Abstract
TLR4 is transmembrane pattern-recognition receptor that initiates signals in response to diverse pathogen-associated molecular patterns especially LPS. Recently, there have been an increasing number of studies about the role of TLRs in the pathogenesis of several disorders as well as the therapeutic potential of TLR intervention in such diseases. Peroxisome proliferator-activated receptor-gamma (PPARγ) is a ligand-activated transcription factor with numerous biological effects. PPARγ has been shown to exert a potential anti-inflammatory effect through suppression of TLR4-mediated inflammation. Therefore, PPARγ agonists may have a potential to combat inflammatory conditions in pathologic states. The current study aims to show the decrease of inflammation by overexpression of PPARγ in a cell reporter model. To reach this goal, recombinant pBudCE4.1 (+) containing encoding sequences of human TLR4 and MD2 was constructed and used to transfect HEK cells. Subsequently, inflammation was induced by LPS treatment as control group. In the treatment group, overexpression of PPARγ prior to inflammation was performed and the expression of inflammatory markers was assessed in this condition. The expression of inflammatory markers (TNFα and iNOS) was defined by quantitative real time PCR and the amount of phosphorylated NF-κB was measured by western blot. Data indicated expression of TNFα and iNOS increased in LPS induced inflammation of stably transformed HEK cells with MD2 and TLR4. In this cell reporter model overexpression of PPARγ dramatically prevented LPS-induced inflammation through the blocking of TLR4/NF-κB signaling. PPARγ was shown to negatively regulate TLR4 activity and therefore exerts its anti-inflammatory action against LPS induced inflammation.
Keywords: HEK, Inflammation, MD2, PPARγ, TLR4
Introduction
Toll-like receptors (TLRs) are categorized as transmembrane pattern-recognition receptors (PRRs) as are capable to initiate cellular responses to a variety of pathogen-associated molecular patterns (PAMPs; Kawai and Akira 2007). TLRs were primarily discovered as mammalian homologues of the Drosophila membrane protein, Toll, as a crucial factor for embryonic development and host defense against fungi in adult flies (Hashimoto et al. 1988; Lemaitre et al. 1996). Following description of Drosophila Toll in host defense against infection, a mammalian homologue was discovered (Medzhitov et al. 1997). Then, a family of proteins structurally relevant to Drosophila Toll was identified, concertedly referred as TLRs (Kawai and Akira 2006). Like Drosophila Toll, human Toll is a type I transmembrane protein with an extracellular domain containing of a leucine-rich repeat (LRR) domain and a cytoplasmic domain homologous to the cytoplasmic domain of the human interleukin (IL)-1 receptor (Wasserman 1993; Hultmark 2006; Belvin and Anderson 1996). One of the members of TLR family is TLR4 which detects lipopolysaccharide (LPS) of gram-negative bacteria. Hence it is important for the activation of the host innate immune system (Poltorak et al. 1998; Qureshi et al. 1999; Hoshino et al. 1999; Chow et al. 1999; Shimazu et al. 1999). Miyake’s group reported an association between myeloid differentiation protein 2 (MD2) with TLR4 which was required for TLR4-mediated LPS-signaling (Wakabayashi et al. 2006). MD2 is an extracellular adaptor protein, associates with the extracellular domain of TLR4 and is indispensable for LPS recognition by TLR4 (Nagai et al. 2002; Schromm et al. 2001; Miyake et al. 2000; Vabulas et al. 2002). The binding of exogenous (e.g. LPS) or endogenous (e.g. members of heat shock protein family and proteoglycans) ligands to TLR4 activates NF-κB signaling pathway and then expression of pro-inflammatory cytokines such as TNF-α, IL-1 β, iNOS and IL-6TLR4 (Tsan and Gao 2004; Kitaoka et al. 2007; Okun et al. 2009). TLR4 has been examined mostly for its broad contribution to immune-related disorders. Evidences suggest that TLR4 not only contributes to cellular pathophysiology, but also plays a vital role in facilitating neurodegenerative diseases such as ischemic stroke, Alzheimer’s disease and multiple sclerosis (Lanza et al. 2013). However, most of the evidence for role of TLR4 is observational, and little is known about its molecular mechanism.
Peroxisome proliferator-activated receptor-gamma (PPARγ) is a ligand-activated transcription factor with numerous biological effects. It also exerts a potential anti-inflammatory effect by suppressing TLR4-mediated inflammation (Zhang et al. 2011; Jung et al. 2012; Ji et al. 2011; Zhao et al. 2011; Wang et al. 2011; Zhang et al. 2010; Ji et al. 2009; Necela et al. 2008; Yin et al. 2013, 2014).
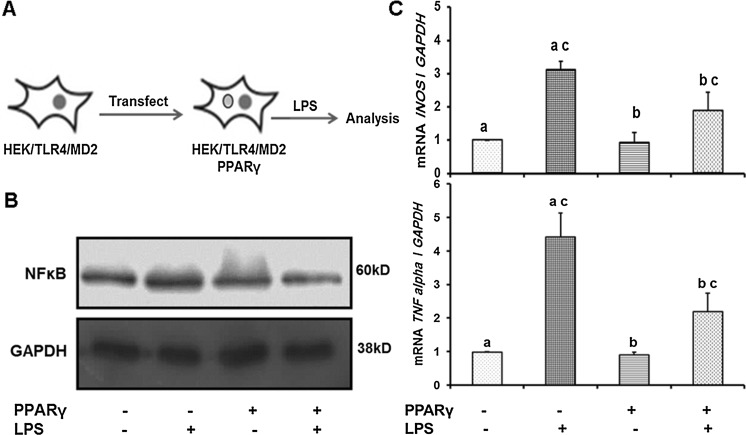
In the present study, to clarify whether anti-inflammatory effect of PPARγ is mediated through TLR4 receptor inhibition, we designed a reporter model cell. At the first step, human coding sequence (CDS) of TLR4 and MD2 were cloned in one vector. This vector was stably inserted to human embryonic kidney (HEK) cell line. By generating such reporter cell, we assessed the direct response of TLR4 to LPS. Furthermore, we transiently transfected an expression vector encoding EGFP-PPARγ in these reporter cells prior to induction of inflammation by LPS. Data have shown that PPARγ could effectively attenuate the inflammatory condition. This approach facilitates the functionality assessment of TLR4 in further studies using numerous therapeutically molecules to inhibit the progress of inflammation and neurodegenerative diseases.
