Abstract
The purpose of this study was to clarify the relationship between neuron cells and astrocyte cells in regulating glutamate toxicity on the 10th and 20th day in vitro. A mixed primary culture system from newborn rats that contain cerebral cortex neurons cells was employed to investigate the glutamate toxicity. All cultures were incubated with various glutamate concentrations, then viability tests and histological analyses were performed. The activities of glutamate transporters were determined by using in vitro voltammetry technique. Viable cell number was decreased significantly on the 10th day at 10−7 M and at 10−6 M glutamate applications, however, viable cell number was not decreased at 20th day. Astrocyte number was increased nearly six times on the 20th day as compared to the 10th day. The peak point of glutamate reuptake capacity was about 2 × 10−4 M on the 10th day and 10−3 M on the 20th day. According to our results, we suggested that astrocyte age was important to maintain neuronal survival against glutamate toxicity. Thus, we revealed activation or a trigger point of glutamate transporters on astrocytes due to time since more glutamate was taken up by astrocytes when glutamate transporters on the astrocyte were triggered with high exogenous glutamate concentrations. In conclusion, the present investigation is the first voltammetric study on the reuptake parameters of glutamate in vitro.
Keywords: Glutamate-uptake, Neurotoxicity, Astrocyte, Neuron, In vitro voltammetry
Introduction
Glutamate is known as the major excitatory neurotransmitter in the brain and present in millimolar concentrations in mammalian central nervous system (Coyle et al. 1981; Choi et al. 1987). Up to 40 % of all synapses are glutamatergic (Fairman and Amara 1999) and these synapses are distributed throughout the nervous system, prominently in the cerebral cortex and limbic regions of the brain (Kim et al. 2011). Increased extracellular glutamate causes nerve cell death in stroke or trauma (Rothman and Olney 1986). Lots of studies have been shown that astrocytes influence on glutamate toxicity in vitro (Aizenman et al. 1990; Brown 1999).
Voltammetry is the technique that measures the concentrations of compounds through their oxidation at an inert electrode, and it has been applied in vitro for measuring the release and reuptake amounts of neurotransmitters (Burmeister et al. 2000). Investigators have determined signaling dynamics of neurotransmitters, such as dopamine, norepinephrine, serotonin, acetylcholine, nitric oxide, glutamate by microelectrodes. Unlike the complimentary technique known as microdialysis, in vitro voltammetric methods allow for very rapid measurement of the dynamic properties of neurochemicals. However, the routine detection limits of such methods, which are in the 25–50 nM range for analytes such as glutamate, do not rival the picomolar detection limits of methods that are used to analyze microdialysis samples. Electrochemistry is really important for investigating high frequency stimulation of neurotransmitter systems and correlating behavioral phenomena with neurochemical changes, for which the utilization of microdialysis would be problematic. Electrochemistry especially in vivo voltammetry has been shown to overcome the limitations of microdialysis (Robinson et al. 2008; Kasasbeh et al. 2013). Thus, in vivo voltammetric methods have higher spatial and temporal resolution compared to microdialysis (Adams 1990; Burmeister et al. 2002; Bortz et al. 2013).
Na+-dependent transport keeps extracellular glutamate at low levels (Nicholls and Attwell 1990), and this transport also terminates synaptic transmission (Kim et al. 2011). A rat brain has three different Na+-dependent transporters: EAAC1, GLT-1 and GLAST. They are membrane-bound pumps. The first one is expressed in neurons and the other two in astrocytes (Storck et al. 1992; Pines et al. 1992; Rothstein et al. 1994; Schmitt et al. 1996; Swanson et al. 1997). Astrocytes play the major role in removal of glutamate from the extracellular compartment. This clearance limits the activation of glutamate receptors and affects the synaptic response (Rothstein et al. 1996; Genoud et al. 2006). The astrocyte-selective glutamate transporter EAAT2 (GLT-1 in rats) has been shown to be chief for keeping extracellular glutamate levels below excitotoxic levels. Astrocyte ratio might be important for the viability of neuron cells.
The nerve terminals from the cerebral cortex and the basal ganglia, retain the ability to release glutamate via exocytosis during development (Sanchez-Prieto et al. 1994). The age-related alterations in L-glutamate regulation may be the main contributor to the changed vulnerability of the aged brain to excitotoxic damages, such as stroke and trauma. In brief, putting the changes of the activities of glutamate transporters by day and astrocyte/neuron ratio together seems to be very important for revealing the age related toxicity of glutamate. In our opinion, this information will serve to understand the relationship between the duration of the culture and the toxic effects of the different glutamate doses on mixed cell cultures. Therefore, we evaluated astrocyte/neuron ratio and its importance on glutamate toxicity with an in vitro voltammetric study for the first time.
