Abstract
Multipotent mesenchymal stem/stromal cells (MSCs) are of great interest to researchers because of the unique properties, such as enhanced proliferation, paracrine activity and multilineage differentiation. Their non-immunogenicity, in combination with immunomodulatory properties, opens up the opportunity for the allogeneic application of MSCs. The MSC immunomodulatory capacity is currently being actively studied in vitro using various experimental designs. However, the results are not always univocal. It was found that the outcome of the stromal/immune cell interaction depends on experimental conditions. In this review we considered the impact of different factors, such as the ratio of stromal/immune cells, interaction time, the path of immune cell activation, etc. on the MSC immunomodulation. We also accentuated the importance of local milieu, in particular, oxygen tension, for the realization of MSC immunosuppressive activity.
Keywords: MSCs, Lymphocytes, Activation, Low oxygen, Cell-to-cell interaction, Immunosuppression
Introduction
Low differentiated stromal cells were first discovered in bone marrow mononuclear cells in the 1970s by A. Friedenstain, who described their ability to form clones from single cell (CFU-F), proliferate rapidly, differentiate toward at least three mesenchymal lineages, transfer the hemopoietic microenvironment, etc. (Friedenstein et al. 1970). The interest in low differentiated stromal cells kindled again due to the works of A. Caplan, who termed them multipotent stem cells (MSCs). Abiding by the recommendation of the International Society of Cell Therapy, these cells are now called multipotent mesenchymal stem (stromal) cells (Dominici et al. 2006).
The recent 20 years have witnessed significant headway in studying the MSCs’ role in organisms and the properties they demonstrate in vitro make them a desirable instrument for cell therapy and regenerative medicine. For instance, the possibility of quickly obtaining a required number of cells that can be committed into osteo- chondro- or adipo-lineages is an incentive for tissue engineering. At the same time, trophic and hematopoiesis-supporting properties (Caplan 2009) render MSCs a useful instrument for therapy of many diseases (Ankrum et al. 2014). MSCs are considered hypoimmunogenic due to the absence of MHC-II (major histocompatibility complex) and co-stimulatory molecules CD80 and CD86 on the MSC surface and, therefore, the inability of these cells to provoke a cytotoxic effect by allogeneic immune cells (Jones and McTaggart 2008). Consequently, MCSs harvested from MHC-mismatched donors can also be used in therapy (Ankrum et al. 2014).
MSCs not only evoke the immune response of allogeneic immune cells, but they are also capable of immunosuppressing. These two properties open up a broad opportunity for allogeneic applications of these cells. There are at least three areas where the immunologic merits of MSCs will be of the utility. The first one is supplementary therapy of pathologies associated with tissue damage. These include cardiovascular diseases, trauma, etc., where MSCs have to be introduced directly (locally or systemically). The second possibility is the MSC use as a third tissue to improve engraftment. The third one is supplementary therapy of autoimmune diseases. All of these applications equally rely on the MSC “invisibility” for the immune system, as well as the ability of allogeneic MSCs to suppress the overreaction of the recipient’s immune cells. The MSC immunomodulatory activity was observed as far back as in Friedenstain’s studies (Friedenstein and Luria 1980). In light of the above, investigation of MSC interaction with immune cells appear to be of the utmost priority.
The phenomenon of immunosuppression has notably been in a focus of interest since the 2002 publication of the report on the immunosuppressive capacities of allogeneic MSCs and the demonstration of the ability of baboon’s MSCs to suppress lymphocyte proliferation in a mixed culture in vitro and to impede the rejection of the skin allograft in vivo (Bartholomew et al. 2002). Then, this phenomenon was carefully examined, resulting in the elucidation of basic cellular and molecular mechanisms of the MSCs immunomodulatory activity in vitro.
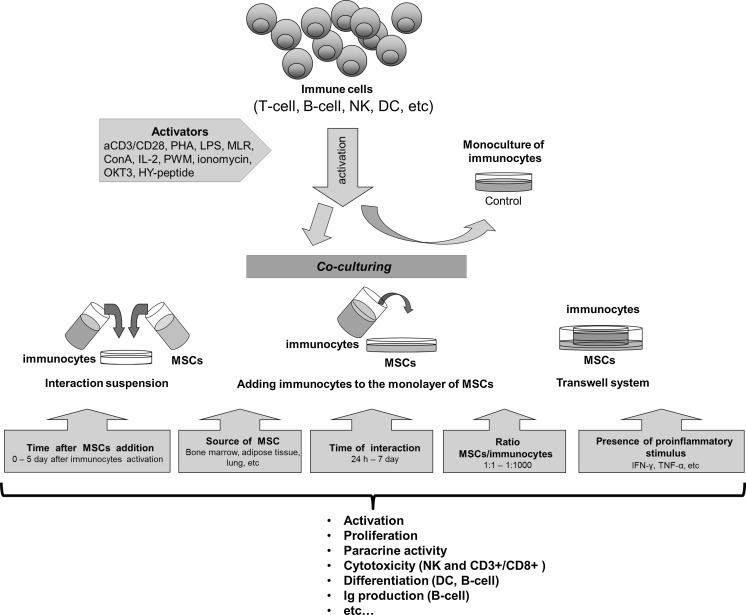
By now, there is no doubt that low differentiated stromal precursors affect virtually all types of immune cells by suppressing the proliferation of T-, B-cells and natural killer (NK) cells, activation of T- and B-cells, reducing NK cells cytotoxicity, etc. (Suva et al. 2008; Yang et al. 2009; Andreeva et al. 2012; Engela et al. 2012; Francois et al. 2012; Gornostaeva et al. 2013). However, the analysis of the broad spectrum of data discloses the dissimilarity and contradiction in the reported results, when suppressive, as well as stimulating, effects were described (Grigoryan et al. 2007; Crop et al. 2010; Saldanha-Araujo et al. 2012). The outcome of the MSCs interaction with the immune cells may depend on various factors which contingently are classified into the following groups: (1) effects depending on the properties of MSC themselves, (2) effects depending on the state of the immune cells, and (3) conditions of interaction, microenvironment, and the origin of immune cell stimulator (Fig. 1). To adequately interpret the results of MSC’s immunosuppression studies for ensuing inoculation of this knowledge in cell therapy protocols the critical analysis of the factors influencing MSC immunomodulatory properties is required, which is the goal of this review.