Materials and methods
Construction of recombinant pBudCE4.1 (+) containing CDS of human TLR4 and MD2
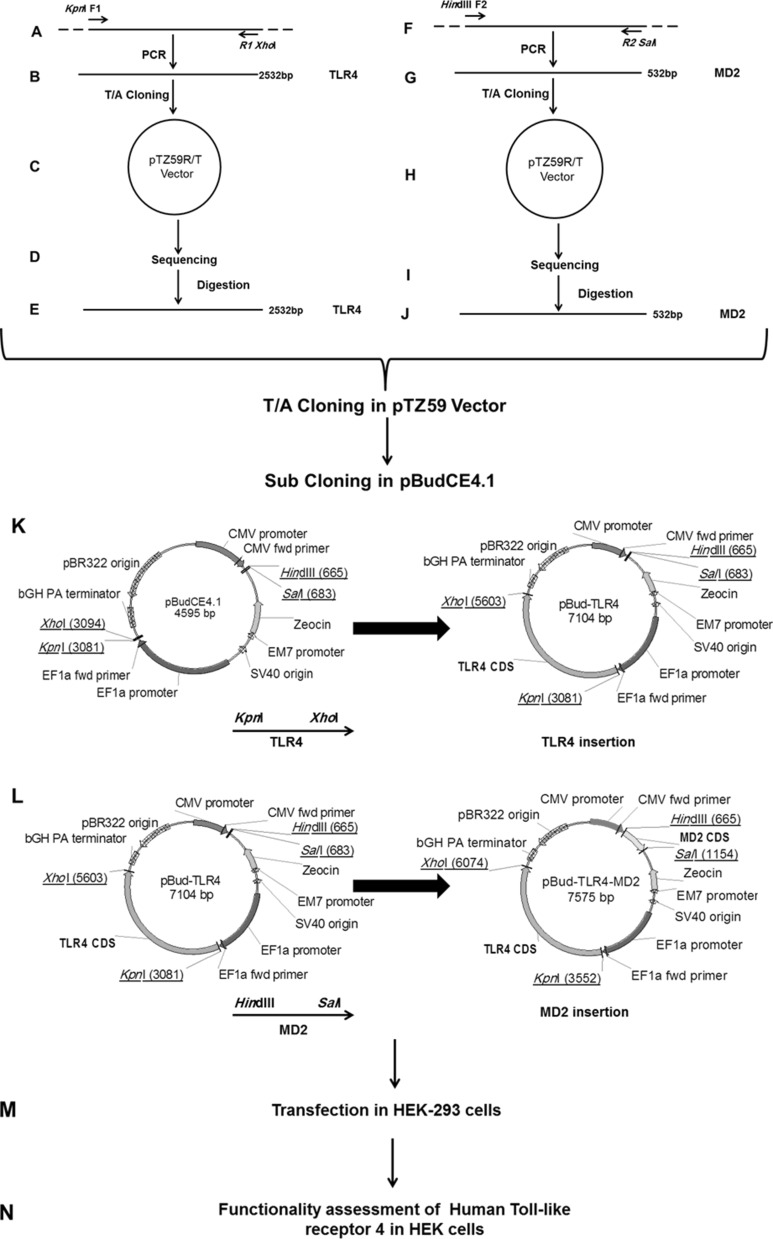
Oligodendrocyte precursor cells (OPCs) were provided by the Royan Institute for Stem Cell Biology and Technology (Tehran, Iran). Total RNA was extracted from OPCs using RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Then, cDNA was constructed (Thermo Scientific, Waltham, MA, USA) with random hexamers according to the manufacturer’s protocol. In order to amplify CDS of TLR4 and MD2, two set of primers were designated based on the sequence data for CDS of TLR4 and MD2 (NCBI; Table 1A) and ordered through Bioneer Company (Daejeon, Korea). Human MD2 and TLR4 CDS were amplified by PCR reactions using OPCs derived cDNA as the template and Dream Taq DNA polymerase (Thermo Scientific). Amplifications of DNA fragments encompassing of TLR4 CDS with the length of 2532 bp were carried out using the specific primers (Fig. 1A, B) under the following conditions: initial denaturation at 95 °C for 5 min, 25 cycles of 95 °C for 1 min, 72 °C for 1 min and 72 °C for 2 min, and a final extension for 10 min at 72 °C. For amplification of 532 bp DNA fragment containing MD2 CDS (Fig. 1F, G), a specific prime pair was used under aforementioned conditions. Both of PCR products were extracted from agarose gel using gel extraction kit (Qiagen) to remove excess primers and templates. The purified products were inserted into pTZ57/R cloning vector (Thermo Scientific) according to the manufacturer’s protocol (Fig. 1C, H). The recombinant vectors were transformed into Ecoli DH5α competent cells. Plasmid extraction was carried out on selected positive colonies by Qiagen Miniprep Kit (Qiagen) and were sent for sequencing (Metabion International, Planegg/Steinkirchen, Germany) to ensure the accurate cloning of CDS of TLR4 and MD2 without any undesired mutation (Fig. 1D, I). In order to construct a recombinant pBudCE4.1 vector expressing TLR4 under the control of human elongation factor 1α-subunit (EF-1α) promoter, the recombinant pTZ57/TLR4 plasmid was double digested with KpnI and XhoI (Thermo Scientific; Fig. 1E). To generate pBudCE4.1-TLR4, KpnI-TLR4 CDS-XhoI fragment was inserted into the same sites in pBudCE4.1 vector (Fig. 1K) using DNA ligation kit (TaKaRa, Otsu, Shiga, Japan). At the next step, this recombinant pTZ57/MD2 plasmid was double digested with HindIII and salI (Thermo Scientific) (Fig. 1J). Purified HindIII-MD2 CDS-SalI was substituted into the same sites in pBudCE4.1-TLR4 (Fig. 1L). The latter generated recombinant vector was named pBudCE4.1-TLR4-MD2 (Fig. 1L).