Materials and methods
Animal cell culture experiments were performed in accordance with the national guidelines for the use and care of laboratory animals and approved by the local animal care committee of the Ataturk University. 30 albino Wistar newborn rats, provided by the Ataturk University Medical Experimental Practice and Research Center, were used.
Neuronal/astrocytic cell cultures
Mixed cell cultures were prepared using rat astrocytes. In these co-cultures, rat astrocyte provides the required support for optimal synaptic function (Goudriaan et al. 2014; Wierda et al. 2007). Primary cultures of cerebral cortex cells were prepared from one-day-old albino Wistar newborn rat (Gepdiremen et al. 2000a, b). The newborn rats were decapitated and their cerebral cortexes were dissected. Cerebral cortex was divided into minor pieces in a petri dish using a scalpel. They were suspended in 5 ml of calcium-free Hank’s balanced salt solution (HBSS, Sigma Co., St. Louis, USA) with 2 ml of trypsin-ethylen diamine tetra acetic acid (0.25 % trypsin- EDTA; Sigma-Aldrich Co. Ltd., Irvine, UK) at 37 °C for 20 min. Trypsin digestion was ended by the addition of 10 ml of HBSS that contained DNAase type 1 (120 units per ml; Sigma Co., St. Louis, MO, USA). After 3 min centrifugation at 800 rpm, the pellet was re-suspended in 10 ml B27 neurobasal medium + 10 % fetal calf serum (Biol. Ind., Haemek, Israel) and was dissociated by repeated pipetting (Gepdiremen et al. 2002). Then the cells were plated at 3 × 105 cells per well in 24 micro titer plates (Corning Inc., Corning, NY, USA). The culture dishes were kept at 37 °C in humidified atmosphere containing 95 % air and 5 % CO2. Culture media were changed twice a week and glutamate neurotoxicity tests were performed on the 10th and 20th days.
The cell cultures were treated with a range of L-glutamate concentrations (10−8, 10−7, 10−6, 10−5 and 10−4 M) for 16 h except the control wells (Zhang and Bhavnani 2006). Sterile saline solution (0.90 % weight/volume of NaCl) was given to control groups. Cell viability was measured via using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) after 4 h. The plates were incubated for 2.5 h with MTT. MTT reduction in live cells by mitochondrial reductase results in the formation of insoluble formazan. Therefore, the amount of formazan dye formed directly correlates to the number of metabolically active cells in the culture. The plates were incubated overnight at 37 °C and absorbance read at 570 nm on the enzyme-linked immunosorbent assay (ELISA, MicroQuant, Reader, BioTek, Winooski, VT, USA). The values obtained for the solutions were compared with the control. Mixed cultures of rat cerebral cortex also contain non-neuronal cells like astrocytes, oligodentroytes, microglias and ependymal cells. These cells, especially astrocytes, are the largest component of the cultures. Cell viability values were shown as sample absorbance/control absorbance rate.
Platinum microelectrode arrays
Microelectrodes
The real-time monitoring of rapid changes in extracellular levels of glutamate and other neuro-active molecules in the central nervous system were provided by fast analytical sensing technology (FAST). We also used S2 type (for rats), glutamate oxidase and nafion-coated multisite ceramic microelectrodes in this study. FAST and microelectrodes were obtained commercially from Pronexus Analytical AB (Stockholm, Sweden). The microelectrodes have platinum (Pt) recording sites with Pt connecting lines.
