Abstract
Endothelial cell activation, injury and dysfunction have been regarded as one of the initial key events in the pathogenesis of atherosclerosis. Lipopolysaccharide (LPS), an important mediator of inflammation, can cause endothelial cell damage and apoptosis. Naringin (Nar), one major flavanone glycoside from citrus fruits, shows various pharmacological actions, but the effect of Nar on LPS-induced damage in human umbilical vein endothelial cells (HUVECs) remains unknown. The present results showed that Nar significantly improved the survival rate of HUVECs, and decreased reactive oxygen species and intracellular Ca2+ levels caused by LPS compared with model group. In addition, Nar obviously decreased cytochrome c release from mitochondria into cytosol. Moreover, Nar significantly down-regulated the protein or mRNA levels of IL-1, IL-6, TNF-α, VCAM-1, ICAM-1, NF-κB, AP-1, cleaved-3,-7,-9, p53, Bak and Bax, and up-regulated the expressions of Bcl-xl, Bcl-2 to suppress inflammation and apoptosis. Furthermore, Nar obviously inhibited phosphorylation levels of JNK, ERK and p38 MAPK. In conclusion, Nar exhibited potent effects against LPS-induced damage in HUVECs through the modulation of oxidative stress, inflammation, apoptosis and MAPK pathways, which should be developed as a potent candidate for the treatment of atherosclerosis in the future.
Keywords: Apoptosis, HUVECs, Inflammation, Lipopolysaccharide, Naringin
Introduction
Atherosclerosis, a multifactorial disease, mainly leads to the thrombus formation, and causes complications such as cerebrovascular disease, heart disease or peripheral arterial disease when an erosion, fissure or rupture of the plaque occurs (Libby 2003). Atherosclerosis, a very common condition related to cardiovascular disorders (CVD), is the principal cause of death around the world. Endothelial dysfunction and inflammation have been considered to play important roles in the thrombotic complications of atherosclerosis and endothelial dysfunction (Muller and Morawietz 2009). Moreover, endothelial cells that inhibit adhering of platelets and monocytes (Jiang et al. 2011) play a vital role in developing atherosclerotic plaques (Ikushima et al. 2006). Endothelial cell injury and dysfunction have been regarded as one of the key factors in the pathogenesis of atherosclerosis. Thus, protecting endothelium cell injury is quite important for the treatment of atherosclerosis.
Lipopolysaccharide (LPS), an endotoxic component of the outer membrane of gram-negative bacteria, is a potent stimuli of innate immunity in mammalian systems by inducing inflammation, disseminated intravascular coagulation, shock and death (Chaby 2004). Some cellular signals activated by gram-negative bacteria are attributed to LPS (Raetz 1990). Many papers have reported that human umbilical vein endothelial cells (HUVECs) exposured to LPS led to the release of numerous pro-inflammatory cytokines which result in a series of endothelial responses including the upregulation of adhesion molecules, cytokines, tissue factor, and even induction of endothelial cell apoptotic death (Lee et al. 2014). In fact, LPS-mediated inflammation associated with vascular diseases has been reported in the pathogenesis of endothelial cell injury and dysfunction (Schlegel et al. 2009; Echeverría et al. 2013). LPS binding to the toll-like receptor 4 (TLR4) on host cell triggers the release of pro-inflammatory mediators, free radicals, and caspases that subsequently contribute to apoptosis which is related to atherosclerosis (Lee et al. 2008).
Traditional Chinese medicines (TCMs) have been used for thousands of years in China, and more and more attentions are focused on them because of their high efficiency and low toxicology (Wang et al. 2012). Many natural products including celastrol from Tripterygium wilfordii (Ni et al. 2014), saikosaponin C from Radix Bupleuri (Tae et al. 2014), ginsenosides from the plant genus Panax (Cho et al. 2013), oroxylin A from Scutellaria baicalensis Georgi (Song et al. 2012) have been shown potent anti-atherosclerotic activities. Thus, it is reasonable to explore new and effective natural products from medicinal plants against LPS-induced HUVECs injury.
Naringin (Nar, shown in Fig. 1a), one natural product, widely exists in some medicinal plants (Bharti et al. 2014). Now pharmacological investigations have shown that Nar has anti-inflammatory (Liu et al. 2012), anti-oxidant (Zielinska-Przyjemska and Lgnatowicz 2008), anti-apoptotic (Gopinath et al. 2011), anti-atherosclerotic (Lee et al. 2009), anti-diabetic (Jung et al. 2004) and hepatoprotective effects (Dong et al. 2015). And its aglycone–naringenin also has anticancer, antimutagenic, anti-inflammatory, antioxidant, antiproliferative and antiatherogenic activities (Patel et al. 2014). However, there have no papers to report the effect of Nar and naringenin on LPS-induced injury in HUVECs to our best knowledge.
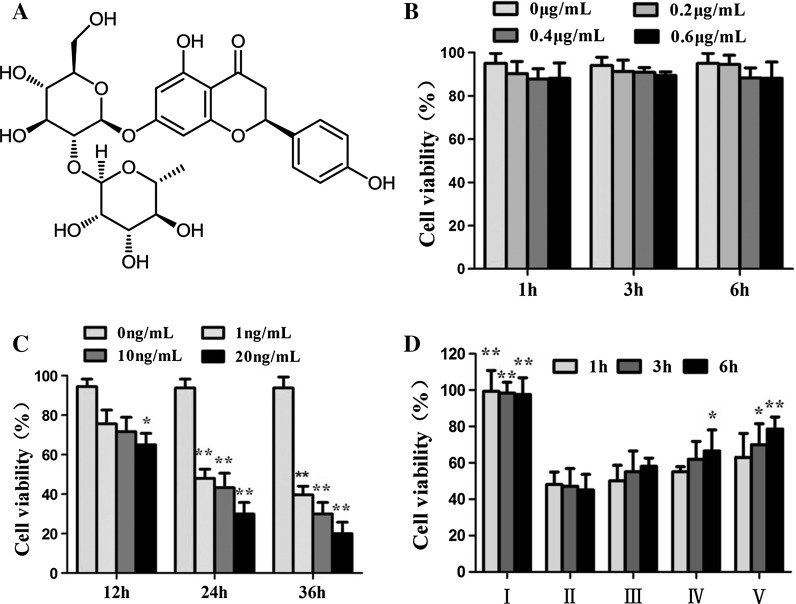
Fig. 1.
Protective effects of the Nar on LPS-induced cell injury in HUVECs. a The chemical structure of naringin; b Effects of Nar on the cell cytotoxicity of HUVECs; c Effects of LPS on the cell cytotoxicity of HUVECs; d Effect of Nar against LPS-induced cell injury in HUVECs. I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL). Values are presented as mean ± SD (n = 5). *p < 0.05 and **p < 0.01 compared with the model group
Therefore, the aim of the present paper was to investigate the protective effect of Nar against LPS-induced damage in HUVECs, and then the possible mechanisms were also studied.
