Abstract
Experiences worldwide reveal that degraded lands restoration projects achieve little success or fail. Hence, understanding the underlying causes and accordingly, devising appropriate restoration mechanisms is crucial. In doing so, the ever-increasing aspiration and global commitments in degraded lands restoration could be realized. Here we explain that arbuscular mycorrhizal fungi (AMF) biotechnology is a potential mechanism to significantly improve the restoration success of degraded lands. There are abundant scientific evidences to demonstrate that AMF significantly improve soil attributes, increase above and belowground biodiversity, significantly improve tree/shrub seedlings survival, growth and establishment on moisture and nutrient stressed soils. AMF have also been shown to drive plant succession and may prevent invasion by alien species. The very few conditions where infective AMF are low in abundance and diversity is when the soil erodes, is disturbed and is devoid of vegetation cover. These are all common features of degraded lands. Meanwhile, degraded lands harbor low levels of infective AMF abundance and diversity. Therefore, the successful restoration of infective AMF can potentially improve the restoration success of degraded lands. Better AMF inoculation effects result when inocula are composed of native fungi instead of exotics, early seral instead of late seral fungi, and are consortia instead of few or single species. Future research efforts should focus on AMF effect on plant community primary productivity and plant competition. Further investigation focusing on forest ecosystems, and carried out at the field condition is highly recommended. Devising cheap and ethically widely accepted inocula production methods and better ways of AMF in situ management for effective restoration of degraded lands will also remain to be important research areas.
Keywords: AMF, ecological restoration, facilitation, inoculation, land degradation, mycorrhiza, monoxenic culture, succession
Introduction
Ecological restoration has emerged to be the central theme of global environmental policies (Aradottir and Hagen, 2013; Jacobs et al., 2015). Restoration of at least 15% of the world’s degraded ecosystems is one of the 20 2011–2020 targets of the UN Convention on Biological Diversity (CBD, 20101). In 2011, world leaders endorsed the “Bonn challenge” which is a global commitment to restore 150 million hectares of deforested and degraded lands by 2020 (Aradottir and Hagen, 2013). In 2014, the New York Declaration on Forests put forward even a bigger global commitment of restoring 350 million hectares of deforested and degraded lands until 2030 (Jacobs et al., 2015). Most importantly, in 2015, the UN concretized these global commitments by adopting the 2030 Sustainable Development Goals which has one of the 17 targets (Target 15) dealing on ecological restoration (UN, 2015). However, restoration experiences so far show that many restoration projects achieve limited success or fail completely (Thomas et al., 2014) and therefore, extra effort is needed to achieve the huge global restoration commitments put on the table. Here we propose AMF inoculation and in situ management for better restoration outcome of degraded lands.
About 93% of flowering plant families (Brundrett, 2009) and 92% of land plant families (Wang and Qiu, 2006) are estimated to have mycorrhizal associations. These associations, based on their structure and physiological relationship with symbionts, are categorized in to seven; of which arbuscular mycorrhiza is one (Brundrett et al., 1996; Barea et al., 2011). Arbuscular mycorrhiza is the most predominant and evolutionarily the ancestor of all the association types (Wang and Qiu, 2006). AMF produce arbuscules, hyphae, and vesicles within host plants’ root cortical cells (Brundrett and Abbott, 2002). However, some species within the family Gigasporaceae do not form vesicles but instead, form auxiliary cells of unknown function (Redecker and Raab, 2006). In few other cases as well, arbuscules develop poorly or may be absent (Koide and Mosse, 2004). AMF are; (1) obligate biotrophs completely depending on host plants for organic carbon (File et al., 2012), (2) evolutionarily intimately associated with plants (Taylor et al., 1995), (3) multiple nucleated, and (4) asexually reproducing eukaryotes (Schüßler et al., 2007).