Calibration
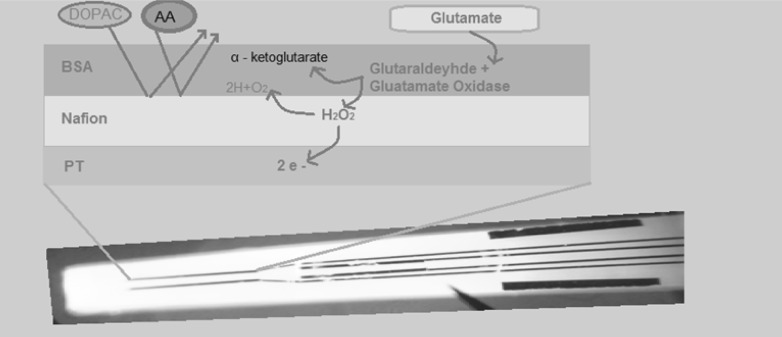
Calibration tests were performed with FAST-16 Data Acquisition Unit (Pronexus Analytical AB, Stockholm, Sweden). We used constant amperometric 0.7 voltage for in vivo voltammetry. The ceramic microelectrode amplifies head stage by being attached to a FAST 16 system. An Ag/AgCl commercial electrode also attaches to the head stage and functions as the reference electrode (Burmeister et al. 2000). Calibration tests involved placement of the electrodes in a stirred 40-ml of 0.1 M phosphate buffered saline (PBS; pH 7.4) solution. Different layers on microelectrode were shown in Fig. 1.
The generated peroxide can be efficiently oxidized by the Pt recordings sites at +0.7 V versus Ag/AgCl. The current generated by H2O2 oxidation on the surface of the electrode corresponded to the concentration of glutamate in solution. Glutamate selectivity versus ascorbic acid, sensitivity (slope) of glutamate detection, linearity of the dose–response curve (R2), and limit of detection (LOD) were evaluated with the microelectrode calibration results. For microelectrode calibration test; selectivity, R2, LOD values are considered acceptable >100:1, >0.990, <1 µM, respectively, in vitro experiments (Hascup et al. 2007). In our calibration test selectivity, R2, LOD results were >100:1, >0.990, <0.1 µM, respectively.
Fig. 1.
Different layers on microelectrode. Platin record site, barriers and enzymes
Reference electrode plating (Ag/AgCl) for in vitro recordings
To obtain records, a Teflon coated silver wire (bare inch AM Systems, Inc. 0008, coated in 0011, 8 inches in length) was used in vitro as a reference electrode. As is necessary in the preparation of a reference electrode, a silver wire was coated with chloride. The voltage source, wire, and Cl− ions were activated, and the silver wire rod was kept in the bath for at least 10 min to ensure proper coating. After removal, a light gray wire tip color was obtained (Hascup et al. 2007).
In vitro voltammetry
Microelectrodes were used to record the reuptake parameters of glutamate from the cell culture wells. Before and after calibration, culture plates were placed in the middle of a circulating water bath that holds the temperature constant at 37 °C. An electrode manipulator that attached to electrode on a stereotaxic frame (Stoelting Co., Wood Dale, IL, USA) was positioned above the culture dish. Our microelectrode had nafion and glutamate oxidase on the platinum surface. Glutamate oxidase converts glutamate to peroxide. On the other hand the nafion blocks interferents. The peroxide can pass through the nafion barrier and can be detected by the platinum side of microelectrode in voltammetry system. The peroxide level is correlated with the glutamate level. Finally, without breaking the tip on the bottom of the plate, the microelectrode is lowered into a culture well with the aid of a stereomicroscope to ensure that the Pt recording sites are immersed in the culture media. The coated tip of the Ag/AgCl reference electrode is placed into the same well (Hascup et al. 2007). Our laboratory used cell culture techniques with enzyme-based multisite microelectrodes to examine L-glutamate uptake in mix cell cultures. By ejecting a solution of different concentration of L-glutamate (10−8–10−4 M) into the culture media, we determined the 80 % of reuptake time after peak point (t80) time of uptake of the exogenously given L-glutamate into cultures. The mean ± SEM counts of reuptake time of glutamate (t80) were determined.
Histology
Cultured cells were fixed in ice-cold methanol for 10 min at room temperature. Then cells were washed twice with ice cold PBS. After that cells were incubated for 10 min with PBS containing 0.25 % Triton X-100. Then cells were washed cells in PBS three times for 5 min. Cells were incubated with 1 % BSA in PBST for 30 min to block unspecific binding of the antibodies. After that cells were incubated in the mixture of two primary antibodies, rabbit anti-microtubule-associated protein (MAP2, Abcam, Cambridge, MA, USA) mouse anti-glial fibrillary acidic protein (GFAP, Abcam, Cambridge, MA, USA) in 1 % BSA in PBST in a humidified chamber for overnight at 4 °C. The mixture solution was decanted and the cells were washed three times in PBS, 5 min for each wash. Cells were incubated with the mixture of two secondary antibodies (goat anti-mouse Ig G (Abcam) and goat anti-rabbit Ig G (Abcam) which were raised in different species) in 1 % BSA for 1 h at room temperature in dark. The mixture of the secondary antibody solution was decanted and washed three times with PBS for 5 min each in dark. Neuron and astrocyte cells for each group were counted in every well with an invert microscope at X20 magnification by a histologist. Astrocyte/neuron cell rates were compared before and after glutamate (10−8–10−4 M doses) additions in 6 random areas in four different wells.