Table 1.
List of primers used in this study
| Name | (5′–3′) | Underlined site |
|---|---|---|
| A. Primers used for amplification of TLR4 CDS and MD2 CDS | ||
| F1-TLR4 | GGTACCATGATGTCTGCCTCGCGCCTGGCTG | KpnI |
| R1-TLR4 | CTCGAGTCAGATAGATGTTGCTTCCTGCCAATT | XhoI |
| F2-MD2 | AAGCTTATGTTACCATTTCTGTTTTTTTCCACCC | HindIII |
| R2-MD2 | GTCGACCTAATTTGAATTAGGTTGGTGTAGGATG | SalI |
| Genes | Forward primer (5′–3′) | Reverse primer (5′–3′) | AT (°C) | Accession no. |
|---|---|---|---|---|
| B. Primers used for gene expression analysis by real time PCR | ||||
| GAPDH | GTTCAACGGCACAGTCAAG | TACTCAGCACCAGCATCAC | 60 | NM_008084.2 |
| TLR4 | CTCAGTGTGCTTGTATC | CTCATTCCTTACCCAGTC | 54 | NM_138554.4 |
| MD2 | GGGAGAGACTGTGAATAC | TAGGTTGGTGTAGGATGAC | 54 | NM_015364.4 |
| TNFα | TAAGAGGGAGAGAAGCAACT | CAGTATGTGAGAGGAAGAGAAC | 60 | NM_000549.3 |
| iNOS | AAGAGACGCACAGGCAGAG | CAGGCACACGCAATGATGG | 58 | NM_012611.3 |
F and R, are forward and reverse, respectively. AT is annealing temperature of PCR
Fig. 1.
Schematic representation of PCR amplification of MD2 and TLR4 CDS and sub-cloning into pBudCE4.1 (+) vector. Description for stages (A–N) of procedure is detailed in the “Materials and methods” section
Human embryonic kidney HEK293T (HEK) cells culture and transfection
HEK293T cells were obtained from the Royan Institute for Stem Cell Biology and Technology (Tehran, Iran) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) containing 10 % heat-inactivated fetal bovine serum (FBS, Clontech, Mountain View, CA, USA) supplemented with 10 µg/mL β-Mercaptoethanol (100 mM, Sigma, St. Louis, MO, USA), 100 μg/mL penicillin (Gibco) and 100 μg/mL streptomycin (Gibco). Cell culture incubation was performed in a humidified atmosphere of 5 % CO2 at 37 °C. Approximately, 6.25 × 105 cells were passaged in each well of six-well plate dish 1 day prior to the transfection. Transfection was carried on the cells with lipofectamine LTX reagent (Invitrogen, Carlsbad, CA, USA) using each of pBudCE4.1-TLR4-MD2, pBudCE4.1-TLR4 or pBudCE4.1 (as Mock) (Fig. 1M).
Isolation of a stable HEK cell line expressing TLR4 and TLR4/MD2
Two days post-transfection, cells were treated with 60 µg/mL Zeocin (Invitrogen) for 2 weeks. Emerged colonies were isolated for further screening by RT-PCR to confirm the presence of TLR4 and MD2 expression. Limiting dilution was performed to isolate homogenous stable cell line in terms of TLR4 and MD2 expression. HEK cells which were stably transformed with pBudCE4.1 were nominated Mock cells. Those cells which were stably transfected with pBudCE4.1-TLR4 were labeled as HEK/TLR4 cells and stably transformed cells expressing both TLR4 and MD2 were termed HEK/TLR4/MD2.
Reconstitution of TLR4-mediated cellular activation upon LPS induced inflammation
Following isolation of cell lines, expression of TLR4 and MD2 was examined by RT-PCR on RNAs extracted from the cell. Then to evaluate the functionality of the TLR4, LPS induced inflammation strategy was implemented. To carry out this experiment, effective concentration of LPS was determined by the MTS assay. Different concentrations of LPS (Santa Cruz Biotech, Santa Cruz, CA, USA) were dissolved in water. MTS assay was carried out with adding 20 µL of [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)/phenazine methosulfate] (MTS/PMS, Promega, Madison, WI, USA) solution to the culture medium. The absorbance of viable cell samples was measured at 490 nm after 3 h incubation by an ELISA reader (Awareness Technology, Palm City, FL, USA) as described (Peymani et al. 2013).The effective concentration of LPS (1 μg/mL) was selected for further experiments as reported by Chow et al. (1999). Cells were treated with LPS for 24 h, and then cells were harvested for expression analysis of two target genes, iNOS and TNFα by quantitative real time PCR analysis and Western blot analysis for NFκB.