Fig. 1.
Factors influencing the MSC-lymphocytes’ interaction
Multipotent mesenchymal stromal cells
MSCs tissue source
To date, it has been clearly demonstrated that MSCs can be isolated from virtually all tissues with abundant vasculature, which is explained by the perivascular location of these cells (Orkin and Zon 2008). Irrespective of tissue source, all MSCs are immunosuppressive (Kronsteiner et al. 2011; Carrade Holt et al. 2014). This activity is exhibited by MSCs derived from the bone marrow (Aggarwal and Pittenger 2005; Suva et al. 2008), adipose, lung, umbilical blood, etc. (Puissant et al. 2005; Jarvinen et al. 2008; Kronsteiner et al. 2011; Carrade Holt et al. 2014). However, immunomodulatory effects of stromal cells from different tissues are not similar. For instance, Carrade Holt et al. (2014) showed that MSCs derived from the bone marrow, adipose, umbilical blood and umbilical cord inhibit lymphocyte proliferation and synthesis of pro-inflammatory cytokines, producing a variety of soluble inhibitors like prostaglandin E2 (PGE2), nitrogen oxide (NO) and IL-6. Yet, the immunomodulatory effect was dependent on MSC tissue source. MSCs isolated from the adipose and umbilical cord inhibited T cell proliferation by the induction of apoptosis, whereas MSCs isolated from the bone marrow and umbilical blood provoked the arrest of the lymphocyte cell cycle. The authors concluded that tissue origin of the MSCs might impact the realization of their immunosuppressive potential. In its turn, this may govern the choice of MSC tissue for cell therapy (Carrade Holt et al. 2014).
It is interesting that different tissue-specific effects of MSCs can be also determined by the properties of lymphocytes they act on. In particular, interaction with bone marrow and adipose-derived MSCs was followed by approximately equal reductions in the proliferative activity of lymphocytes stimulated by phytohemagglutinin (PHA), Concanavalin A (ConA) and OKT3-antibody. For lymphocytes induced by pokeweed mitogen (PWM), the suppressive effect was observed in only five of the seven experiments with bone marrow-derived MSCs and in all experiments with adipose-derived MSCs (Puissant et al. 2005).
Autologous versus allogeneic MSCs
It can hardly be conceived that in the evolutionary process, the immunomodulatory capacities of stromal cells (not only of MSCs but also cognate cells, fibroblasts specifically) could emerge as a reaction with allogeneic immune cells. Indeed, MSCs are able to modulate the activity of both autologous and allogeneic immune cells; however, the strength of the suppressive effect may be different. It was shown that allogeneic MSCs inhibited NK cells proliferation more effectively (in five times) than autologous (in 1.5 times). However, in relation to dendritic cells (DC), autologous and allogeneic MSCs were equally effective in the inhibition of differentiation stimulated by mixed lymphocytes reaction (MLR). After 5 days of co-culturing, the total DC number showed an 80 % decrease due to reduction of the CD11+ fraction (pro-inflammatory DC1), while CD123+ (regulatory DC2) did not change (Maccario et al. 2005). According to some other authors, both allogeneic and autologous MSCs had the same antiproliferative effect when the lymphocytes were stimulated by ConA, PHA and protein A. In the case of PWM stimulation, the proliferative effect was inhibited by allogeneic MSCs only in four and by autologous MSCs in two of six experiments (Le Blanc et al. 2003a).
MSCs from different species
Immunomodulation is a stromal cell feature of that is displayed irrespective of species appliances. In vitro experiments are most commonly conducted with MSCs of humans (Crop et al. 2010; Kronsteiner et al. 2011; Saldanha-Araujo et al. 2012; Lee et al. 2013), rodents (mice and rats) (Deng et al. 2005; Krampera et al. 2006; Yang et al. 2009) and, less frequently, hoofed animals (Pigott et al. 2013; Carrade Holt et al. 2014). MSCs of different tissue were found to have a similar effect on both autologous and allogeneic/xenogeneic immune cells (suppressing activation, inhibiting proliferation, etc.) (Glennie et al. 2005; Puissant et al. 2005; Jarvinen et al. 2008; Yang et al. 2009; Carrade Holt et al. 2014). However, there are diverse mechanisms employed by different MSCs to carry out the immunomodulatory effect. Thus, under same culture conditions the immunosuppressive effect of human MSCs involved indolamine-2,3-dioxygenase (IDO), whereas mice MSCs implied NO (Ren et al. 2009). Up to now NO-mediated immunosuppression was peculiar to mice only (Chabannes et al. 2007; Ren et al. 2009) while IDO-mediated suppression was demonstrated in all examined species including humans (Krampera et al. 2006; Spaggiari et al. 2006; Ryan et al. 2007).
