and migration of human microvascular endothelial cells (Kobayashi-Sakamoto et al. 2008), and up-regulates the expression of the cell adhesion molecule angiopoietin-2 in TNF-α-activated cells (Mangan et al. 2007). Furthermore, OPG treatment strongly increases the number of adherent human CD14+ monocytes, with an activation of the Akt/PI3-kinase signaling pathway in these cells (Baud’huin et al. 2013) and induces leukocyte adhesion to endothelial cells within 15 min (Zauli et al. 2007). These reports, in combination with our findings that exposure of preosteoclasts to OPG stimulates integrin β3 expression and actin ring formation at the cell periphery and also strongly enhances the growth of osteoclast precursors within 24 h of exposure, suggests that OPG enhances adhesion by modulating the expression of the proteins within podosomes and inhibits preosteoclast apoptosis by binding to TRAIL.
In the presence of M-CSF and RANKL, RAW264.7 cells give rise to TRAP+ mono-nucleated preosteoclasts, which fuse spontaneously within 3 days to form multinucleated osteoclasts with the expression of osteoclast phenotypic markers such as RANK and TRAP (Hofbauer et al. 2001). To maintain the normal metabolism of osteoclasts, the medium of RAW264.7 cells incubated for 3 days in the presence of 30 ng/mL RANKL and 25 ng/mL M-CSF was changed every other day. In our study of growth curves using the xCELLigence system, the CI always declined after each changing of the medium, owning to the fact that RAW264.7 cells, preosteoclasts and apoptotic cells still adhered to the bottom of the wells can be easily detached from the substrate with a little mechanical force. Therefore, there was significant change of growth curve during earlier culturing (from the 70-th to 140-th hour) in cells incubated for 7 days (Fig. 1D, panel d). However, we only analysed the NCI after OPG treatment i.e. CI, normalized to the time of treatment, giving a relative measurement of cellular response to OPG treatment. In that case, OPG clearly reduced the NCI of cells incubated for 7 days. TRAP can serve as a marker of osteoclast bone resorption, and TRAP expression positively correlates with the extent of bone resorption (Price et al. 1995). In our study, TRAP was highly expressed in terminally differentiated RAW264.7 cells. OPG reduces the RANK mRNA levels in osteoclasts (Fu et al. 2013a) and RANK is highly expressed in osteoclast precursors at the mRNA level (Wright et al. 2009). However, directly contradicting these findings, our experiments revealed little expression of RANK in preosteoclasts and an OPG-induced enhancement of this expression.
Osteoclastogenesis includes several steps, including the formation of TRAP+ mono-nucleated preosteoclasts, fusion to form multinucleated osteoclasts, and activation to produce cells capable of bone resorption (Xing et al. 2012). However, osteoclasts are heterogeneous with respect to size (Hu et al. 2008). Fusion initiates at an early stage during osteoclastogenesis, and then large osteoclasts form. In our study, we determined day 1 cells to contain a mix of precursors and preosteoclasts, day 3 cells to be those in the early differentiation stage, day-5 cells as those in the mid-differentiation stage, and day 7 cells as those in the terminal differentiation stage, based on TRAP staining. Several studies have shown that small osteoclasts showed a decreased capacity for resorption and a stronger adherence to prothrombin and thrombin than large osteoclasts (Lees and Heersche 1999). Osteoclasts polarize not only on dentine slices, but also on plastic plates (Nakamura et al. 1996). On plastic plates, osteoclasts form actin rings and generate an acidic microenvironment (pH 3.0 or less) in the attachment zone between the cell and the base of culture dish (Silver et al. 1988). Protons are produced by CA II from carbon dioxide and water in the cytoplasm, and are secreted into the extracellular space by V-ATPases (Tanabe et al. 2004). Osteoclasts degrade bone by the polarized secretion of proteolytic enzymes (e.g., cathepsin K) and acids, which hydrolyze and solubilize the organic and inorganic components of bone, respectively (Boyce 2013). MMP-9 released from the ruffled border is highly expressed at early stages of osteoclast development and in mature osteoclasts, and helps regulate the osteoclast migration required for bone resorption (Engsig et al. 2000; Reponen et al. 1994). It is reported that OPG blocks the later stages of osteoclast differentiation in mice and suppresses the activation of mature osteoclasts (Simonet et al. 1997). These findings are confirmed by our observations that OPG had a greater effect on the proteins regulating bone resorption when cells at a later stage of differentiation were treated with this decoy receptor (cells cultured up to days 5–7 compared to cells cultured up to day 3).
In conclusion, the biological effects of OPG exposure at the various stages of osteoclast differentiation are varied and include the enhanced adhesion and survival of preosteoclasts, the block of differentiation from the early to the terminal stages of osteoclastogenesis, and suppression of mature osteoclast activation following OPG treatment during the terminal differentiation stage, suggesting that the effects of OPG differ based on the stage of differentiation. The findings will be of relevance in the development and improvement of therapies using OPG to treat osteopenic disorders characterized by excessive osteoclast activity.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Numbers 31172373, 31302154, 31372495), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant Number 20113250110003), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Graduate Innovation Project of Jiangsu Province (Grant Number CXZZ12_0917).
Footnotes
Hongyan Zhao and Jianhong Gu have contributed equally to this work.