RT-PCR and quantitative real time PCR for quantification of gene expression
Total RNA was extracted from transfected HEK293T cells according to the protocol using TRizol reagent (Invitrogen) based on manufacturer instruction. Approximately, 1 µg of total RNA was used to synthesize cDNA by Revert Aid First Strand cDNA synthesis Kit which was purchased from Thermo Scientific utilizing random hexamer primers. Final step of RT-PCR was completed with the specifically designed primers for TLR4 and MD2, iNOS and TNFα CDSs (Table 1A). The primers were ordered from Bioneer Company. PCR products were electrophoresed in 1 % agarose gel containing ethidium-bromide and bands were visualized with UV light (Uvico, Rapp Optoelectronic, Hamburg, Germany). Quantitative real time PCR was carried out using specific primer pairs (Table 1B) in a step One Plus thermal cycler (Applied Biosystems, Carlsbad, CA, USA) as defined by the manufacturer’s protocol. All reactions were carried out in triplicate. The expression level of each target gene was normalized by expression of the house keeping gene, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Final data were calculated based on 2−ΔΔCt.
Transient expression of PPARγ into HEK/TLR4/MD2 cells to attenuate inflammatory conditions of LPS treatment
HEK/TLR4/MD2 cells were transiently transfected with pEGFP-C1/EGFP-PPARγ (Ghasemi et al. 2010) similar to aforementioned conditions. Two days post-transfection, transfection efficiency was estimated by direct green fluorescence observation of the cells without cell fixation. Then transiently transfected cells were implemented for LPS treatment as described earlier.
Immunofluorescence and western blot analyses
To confirm the results of real time PCR technique, expression of TLR4 at the protein level was also observed using indirect immunofluorescence staining. To do the experiment, cells were fixed in 4 % paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 30 min, permeabilized with 0.2 % Triton X-100 (Sigma-Aldrich) for 40 min. The primary antibody was mouse anti-TLR4 antibody (Abcam, Cambridge, U.K.; final concentration 1:200 for 60 min) and the secondary antibody was Alexa Fluor 488 Goat Anti-Mouse IgG (H + L) (Molecular probes, Eugene, OR, USA; final concentration 1:50 for 60 min). Both antibodies were diluted in blocking buffer [10 mg/mL bovine serum albumin (BSA; Bio-Rad, Hercules, CA, USA) in phosphate buffered saline (PBS)] and incubated with cells at room temperature. Cells were washed with PBS before and after addition of antibodies. To observe expression of EGFP-PPARγ in transfected cells, green fluorescence was observed without fixation. Meanwhile, nuclei were counter stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich). Omission of primary antibody was used as a control for all markers. The cells were analyzed with a fluorescentmicroscope (Olympus, Tokyo, Japan) and images were acquired with an Olympus D70 camera (Olympus).
To perform immunoblotting, cells were lysed with TRizol reagent according to the manufacturer’s protocol. A solubilized protein fraction of each sample (30 µg) was subjected to SDS-PAGE electrophoresis and transferred to polyvinylidenedifluoride (PVDF) membranes. After blocking with 5 % skim milk, membranes were labeled with rabbit polyclonal antibody against phosphorylated NFкB p65 (Abcam, dilution 1:2000), mouse anti-GAPDH antibody (Santa Cruz, dilution 1:5000), then membranes were treated with horseradish peroxidase (HRP)-conjugated goat anti rabbit (Dako, Carpinteria, CA, USA, dilution 1:16000) and goat anti-mouse IgG (Dako, dilution 1:5000) respectively. HRP-conjugated IgG bound to each protein band was visualized by an Amersham ECL Advanced Western Blotting Detection Kit (GE Healthcare, Pittsburgh, PA, USA).
Statistical analysis
Microsoft Excel (2007) and SPSS (version 17) were used to express data as mean ± SEM obtained from three independent treatments of replicated observations. One-way analysis of variance (ANOVA) was performed to identify statistical differences between treatments, which were considered to be significant at p < 0.05 (*).
Results
Ectopic expression of TLR and TLR/MD2 CDS in HEK cells
As depicted in Fig. 1 and described in materials and methods, several sets of PCR were carried out using designated primer pairs for amplification of human TLR4 and MD2 CDS. Amplified fragments were extracted from agarose gel and inserted into pTZ59R/T vector and sent for sequencing (Fig. 1A–J). Results of sequences showed 100 % identity to MD2 and TLR4 CDS (Data not shown). In the next stage TLR4 CDS was subcloned into pBudCE4.1 vector under regulation of EF1a promoter which resulted in generation of pBudCE4.1-TLR4 (Fig. 1K). Next, MD2 CDS was subcloned into pBudCE4.1-TLR4 under regulation of CMV promoter. Hence, the new recombinant vector, pBudCE4.1-TLR4-MD2 encompassed two expression cassettes for simultaneous expression of MD2 and TLR4 proteins (Fig. 1L).