Statistical analysis
SPSS 18.0 (Statistical Package Program, Chicago, IL, USA) was used for the statistical analysis. The mean ± SEM counts of t80, viability rates, astrocyte/neuron cell number ratio were determined. The data were analyzed statistically using ANOVA.
Results
Cell viability
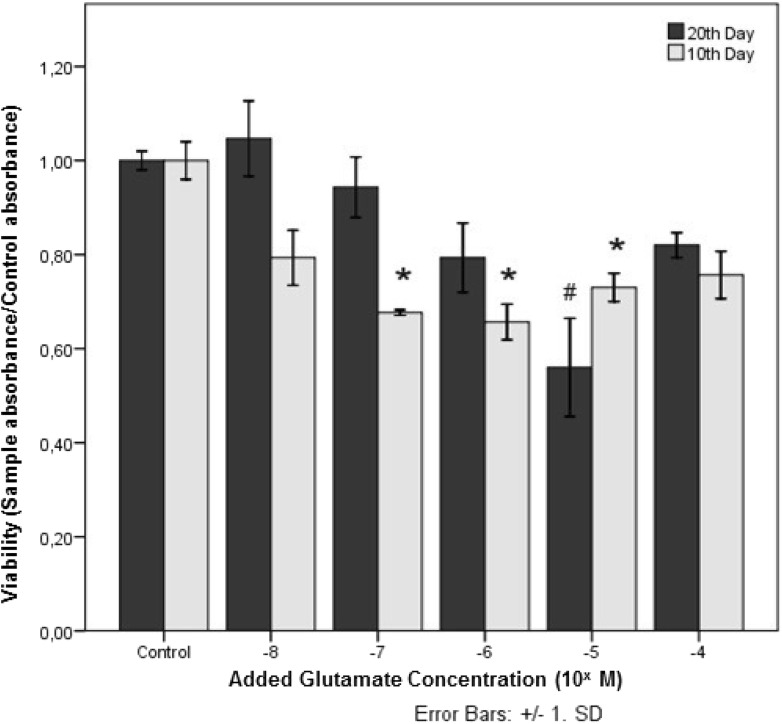
The total cell numbers in all groups were compared with control groups in Fig. 2. Viability (sample absorbance/control absorbance) values decreased in the groups from 10−8 to 10−6 M glutamate on the 10th day cultures and in groups from 10−8 to 10−5 M glutamate on the 20th day cultures. The most toxic dose of glutamate was 10−6 and 10−5 M, for the 10th and 20th days, respectively. Surprisingly, in both cases, higher concentrations of glutamate resulted in increased viability values. Significant differences in viability values were not found at 10−8 and 10−7 M glutamate supplemented groups on the 20th day.
Fig. 2.
Viability rates in newborn rat cerebral cortex cells after exposure to 10−8–10−4 M glutamate doses for 16 h
* p < 0.0001 for 10th day, # p < 0.0001 for 20th day are considered significant. Data were compared between the control group and the other concentration groups (n = 6)
Voltammetric studies
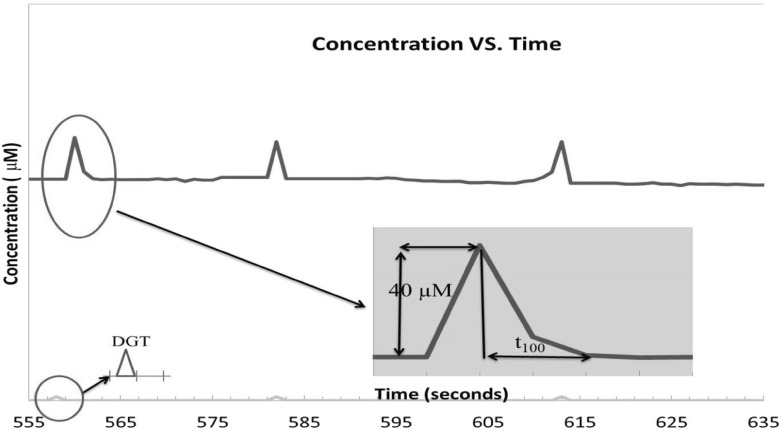
In vitro voltammetry was used to understand the uptake time of glutamate in cultured cells. Glutamate was given in different concentrations to the different wells. The results of t80 are given in Table 1. The t80 time is related to 80 % reuptake time of supplemented glutamate (Fig. 3). The reuptake time was increased by concentrations (<10−6 M) on the 10th day. But at 10−5 M glutamate concentration, t80 time was higher than at 10−6 M glutamate concentration for both 10th and 20th days. At 10−4 M, the t80 time was prolonged to 19.5 ± 4.9 s. At levels higher than 2 × 10−4 M of glutamate concentrations, glutamate amplitude did not return on the 10th day. The t80 time increment was found at 2 × 10−4 M glutamate on the 20th day (Table 1).