Arbuscular mycorrhizal fungi are keystone organisms with myriads of ecosystem roles. The external hyphae network (extraradical mycelium) of the fungi permeate in to the microsites of rocks and soils surrounding the plant roots (Finlay, 2008; Barea et al., 2011) increasing the root absorbing surface area 100 or even 1000 fold (Larcher, 1995). Therefore, AMF increase plants’ nutrient and water relation (e.g., Birhane et al., 2012, 2015; Banerjee et al., 2013), and can improve plants’ field survival and establishment (e.g., Pouyu-Rojas and Siqueira, 2000; Habte et al., 2001; Ouahmane et al., 2006; Dag et al., 2009; Kapulnik et al., 2010; Karthikeyan and Krishnakumar, 2012; Manaut et al., 2015). AMF improve soil structure, soil water relation, plants’ tolerance to biotic and abiotic stresses, increase plants’ nutrient supply, plants’ growth, yield and reproductive success and reduce fertilizer requirement (Finlay, 2008; Gianinazzi et al., 2010; Simard and Austin, 2010; Barea et al., 2011; Al-Karaki, 2013; Soka and Ritchie, 2014). AMF influence plant community structure (Van der Heijden et al., 1998; Hartnett and Wilson, 1999; Renker et al., 2004; Heneghan et al., 2008; Lin et al., 2015) and are considered to have a pivotal role in plant community assembly and succession (Janos, 1980; Renker et al., 2004; Kikvidze et al., 2010). Therefore, AMF have significant role in ecological restoration.
The potential role of AMF in ecological restoration has been well recognized even before restoration ecology emerged as a scientific field of study (see Janos, 1980 and the references there). However, as of yet, there is no report available to confirm that AMF inoculation has grown to be a biotechnological tool that is widely applicable in ecological restoration. Review articles dealing on the subject are also very few. To our knowledge, those review articles that dealt on the subject are Skujins and Allen (1986), Brundrett and Abbott (2002), Jeffries et al. (2002) and Renker et al. (2004). Other reviews (e.g., Perry et al., 1987; Quoreshi, 2008; Sanon et al., 2010; Al-Karaki, 2013) did not deal on AMF specifically and the one by Koide and Mosse (2004) allocated some paragraphs for the topic. Therefore, although there is a large number of articles and sufficient knowledge on the ecosystem role of AMF, their role in the restoration of degraded lands is relatively little reviewed. Hence, the purpose of this review article is to gather data from published articles and assess the effects AMF have on measurable ecological restoration attributes and ecological processes.
Features of Degraded Lands
There is no single internationally approved definition of land degradation (World Resource Institute, 20152). However, land degradation is often defined as a long-term loss of ecosystem function and productivity caused by disturbances from which the land cannot recover unaided (e.g., Bai et al., 2008). Meanwhile, reduction in net primary productivity has commonly been used to measure the level of land degradation and restoration (Bai et al., 2008). The Society for Ecological Restoration (SER), however, recommends nine attributes to measure restoration success (SER, 2004). Earlier, Aronson et al. (1993) adapted Odum (1969) succession traits to formulate their own restoration attributes. The SER restoration attributes are excellent parameters (Ruiz-Jaen and Aide, 2005), however, it is the Aronson et al. (1993) restoration attributes that have commonly been used by restoration ecologists (Choi, 2004; Ruiz-Jaen and Aide, 2005). Therefore, in this article, the attributes listed by Aronson et al. (1993) are adapted and used to safely characterize degraded lands (Table 1).
Table 1.