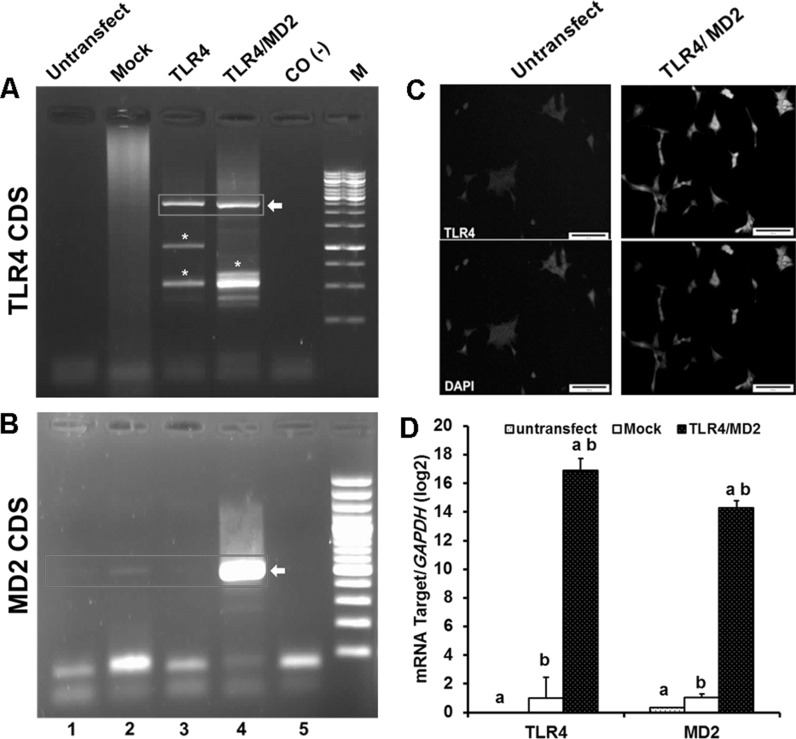
Recombinant vectors (pBudCE4.1-TLR4; TLR4 and pBudCE4.1-TLR4-MD2; TLR4/MD2) were transfected along with the pBudCE4.1 into the HEK cells (Mock) separately. Transfected cells were further expanded for colony formation under antibiotic treatment (Fig. 1M, N). Numerous resistant colonies were grown and finally among ten isolated colonies (Mock, TLR4, and TLR4/MD2), one colony with higher expression of the insert was selected for further experiments (Data not shown). RT-PCR data showed high expression level of MD2 in TLR4/MD2 cells (Fig. 2b, lane 4) and TLR4 in both TLR4 and TLR4/MD2 cells (Fig. 2a, lanes 3, 4). Meanwhile, a faint product band was observed for MD2 in Mock and untransfected and TLR4 cells (Fig. 2b, lanes 1–3), indicating a basal level of MD2 transcription in HEK cells.
Fig. 2.
Characterization of stable HEK cell lines expressing MD2 and TLR4 proteins. RT-PCR analysis of HEK cell lines to identify the expression of TLR4 (A) and MD2 (B). The respective bands are shown in red box. Star represents the artificial nonspecific bands. M is DNA marker. Lanes 1–5 are referred as Untransfect: untransfected cell line, Mock: pBudCE4.1-TLR4-MD2 transformant cell line, TLR4: pBudCE4.1-TLR4 transformant cell line, TLR4/MD2: pBudCE4.1-TLR4-MD2 transformant cell line, and CO-: no template PCR reaction, respectively. C Immunofluorescence staining of TLR4 in HEK cells. The nuclei were counterstained with DAPI. Bar, 200 µm. D Relative expression levels of TLR4 and MD2 of untransfected cell line (untransfect) and pBudCE4.1(+) transformant cell line (Mock) and pBudCE4.1-TLR4-MD2 transformant cell line (TLR4/MD2) were compared. The number of independent repeats were 3 for each experiment (n = 3). Similar alphabets indicate significant difference between same samples at p < 0.001. (Color figure online)
Quantitative real-time PCR confirmed the data obtained by RT-PCR (Fig. 2d). Furthermore, indirect immunofluorescence staining indicated a vast range of TLR4 expression in TLR4/MD2 (Fig. 2c, right panel) and TLR4 cells (Data not shown) dissimilar to those observed in untransfected HEK (Fig. 2c, left panel) and Mock cells (Data not shown).These results confirmed the RT-PCR data indicating a reasonable production of ectopic TLR4 in transfected cells.
Functionality assessment of TLR4-mediated cellular responses to LPS treatment
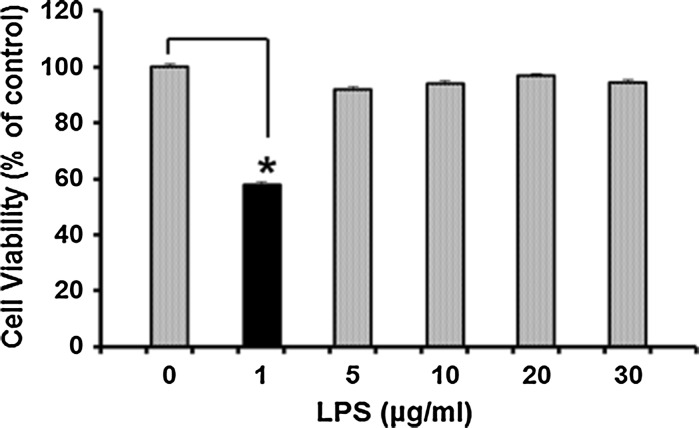
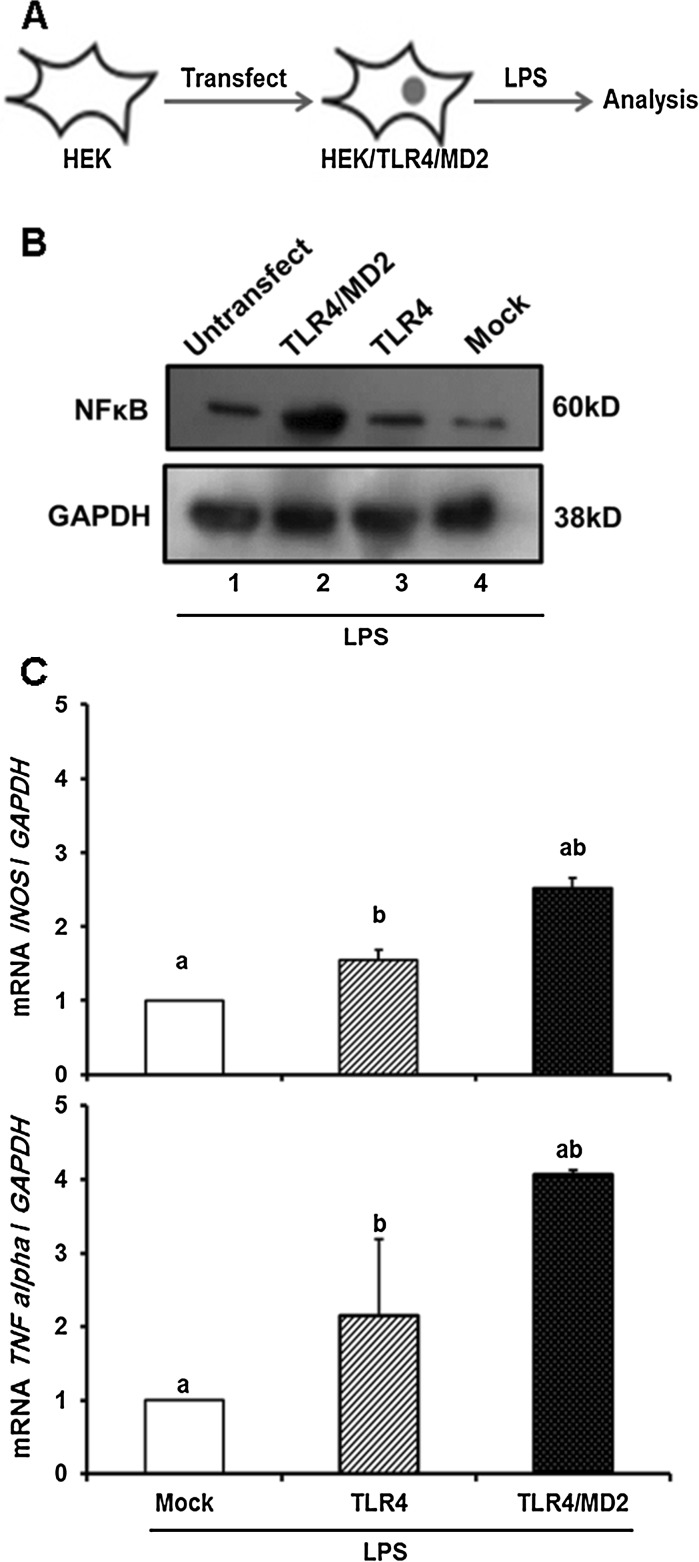
To estimate the effective amount of LPS required for induction of TLR4-mediated cellular responses, TLR4/MD2 cell viability assessment was carried out following treatment with several concentrations of LPS (0–30 µg/mL). MTS results revealed that LPS significantly decreased cell viability at concentration of 1 µg/mL. Interestingly higher amounts of LPS could actually inhibit this system (Fig. 3). Therefore, experiments were carried out with 1 µg/mL of LPS. As depicted in Fig. 4a, LPS