Because of dissimilarity of executive mechanisms, MSCs of different species may differ in their effects. For instance, the population of apoptotic lymphocytes increased significantly after co-culturing with mice MSCs (Yang et al. 2009; Park et al. 2011), but did not alter after interaction with human MSCs (Benvenuto et al. 2007). Lymphocyte apoptosis in the mice model and its absence in case of human cells can be explained by the fact that a high NO concentration down-regulates the signal transduction and activates STAT-5 transcription, provoking the induction of apoptosis in immune cells, whereas IDO arrests proliferation as a consequence of tryptophan depletion (Shi et al. 2012). The data of Kim et al. shed light on the differences in the mechanism of immunosuppression of MSCs from different species. Both murine and human-activated lymphocytes provoked pro-inflammatory activation of human adipose MSCs, resulting in the inhibition of the proliferative response of both murine and human lymphocytes to the same extent. After stimulation with the conditioning medium from human lymphocytes the paracrine profile of MSCs included COX-2, PD-L1, CXCL-9, CXCL-12, IDO, PD-L2 and CXCL-10. In the case of murine lymphocytes, production of these factors was reduced by one half with the total absence of IDO expression. The point is that murine and human IFN-γ is only 50 % homologous and that human MSCs produce IDO only in response to human IFN-γ. The experiments with inhibitors of these factors showed that after murine lymphocyte stimulation, human MSCs provided immunosuppression mainly through COX-2 secretion (Kim et al. 2013, 2014).
To summarize, though MSCs can inhibit the activity of auto- and allogeneic as well as xenogeneic immune cells, certain differences in pathways of their effects may exist in different mammalian species.
MSCs’ functional state
Culture conditions and MSC immunosuppression
Since this review is focused on the MSC immunomodulatory properties in vitro, it is necessary to discuss how cultivation parameters may affect this activity. It is well known that cell growth in vitro is described by the growth curve, including a lag-phase during which cells adhere and spread, a log-phase (exponential growth) when cells divide actively, and a plateau when cells form a monolayer. Apparently, the MSC state, including the anti-inflammatory activity, is growth phase-dependent. For instance, when MSCs were seeded with different density and cultivated during the same time, MSCs with 50 % confluence predominantly expressed genes responsible for proliferation, cell cycle, nucleic acid metabolism and cell structure, such as UBE2C, ESM1, TOP2A, CDC45L, AURKA, PRC1, KIFC1, PTTG1, AURKB, KIF23, KIF11, KIF20B, CENPE, ASPM, TTK, MAD2L1, NUF2, CDC20, CCNA2 and CCNB2. The expression of pro-inflammatory cytokines, chemokines and growth factor genes, such as IL1B and IL6, CXCL1, CXCL2, CXCL5, CXCL6, IL8, CXCL16, CCL2, CCL8 and VEGF were not upregulated. Expression of these genes was high in MSCs that reached a 90 % monolayer (Kim et al. 2014). It is reasonable to suppose that MSCs with a higher level of confluence may possess more pronounced immunosuppressive properties. Indeed, a population of dividing lymphocytes was shown to decrease by 82 % after co-culturing with a MSCs monolayer (90 %) and only by 40 % when interacting with growing MSCs (50 % confluence). The higher expression of PTGES (Prostaglandin E synthase) and ULBP1 (UL16 binding protein) genes directly involved in the immunomodulatory process was evidenced in the monolayered MSCs (Lee et al. 2013).
Another crucial factor for MSC suppression is the number of passages in vitro. Freshly isolated (P0) stromal precursors had no anti-proliferative effect, whereas after one to four passages, the MSCs considerably inhibited the proliferation of stimulated lymphocytes (McIntosh et al. 2006). MSCs from a patient with systemic lupus erythematosus after the third passage ensured a decreased number of Th17 cells, causing a reduction in the pro-inflammatory IL-17 production. The eighth passage MSCs had a converse effect, that was Th17 cells grew in number and, therefore, the IL-17 concentration also increased (Lin et al. 2012).
Besides the growth phase, it was important for immunosuppression whether MSCs were in a suspension or attached to the substrate. In our lab we performed a comparative analysis of the immunosuppressive effects of the adhered and suspended MSCs. It turned out that suspended cells have a stronger anti-proliferative effect on the T-lymphocytes, but their ability to down-regulate lymphocyte activation does not differ from that of adhered MSCs (Gornostaeva et al. 2011, 2013).
An important factor that may determine the immunomodulatory potential of MSCs is the differentiation commitment. Le Blanc et al. (2003b) showed that after induction into osteo-, adipo- or chondrogenic directions, MSCs retained the ability to suppress the proliferative response of lymphocytes as effectively as they did in the non-differentiated state.
It is known that the production of immunosuppressive mediators by MSCs is not constitutive and that these substances are absent or are minimal in the conditioned medium of MSC in the monoculture (Meisel et al. 2004; Sotiropoulou et al. 2006; Krampera et al. 2006). To induce secretion of these substances, the cells had to be activated. At the present, the most well studied mechanism is the so-called “proinflammatory activation” or “priming” of MSCs by metabolites of stimulated immune cells. These metabolites are capable to induce immunosuppressive factors in MSCs. For instance, TNF-α is known as an activator of PGE2 synthesis, while IFN-γ induces IDO production (Meisel et al. 2004; Sotiropoulou et al. 2006). In vitro non-stimulated human bone marrow MSCs (bmMSCs) inhibited proliferation of T-cells and NK cells but not B-cells. The addition of IFN-γ increased the anti-proliferative effect of MSCs on T-cells and NK cells; besides, the effect was also revealed in B-cells. These data are explainable by the fact that NK cells and T-cells generate IFN-γ themselves and activate MSCs while interacting with them. On the contrary, B-cells do not synthesize this cytokine, which necessitates MSC priming (Krampera et al. 2006).