Features of degraded lands compared to reference climax ecosystems (Based on Aronson et al., 1993).
| Structural indicators of degraded lands | Functional indicators of degraded lands | ||
|---|---|---|---|
| Low total plant cover | Low biomass productivity | ||
| Low perennial and annual plant species richness | Low soil organic matter | ||
| Low aboveground phytomass | Poor soil water relation | Lowered soil water reserves | |
| Low beta diversity (species turnover along environmental gradient) | Low coefficient of rainfall efficiency (the amount of water infiltrating to middle and deep soil layers) | ||
| Decreased life form spectrum (Decreased number of species with different modes of adaptation) | Reduced length of water availability period | ||
| Reduced number of keystone species | Low rain use efficiency (RUE) | ||
| Low soil microbial biomass | Poor nutrient cycling index (the ratio of the amount of nutrients mainly N&P recycled to the amount leaching or lost) | ||
| Low soil microbial diversity | Low nitrogen use efficiency (NUE) | ||
Degraded lands are characterized by low levels of AMF abundance and diversity. An experiment carried out in Brazil (Cardozo-Junior et al., 2012) compared the abundance and diversity of AMF on lands of differing degradation levels and also determined the same on a young restoring site. The result of the observation clearly showed that as the scale of degradation increases, the abundance and diversity of AMF reduces and when restoration presumes both AMF abundance and diversity increase (Cardozo-Junior et al., 2012). Elsewhere also, it was reported that an effective exclosure increased AMF abundance (Birhane et al., 2010). These are in agreement to the remarks by Abbott and Robson (1991) and Schnoor et al. (2011) which indicated that although AMF are ubiquitous, the very few conditions where a natural ecosystem can be devoid of AMF is in areas that are severely eroded or disturbed.
A greenhouse experiment with simulated erosion was able to demonstrate that erosion of soil beyond 7.5 cm could make soil loose AMF completely (Habte, 1989). Likewise, Jasper et al. (1989) were able to experimentally determine that, while AMF maintained their infective potential in extremely dry soil conditions, their infective potential was significantly lowered when the soil was disturbed. Hyphae are important source of inoculum but are highly susceptible to disturbance and hence, disturbance leads to lowered infective potential of AMF (Brundrett and Abbott, 2002).
Furthermore, several studies conducted in agricultural fields have shown that disturbance not only reduces AMF abundance, diversity and infectivity but also results in drastic shift in the AMF community (Schnoor et al., 2011). Most species of the most common AMF families (Glomeraceae, Acaulosporaceae, and Gigasporaceae) have distinctive biomass allocation strategies whereby species of the Glomeraceae allocate most of their biomass in the intraradical hyphae while species of the Gigasporaceae allocate most of their biomass in the extraradical hyphae and species of the Acaulosporaceae produce low biomass both intra and extraradically (Maherali and Klironomos, 2007). Similarly, these distinctive fungal groups have distinctive life history with most species of the Glomeraceae being ruderals while that of Gigasporaceae and Acaulosporaceae are competitors and stress tolerators respectively (Chagnon et al., 2013). Ruderal AMF species are disturbance tolerant since they have shorter extraradiacal mycelium and have the following life history strategy viz., grow faster, have short life cycle and invest earlier and more abundantly in spore formation, fuse fragmented hyphae more readily, and form cross-walls that enable infected root pieces and severed hyphal fragments to heal and re-colonize host roots (Chagnon et al., 2013). Meanwhile, AMF communities of disturbed sites are characteristically dominated by disturbance tolerant species of the family Glomeraceae and more specifically the genus Glomus (Chagnon et al., 2013).
The number of surviving propagules of AMF in soils also declines with time in the absence of host plants (Brundrett and Abbott, 2002). Alexander et al. (1992) have reported that heavy logging in a Malaysian forest significantly reduced (75% reductions) the abundance and infectivity of AMF propagules. Therefore, considering the fact that land degradation significantly reduces plant cover, increases soil disturbance and erosion, low levels of AMF abundance, diversity and infective potential can be considered as a peculiar feature of degraded lands. Degraded lands are also prone to invasion by exotic alien species. This is because, the low level of native plants diversity can potentially provide vacant niche for invasives (Mack et al., 2000).
What is Ecological Restoration?