Methods
Chemicals and reagents
Naringin (Nar, shown in Fig. 1a) with purity >98 %, Naringenin (shown in Fig. 2a) with purity >98 %, lipopolysaccharide (LPS), dimethyl sulfoxide (DMSO) and 4,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Acridine orange (AO), ethidium bromide (EB) fluorescent dyes, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Nanjing KeyGen Biotech. Co. LTD. (Nanjing, China). Fluo-3 AM, Reactive Oxygen Species Assay kit and lysis buffer were all purchased from Beyotime Institute of Biotechnology (Shanghai, China). Fetal Bovine Serum (FBS) and Deulbecco’s Modified Eagle Medium (DMEM) were purchased from THERMO (Beijing, China). Cell and Apoptosis Analysis Kits were purchased from Invitrogen (California, USA). All the primary antibodies and secondary antibodies were purchased from Beyotime Biotechnology (China).
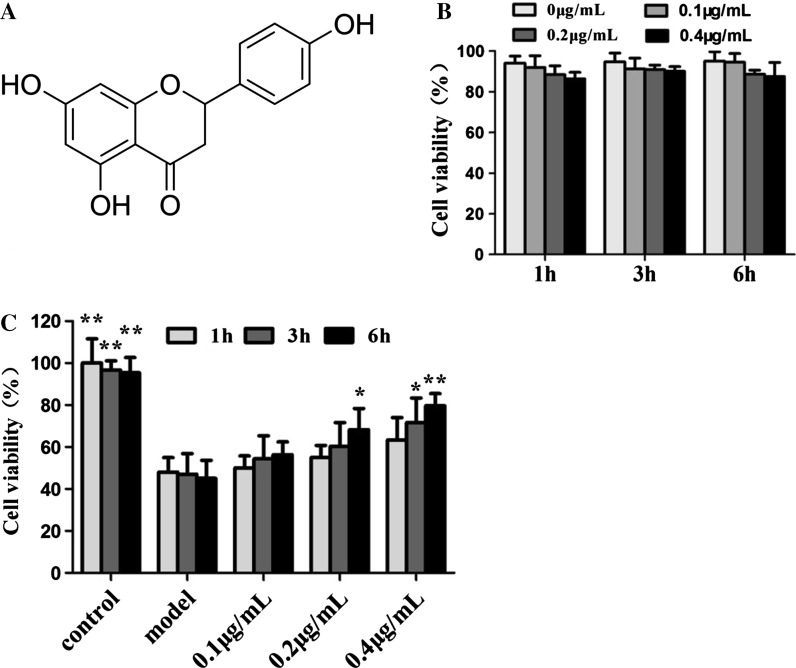
Fig. 2.
Protective effects of the Naringenin on LPS-induced cell injury in HUVECs. a The chemical structure of naringenin; b Effects of naringenin on the cell cytotoxicity of HUVECs; c Effect of naringenin against LPS-induced cell injury in HUVECs. *p < 0.05 and **p < 0.01 compared with model group
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from the Institute of Biochemistry Cell Biology (Shanghai, China). The cells were cultured in DMEM at 37 °C in a humidified incubator supplied with 5 % CO2 and 95 % air. The culture medium was supplemented with 10 % foetal bovine serum (FBS) and 1 % penicillin/streptomycin (100 U/mL/100 μg/mL) (Gibco, Big Cabin, Oklahoma, ME, USA). HUVECs were allowed to adhere in the logarithmic growth phase for 24 h before treatment.
Cell viability assay
The HUVECs were cultured in 96-well plates at a density of 1 × 105 cells/well. Different concentrations of Nar (0.2, 0.4 and 0.6 μg/mL) and naringenin (0.1, 0.2 and 0.4 μg/mL) were added to each well for different times (1, 3 and 6 h), then 10 ng/mL LPS was added into each well for 24 h MTT (10 μL, 5 mg/mL) solution was added to the medium and the cells were incubated for another 4 h. The supernatant was removed and 150 μL DMSO was added in each well to solubilize the formazan crystals. At last, the absorbance of the sample was measured at 490 nm using a microplate reader (Thermo, USA).
AO/EB and DAPI staining
The HUVECs were cultured in 6-well plates at a density of 1 × 106 cells/well and treated by different concentrations of Nar (0.2, 0.4 and 0.6 μg/mL) for 6 h, and also treated by the Nar at the dose of 0.6 μg/mL under different treatment times (1, 3, and 6 h). The cultured cells also were treated by different concentrations of naringenin (0.1, 0.2 and 0.4 μg/mL) for 6 h, and treated by the naringenin at the dose of 0.4 μg/mL under different treatment times (1, 3, and 6 h). Then, 10 ng/mL LPS was added into each well for 24 h. The cells were washed twice with cold PBS, then the mixture containing same volume of AO (100 μg/mL in PBS) and EB (100 μg/mL in PBS) was put onto the cells. Finally, the stained cells in the plate were photographed by using inverted fluorescence microscopy (Olympus BX63, Tokyo, Japan). In addition, the cells were treated as above and stained with DAPI (1 μg/mL) solution for 10 min at 37 °C, then washed twice with PBS. At last, the images of the cells were acquired by using an inverted fluorescence microscope (Olympus BX63, Tokyo, Japan).
Detection of intracellular ROS accumulation and Ca2+level
The HUVECs were plated in 6-well plates at a density of 1 × 106 cells/well. After being pretreated as described above, the cells were harvested and then re- suspended in 500 μL of DCFH-DA (10 μM) for detecting ROS and in 500 μL of Fluo-3/AM (2.5 μM) for detecting the level of Ca2+, which were all analyzed by flow cytometry (FACSCalibur, Becton Dickson, San Diego, CA, USA).
Cell apoptosis assay
Extent of apoptosis was measured by Annexin VFITC/propidium iodide (PI) apoptosis detection kit. After being pretreated as described above, the cells were harvested, washed in cold PBS, and then used for assay according to the manufacturer’s instructions. Finally, the stained cells were detected by flow cytometry (FACSCalibur, Becton Dickson, San Diego, CA, USA). The fraction of cell population in different quadrants was analyzed by quadrant statistics.
Detection of cytochrome c release
The HUVECs were plated in six-well incubated overnight to detect cytochrome c release. After pretreatment, the cells washed with PBS again, fixed with 4 % paraformaldehyde for 15 min at 4 °C, washed with PBS again, permeabilized with 0.2 % Triton-100 for 8 min. Non-specific binding was blocked by incubating the cells in 3 % BSA (Bovine Serum Albumin) for 45 min at 37 °C, and the cells were hatched with the primary antibody overnight at 4 °C. Then, the plates were washed twice with PBS. The cells were incubated with secondary antibody for 1 h at 37 °C, washed with PBS, then stained with DAPI (5.0 μg/mL) for 5 min. The stained cells in the plate were imaged by using a laser scanning confocal microscope (Leica, TCS SP5, Germany).
Quantitative real-time PCR assay
Total RNA samples from the HUVECs were obtained by using RNAiso Plus reagent following the manufacturer’s protocol. Reverse transcription polymerase chain reaction (RT-PCR) was performed using PrimeScript® RT reagent Kit following the manufacturer’s instructions with a TC-512 PCR system (TECH-NE, Stone, UK). The levels of mRNA expression were quantified by real-time PCR with SYBR® PremixEx Taq™II (Tli RNaseH Plus) and ABI 7500 real time PCR system (Applied Biosystems, Carlsbad, CA, USA), and the data was analyzed by System SDS software (Applied Biosystems). The sequences of the primers were as shown in Table 1. A no-template control was analyzed in parallel for each gene, and we selected GAPDH gene as the housekeeping gene. Lastly, the unknown template was calculated through the standard curve for quantitative analysis.