The implication of the MSCs pro-inflammatory activation was demonstrated in experiments with a conditioned medium. Supernatant from nonstimulated MSCs had no effect on NK cells and B-lymphocytes proliferation, NK cells cytotoxicity or differentiation of DC (Corcione et al. 2006; Sotiropoulou et al. 2006). Maturation of dendritic cells decreased only after the addition of the conditioned medium from MSCs co-cultured with monocytes (Nauta et al. 2006). These data conclusively demonstrate that effective immunosuppression requires pro-inflammatory induction that takes place in the course of a “dialogue” of MSCs with activated immune cells.
The other pathways of MSC pro-inflammatory activation that are not linked directly with the immune cells could not be excluded. In the sites of tissue injury, non blood-born cells, such as activated endothelium, the elements of extracellular matrix remodeling or degradation (connective tissue molecules, matrix-associated enzymes as MMPs), etc. may be considered as presumable triggers of MSC priming. This issue needs further investigation.
Immune cells
Activation of immune cells
In the in vitro studies of the MSC immunosuppression, both the whole population of white blood cells (WBCs) or fractionated WBC subpopulations (T-, B-cells, NK cells, dendritic cells, monocytes) are implicated as effector cells. These cells can be activated via different pathways, i.e. by lectins, peptides, antibodies etc. or allogeneic immune cells. The choice of a stimulator is critical, as MSC immunomodulatory effects may depend on the nature of the activating agent.
Thus, MSC co-cultured with phytohemagglutinin (PHA)-activated T-cells resulted in significant reduction of the CD25+ population, while the antibodies αCD3/αCD28 caused no changes (Kronsteiner et al. 2011). A comparative study of T-cell proliferation after activation with CD3/CD28 or PHA in the presence/absence of IL-2 showed the MSC-mediated suppression of proliferation in the presence of IL-2, while there was no effect without IL-2. The same relationship was observed in the absence of a direct cell contact between MSCs and T-cells (Suva et al. 2008). According to others, MSCs were able to suppress lymphocyte proliferation effectively when only PHA was used for activation (Puissant et al. 2005; Kronsteiner et al. 2011; Gornostaeva et al. 2011, 2013).
The extent of the immunosuppressive response of MSCs from different tissues may differ depending on which mitogen was used to activate the lymphocytes. For instance, MSCs derived from bone marrow and adipose tissue had an identical anti-proliferative effect on PHA, ConA and OKT3-activated lymphocytes; the suppressive effect of the bmMSCs on PWM-stimulated lymphocytes was revealed only in some experiments (Puissant et al. 2005).
The effect of MSCs on the viability of lymphocytes and its cytokine profile also is determined by a path of immune cell activation. For instance, the IFN-γ production by PHA-, ConA- or MLR-activated lymphocytes after interaction with MSCs was low (Glennie et al. 2005; Prasanna et al. 2010; Kronsteiner et al. 2011). On the contrary, IFN-γ synthesis increased in the co-culture of MSCs and αCD3/αCD28-stimulated lymphocytes (Kronsteiner et al. 2011). According to our data, in the presence of MSCs, the IFN-γ and IL-10 production by PHA-stimulated lymphocytes increased and TNF-α decreased (Gornostaeva et al. 2011, 2013). In the co-culture with MSCs, the CD3/CD28-activated T-cells demonstrated a significant increase in apoptotic cells (Yang et al. 2009; Park et al. 2011); at the same time, the share of apoptotic cells did not change among ConA and PHA-stimulating lymphocytes (Zappia et al. 2005; Gornostaeva et al. 2011, 2013).
Hence, the outcome of the MSC-lymphocyte interaction in vitro is depended on lymphocyte activation path. MSCs effectively suppressed the proliferative activity but slightly affected the activation and cytokine profile of the lymphocytes stimulated with CD3/CD28. PHA-stimulated lymphocytes in co-culture with MSCs displayed a significant decrease in activation and pro-inflammatory cytokine production, while proliferation changes were less pronounced.
Terms of interaction
MSCs/immune cells ratio
The MSCs/immune cells ratio is an important factor defining the efficiency of MSC-mediated immunosuppression. Significance of this parameter was demonstrated in several papers where the interaction between MSCs and immune cells in different ratio was examined (Table 1). Thus, the inhibition of T-cell proliferation was directly proportional to the MSC number in culture, i.e. maximum inhibition was achieved at 1:1 and the weakest, at 1:1000 (Deng et al. 2005; Puissant et al. 2005; McIntosh et al. 2006; Jarvinen et al. 2008; Ramasamy et al. 2008; Yang et al. 2009). It should be noted that the effect of the MSCs/T-cells ratio on the outcome of interaction is also a function of the lymphocyte stimulator applied. When lymphocytes were activated with CD3/CD28, the maximum suppression of proliferation was observed at the 1:1 ratio and did not change when the ratio was low (1:100) (Ramasamy et al. 2008). With PHA as a stimulator, the T-cells’ proliferative activity was suppressed considerably even when the MSCs concentration was very low (1:1000) (Jarvinen et al. 2008). Suppression of MLR-activated cells was distinct only when the MSCs/lymphocytes ratio was as high as 1:1, 1:2 and 1:4 (McIntosh et al. 2006). Our findings show that MSCs are capable of effectively suppressing the proliferation of PHA-stimulated lymphocytes at the ratio of 1:10 (Gornostaeva et al. 2011, 2013) (Table 1).
Table 1.