Ecological restoration, according to Hobbs et al. (2007) is a process of assembly and succession mediated by disturbance. Succession refers to the more or less regular and predictable replacement of seral communities while community assembly refers to the species dynamics of each seral community. Community assembly is typically viewed as a hierarchical process with local species assemblages representing subsets of a larger species pool (Kikvidze et al., 2015). There are three level filters that result in a particular seral community assemblages viz. (1) speciation, extinction and migration, (2) dispersal, and (3) habitat filters (abiotic factors) and biotic filters like competition and facilitation (Gotzenberger et al., 2011). A typical community assembly was observed by Gleason (1927) whereby ponds at similar locality with similar environmental condition formed different kinds of wetland communities.
Restoration strategies of degraded terrestrial systems usually center on manipulation of species order of arrival and modification of filters to accelerate succession and/or jump start succession (Young et al., 2001; Hobbs et al., 2007; Gómez-Aparicio, 2009). The seral community assemblage is very important in ecological succession since it can determine the latter seral community assemblage and hence, succession trajectory. Egler (1954), argued that ecosystem development can be accelerated by controlling initial species composition and succession to achieve the desired end point. Some field observations (e.g., Cortines and Valcarcel, 2009) have shown that this has practical application in ecological restoration. This phenomenon is known as the priority effect (Young et al., 2005; Zedler, 2005). Hence, designing initial species composition and ensuring their survival and establishment is an important step in ecological restoration (Angelini et al., 2011). With the growing appreciation to plant–plant facilitation interaction and due to better adaptation to resource limited conditions, early/mid successional shrubs are gaining preference to late successional tree/shrubs to startup restoration process of degraded lands (Gómez-Aparicio, 2009; Padilla et al., 2009).
Restoration ecologists not only provide appropriate conditions for desired species to establish but they also, in the meantime, devise ways of preventing the establishment by invasives (Zedler, 2005). Disturbance is also an important factor in ecological restoration since it can modify filters and community assemblage (Choi, 2004). Restoration ecologists have observed that some low level of natural disturbance (e.g., logging, fire, flooding, etc.) can enhance biological diversity and hence, ecological restoration (Palmer et al., 1997).
Based on the scope and complexity of intervention, ecological restoration ranges from species reintroduction to population restoration to community restoration (Young et al., 2001). Based on the restoration goal, it ranges from reclamation to rehabilitation to true restoration. An ecosystem that is slightly disturbed can restore back to its pre-disturbance status (true restoration). As the magnitude of disturbance increases the return to pre-disturbance status may be impossible and hence, return to an intermediate successional status of the given community (an alternative steady state) may be achieved (rehabilitation). When the disturbance is severe, the threshold of irreversibility is passed and the return to pre-disturbance community status or intermediate successional status will be completely impossible and hence, restoration can only result in a novel community stature (reclamation) (Aronson et al., 1993). In the advent of climate change, to have reclamation as a restoration goal is considered to be relevant since novel climatic conditions are anticipated in the future (Choi, 2004).
Considering the wide range of concepts embedded in ecological restoration, as shown above, a comprehensive definition is crucial. Hence, the SER defined ecological restoration as, the process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed (SER, 2004). This is the most widely accepted definition of ecological restoration (Harris et al., 2006; Higgs et al., 2014). Likewise, for this article, this definition is adopted.
In tropical lands ecological restoration, tree planting (Lamb et al., 2005; Holl et al., 2010; Aerts and Honnay, 2011) and re-vegetation/reforestation (Cortines and Valcarcel, 2009; Al-Karaki, 2013) are known to be the most effective and widely used biological measures. Accordingly, in this article ecological restoration is considered to be the re-vegetation of degraded sites mainly through tree/shrub planting.