Table 1.
The voltammetry results in glutamate-treated mixed neuronal cultures after 10 and 20 days
| Drug concentrations | 10th day t80 (s) | 20th day (s) |
|---|---|---|
| Control | 2.67 ± 0.58 | 2.33 ± 0.58 |
| 10−8 M | 3.75 ± 0.96 | 2.50 ± 0.58 |
| 10−7 M | 3.50 ± 0.71 | 2.50 ± 0.71 |
| 10−6 M | 5.75 ± 1.25* | 4.25 ± 0.50 |
| 10−5 M | 2.60 ± 0.55 | 2.60 ± 0.90 |
| 10−4 M | 19.50 ± 4.90** | 3.50 ± 0.70 |
| 2 × 10−4 M | ∞** | 60.50 ± 13.44** |
| 5 × 10−4 M | ∞** | 92.00 ± 16.98** |
| 10−3 M | ∞** | ∞** |
t80: 80 % of total reuptake time (t100) of given glutamate; s: second
* p < 0.05; ** p < 0.0001
Fig. 3.
Representative example of some signals produced acutely by exogenous glutamate application
t80: 80 % of total reuptake time after peak (t100) of given glutamate. Ejection times: 556, 581, 613 s on the 20th day. DGT: Drug given time
Histological analysis
The astrocyte and neuron cell numbers and ratios were higher at days in vitro (DIV) 20 compared to DIV 10. Astrocyte number was increased nearly 6 times in DIV 20 according to DIV 10 but there was no change in neuron cell number. We detected the highest difference between ratios DIV 20 and DIV 10 at 10−6 M glutamate concentration (Table 2).
Table 2.
Cell numbers and rates (astrocyte/neuron) in treatments with different glutamate concentrations on the 10th and 20th DIV
| Glutamate concentrations | 10th day | 20th day | ||||
|---|---|---|---|---|---|---|
| Neuron | Astrocyte | Ratio | Neuron | Astrocyte | Ratio | |
| Control | 5.3 ± 3.3 | 38.0 ± 16.0 | 7.1 | 6.0 ± 0.8 | 174.0 ± 23.7* | 29.0 |
| 10−8 M | 2.0 ± 0.8 | 40.0 ± 16.3 | 20.0 | 3.5 ± 0.6 | 175.0 ± 28.9* | 50.0 |
| 10−7 M | 2.0 ± 0.8 | 15.0 ± 6.1 | 7.5 | 6.0 ± 0.8* | 171.0 ± 23.3* | 28.6 |
| 10−6 M | 2.3 ± 1.0 | 17.0 ± 7.2 | 7.6 | 1.5 ± 0.6 | 150.0 ± 57.7* | 100.0 |
| 10−5 M | 2.0 ± 0.8 | 20.0 ± 8.2 | 10.0 | 3.5 ± 1.3 | 175.0 ± 64.5* | 50.0 |
| 10−4 M | 5.6 ± 2.2 | 22.0 ± 8.3 | 3.8 | 3.4 ± 1.3 | 107.0 ± 39.5* | 31.5 |
Data were compared between the 10th and the 20th days
n = number of wells; for each well six random areas were calculated
* p < 0.05
Discussion
Glutamate levels are regulated in central nervous system synapses. There is equilibrium between the glutamate release and reuptake. Basal extracellular glutamate is maintained by its release from neurons and reuptake by astrocytes and neurons (Day et al. 2006; Robert 2011). Exogenous glutamate is also removed by both uptake and reuptake mechanisms (Rothstein et al. 1995). These mechanisms play an important role in the glutamate toxicity. In this study, a cerebral cortex mixed neuronal cultures of newborn rats were used to determine maximum toxicity levels of exogenous glutamate and relationship between the duration of cell culture and the exogenous glutamate uptake time.