Changes in the proliferative activity of T cells
| MSCs source | Co-culturing time | T-cell stimulator | MSC/T-cell ratio | Effect | References | |
|---|---|---|---|---|---|---|
| Cell to cell contact | Transwell | |||||
| Human bone marrow | 5 d | PHA CD3/CD28 + IL-2 PHA + IL-2 |
1:1 | Increase Decrease Decrease |
Unchanged Decrease Unchanged |
Suva et al. (2008) |
| Rat bone marrow | 24 h 36, 72 h |
MLR | 1:20, 1:200 1:2000 1:20, 1:200, 1:2000 |
Decrease Unchanged Decrease |
Decrease (1:20) | Yang et al. (2009) |
| Rat bone marrow | 5 d | MLR | 1:1, 1:10, 1:100, 1:1000, | Increase, maximum effect at 1:1 ratio |
– | Grigoryan et al. (2007) |
| Human adipose tissue and bone marrow | 7 h | MLR | 1:4, 1:2, 1:1 | Decrease, maximum effect at 1:1 ratio | – | McIntosh et al. (2006) |
| Human adipose tissue | 7 d | MLR | 1:10 | Decrease | – | Crop et al. (2010) |
| Human bone marrow | 3 d | PHA | 1:10 | Decrease | – | Aggarwal and Pittenger (2005) |
| Rat bone marrow | 1 d 2, 3 d |
ConA | 1:10 | Unchanged Decrease |
– | Glennie et al. (2005) |
| Rat bone marrow | 3 d | ConA | 1:10, 1:5 | Decrease, maximum effect at 1:5 ratio | – | Deng et al. (2005) |
| Human bone marrow | 5 d | CD2/CD3/CD28 | 1:10 | Decrease | – | Saldanha-Araujo et al. (2012) |
| Human bone marrow and umbilical blood | 6 d | PHA CD3/CD28 |
1:10 | Decrease Decrease |
– Decrease |
Kronsteiner et al. (2011) |
| Human adipose tissue and bone marrow Human adipose tissue Human bone marrow |
4 d 72, 96, 113 and 120 h 5 d |
MLR, OKT3,PHA, ConA, PHA PHA PWM |
1:1 1:1 1:1, 1:10, 1:100, 1:1000 |
Decrease, maximum effect at PHA-stimulation Decrease, maximum effect—120 h Decrease, maximum effect at 1:1 ratio Decrease– 5 experiments Unchanged– 2 experiments Decrease |
Decrease (PHA) – – – – |
Puissant et al. (2005) |
| Human bone marrow | 3 d | CD3/CD28 | 1:1, 1:5, 1:10, 1:100. |
Decrease Unchanged |
– – |
Ramasamy et al. (2008) |
| Human lung | 4 d | PHA | 1:10, 1:50, 1:100, 1:500, 1:1000 | Decrease, maximum effect at 1:10 ratio | Decrease <(1:10) |
Jarvinen et al. (2008) |
| Human adipose tissue | 3 d | PHA | 1:10 | Decrease | Decrease | Gornostaeva et al. (2011, 2013) |
The anti-proliferative effect of MSCs on B-cells is also dose-dependent. A considerable inhibition of B-lymphocyte division was demonstrated only at 1:1 when B-cells were stimulated with antibodies (Corcione et al. 2006). With B-lymphocytes activated by lipopolysaccharide, the anti-proliferative effect was manifested at a ratio of MSCs: B-cells of 1:5 and 1:10, but not at 1:100 (Deng et al. 2005).
The maximum reduction of immunoglobulin production by murine B-cells was detected at the MSCs/B-cells ratio of 1:5; the effect became less evident with a decrease in the number of MSCs in the culture (1:10, 1:50, 1:100) (Deng et al. 2005). In the case of human MSCs, IgM, IgG and IgA levels declined significantly at a 1:1 MSCs:B-cells ratio only. The MSCs ability to modulate the expression of chemokine receptors (CD40, CXCR4, CXCR5 and CCR7) by B-cells was also in direct connection with the number of stromal precursors in the culture (Corcione et al. 2006).
After the interaction between MSCs and DC precursors at a high MSC/DC ratio (1:10–1:30), a larger part of the DCs demonstrated an immature cells’ phenotype (CD14+CD1a−), mature DC (CD14−CD1a+) prevailed in the culture with a lower ratio (1:50–1:100) (Nauta et al. 2006).
It was shown that MSCs inhibited proliferation of stimulated NK cells in a dose-dependent manner. The suppression was demonstrated at the MMSC/NK cells ratio from 1:1000 and above; maximum inhibition was detected at 1:10 (Maccario et al. 2005; Krampera et al. 2006; Cappellesso-Fleury et al. 2010). The anti-proliferative effect, in relation to NK cells, remained even after their repeated stimulation when the MSC number in the co-culture was sufficient. The NK cells were stimulated in the MLR and then, after interaction with MSCs, were activated once again by the PHA. In the presence of MSCs, the number of dividing NK cells in the standard MLR decreased significantly and the effect was dose-dependent. After the second PHA-activation, NK cells proliferation recovered fully at the 1:10 ratio and partly at a 1:1 ratio (Maccario et al. 2005). After fast activation (20 h), the NK cells proliferative activity was reduced irrespectively of the ratio (from 1:1 to 1:16). After prolonged activation (7 days), the effect was dose-dependent and was detected within the whole range of ratios, except for the minimum ratio (1:16), when the MSC number in the culture seemed to be too low to significantly affect NK cells (Spaggiari et al. 2006). A decline in the NK cells cytolytic activity against the target cells was at the maximum at a MSC/NK cells ratio of 1:1 (cytotoxicity was halved, whereas this effect was negligible at 1:10) (Maccario et al. 2005; Sotiropoulou et al. 2006).