AMF and the Mycorrhizosphere Ecology
The rhizosphere is a narrow zone of soil affected by the presence of plant roots (Hrynkiewicz and Baum, 2011). It is extremely important and active area for root activity and metabolism (Saharan and Nehra, 2011). Roots release a multitude of organic compounds (e.g., exudates and mucilage) derived from photosynthesis and other plant processes making the rhizosphere a hot spot of microbial activities mainly that of fungi and bacteria (Hrynkiewicz and Baum, 2011). The physical, chemical and biological environment of the rhizosphere is hence, clearly distinct from the bulk soil (Barea et al., 2002).
Similarly, the rhizosphere of the mycorrhizal plant can be referred to as the mycorrhizosphere (Barea et al., 2002). Mycorrhizosphere comprises both the root and hyphae influence zones or the rhizosphere and hyphosphere (Timonen and Marschner, 2006). Mycorrhizal hyphal growth in soils is extensive, with mycelial lengths reaching 111 m cm-3 or 0.5 mg g-1 or 900 kg ha-1 of soil (Simard and Austin, 2010). Hence, the mycorrhizosphere provide a critical link between plants, other microorganisms and the soil (Hrynkiewicz and Baum, 2011).
Intricate interactions take place within the mycorrhizosphere. The most important ones could be interactions between; AMF and the plant, AMF and bacteria, AMF and other fungi, and among AMF themselves. These interactions commence when plant roots exude strigolactones (SLs) (Parniske, 2008; Gutjahr, 2014). Under phosphate or nitrogen limiting conditions plants exude elevated amounts of SLs into the rhizosphere (Gutjahr, 2014). SLs are carotenoid-derived plant hormones (Gutjahr, 2014) that induce AMF spore germination and hyphal branching (Parniske, 2008). They are also known to induce seed germination in parasitic plants, such as Striga (Parniske, 2008) and are also involved in suppression of shoot branching and shaping root architecture (Gutjahr, 2014).
The AMF on their part, produce mycorrhiza (Myc) factors. Myc-factors induce calcium oscillations in root epidermal cells and also activate plant symbiosis-related genes (Parniske, 2008). Then the AMF form special type of appressoria called hyphopodia which develops from mature hyphae (Parniske, 2008). As a consequence of sequential chemical and mechanical stimulation, plant epidermal cells produce a pre-penetration apparatus (PPA) (Parniske, 2008). Subsequently, a fungal hypha that extends from the hyphopodium enters the PPA, which guides the fungus through root cells toward the cortex. The fungus then leaves the plant cell and enters the apoplast, where it branches and grows laterally along the root axis (Parniske, 2008; Gutjahr, 2014). These hyphae induce the development of PPA-like structures in inner cortical cells, subsequently enter these cells, and branch to form arbuscules (Parniske, 2008). Upon getting nourished via the arbuscules the fungi will develop extraradical mycelium whose leading tips form new spores to continue the lifecycle of the fungi (Parniske, 2008). Vesicles, which are proposed to function as storage organs of the fungus, when applicable, are formed in the apoplast (Parniske, 2008).
Arbuscular mycorrhizal fungi have a significant role in plants’ P nutrition and sometimes, 100% of the P may be provided by the AMF (Smith et al., 2011). In return, plants allocate, according to most authors, 4–20% of the photosynthate to the AMF (Lerat et al., 2003). It has been shown that plants preferentially allocate more carbon in favor of the more beneficial fungi (e.g., Bever et al., 2009). Moreover, plants allow the arbuscules to live in their cells as long as the AMF is delivering phosphorous and maybe other nutrients efficiently (Parniske, 2008). The observation that mutation of the arbuscule specific phosphate transporter PT4 results in premature degradation of arbuscules suggests that the lifetime of arbuscules is influenced by their ability to deliver phosphate and probably other nutrients (Parniske, 2008). This provides the plant with a means to maintain efficient arbuscules and penalize inefficient ones with early degradation. Conceptually, this mechanism allows the plant not only to discriminate between efficient and inefficient fungal species but also allows to remove potentially ‘good’ fungal symbionts that are attached to a poor phosphate source. This concept allows fungal clones and species to compete for arbuscule formation, which allows succession in an established root system (Parniske, 2008). Meanwhile, the formation of fungal colonization structures and the extent of root colonization are largely under plant control (Gutjahr, 2014).