Table 1.
The primer sequences used for real-time PCR assay
| Gene | Forward primer (5′—3′) | Reverse primer (5′—3′) |
|---|---|---|
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| IL-1β | CCTGTCCTGCGTGTTGAAAGA | GGGAACTGGGCAGACTCAAA |
| IL-6 | TGGCTGAAAAAGATGGATGCT | TCTGCACAGCTCTGGCTTGT |
| TNF-α | TGTAGCCCATGTTGTAGCAAACC | GAGGACCTGGGAGTAGATGAGGTA |
| ICAM-1 | CACAGTCACCTATGGCAACGA | GGAAAGCTGTAGATGGTCACTGTCT |
| VCAM-1 | TCATGAAGTTTGTCTCAACCCTGAA | AGACAGCGAGGCACATCAGGTA |
Western blotting assay
The HUVECs were grown in the 6-well plate, treated by different concentrations of Nar (0.2, 0.4 and 0.6 μg/mL) for 6 h, and 10 ng/mL LPS was added into each well for 24 h. The total proteins form different groups were extracted by a cold lysis buffer (RIPA, 200 µM PMSF) for 15 min at 4 °C, and the mixtures were centrifuged at 12,000 g for 5 min to produce the total protein. Nuclear and cytoplasmic proteins were extracted by using the extraction kit. Then, the protein content was determined by Coomassie brilliant blue G. Western blotting assay was performed as follows: protein (5 mg/mL) was denatured by mixing with an equal volume of 2 × sample loading buffer and then boiling at 100 °C for 5 min. An aliquot (50 μg protein) of the supernatant was fractionated by an electrophoresis on 10–15 % SDS-PAGE and transferred onto a PVDF membrane. After blocking non-specific binding sites for 3 h with 5 % non-fat milk in TTBS (TBS with Tween 20), the membranes were individually incubated for overnight with primary antibody including rabbit anti-Bak (1:1000), rabbit anti-Bax (1:1000), rabbit anti-Bcl-xl (1:1000), rabbit anti-Bcl-2 (1:1000), rabbit anti-Cleaved-Caspase-3 (1:1000), rabbit anti-Cleaved-Caspase-7 (1:1000), rabbit anti-Cleaved-Caspase-9 (1:1000), rabbit anti-p53 (1:1000), rabbit anti-p-JNK (1:500), rabbit anti-JNK (1:500), rabbit anti-p-p38 (1:500), rabbit anti-p38 (1:500), rabbit anti-p-ERK (1:500), rabbit anti-ERK (1:500), rabbit anti-AP-1 (1:1000), rabbit anti-NF-κB (1:1000), rabbit anti-COX-2 (1:1000), rat anti-GAPDH (1:2000), rat anti-Cytochrome C (1:1000). Then the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or horseradish peroxidase- conjugated goat anti-rabbit IgG for 2 h at room temperature. Detection was performed by an enhanced chemiluminescence (ECL) method and imaged by using a BioSpectrum Gel Imaging System (UVP, Upland, CA, USA). In order to eliminate variations caused by protein quantity and quality, the data were adjusted to GAPDH expression (IOD of objective protein versus IOD of GAPDH protein) (Liu et al. 2015).
Statistical analysis
All results for each group were presented as mean and standard deviation (SD), and analysed by one-way analysis of variance (ANOVA), followed by LSD in Post Hoc Multiple Comparisons using the SPSS Statistics 15.0 (IBM, New York, NY, USA) software. A significance of p < 0.05 was considered statistically significant.
Results
Effects of Nar, Naringenin and LPS on the cell cytotoxicity of HUVECs
Firstly, the effects of Nar, naringenin and LPS on the cell cytotoxicity were investigated by MTT assay. As shown in Fig. 1b, we found that Nar in the range of 0.2–0.6 μg/mL didn’t affect the cell proliferation of HUVECs. And naringenin in the range of 0.1–0.4 μg/mL didn’t affect the cell proliferation of HUVECs as well (Fig. 2b). However, the cell viability was significantly decreased when the HUVECs were treated by LPS in the range of 1–20 ng/mL (Fig. 1c). Based on the results, in the present paper, the cell viability was significantly decreased to 46.2 ± 3.12 % when the HUVECs were treated with 10 ng/mL of LPS for 24 h. Therefore, 10 ng/mL of LPS under 24 h treatment was selected to treat the cells in the following experiments, as a model group.
Effect of Nar and Naringenin against LPS-induced cell injury in HUVECs
In model group, the cell viability was 48.1 ± 2.73 %. After pretreated with different concentrations of Nar (0.2, 0.4 and 0.6 μg/mL) for 6 h, the cell viability was increased to 59.2 ± 7.14, 67.6 ± 7.63, 78.3 ± 5.95 %, respectively. In addition, the HUVECs were pretreated with 0.6 μg/mL of Nar under different treatment times (1, 3 and 6 h). The results showed that the cell viability continuously increased with time and the best protective effect was observed when the pretreatment time was set at 6 h. And after pretreated with different concentrations of naringenin (0.1, 0.2 and 0.4 μg/mL) for 6 h, the cell viability was increased to 57.2 ± 8.16, 69.4 ± 8.24, 80.3 ± 4.87 %, respectively. In addition, the HUVECs were pretreated with 0.4 μg/mL of naringenin under different treatment times (1, 3, and 6 h). The results also showed that the cell viability continuously increased with time and the best protective effect was observed when the pretreatment time was set at 6 h. In the light of these results, we found that Nar and naringenin significantly attenuated LPS-induced cell injury in a dose- and time-dependent manner (Fig. 1d, 2c).