The inhibition of cytokine secretion by NK cells after interaction with MSCs was pronounced at a high MSC number in the co-culture (Krampera et al. 2006). However, whereas IFN-γ and IL-10 concentrations decreased in a broad range of MSC/NK cells ratios (from 1:1 to 1:100), TNF-α—only decreased at 1:1 (Sotiropoulou et al. 2006). This effect was particularly obvious in the absence of direct cell-to-cell contact, as in this case, the MSCs’ immunosuppressive properties revealed themselves only at a high ratio (1:1, 1:10) (Jiang et al. 2005; Nauta et al. 2006; Sotiropoulou et al. 2006). A higher MSC immunosuppressive effect was directly proportional to its number in the co-culture. The optimal MSCs/immune cells ratio was within the range of 1:1–1:10. With a lower MSC number, the immunomodulatory effect either slacks or was entirely absent. This consistent pattern was characteristic of the interactions with any type of immune cells.
Time of interaction
The duration of the MSCs/immune cells interaction is the other important issue. For instance, MSCs did not affect lymphocyte proliferation after 24 h of co-culturing, and reduced this activity 48 and 72 h later (Glennie et al. 2005). According to our data, effective inhibition of PHA-activated lymphocytes proliferation also occurs after 72 h of interaction with MSCs (Gornostaeva et al. 2011, 2013). Inhibition of T-cell activation becomes more distinct with an extension of the co-culturing time (Puissant et al. 2005; Yang et al. 2009).
The other essential parameter determining the MSC immunosuppressive effect is how soon after lymphocyte activation the MSCs would be introduced into the co-culture. MSCs were added to the MLR-activated mononuclears immediately after stimulation, after 7, 24 and 48 h (Puissant et al. 2005), or on days 0, 3 and 5 (Le Blanc et al. 2003a). Maximum proliferation inhibition was observed when MSCs were introduced simultaneously with lymphocyte induction. The more delayed the introduction, the weaker the effect.
Interestingly, dose-dependent suppressive effects of the MSCs are more evident after a short co-culture duration. Thus, after 24 h, the proliferative activity of the T-cells decreased at the MSCs/lymphocytes ratio of 1:20 and did not change at a lower ratio, whereas after 72 h, the inhibitory effect was found at every ratio, though a maximum inhibition was still documented at 1:20 (Yang et al. 2009). After 3 days of MSCs-T-lymphocytes interaction, the CD3+/CD25+ level did not alter (Deng et al. 2005); however, after 4 days, the number of CD25-positive T-cells demonstrated a threefold decrease, while the number of CD3+/CD69+ cells decreased slightly (Le Blanc et al. 2004). A more extended interaction (5 days) resulted in suppression of activation on both markers (CD25 and CD69) (Cappellesso-Fleury et al. 2010).
To summarize, a more profound inhibition of the immune response (e.g. decrease of proliferation and activation) requires a longer duration of lymphocytes/MSCs co-culturing (72 h or longer).
Cell-to-cell/paracrine interactions
It has been mentioned already that the MSC immunosuppressive effect may be different depending on the presence/absence of direct MSC/immune cells contact. Essential is the fact that MSCs are capable of providing the immunomodulatory properties, even in the absence of physical contact with lymphocytes (through the paracrine factors only). In order to exclude direct interaction, cells are separated by a semipermeable porous membrane (transwell). In this situation, MSCs can considerably inhibit T-cell proliferation; however, there is no similarity of data on the magnitude of the effect. Some investigators observed slackening of suppression in the absence of direct interaction (Jarvinen et al. 2008; Suva et al. 2008), while others showed that the anti-proliferative effect on B- and T-cells did not depend on whether cells had or had no contact (Puissant et al. 2005; Corcione et al. 2006; Yang et al. 2009; Gornostaeva et al. 2011, 2013).
MSCs were capable of effectively modulating the T-cell subpopulations, by paracrine factors, for example CD4+CD25+Foxp3+Treg cells (Tasso et al. 2012). On the other hand, cell contacts play an important role in the MSCs’ interaction with this T-reg subpopulation. It was shown that CD4+/CD25+ cells being attached to MSCs expressed a higher level of Foxp3 (regulatory transcription factor) than did those in a suspension (Quaedackers et al. 2009). This suggests a switch in T-cell subpopulations toward the regulatory phenotype responsible for the weakening of the immune response. The secretion of the anti-inflammatory factors evidenced that the IFN-γ level decreased equally in the course of direct and contactless interaction (Groh et al. 2005). In contrast, secretion of the soluble molecule HLA-G5, essential for suppression of T-cell proliferation, grew in MSCs in the presence of cell contacts (Selmani et al. 2008).
Inhibition of DC maturation may be realized even without cell contacts, which can be evidenced from the decrease in the subpopulation of CD1a+ (mature DCs) and increase in CD14+ cells after the MSCs co-culturing with the DC precursors in the transwells (Jiang et al. 2005). However, the paracrine factors only were not sufficient to affect the CD56 expression by NK cells in transwells (Sotiropoulou et al. 2006). The NK cells cytolytic activity against the target cells also declined only following direct contact with the MSCs. Effective inhibition of the NK cells proliferative activity was also observed in the absence of interaction (Sotiropoulou et al. 2006).
Hence, cell-to-cell contacts play an important role in the realization of the MSC immunosuppressive properties; their occurrence is mandatory to reach a full-value effect for some types of immune cells (e.g. NK cells), while paracrine regulation alone is sufficient for others (T-, B-cells, DC).