treatment increased expression of NFκB, a key regulator of immune responses inTLR4/MD2 cells (Fig. 4b, lane 2). Such induction was not observed in Mock and untransfecetd cells and even in TLR4 cells (Fig. 4b, lane 3) thereby, demonstrating that endogenous levels of MD2 in HEK cells are not sufficient to trigger TLR4-mediated cellular responses to LPS treatment. To further explore NFκB ability as rapid-acting primary transcription factor through the exogenous TLR4 in TLR4/MD2 cells, the relative expression of two target genes, iNOS and TNFα was quantified by real time PCR analysis. Data indicated increased expression of these factors in TLR4/MD2 cells compared to the Mock and TLR4 cells under treatment of LPS (Fig. 4c). These results indicated that expression of human TLR4 and MD2 was sufficient for the activation of the TLR4 by LPS in HEK cells.
Fig. 3.
Cell viability decrease induced by LPS.TLR4/MD2 cells were pretreated with various concentrations of LPS for 24 h. Star indicates the significant difference between samples and control at p < 0.05. The number of repeats was 3. Control symbols HEK cells which were not treated by LPS
Fig. 4.
LPS induced TLR4-mediated cellular responses in TLR4/MD2 cells. A Schematic representation of TLR4/MD2 cells upon LPS (1 µg/mL) treatment. B LPS increased amount of NFκB, a key regulator in the immune responses of the cells, in TLR4/MD2 cells. Lanes 1–4 are referred as Untransfect: untransfected cell line, Mock: pBudCE4.1-TLR4-MD2 transformant cell line, TLR4: pBudCE4.1-TLR4 transformant cell line, TLR4/MD2: pBudCE4.1-TLR4-MD2 transformant cell line, respectively. Furthermore, LPS induced activation of NFκB as assessed by enhancement of the relative expression of two target genes, TNFα (B) and iNOS (C). All expressions were quantified and normalized with GAPDH. The number of independent repeats was 3 for each experiment (n = 3). Common letters show significant difference between the respective samples at p < 0.05
Ectopic expression of PPARγ efficiently attenuated the LPS induced inflammation
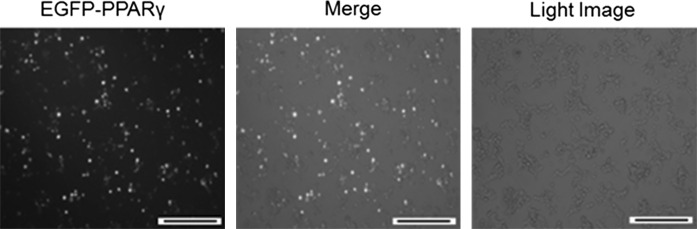
As described in materials and methods, pEGFP-C1/EGFP-PPARγ was used for transfection of HEK/TLR4/MD2 cells. Transfection efficiency was estimated by direct observation of green fluorescent cells which were around 70 % (Fig. 5). Transiently EGFP-PPARγ expressing cells were treated with LPS (1 μg/mL) (Fig. 6a). As indicated in Fig. 6b, PPARγ attenuated activation of NF-κB compared to untransfected cells. This observation was confirmed by quantitative analysis of transcript levels of two target genes (iNOS and TNFα) of NF-κB. A decrease in transcription of iNOS and TNFα was evident in transfected cells under inflammatory condition in comparison with untransfected cells (Fig. 6c).
Fig. 5.
Transient expression of EGFP-PPARγ in HEK/TLR4/MD2 cells. Numerous green fluorescent cells were observed in the culture dish. Magnification is 100×, bar: 20 µm
Fig. 6.