Effect of Nar and Naringenin on LPS-induced morphological changes of HUVECs
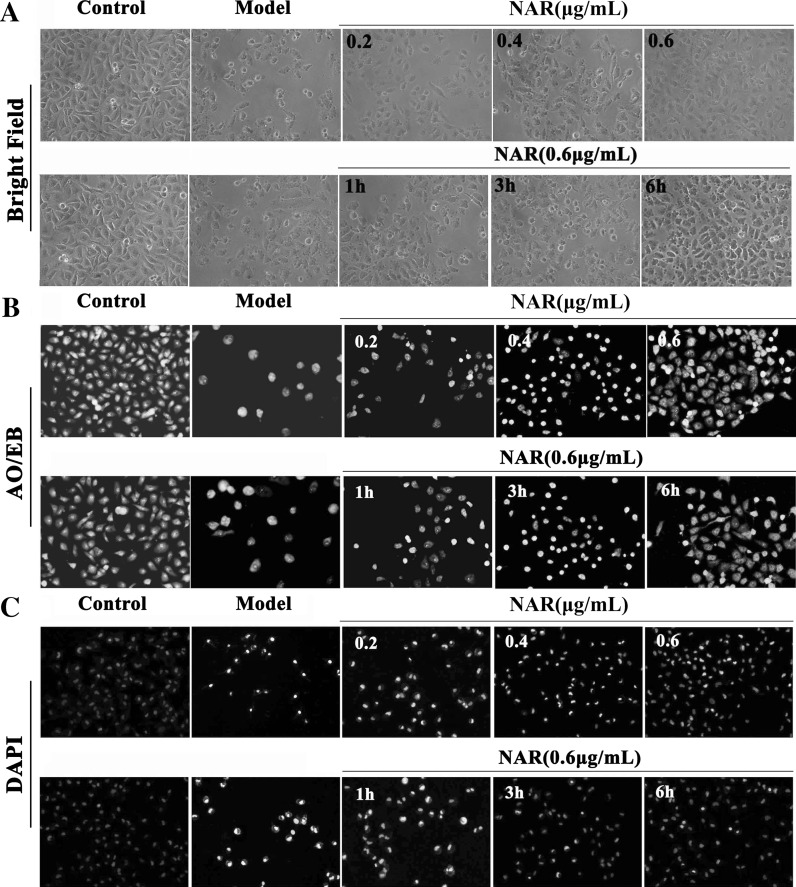
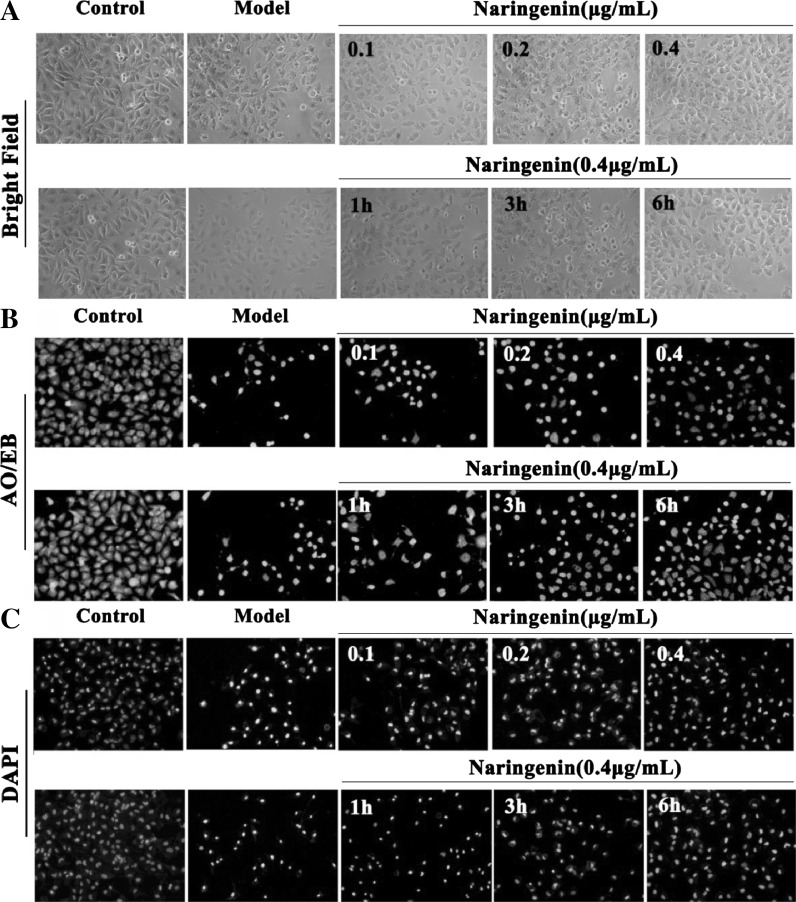
As shown in Figs. 3a and 4a, the conspicuous morphological changes including loss of originally confluent monolayer and cell shrinkage caused by LPS were observed in the bright field images. After pretreated with Nar and naringenin, respectively, these changes were improved. As shown in Figs. 3b and 4b, based on AO/EB staining, the viable cells were bright green, and the apoptotic or necrotic cells were stained jacinth. In Nar and naringenin treated groups, the numbers of apoptotic or necrotic cells were markedly decreased. As shown in Figs. 3c and 4c, in model group, the nucleus of the cells was condensed and nuclear apoptotic bodies appeared after DAPI staining, which was restored by Nar and naringenin, respectively.
Fig. 3.
Morphological and fluorescence images of HUVECs pretreated with different concentrations of Nar (0.2, 0.4, and 0.6 μg/mL) for 6 h and the dose of 0.6 μg/mL under different treatment times (1, 3, and 6 h). a Bright field images; b AO/EB stained images; c DAPI stained images. (×100, final magnification)
Fig. 4.
Morphological and fluorescence images of HUVECs pretreated with different concentrations of naringenin (0.1, 0.2, and 0.4 μg/mL) for 6 h and the dose of 0.4 μg/mL under different treatment times (1, 3, and 6 h). a Bright field images; b AO/EB stained images; (c) DAPI stained images. (×100, final magnification)
Nar decreased the ROS levels caused by LPS
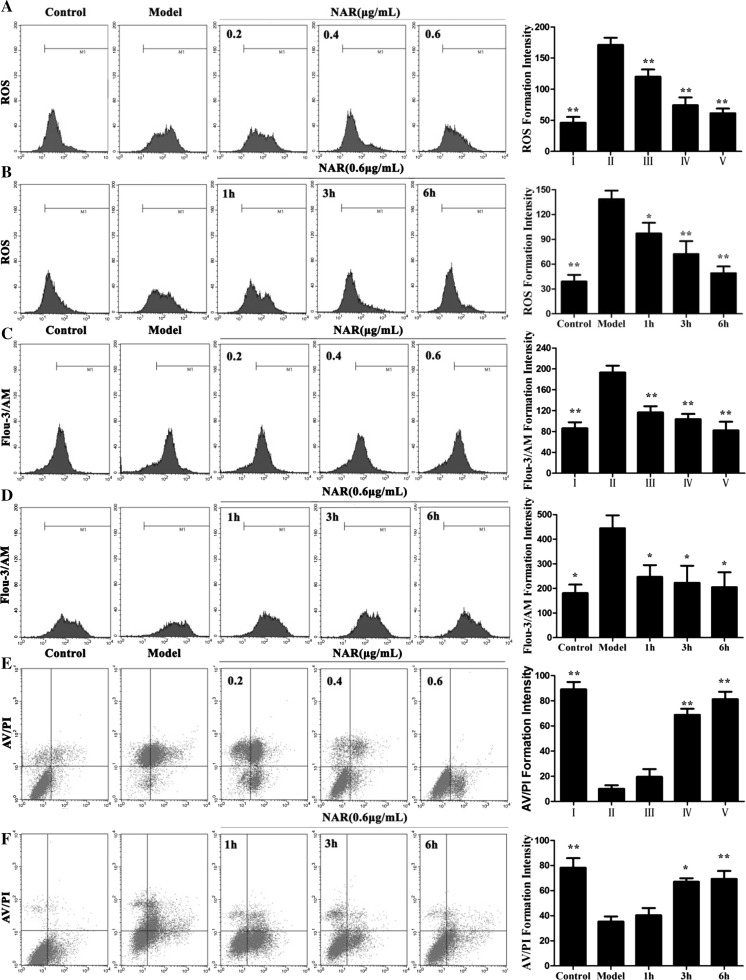
In the present study, we measured the intracellular ROS generation. As shown in Fig. 5a, the ROS level in model group was marked enhanced. However, after pretreated with Nar (0.2, 0.4, and 0.6 μg/mL) for 6 h, the ROS levels were significantly decreased from 171.84 ± 8.72 to 120.48 ± 6.54 %, 74.7 ± 4.81, 62.3 ± 5.82 %, respectively. Meanwhile, after pretreated with Nar at the concentration of 0.6 μg/mL under different treatment times (1, 3, and 6 h), the ROS levels were also significantly decreased with a time-dependent manner (Fig. 5b).