Microenvironmental factors
The specific factors of the local milieu where the MSCs/immune cells interaction would occur in vivo should be taken into consideration while the co-culture experiments are carried out. The oxygen tension is one of the key factors of in vivo microenvironment. Nevertheless, the peculiar properties of MSC immunomodulation at the tissue-related oxygen concentration are practically unrevealed. Study of the MSC/lymphocyte interaction are usually performed with the standard O2 concentration (20 %), whereas oxygen as low as 2–7 % characterizes the MSC in vivo niche and a probable site of their interaction with lymphocytes in tissue. Besides, it was also demonstrated in our lab that hypoxia in vitro modifies the MSC properties substantially; this includes an increase of their proliferative activity and number of CFU-F, inhibition of osteo- and adipogenic lineage commitment and, on the contrary, acceleration of chondrodifferentiation (Grayson et al. 2007; Fehrer et al. 2007; Buravkova et al. 2009; Nekanti et al. 2010). It is reasonable to suppose that low O2 may influence the MSC ability to modulate the immune response.
Our lab was the first where the evaluation of the oxygen concentration as a factor that may impact the MSC immunomodulation was conducted. It was determined that both at standard (20 %) and at low (5 %) O2, human adipose tissue-derived MSCs maintain their immunomodulatory properties, i.e. effectively suppress late T-cell activation, revealing a decrease in HLA-DR-positive T-cells (Andreeva et al. 2012; Gornostaeva et al. 2013), inducing an anti-inflammatory shift in the lymphocytes’ cytokine profile by suppressing TNF-α and IL-6 production and increasing IL-10 secretion (Gornostaeva et al. 2013). These do not affect viability of the PHA-stimulated lymphocytes (Gornostaeva et al. 2011). The anti-proliferative effect of MSCs was enhanced at low O2 (Andreeva et al. 2012; Gornostaeva et al. 2011, 2013). When direct MSCs/immune cells contact was absent at low O2, the activation and proliferation of the lymphocytes and production of pro-inflammatory cytokines also were down-regulated, and anti-inflammatory IL-10—was up-regulated (Gornostaeva et al. 2013). However, in this case, the anti-proliferative effect was the same as at atmospheric O2. Based on these findings, we conclude that oxygen concentration may be an essential factor in the governing of the MSC immunomodulatory properties.
Conclusions
Despite the already fairly wealthy records of using MSCs to control the recipient’s immune response in experimental and clinical conditions, the efficacy of these procedures does not always meet expectations (Le Blanc et al. 2008; Niemeyer et al. 2008; Huang et al. 2010). In vitro data analysis brings to light key points one has to be mindful of when attempting to increase the MSCs efficacy.
Briefly these data could be summarized as follows:
Immunomodulatory activity in vitro has been demonstrated by MSCs from all mammals under study, including humans.
Immunosuppressive efficacy of MSCs from different tissue sources may be unequal. MSCs can inhibit the immune response of auto-, allo- and xenogeneic immune cells.
Immunomodulation is not an intrinsic MSCs property; to initiate this activity, MSCs had to be activated. The most well defined mechanism today is the so-called “pro-inflammatory activation” or “priming” of MSCs by biologically-active metabolites of activated immune cells. Still, we should not rule out other pathways of initiating the MSC immunomodulatory activity related to other factors rather than immune cells directly. Besides, for interpretation of the in vitro data, it is also important to know how immune cells were activated.
Ex vivo expansion of MSCs prior to in vivo application is carried out almost in 100 % of the cases irrespective of whether auto- or “off-shelf”-allogeneic cells are intended to be used. Modulation of MSC immunosuppressive activity with cell culture conditions can be very promising tool. The phase of cell growth, the number of passages, the extent of commitment and microenvironmental factors, and specifically, oxygen concentration, can significantly impact the immunomodulatory potential of MSCs and, therefore, determine the effectiveness of further application.
In vitro experiments demonstrated that either properties of MSCs and immune cells, or parameters of their interaction notably contribute to the realization of the MSC immunomodulatory potential. MSCs are capable of inhibiting the activity of innate and adaptive immunity cells. The extent of the MSC effects depends on several factors including the MSC/immune cells ratio, duration of interaction, presence/absence of direct cell-to-cell contacts, etc.
Thus, the MSC immunosuppressive potential in vitro can be affected by many factors that, in different combinations, may provoke a different outcome. This fact should be kept in mind while designing experiments and analyzing their data. Dependence of the MSC immunomodulatory properties on their physiological state, tissue origin, donor species, growth phase, lymphocyte stimulator and microenvironment requires further investigations. This would help to clarify the mechanisms for implementing the immunosuppressive effects of MSCs, and also make it possible to select the parameters for optimal immunosuppression.
There can be no doubt that factors of the tissue microenvironment, such as extracellular matrix fibers and proteoglycans, non-inflammatory cells, biologically-active molecules, and oxygen partial pressure also may affect the MSC immunosuppressive properties. These investigations are also needed to more elucidation of the mechanisms of stromal precursors and immune cell interaction.
Funding
This study was funded by Grant RFBR 13-04-00791, Grant RFBR 14-04-31362 mol_a and grant of the President of the Russian Federation SP-3502.2015.4.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Aleksandra Gornostaeva, Phone: 8-499-195-68-16, Email: HindIII@yandex.ru.
Elena Andreeva, Phone: 8-499-195-63-01, Email: andreeva_er@mail.ru.