In a toxicity study, it was shown that 5 × 10−4 M glutamate had no toxic effect on immature cortical neurons and glia (Choi et al. 1987). The cultured neurons expressed functional glutamate receptors and they became vulnerable to L-glutamate toxicity after 8 days (Mattson et al. 1993; Domoki et al. 2010). But they could not show glutamate transporter activity directly. In our study, we showed that glutamate was toxic at 10−5, 10−6, and 10−7 M doses on the 10th day and at 10−5 M on the 20th day. Interestingly, viability increased at concentrations above 10−5 M doses of glutamate in both cultures. In histological assessments this cell increment was related with astrocyte proliferation and they were more resistant to glutamate neurotoxicity (Table 2). This event could be explained by McKenna since he demonstrated that when the exogenous glutamate concentration increased above 10−4 M levels, the percent of glutamate which was oxidized in astrocytes via the tricarboxylic acid cycle increased (McKenna 2013).
Mixed cultures of rat cerebral cortex also contain non-neuronal cells like astrocytes, oligodentrocytes, microglia, and ependymal cells. These cells, especially astrocytes, are the largest component of the cultures, contributing up to 80–90 % of the total cellular mass at 3–5 weeks in vitro (Sinor et al. 2000). In another study glutamate administration caused hypertrophy and hyperplasia in microglia and astrocytic cells (Martinez-Contreras et al. 2002). In our study from 10−5 to 10−4 M concentration we saw cell viability increase in MTT analyses.
Araque et al. (1998) showed that astrocyte stimulation reduced excitatory and inhibitory synaptic transmissions through the activation of selective presynaptic metabotropic glutamate receptors in mixed cultures that include astrocytes and neurons (Araque et al. 1998). Additionally, another report showed that extracellular glutamate levels in the neonatal cortex were significantly elevated in GLT1 knock-out mice (Takasaki et al. 2008). GLT-1 transporters are especially placed on astrocytes. It was postulated that when the concentration of glutamate exceeds a threshold level, astrocytic glutamate transporters were activated, and there was a decrease in the extracellular glutamate concentration. This threshold was probably changing with the days in vitro. It was found that this threshold was 10−6 and 10−5 M, on the 10th and 20th day, respectively (Fig. 2). To detect the threshold levels we used in vitro voltammetry technique and we confirmed the results at the 10th day (Table 1).
In another study, a significant increase was found in the V max parameter of glutamate uptake in older astrocyte cultures (Pertusa et al. 2007). Uptake rates by with [3H] a radioactive labeling method were found to be higher in 10−3 M glutamate than in 10−4 M glutamate on the 10th day (Pertusa et al. 2007). These findings support the important increase in viability rates at a glutamate concentration of 10−5 and 10−4 M at DIV 20 and decreased viability rates at DIV 10 (Fig. 2). Reuptake parameters of glutamate could not be understood clearly in astrocyte cultures. There can be two possible explanations for this; glutamate transporter expression may be higher in older cultures and/or their activity may be higher at higher concentrations. Thus, when glutamate transporters were triggered, more glutamate can be taken up by 20 day cultures. Moreover, transporter development on astrocyte continued between the 10th and the 20th days. Immunofluorescence studies demonstrated that GLT and GLAST proteins are present in the CNS from early developmental stages at very low concentrations initially. However, immunoblotting studies have shown that the expression of both transporters increases, and show adult levels nearly 20 days after birth (Danbolt 2001; Kugler and Schleyer 2004). In a long time period, in vitro oxidant parameters can harm neurons (Pertusa et al. 2007), so our experiments were performed on the 10th day (after functional glutamate receptors were expressed) and on the 20th day (before oxidant harm occurs).
In our study, it was thought that the threshold point for glutamate, which triggers the transporters, was elevated and that transporter activities were also accelerated in older cells. In other words, transporter activity starts at high glutamate levels but may be more accelerated in older cells. For clarifying this point, we used in vitro voltammetry technique on different days to detect glutamate reuptake time (t80).