Fig. 5.
Effects of Nar on the ROS levels (a, b), Ca2+ levels (c, d) and cell apoptosis (e, f) caused by LPS in HUVECs. I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL). *p < 0.05 and **p < 0.01, compared with model group
Nar affected Ca2+ levels caused by LPS
As shown in Fig. 5c, d, the Ca2+ level in model group was significantly increased more than 2.4-fold compared with control group (p < 0.01). However, after pretreated with Nar (0.2, 0.4, and 0.6 μg/mL) for 6 h, the levels of Ca2+ were obviously decreased.
Nar inhibited LPS-induced cell apoptosis
Flow cytometric analysis was adopted to evaluate the protective effect of Nar against LPS-induced cell apoptosis. As shown in Fig. 5e, f, the percentage of the apoptotic cells was significantly increased in model group. After pretreated with Nar (0.2, 0.4, and 0.6 μg/mL) for 6 h, or pretreated with the NAR at the concentration of 0.6 μg/mL for different times (1, 3, and 6 h), the percentages of the apoptotic cells were significantly decreased compared with model group. The results showed that Nar attenuated LPS-induced cell apoptosis.
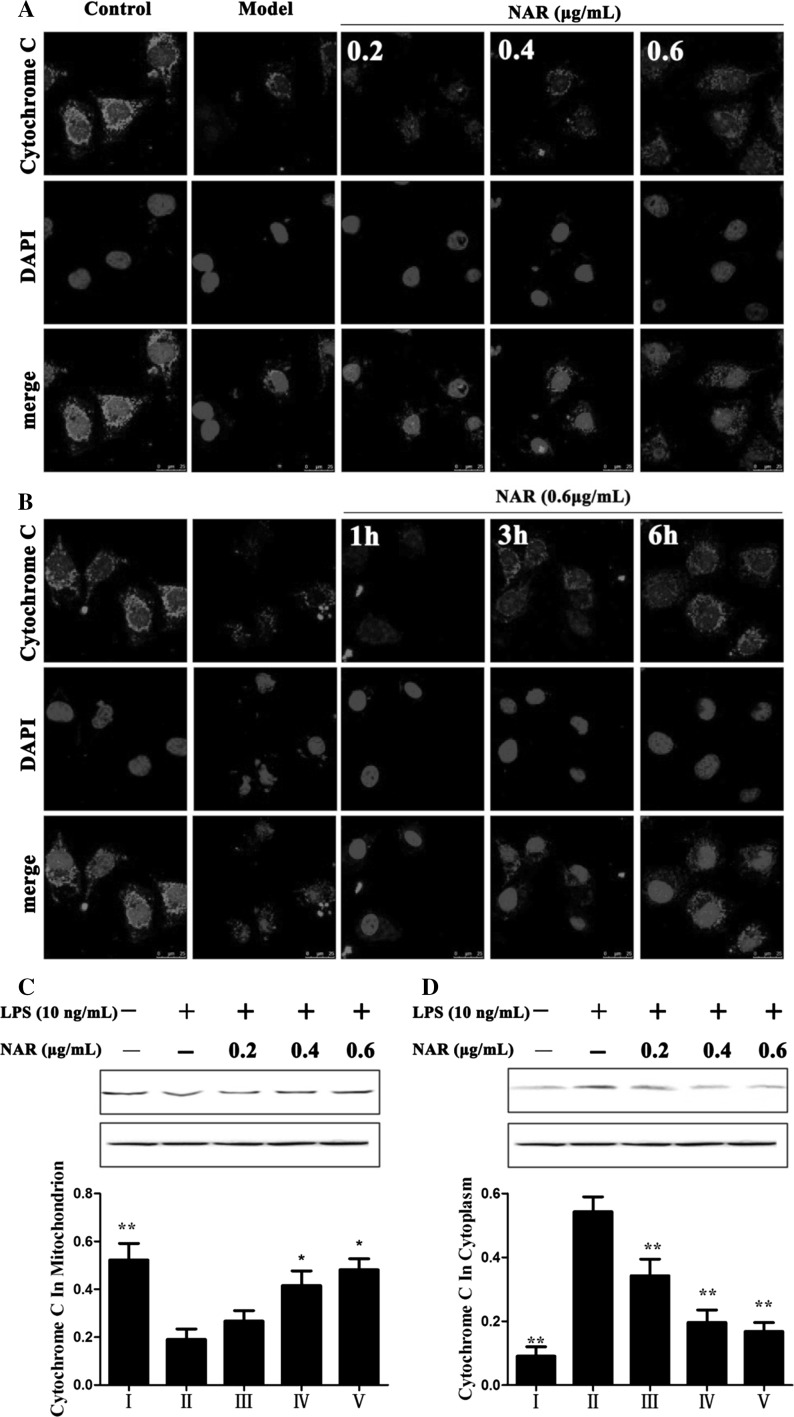
Nar inhibited cytochrome c release
As shown in Fig. 6a, b, we observed that there was a point or massive staining pattern in control group while a diffuse cytoplasmic staining pattern in model group. These results demonstrated that Nar inhibited the release of cytochrome c from mitochondria to cytoplasm. As shown in Fig. 6c, d, Nar treatment significantly decreased the expression level of cytochrome c in cytoplasm and increased its expression in mitochondria compared with LPS-treated group.
Fig. 6.
Effects of Nar on Cytochrome C release (magnification, ×200) (a, b); Effects of Nar on the protein expressions of Cytochrome C in mitochondria and cytoplasm (c, d). I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL)
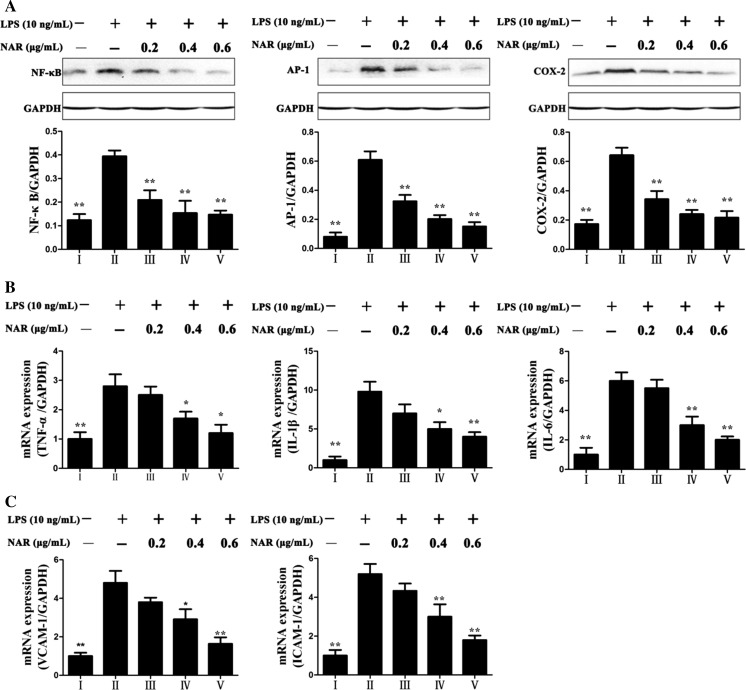
Nar rehabilitated LPS-induced inflammation
As shown in Fig. 7a, the expression levels of inflammation-related proteins including AP-1, NF-κB and COX-2 were significantly down-regulated by Nar compared with model group. In addition, the mRNA levels of IL-1β, IL-6, ICAM-1, VCAM-1 and TNF-α were obviously increased in LPS-treated group (Fig. 7b, c) by 9.8-, 6.0-, 2.4-, 2.5- and 2.8-fold (p < 0.01) compared with control group, which were all markedly restored by Nar.