Ludmila Buravkova, Phone: 8-499-195-68-76, Email: buravkova@imbp.ru.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Andreeva ER, Grigorieva OV, Andrianova IV, Gornostaeva AN, Buravkova LB. Human stromal and blood-born cells interaction under different O2 tension. part I. Immunocupressive effects. Technol Living Syst. 2012;9:13–18. [Google Scholar]
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- Buravkova LB, Grinakovskaia OS, Andreeva EP, Zhambalova AP, Kozionova MP. Characteristics of human lipoaspirate-isolated mesenchymal stromal cells cultivated under a lower oxygen tension. Tsitologiia. 2009;51:5–11. [PubMed] [Google Scholar]
- Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellesso-Fleury S, Puissant B, Apoil PA, et al. Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J Clin Immunol. 2010;30:607–619. doi: 10.1007/s10875-010-9415-4. [DOI] [PubMed] [Google Scholar]
- Carrade Holt DD, Wood JA, Granick JL, et al. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. 2014;23:1258–1265. doi: 10.1089/scd.2013.0537. [DOI] [PubMed] [Google Scholar]
- Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Crop M, Baan CC, Korevaar SS, et al. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19:1843–1853. doi: 10.1089/scd.2009.0368. [DOI] [PubMed] [Google Scholar]
- Deng W, Han Q, Liao L, et al. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24:458–463. doi: 10.1089/dna.2005.24.458. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Engela AU, Baan CC, Dor Frank JMF, et al. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front Immunol. 2012;3:126–134. doi: 10.3389/fimmu.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Gornostaeva AN, Andreeva ER, Andrianova IV, Buravkova LB. Immunosuppressive effects of multipotent mesenchymal stromal cells in cultures with different O2 content in the medium. Bull Exp Biol Med. 2011;151:526–529. doi: 10.1007/s10517-011-1372-2. [DOI] [PubMed] [Google Scholar]
- Gornostaeva AN, Andreeva ER, Buravkova LB. Human MMSC immunosuppressive activity at low oxygen tension: direct cell-to-cell contacts and paracrine regulation. Hum Physiol. 2013;39:31–42. doi: 10.1134/S0362119713020059. [DOI] [PubMed] [Google Scholar]
- Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Grigoryan AS, Tsupkina NV, Sergeev VS, et al. Characterization of bone marrow stromal cells in the mixed lymphocyte reaction. Cell Transplantol Tissue Eng. 2007;2:62–66. [Google Scholar]
- Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- Ja Friedenstein A, Luria EA. Cellular bases of hemopoietic microenviroment. Moscow: Meditsina; 1980. [Google Scholar]
- Jarvinen L, Badri L, Wettlaufer S, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181:4389–4396. doi: 10.4049/jimmunol.181.6.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Jones BJ, McTaggart J. Immunosuppression by mesenchymal stromal cells: From culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YT, Hong JM, Hwang YI. Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat Cell Biol. 2013;46:262–271. doi: 10.5115/acb.2013.46.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Lee MW, Yoo KH, et al. gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014;9:e83363. doi: 10.1371/journal.pone.0083363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- Kronsteiner B, Wolbank S, Peterbauer A, et al. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011;20:2115–2126. doi: 10.1089/scd.2011.0031. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, et al. b) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/S0301-472X(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Gotherstrom C, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Lee MW, Kim DS, Ryu S, et al. Effect of ex vivo culture conditions on immunosuppression by human mesenchymal stem cells. Biomed Res Int. 2013;2013:154919. doi: 10.1155/2013/154919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Lin G, Lue TF. Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev. 2012;12:2770–2778. doi: 10.1089/scd.2012.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4 + T-cell subsets expressing a regulatory/suppressive phenotype. Stem Cell Transplant Haematol. 2005;90:516–525. [PubMed] [Google Scholar]
- Mcintosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells. 2006;24:1246–1253. doi: 10.1634/stemcells.2005-0235. [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase–mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Kruisselbrink AB, Lurvink E, et al. Mesenchymal stem cells inhibit generation and function of both CD34-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- Nekanti U, Dastidar S, Venugopal P, et al. Increased proliferation and analysis of differential gene expression in human wharton’s jelly-derived mesenchymal stromal cells under hypoxia. Cell. 2010;6:499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer P, Vohrer J, Schmal H, et al. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MJ, Shin JS, Kim YH, et al. Murine mesenchymal stem cells suppress T lymphocyte activation through IL-2 receptor α (CD25) cleavage by producing matrix metalloproteinases. Stem Cell Rev. 2011;7:381–393. doi: 10.1007/s12015-010-9203-9. [DOI] [PubMed] [Google Scholar]
- Pigotta JH, Ishiharaa A, Wellmanb ML, et al. Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol. 2013;156:99–106. doi: 10.1016/j.vetimm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-Inflammatory cytokines, IFNγ and TNFa, influence immune properties of human bone marrow and wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol. 2009;39:3436–3446. doi: 10.1002/eji.200939584. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Tong CK, Seow HF, et al. The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T-cell proliferation but not its effector function. Cell Immunol. 2008;251:131–136. doi: 10.1016/j.cellimm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha-Araujo F, Haddad R, Kelen CR, et al. Mesenchymal stem cells promote the sustained expression of CD69 on activated T lymphocytes: roles of canonical and non-canonical NF-kB signaling. J Cell Mol Med. 2012;16:1232–1244. doi: 10.1111/j.1582-4934.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress t lymphocyte and natural killer function and to induce CD4-CD25 high FOXP3- regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- Suva D, Passweg J, Arnaudeau S, et al. In vitro activated human t lymphocytes very efficiently attach to allogenic multipotent mesenchymal stromal cells and transmigrate under them. J Cell Physiol. 2008;214:588–594. doi: 10.1002/jcp.21244. [DOI] [PubMed] [Google Scholar]
- Tasso R, Ilengo C, Quarto R, et al. Mesenchymal stem cells induce functionally active T-regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Park MJ, Yoon IH, et al. Soluble mediators from mesenchymal stem cells supress T cell proliferation by inducing IL-10. Exp Mol Med. 2009;41:315–324. doi: 10.3858/emm.2009.41.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]