References
- Baud’huin M, Lamoureux F, Duplomb L, Redini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–2350. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud’huin M, Duplomb L, Teletchea S, Lamoureux F, Ruiz-Velasco C, Maillasson M, Redini F, Heymann MF, Heymann D. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24:401–409. doi: 10.1016/j.cytogfr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoux E, Houde N, L’Eriger K, Roux S. Osteoprotegerin decreases human osteoclast apoptosis by inhibiting the TRAIL pathway. J Cell Physiol. 2008;216:536–542. doi: 10.1002/jcp.21430. [DOI] [PubMed] [Google Scholar]
- Chen J, He J-Q, Zhen S-Y, Huang I-Q. OPG inhibits gene expression of RANK and CAII in mouse osteoclast-like cell. Rheumatol Int. 2012;32:3993–3998. doi: 10.1007/s00296-011-2338-4. [DOI] [PubMed] [Google Scholar]
- Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborah V, Novack RF. Osteoclast motility: putting the brakes on bone resorption. Ageing Res Rev. 2011;10:54–61. doi: 10.1016/j.arr.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR, Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol. 2000;151:879–889. doi: 10.1083/jcb.151.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY, Yuan Y, Liu XZ, Bian JC, Liu ZP. Inhibitory effects of osteoprotegerin on osteoclast formation and function under serum-free conditions. J Vet Sci. 2013;14:405–412. doi: 10.4142/jvs.2013.14.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY, Yuan Y, Liu XZ, Bian JC, Liu ZP. Osteoprotegerin influences the bone resorption activity of osteoclasts. Int J Mol Med. 2013;31:1411–1417. doi: 10.3892/ijmm.2013.1329. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer. 2001;92:460–470. doi: 10.1002/1097-0142(20010801)92:3<460::AID-CNCR1344>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ek-Rylander B, Karlstrom E, Wendel M, Andersson G. Osteoclast size heterogeneity in rat long bones is associated with differences in adhesive ligand specificity. Exp Cell Res. 2008;314:638–650. doi: 10.1016/j.yexcr.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi-Sakamoto M, Isogai E, Hirose K, Chiba I. Role of alphav integrin in osteoprotegerin-induced endothelial cell migration and proliferation. Microvasc Res. 2008;76:139–144. doi: 10.1016/j.mvr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C. Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet. 2000;9:2059–2063. doi: 10.1093/hmg/9.13.2059. [DOI] [PubMed] [Google Scholar]
- Lees RL, Heersche JN. Macrophage colony stimulating factor increases bone resorption in dispersed osteoclast cultures by increasing osteoclast size. J Bone Miner Res. 1999;14:937–945. doi: 10.1359/jbmr.1999.14.6.937. [DOI] [PubMed] [Google Scholar]
- Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci USA. 1990;87:5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan SH, Van Campenhout A, Rush C, Golledge J. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor-alpha associated with induction of angiopoietin-2. Cardiovasc Res. 2007;76:494–505. doi: 10.1016/j.cardiores.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura I, Takahashi N, Sasaki T, Jimi E, Kurokawa T, Suda T. Chemical and physical properties of the extracellular matrix are required for the actin ring formation in osteoclasts. J Bone Miner Res. 1996;11:1873–1879. doi: 10.1002/jbmr.5650111207. [DOI] [PubMed] [Google Scholar]
- Neville-Webbe HL, Cross NA, Eaton CL, Nyambo R, Evans CA, Coleman RE, Holen I. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86:269–279. doi: 10.1023/B:BREA.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- Okada Y, Naka K, Kawamura K, Matsumoto T, Nakanishi I, Fujimoto N, Sato H, Seiki M. Localization of matrix metalloproteinase 9 (92-kilodalton gelatinase/type IV collagenase = gelatinase B) in osteoclasts: implications for bone resorption. Lab Invest. 1995;72:311–322. [PubMed] [Google Scholar]
- Price CP, Kirwan A, Vader C. Tartrate-resistant acid phosphatase as a marker of bone resorption. Clin Chem. 1995;41:641–643. [PubMed] [Google Scholar]
- Reponen P, Sahlberg C, Munaut C, Thesleff I, Tryggvason K. High expression of 92-kD type IV collagenase (gelatinase B) in the osteoclast lineage during mouse development. J Cell Biol. 1994;124:1091–1102. doi: 10.1083/jcb.124.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Everts V, Jones S, Boyde A, Wehmeyer O, Suter A, Figura KV. Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice. Adv Exp Med Biol. 2000;477:293–303. doi: 10.1007/0-306-46826-3_32. [DOI] [PubMed] [Google Scholar]
- Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–916. [PubMed] [Google Scholar]
- Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Tanabe N, Ito-Kato E, Suzuki N, Nakayama A, Ogiso B, Maeno M, Ito K. IL-1alpha affects mineralized nodule formation by rat osteoblasts. Life Sci. 2004;75:2317–2327. doi: 10.1016/j.lfs.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrant Y, Couillaud S, Theoleyre S, Dunstan C, Heymann D, Rédini F. Osteoprotegerin differentially regulates protease expression in osteoclast cultures. Biochem Biophys Res Commun. 2002;293:38–44. doi: 10.1016/S0006-291X(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2:56–64. doi: 10.1007/s12178-009-9046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Xiu Y, Boyce BF. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J Orthop. 2012;3:212–222. doi: 10.5312/wjo.v3.i12.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Huang Y, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404–1418. doi: 10.1359/jbmr.2003.18.8.1404. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Troen B, Moonga BS, Abe E. Cathepsin K, osteoclastic resorption, and osteoporosis therapy. J Bone Miner Res. 2001;16:1747–1749. doi: 10.1359/jbmr.2001.16.10.1747. [DOI] [PubMed] [Google Scholar]
- Zauli G, Corallini F, Bossi F, Fischetti F, Durigutto P, Celeghini C, Tedesco F, Secchiero P. Osteoprotegerin increases leukocyte adhesion to endothelial cells both in vitro and in vivo. Blood. 2007;110:536–543. doi: 10.1182/blood-2007-01-068395. [DOI] [PubMed] [Google Scholar]