Fig. 7.
Effects of Nar on the protein expressions of NF-κB, AP-1, COX-2 (a) and the gene expressions of TNF-α, IL-1β, IL-6, VCAM and ICAM (b, c). I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL). *p < 0.05 and **p < 0.01, compared with model group
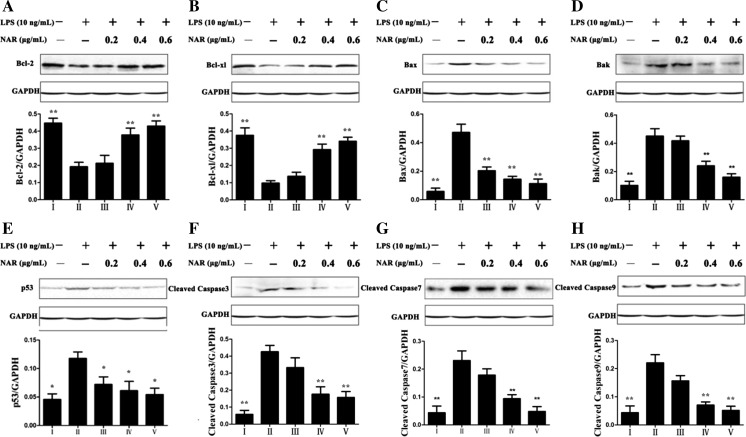
Nar adjusted mitochondria signaling pathway
As shown in Fig. 8, the expression levels of the anti-apoptotic proteins including Bcl-xl and Bcl-2 were detected, and the results showed that their expressions were obviously decreased in model group, which were significantly up-regulated by Nar. The expression levels of pro-apoptotic proteins including Bax, Bak and p53 were significantly up-regulated in LPS-treated group by 9.01-, 4.25- and 3.86-fold (p < 0.01) compared with control group. However, Nar at the concentration of 0.6 μg/mL markedly attenuated the expression levels by 25.5, 36.5, and 31.6 %, respectively, compared with the model group. Meanwhile, the levels of apoptotic-proteins including cleaved caspase-3, -7, and -9 were also significantly down- regulated by Nar compared with LPS-treated group.
Fig. 8.
Effects of Nar on the protein expressions of Bcl-2 (a), Bcl-xl (b), Bax (c), Bak (d), p53 (e), Cleaved Caspase 3 (f), Cleaved Caspase 7 (g), Cleaved Caspase 9 (h). I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL). *p < 0.05 and **p < 0.01, compared with model group
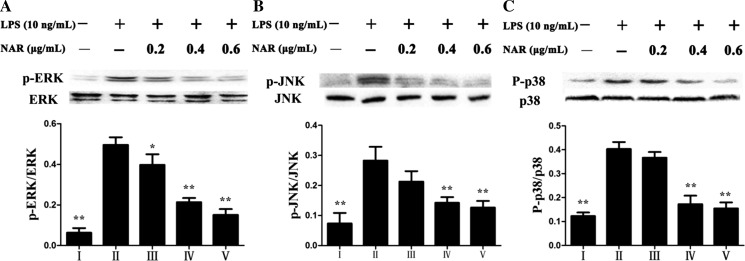
Nar affected MAPKs signaling pathway
As shown in Fig. 9, the effects of Nar on the phosphorylation levels of MAPKs were investigated, and the results indicated that the levels of p-ERK, p-JNK and p-p38 were significantly increased in model group by 7.9-, 3.9-, and 3.3-fold compared with control group. However, Nar at the concentration of 0.6 μg/mL markedly attenuated the expression levels by 38.5, 44.8, and 45.7 %, respectively, compared with model group.
Fig. 9.
Effects of Nar on the protein expressions of p-ERK (a), p-JNK (b) and p-p38 (c). I: Control; II: Model; III: Nar (0.2 μg/mL); IV: Nar (0.4 μg/mL); V: Nar (0.6 μg/mL). *p < 0.05 and **p < 0.01, compared with model group
Discussion
As one flavonoid glycoside from Citrus aurantium L., the protective effect of Nar against LPS-induced damage in HUVECs was investigated in the present paper for the first time. HUVECs only explored to 10 ng/mL LPS for 24 h showed the cell viability was significantly decrease in cell viability. However, the cell viability was significantly increased by Nar and naringenin with the increased concentration and treatment time. From morphological observation, there were several changes in bright field images, AO/EB and DAPI staining. However, after pretreated with Nar and naringenin, these changes were markedly improved. Accordingly, these results suggested that Nar and naringenin had potent effect against cell injury induced by LPS. And naringenin is more effective than Nar.
Calcium ion (Ca2+), an important signaling ion, is related with cell migration, proliferation and cell death, and Ca2+ overload is the key factor in atherosclerosis (Koliakos et al. 2007). High level of Ca2+ mediates a set of biological processes and activates NADPH oxidase indirectly, thereby reactive oxygen species (ROS) is increased (Sarmiento et al. 2014). Ca2+ influx, from the outside of the cell to cytoplasm mediated by a number of Ca2+ channels, promotes cell migration and triggers apoptosis (Boehning et al. 2003). Accordingly, in our study, the level of Ca2+ in the cytoplasm was increased by LPS, which was significantly restored by Nar. Thus, the effect of Nar against LPS-induced damage in HUVECs may be associated with the decreased Ca2+ level.
ROS, normal metabolic product of intracellular oxidation–reduction reaction, can be stimulated by LPS as well as hydrogen peroxide (H2O2) and oxidized low density lipoprotein (ox-LDL), which has been proposed as one important signaling molecule in many biological events including inflammation and cell apoptosis. ROS is mainly produced by NADPH oxidase and is regarded as a key modulator of immune signal transduction, which can regulate the production of pro-inflammatory cytokines (Cheret et al. 2008). Meanwhile, ROS targets cysteine and methionine residues on these proteins as an intracellular second messenger, thereby to modulate a few major signal cascades including MAPK pathway and downstream transcription factors such as Nuclear Factor Kappa B (NF-κB) (Hui et al. 2014). An excess production of ROS can cause inflammatory diseases including atherosclerosis, rheumatoid arthritis, diabetes, septic shock and multiple sclerosis (Hsu et al. 2013). In the present study, the ROS level in HUVECs was remarkably increased by LPS, which was significantly decreased by Nar. These results suggested that Nar inhibited oxygen free radicals to inhibit LPS-induced cell apoptosis.