There were no significant difference in glutamate reuptake time (t80) at lower glutamate concentrations (<10−6 M) when compared to control groups on the 10th day. At a concentration of 10−6 M, t80 time (5.75 ± 1.25 s) was increased when compared to the control groups on the 10th day. When we increased the glutamate dose to 10−5 M, a reuptake trigger mechanism may have played a role, perhaps due to the transporters. The acceleration on reuptake time at 10−5 M of glutamate were seen (2.60 ± 0.55 s). When we increased the concentration of glutamate to 10−4 M, we saw the t80 times extend to 19.5 ± 4.9. Moreover, when the concentration was passed to 2 × 10−4 M, the t80 time could not return to basal glutamate levels. Voltammetric results on the 20th day showed that a delay in t80 time started at the doses two times higher (2 × 10−4 M) compared to the recordings on the 10th day. At 5 × 10−4 M concentrations, glutamate amplitude returned to basal levels lately (92 ± 16.98 s). At the concentrations of 10−3 M of glutamate, t80 became infinite on the 20th day (Table 1).
The astrocyte/neuron rate in vitro was nearly four times higher on the 20th day compared to the 10th day. Astrocyte numbers were shown to increase compared to neurons between the 10th and the 20th days (Table 2). It was hypothesized that astrocytes were activated at 10−6 M concentration of glutamate on the 10th day culture and 10−5 M on the 20th day culture, and they took up the toxic glutamate from the synaptic cleft (Table 1). In conclusion, astrocyte age was important to maintain neuronal survival; thus, it was proposed that the activation point or trigger point of glutamate transporters on astrocytes changes time dependently. These changes were shown via in vitro voltammetry in cultured cells for the first time.
Acknowledgments
This study was supported by grants from The Scientific and Technological Research Council of Turkey (TUBİTAK-Project No: 107S067) and Atatürk University BAP (Project No: 2005/160 and 2011/270-271)
Conflict of interest
The authors declared that there are no conflicts of interest.
References
- Adams RN. In vivo electrochemical measurements in the CNS. Prog Neurobiol. 1990;35:297–311. doi: 10.1016/0301-0082(90)90014-8. [DOI] [PubMed] [Google Scholar]
- Aizenman E, White WF, Loring RH, Rosenberg PA. A 3,4-dihydroxyphenylalanine oxidation product is a non-N-methyl-D-aspartate glutamatergic agonist in rat cortical neurons. Neurosci Lett. 1990;116:168–171. doi: 10.1016/0304-3940(90)90404-W. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Bortz DM, Mikkelsen JD, Bruno JP. Localized infusions of the partial alpha 7 nicotinic receptor agonist SSR180711 evoke rapid and transient increases in prefrontal glutamate release. Neuroscience. 2013;255:55–67. doi: 10.1016/j.neuroscience.2013.09.047. [DOI] [PubMed] [Google Scholar]
- Brown DR. Neurons depend on astrocytes in a coculture system for protection from glutamate toxicity. Mol Cell Neurosci. 1999;13:379–389. doi: 10.1006/mcne.1999.0751. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Moxon K, Gerhardt GA. Ceramic-based multisite microelectrodes for electrochemical recordings. Anal Chem. 2000;72:187–192. doi: 10.1021/ac9907991. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods. 2002;119:163–171. doi: 10.1016/S0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Bird SJ, Evans RH, Gulley RL, Nadler JV, Nicklas WJ, Olney JW. Excitatory amino acid neurotoxins: selectivity, specificity, and mechanisms of action. Based on an NRP one-day conference held June 30, 1980. Neurosci Res Program Bull. 1981;19:1–427. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Domoki F, Kis B, Gáspár T, Snipes JA, Parks JS, Bari F, Busija DW. Rosuvastatin induces delayed preconditioning against L-glutamate excitotoxicity in cultured cortical neurons. Neurochem Int. 2010;56:404–409. doi: 10.1016/j.neuint.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman WA, Amara SG. Functional diversity of excitatory amino acid transporters: ion channel and transport modes. Am J Physiol. 1999;277:F481–F486. doi: 10.1152/ajprenal.1999.277.4.F481. [DOI] [PubMed] [Google Scholar]
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4:e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepdiremen A, Düzenli S, Hacimüftüoglu A, Bulucu D, Süleyman H. The effects of melatonin in glutamate-induced neurotoxicity of rat cerebellar granular cell culture. Jpn J Pharmacol. 2000;84:467–469. doi: 10.1254/jjp.84.467. [DOI] [PubMed] [Google Scholar]