Transient expression of EGFP-PPARγ in HEK/TLR4/MD2 cells attenuated the LPS induced inflammatory conditions. a EGFP-PPARγ expressing cells were treated with LPS. b Ectopic amounts of PPARγ in transfected cells attenuated activation of NF-κB compared with untransfected cells. c Quantitative real time PCR analysis of transcript levels of two target genes (iNOS and TNFα). All expressions were quantified and normalized with GAPDH. The number of independent repeats was 3 for each experiment (n = 3). Common letters show significant difference between the respective samples at p < 0.05
Discussion
In this study, we have established a HEK stable cell line (HEK/TLR4/MD2) as an appropriate model to study inflammation, which could be used to study the enormous therapeutic approaches to inhibit the progress of inflammation especially in neurodegenerative diseases. Since HEK cells do not express endogenous TLR4 (Muta and Takeshige 2001), therefore this system would be helpful to assess the direct stimulation of TLR4 mediated signaling without interplaying with other molecules that may mediate the signaling in LPS-responsive cells.
Our established system is similar to those previously published (Yang et al. 2000; Latz et al. 2002). Here we showed that the endogenous amount of MD2 is not adequate for TLR4-mediated cellular responses. Therefore, coexpression of both TLR4 and MD-2 is required for triggering the cellular responses against LPS to augment the MAP kinase pathways, Elk-1 stimulation, and IL-8 generation (Yang et al. 2000). Nuclear translocation of activated NF-κB mediates the increased transcription levels of a vast range of proteins involved in cell survival, proliferation, inflammatory response, and anti-apoptotic factors. Here we showed upregulated in expression of two target genes of NF-κB in TLR4/MD2 cells but not in TLR4 cells. This striking difference reflects the different approach which was used in our study to generate a stable cell line. In our study we compared TLR4/MD2 cells with TLR4 cells and both cells were isolated after stable transfection of MD2 and TLR4 expression vectors. Previous studies were performed using 2 vectors for co-transfection of MD2 and TLR4 (Muta and Takeshige 2001), whereas our study was carried out with only one recombinant pBudCE4.1 (+) containing both CDS of TLR4 and MD2. Our results indicated that stable expression of human TLR4 and MD2 is sufficient for the activation of the TLR4 in the HEK cells through LPS. The reconstitution system we have established, allows a direct and extensive comparison of the responses of TLR4 to LPS. Numerous studies have indicated ameliorating properties of PPARγ in LPS-induced inflammatory conditions in a variety of cells (Zhang et al. 2010 and 2011; Jung et al. 2012; Ji et al. 2009 and 2011; Zhao et al. 2011; Wang et al. 2011; Necela et al. 2008; Yin et al. 2013, 2014). To clarify whether anti-inflammatory effects of PPARγ are mediated via TLR4 receptor inhibition, we implemented such reporter cell. This cell reporter system enabled us to examine this phenomenon without interfering undesired cellular components. Previous studies have indicated suppressive property of PPARγ on NF-κB in various tissues (Sasaki et al. 2005; Chen et al. 2003; Remels et al. 2009). One suggestive mechanism is inhibition of the IкB kinases (Sasaki et al. 2005). An alternative mechanism is phosphorylation of PPARγ which physically interacts with p65 and subsequently decreases the transcriptional activity of NF-κB (Chen et al. 2003). Therefore, further experiments are required to define which mechanism mediates ameliorative effects of PPARγ on inflammation.
Conclusion
Here we reported the establishment of a stable HEK cell line expressing both human TLR4 and MD2 proteins, which is suitable for monitoring the studies of inflammation status of the cells. The functionality of this system was approved by direct overexpression of PPARγ which attenuated the LPS induced cellular inflammatory responses. This cell reporter model would be an ideal system to study the inflammatory responses involved in a variety of pathologic conditions. Moreover, it facilitates to understand how innate immune system triggers antipathogen signaling cascades.
Conflict of interest
None of the authors has any conflicts of interest to disclose and all authors support submission to this journal.
Abbreviations
- BSA
Bovine serum albumin
- CDS
Coding sequence
- DAPI
4,6-Diamidino-2-phenylindole
- DMEM
Dulbecco’s modified Eagle’s medium
- EF-1α
Elongation factor 1α-subunit
- FBS
Fetal bovine serum
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HEK
Human embryonic kidney
- IL
Interleukin
- LPS
Lipopolysaccharide
- LRR
Leucine-rich repeat
- MD2
Myeloid differentiation protein 2
- MTS/PMS
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)/phenazine methosulfate
- OPC
Oligodendrocyte precursor cell
- PAMP
Pathogen-associated molecular patterns
- PBS
Phosphate buffered saline
- PRR
Pattern-recognition receptor
- PVDF
Polyvinylidenedifluoride
- TLR
Toll-like receptor
Contributor Information
Mir Davood Omrani, Phone: +98 2123872572, Email: davood_omrani@sbmu.ac.ir.
Kamran Ghaedi, Phone: +98 31 95015694, Email: kamranghaedi@royaninstitute.org.
Mohammad Hossein Nasr-Esfahani, Phone: +98 31 95015694, Email: mh_nasr@royaninstitute.org.