It is well known that inflammation, one important biological process, has been involved in atherogenesis. Some genes are involved in LPS-induced inflammation reaction including NF-kB, activator Protein-1 (AP-1), cyclooxygenase-2 (COX-2), tumor necrosis factor-a (TNF-α), interleukin-1 (IL-1β), interleukin-6 (IL-6), intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). NF-κB, a nuclear transcription factor, plays a key role in the transcriptional regulation of inflammatory mediators, including IL-1β, IL-6 and TNF-α (Zhang et al. 2015). Meanwhile, NF-kB activates the transcriptions of several inflammatory enzymes, such as COX-2 (Kim et al. 2013). NF-κB signaling pathway is considered to play important roles in inflammatory response and cell death (Zeng et al. 2014). AP-1, a transcription factor, is also involved in the production of inflammatory mediators including IL-1β, IL-6 and TNF-α, which can up-regulate the transcriptions of pro-inflammatory genes (Zenz et al. 2008). COX-2 is the product of an immediate early gene induced by LPS in macrophages, which can increase vascular permeability, vasodilation and control cellular migration through the production and release of pro-inflammatory cytokines, thereby promote inflammation (Kim et al. 2004). IL-1β and TNF-α have been regarded as the primary inflammatory mediators to induce the synthesis and secretion of IL-6, which can mediate both pro- and anti-inflammatory effects (Scorei et al. 2010). VCAM-1 and ICAM-1, on the surfaces of cells, play the key roles in cell adhesion to the vascular endothelium which is required in leukocyte extravasation during inflammation (Lee et al. 2014). In the present work, we found that Nar remarkably decreased the mRNA levels of IL-1β, IL-6, TNF-α, ICAM-1, VCAM-1 and the protein expressions of COX-2, NF-κB and AP-1, which showed that Nar attenuated inflammation caused by LPS. These results suggested that Nar can inhibit LPS-induced damage in HUVECs by suppressing inflammatory reaction
Apoptosis, a cell-suicide program, is activated in physiological processes under pathophysiological conditions. Any change of the mitochondrial membrane permeability is related to an early event in apoptosis. Mitochondria swelling and rupture may release a set of apoptosis promoting factors containing cytochrome c and apoptosis-inducing factors (Wang 2001). ROS can directly activate p53 which plays an important role in regulating apoptosis (Simbula et al. 2007). Meanwhile, p53 induces the expression of pro-apoptotic protein Bax, one of the members of the Bcl-2 family, which is able to enter the mitochondria and trigger apoptosis (Liu et al. 2014). Bcl-2 family mainly includes Bcl-2, Bax, Bcl-xl and Bak, which are the key regulators of apoptosis in mitochondria dependent apoptosis. Bcl-2 and Bcl-xl, the anti-apoptotic proteins, protect cells from apoptosis by protecting membrane integrity and inhibiting Cytochrome C released. Bax, the Bcl-2-associated X protein, can increase mitochondrial membrane permeability, thereby promote apoptotic (Adams and Cory 2007). Bak, also a pro-apoptotic member, can disrupt the integrity of the outer mitochondrial membrane and increase its permeability resulting in the release of Cytochrome C. Furthermore, the ratio between Bcl-2 and Bax regulates Cytochrome C and other pro-apoptotic factors released into the cytoplasm downstream (Huang et al. 2014). In addition, Cytochrome C release is a main event in the activation of caspase family which is a pivotal downstream step in apoptosis initiation (Ghibelli and Diederich 2010). And the activation of caspase-3 and caspase-9 has been regarded as the final enforcer of cellular apoptotic death (Cryns and Yuan 1998). Mitochondria-dependent death signals recruit and cleave initiator caspase-9, which also activate caspase-3 and -7 (Guan et al. 2015). In our study, we found that the expressions of Bax, Bak, cleaved caspase-3, -7, -9, and p53 were significantly increased in HUVECs by LPS, while Bcl-xl and Bcl-2 were dramatically suppressed. After pretreated with Nar, the levels of Bax, Bak, cleaved caspase-3, -7, -9, and p53 were up- regulated, while Bcl-xl and Bcl-2 were down-regulated. These results indicated that the effect of Nar on inhibiting LPS-induced apoptosis may be through regulating mitochondrial signaling pathway.
LPS is the inflammatory stimuli to activate mitogen-activated protein kinase (MAPK) proteins in cellular responses (Hsu and Wen 2002). The three major members of MAPK family are extracellular signal-regulated kinases (ERK1/2), c-jun N-terminal or stress-activated protein kinase (JNK) and p38 protein kinase, which play important roles in regulating cell growth, cellular responses to inflammation and cell apoptosis, and MAPKs are considered as potential targets as the anti-inflammatory therapeutic (Kaminska 2005). In this study, our results demonstrated that LPS up-regulated the levels of p-p38, p-ERK and p-JNK, which were significantly inhibited by Nar. These results indicated that the effect of Nar against LPS-induced damage in HUVECs may be through regulating MAPKs signaling pathway.
Conclusions
In conclusion, our study clearly showed that Nar attenuated LPS-induced damage in HUVECs by inhibiting intracellular ROS, releasing Ca2+, suppressing endothelial cell death, rehabilitated inflammation, activating mitochondrial and MAPK signal pathways. These findings suggest that Nar is an effective natural product for attenuating injury caused by LPS.
Acknowledgments
This work was finally supported by the Natural Science Foundation of Liaoning Province (No. 2014023033).