- Gepdiremen A, Hacimüftüoglu A, Düzenli S, Oztaş S, Süleyman H. Effects of salicylic acid in glutamate- and kainic acid-induced neurotoxicity in cerebellar granular cell culture of rats. Pharmacol Res. 2000;42:547–551. doi: 10.1006/phrs.2000.0717. [DOI] [PubMed] [Google Scholar]
- Gepdiremen A, Hacimuftuoglu A, Buyukokuroglu ME, Suleyman H. Nitric oxide donor sodium nitroprusside induces neurotoxicity in cerebellar granular cell culture in rats by an independent mechanism from L-type or dantrolene-sensitive calcium channels. Biol Pharm Bull. 2002;25:1295–1297. doi: 10.1248/bpb.25.1295. [DOI] [PubMed] [Google Scholar]
- Goudriaan A, Camargo N, Carney KE, Oliet SH, Smit AB, Verheijen MH. Novel cell separation method for molecular analysis of neuron-astrocyte co-cultures. Front Cell Neurosci. 2014;8:12. doi: 10.3389/fncel.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup KN, Rutherford EC, Quintero JE, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Gerhardt GA. Second-by-second measures of L-glutamate and other neurotransmitters using enzyme-based microelectrode arrays. In: Michael AC, Borland LM, editors. Electrochemical methods for neuroscience. Boca Raton, FL: CRC Press; 2007. pp. 407–450. [PubMed] [Google Scholar]
- Kasasbeh A, Lee K, Bieber A, Bennet K, Chang SY. Wireless neurochemical monitoring in humans. Stereotact Funct Neurosurg. 2013;91:141–147. doi: 10.1159/000345111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–2493. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P, Schleyer V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus. 2004;14:975–985. doi: 10.1002/hipo.20015. [DOI] [PubMed] [Google Scholar]
- Martínez-Contreras A, Huerta M, Lopez-Perez S, García-Estrada J, Luquín S, Beas Zárate C. Beas Zarate C. Astrocytic and microglia cells reactivity induced by neonatal administration of glutamate in cerebral cortex of the adult rats J Neurosci Res. 2002;67:200–210. doi: 10.1002/jnr.10093. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Zhang Y, Bose S. Growth factors prevent mitochondrial dysfunction, loss of calcium homeostasis, and cell injury, but not ATP depletion in hippocampal neurons deprived of glucose. Exp Neurol. 1993;121:1–13. doi: 10.1006/exnr.1993.1066. [DOI] [PubMed] [Google Scholar]
- McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-V. [DOI] [PubMed] [Google Scholar]
- Pertusa M, García-Matas S, Rodríguez-Farré E, Sanfeliu C, Cristòfol R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J Neurochem. 2007;101:794–805. doi: 10.1111/j.1471-4159.2006.04369.x. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Robert LS., Jr Glutamate transporter activators as anti-nociceptive agents. Eurasian J Med. 2011;43:182–185. doi: 10.5152/eajm.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol. 1986;19:105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prieto J, Herrero I, Miras-Portugal MT, Mora F. Unchanged exocytotic release of glutamic acid in cortex and neostriatum of the rat during aging. Brain Res Bull. 1994;33:357–359. doi: 10.1016/0361-9230(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Jons T, Kugler P. Expression of the glutamate transporter GLT1 in neural cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience. 1996;71:989–1004. doi: 10.1016/0306-4522(95)00477-7. [DOI] [PubMed] [Google Scholar]
- Sinor JD, Du S, Venneti S, Blitzblau RC, Leszkiewicz DN, Rosenberg PA, Aizenman E. NMDA and glutamate evoke excitotoxicity at distinct cellular locations in rat cortical neurons in vitro. J Neurosci. 2000;20:8831–8837. doi: 10.1523/JNEUROSCI.20-23-08831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Cogn Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki C, Okada R, Mitani A, Fukaya M, Yamasaki M, Fujihara Y, Shirakawa T, Tanaka K, Watanabe M. Glutamate transporters regulate lesion-induced plasticity in the developing somatosensory cortex. J Cogn Neurosci. 2008;28:4995–5006. doi: 10.1523/JNEUROSCI.0861-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in neuronal cells is mediated via caspase-dependent and independent mechanisms involving calpain and caspase-3 proteases as well as apoptosis inducing factor (AIF) and this process is inhibited by equine estrogens. BMC Neurosci. 2006;7:49. doi: 10.1186/1471-2202-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]