References
- Belvin MP, Anderson KVA. Conserved signaling pathway: the Drosophila Toll–dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Chen F, Wang M, O’Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARgamma via active ERK1/2 leads to its physical association with p65 and inhibition of NF-kappa/beta. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Ghasemi S, Ghaedi K, Nasr Esfahani MH, Tanhaei S, Rabeei F, Karbalaii K, Baharvand H, Esmaeili A. Intra-nuclear localization of EGFP-mouse PPARγ1 in bovine fibroblast cells. Yakhteh Med. 2010;J12:97–104. [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- Hultmark D. Insect immunology: ancient relationships. Nature. 2006;367:116–117. doi: 10.1038/367116a0. [DOI] [PubMed] [Google Scholar]
- Ji Y, Liu J, Wang Z, Liu N, Gou W. PPAR gamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Invest. 2009;89:887–902. doi: 10.1038/labinvest.2009.45. [DOI] [PubMed] [Google Scholar]
- Ji Y, Liu J, Wang Z, Li Z. PPARγ agonist rosiglitazone ameliorates LPS-induced inflammation in vascular smooth muscle cells via the TLR4/TRIF/IRF3/IP-10 signaling pathway. Cytokine. 2011;55:409–419. doi: 10.1016/j.cyto.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Torrejon C, Chang CL, Hamai H, Worgall TS, Deckelbaum RJ. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: a role for PPARγ. Arterioscler Thromb Vasc Biol. 2012;32:2929–2937. doi: 10.1161/ATVBAHA.112.300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signalling. Cell Death Diff. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kitaoka Y, Munemasa Y, Nakazawa T, Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain Res. 2007;1142:247–255. doi: 10.1016/j.brainres.2007.01.097. [DOI] [PubMed] [Google Scholar]
- Lanza AM, Kim DS, Alper HS. Evaluating the influence of selection markers on obtaining selected pool s and stable cell lines in human cells. Biotechnol J. 2013;8:811–821. doi: 10.1002/biot.201200364. [DOI] [PubMed] [Google Scholar]
- Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus control the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/S0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miyake K, Ogata H, Nagai Y, Akashi S, Kimoto M. Innate recognition of lipopolysaccharide by Toll-like receptor 4/MD-2 and RP105/MD-1. J Endotoxin Res. 2000;6:389–391. doi: 10.1177/09680519000060051001. [DOI] [PubMed] [Google Scholar]
- Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem. 2001;268:4580–4589. doi: 10.1046/j.1432-1327.2001.02385.x. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Shimazu R, Ogata H, Akashi S, Sudo K, Yamasaki H, Hayashi S, Iwakura Y, Kimoto M, Miyake K. Requirement for MD-1 in cell surface expression of RP105/CD180 and B-cell responsiveness to lipopolysaccharide. Blood. 2002;99:1699–1705. doi: 10.1182/blood.V99.5.1699. [DOI] [PubMed] [Google Scholar]
- Necela BM, Su W, Thompson EA. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor gamma and nuclear factor-kappa B in macrophages. Immunology. 2008;125:344–358. doi: 10.1111/j.1365-2567.2008.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peymani M, Ghoochani A, Ghaedi K, Karamali F, Karbalaie K, Kiani-Esfahani A, Rabiee F, Nasr-Esfahani MH, Baharvand H. Dual effects of peroxisome proliferator-activated receptor γ on embryonic stem cell self-renewal in presence and absence of leukemia inhibitory factor. Eur J Cell Biol. 2013;92:160–168. doi: 10.1016/j.ejcb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remels AH, Langen RC, Gosker HR, Russell AP, Spaapen F, Voncken JW, Schrauwen P, Schols AM. PPAR gamma inhibits NF-kappa B-dependent transcriptional activation in skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E174–E183. doi: 10.1152/ajpendo.90632.2008. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:3. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schromm AB, Lien E, Henneke P, Chow JC, Yoshimura A, Heine H, Latz E, Monks BG, Schwartz DA, Miyake K, Golenbock DT. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol. 2004;286:C739–C744. doi: 10.1152/ajpcell.00364.2003. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin V 1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Kobayashi M, Akashi-Takamura S, Tanimura N, Konno K, Takahashi K, Ishii K, Mizutani T, Iba H, Kouro T, Takaki S, Takatsu K, Oda Y, Ishihama Y, Saitoh S, Miyake K. A protein associated with Toll-like receptor 4 regulates cell surface expression of TLR4. J Immunol. 2006;177:1772–1779. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Zhang Y, Li XD, Hu Y, Fang ZG, Lin DJ, Xiao RZ, Huang RW, Huang HQ, Liu PQ, Liu JJ. PPARγ agonist suppresses TLR4 expression and TNF-α production in LPS stimulated monocyte leukemia cells. Cell Biochem Biophys. 2011;60:167–172. doi: 10.1007/s12013-010-9136-6. [DOI] [PubMed] [Google Scholar]
- Wasserman SAA. Conserved signal transduction pathway regulating the activity of rel-like proteins dorsal and NF-кB. Mol Biol Cell. 1993;4:767–771. doi: 10.1091/mbc.4.8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861–20866. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- Yin Y, Hou G, Li ER, Wang QY, Kang J. Regulation of cigarette smoke-induced toll-like receptor 4 expression by peroxisome proliferator-activated receptor-gamma agonists in bronchial epithelial cells. Respirology. 2013;18:30–39. doi: 10.1111/resp.12167. [DOI] [PubMed] [Google Scholar]
- Yin Y, Hou G, Li E, Wang Q, Kang J. PPAR Gamma agonists regulate tobacco smoke-induced toll like receptor 4 expression in alveolar macrophages. Respir Res. 2014;15:28. doi: 10.1186/1465-9921-15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Liu F, Yan M, Ji H, Hu L, Li X, Qian J, He X, Zhang L, Shen A, Cheng C. Peroxisome proliferator-activated receptor-gamma agonists suppress iNOS expression induced by LPS in rat primary Schwann cells. J Neuroimmunol. 2010;218:36–47. doi: 10.1016/j.jneuroim.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC. PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells. Cardiovasc Res. 2011;92:484–493. doi: 10.1093/cvr/cvr238. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang L, Zhang M, Wang P, Zhang L, Yuan C, Qi J, Qiao Y, Kuo PC, Gao C. Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter. J Biol Chem. 2011;286:5519–5528. doi: 10.1074/jbc.M110.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]