Author contributions
Cheng Bi performed experiments and Yinong Jiang, Tingting Fu, Yu Hao, Xifang Zhu analyzed the data. Cheng Bi and Yan Lu designed the experiments and wrote the manuscript. Yan Lu edited the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti S, Rani N, Krishnamurthy B, Arya DS. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 2014;80:437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1, 4, 5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Chaby R. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. Cell Mol Life Sci. 2004;61:1697–1713. doi: 10.1007/s00018-004-4020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. Neuroscience. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Kim CH, Ha TS, Lee SJ, Ahn HY. Ginsenoside Rg2 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell. Korean J Physiol Pharmacol. 2013;17:133–137. doi: 10.4196/kjpp.2013.17.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Dong D, Xu L, Yin L, Qi Y, Peng J. Naringin prevents carbon tetrachloride-induced acute liver injury in mice. J Funct Foods. 2015;12:179–191. doi: 10.1016/j.jff.2014.11.020. [DOI] [Google Scholar]
- Echeverría C, Montorfano I, Sarmiento D, Becerra A, Nunez-Villena F, Figueroa XF, Cabello-Verrugio C, Elorza AA, Riedel C, Simon F. Lipopolysaccharide induces a fibrotic-like phenotype in endothelial cells. Cell Mol Med. 2013;17:800–814. doi: 10.1111/jcmm.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibelli L, Diederich M. Multistep and multitask bax activation. Mitochondrion. 2010;10:604–613. doi: 10.1016/j.mito.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Gopinath K, Prakash D, Sudhandiran G. Neuroprotective effect of naringin, a dietary flavonoid against 3-nitropropionic acid-induced neuronal apoptosis. Neurochem Int. 2011;59:1066–1073. doi: 10.1016/j.neuint.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Guan F, Wang Q, Wang M, Shan Y, Chen Y, Yin M, Zhao Y, Feng X, Liu F, Zhang J. Isolation, identification and cytotoxicity of a new noroleanane-type triterpene saponin from Salicornia bigelovii Torr. Molecules. 2015;20:6419–6431. doi: 10.3390/molecules20046419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1gene expression. Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Lien JC, Chang CW, Chang CH, Kuo SC, Huang TF. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem Pharmacol. 2013;85:385–395. doi: 10.1016/j.bcp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Huang WR, Zhang Y, Tang X. Shikonin inhibits the proliferation of human lens epithelial cells by inducing apoptosis through ROS and caspase-dependent pathway. Molecules. 2014;19:7785–7797. doi: 10.3390/molecules19067785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui B, Yao X, Zhou QH, Wu ZY, Sheng P, Zhang LP. Pristimerin, a natural anti-tumor triterpenoid, inhibits LPS-induced TNF-α and IL-8 production through down-regulation of ROS-related classical NF-κB pathway in THP-1 cells. Int Immunopharmacol. 2014;21:501–508. doi: 10.1016/j.intimp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, Chihara Y, Kida I, Oqihara T. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339:827–832. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang Z, Chandrakala AN, Pressley D, Parthasarathy S. Oxidized low density lipoproteins–do we know enough about them? Cardiovasc Drugs Ther. 2011;25:367–377. doi: 10.1007/s10557-011-6326-4. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kim JW, Zou Y, Yoon S, Lee JH, Kim YK, Yu BP, Chung HY. Vascular aging: molecular modulation of the pros-tanoid cascade by calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:B876–B885. doi: 10.1093/gerona/59.9.B876. [DOI] [PubMed] [Google Scholar]
- Kim DH, Chung JH, Yoon JS, Ha YM, Bae SJ, Lee EK, Jung KJ, Kim MS, Kim YJ, Kim MK, Chung HY. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kB in LPS-stimulated RAW264.7 cells and mouse liver. J Ginseng Res. 2013;37:54–63. doi: 10.5142/jgr.2013.37.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliakos C, Befani K, Paletas M, Kaloyianni M. Effect of endothelin on sodium/hydrogen exchanger activity of human monocytes and atherosclerosis- related functions. Ann NY Acad Sci. 2007;1095:274–291. doi: 10.1196/annals.1397.031. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:1–14. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kim DI, Kim WJ, Moon SK. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-alpha-induced vascular smooth muscle cells. Mol Nutr Food Res. 2009;53:1582–1591. doi: 10.1002/mnfr.200800210. [DOI] [PubMed] [Google Scholar]
- Lee WH, Ku SK, Min BW, Lee SK, Jee JG, Kim JA, Bae JS. Vascular barrier protective effects of pellitorine in LPS-induced inflammation in vitro and in vivo. Fitoterapia. 2014;92:177–187. doi: 10.1016/j.fitote.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/S0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Su WW, Wang S, Li PB. Naringin inhibits chemokine production in an LPS-induced RAW 264.7 macrophage cell line. Mol Med Rep. 2012;6:1343–1350. doi: 10.3892/mmr.2012.1072. [DOI] [PubMed] [Google Scholar]
- Liu M, Xu YW, Han X, Liang C, Yin LH, Xu LN, Qi Y, Zhao YY, Peng JY, Sun CK. Potent effects of flavonoid-rich extract from rosa laevigata michx fruit against hydrogen peroxide-induced damage in PC12 cells via attenuation of oxidative stress, inflammation and apoptosis. Molecules. 2014;19:11816–11832. doi: 10.3390/molecules190811816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xu LN, Yin LH, Qi Y, Xu YW, Han X, Zhao YY, Sun HJ, Yao JH, Lin Y, Liu KX, Peng JY. Potent effects of dioscin against obesity in mice. Sci Rep. 2015;5:7973. doi: 10.1038/srep07973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–1731. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- Ni H, Zhao W, Kong X, Li H, Ouyang J. Celastrol inhibits lipopolysaccharide-induced angiogenesis by suppressing TLR4-triggered nuclear factor-kappa B activation. Acta Haematol. 2014;131:102–111. doi: 10.1159/000354770. [DOI] [PubMed] [Google Scholar]
- Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2014;12:1–13. doi: 10.1016/S2095-4964(14)60008-X. [DOI] [PubMed] [Google Scholar]
- Raetz CR. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Sarmiento D, Montorfano I, Cáceres M, Echeverría C, Fernández R, Cabello-Verrugio C, Cerda O, Tapia P, Simona F. Endotoxin-induced vascular endothelial cell migration is dependent on TLR4/NF-κB pathway, NAD(P)H oxidase activation, and transient receptor potential melastatin 7 calcium channel activity. Int J Biochem Cell Biol. 2014;55:11–23. doi: 10.1016/j.biocel.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Schlegel N, Baumer Y, Drenckhahn D, Waschke J. Lipopolysaccharide induced endothelial barrier breakdown is cyclic adenosine monophosphate dependent in vivo and in vitro. Crit Care Med. 2009;37:1735–1743. doi: 10.1097/CCM.0b013e31819deb6a. [DOI] [PubMed] [Google Scholar]
- Scorei RI, Ciofrangeanu C, Ion R, Cimpean A, Galateanu B, Mitran V, Iordachescu D. In vitro effects of calcium fructoborate upon production of inflammatory mediators by LPS-stimulated raw 264.7 macrophages. Biol Trace Elem Res. 2010;135:334–344. doi: 10.1007/s12011-009-8488-5. [DOI] [PubMed] [Google Scholar]
- Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A. Increased ROS generation and p53 activation in alpha-lipoic acid induced apoptosis of hepatoma cells. Apoptosis. 2007;12:113–123. doi: 10.1007/s10495-006-0487-9. [DOI] [PubMed] [Google Scholar]
- Song XM, Chen Y, Sun YJ, Lin BQ, Qin YS, Hui H, Li ZY, You QD, Lu N, Guo QL. Oroxylin A, a classical natural product, shows a novel inhibitory effect on angiogenesis induced by lipopolysaccharide. Pharmacol Rep. 2012;64:1189–1199. doi: 10.1016/S1734-1140(12)70915-5. [DOI] [PubMed] [Google Scholar]
- Tae HL, Jihoon C, Byeong MK. Saikosaponin C inhibits lipopolysaccharide-induced apoptosis by suppressing caspase-3 activation and subsequent degradation of focal adhesion kinase in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2014;445:615–621. doi: 10.1016/j.bbrc.2014.02.046. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wang SP, Wu X, Tan M, Gong J, Tan W, Bian BL, Chen M, Wang Y. Fighting fire with fire: poisonous Chinese herbal medicine for cancer therapy. Ethnopharmacol. 2012;140:33–45. doi: 10.1016/j.jep.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Zeng L, Zhen YL, Chen YM, Zou L, Zhang Y, Hu F, Feng JQ, Shen JH, Wei B. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int J Oncol. 2014;45:1929–1936. doi: 10.3892/ijo.2014.2617. [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, Kenner L, Tschachler E, Wagner EF. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:1–10. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Han X, Yin LH, Xu LN, Qi Y, Xu YW, Sun HJ, Lin Y, Liu KX, Peng JY. Potent effects of dioscin against liver fibrosis. Sci Rep. 2015;5:9713. doi: 10.1038/srep09713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska-Przyjemska M, Lgnatowicz E. Citrus fruit flavonoids influence on neutrophil apoptosis and oxidative metabolism. Phytother Res. 2008;22:1557–1562. doi: 10.1002/ptr.2449. [DOI] [PubMed] [Google Scholar]