Arbuscular mycorrhizal fungi are known to play role in plant nutrition as long as they collaborate with other soil microbes. It was experimentally proven that mechanisms underlying the increased P-uptake in arbuscular mycorrhizal plants were solely due to AMF synergistic interactions with P-solubilizing microorganisms and/or greater soil volume explored by the AMF hyphae (Antunes et al., 2007). Otherwise, the AMF unlike ectomycorrhiza are not able to neither solubilize phosphate nor decompose organic matter (Simard and Austin, 2010). The well-known activities of nitrogen-fixing bacteria and P-solubilizing microorganisms improving the bioavailability of the major plant nutrients N and P are very much enhanced in the mycorrhizosphere where synergistic interactions of such microorganisms with mycorrhizal fungi have been demonstrated (Barea et al., 2002). In particular, mycorrhizal inoculation improved the establishment of both inoculated and indigenous P-solubilizing rhizobacteria and, again P-solubilizing rhizobacteria usually behave as mycorrhiza-helper-bacteria, promoting mycorrhiza establishment by both the indigenous and the inoculated mycorrhizal fungi (Barea et al., 2002).
Arbuscular mycorrhizal fungi also interact with decomposer fungi (Soka and Ritchie, 2014) and phosphate solubilizing fungi (PSF) synergistically (Osoria and Habte, 2001). Accordingly, presence of mycorrhizal fungi is known to alter the rates of above and below ground litter decomposition due to chemical changes in the roots and interactions with the decomposer fungi (Soka and Ritchie, 2014). PSF were also observed to have lesser effect in plant nutrition when applied alone and maximum effect took place when both AMF and PSF were inoculated showing the synergistic interaction between the AMF and PSF (Osoria and Habte, 2001). In the meantime, AMF are also known to have antagonistic relationship with root pathogens (Soka and Ritchie, 2014) and even leaf pathogens (Parniske, 2008).
Arbuscular mycorrhizal fungi may also interact with each other synergistically. It was experimentally found out that AMF effects are greater when AMF consortia inoculums are applied than single AMF (Banerjee et al., 2013). After long years of observation, Barea et al. (2011) concluded that the use of native AMF consortia has the maximum effect. A meta-analysis on 306 studies also indicated that plant response was substantially lower when plants were inoculated with single AMF species, compared with inoculations with multiple AMF species (Hoeksema et al., 2010). This could be due to synergistic interaction between the various AMF species. Different species of AMF have different hyphal growth patterns, anastomoses and branching frequencies (Parniske, 2008). These differences probably reflect different strategies and the occupation of different niches within the soil (Parniske, 2008).
AMF and Measurable Restoration Attributes
Improved plant fitness (survival, growth and reproduction), nutrient uptake and accumulation, tolerance of adverse conditions (biotic and abiotic stresses) and altering plant community structure [competition/facilitation, diversity (richness and evenness) and succession] and that of animal communities (Direct effects on organisms which feed on fungi and indirect effects due to changes in plant fitness) were identified to be the pivotal role AMF play in ecological restoration (Brundrett and Abbott, 2002).
Based on Aronson et al. (1993), the functional and structural attributes to measure ecological restoration include; soil organic matter, soil water relation, nutrient cycling index, plant diversity and soil microbial diversity and abundance, and plant productivity. Therefore, the role of AMF in soil organic matter content, soil water relation, nutrient cycling index, plant stress tolerance, plants survival, establishment and growth on degraded soils, plant diversity, soil microbial diversity and abundance, and plant succession, competition/facilitation and productivity is highlighted below.
AMF Improve Soil Aggregation; Hence Increase Soil Organic Matter and Soil Water Relation
Fungi and most importantly AMF may be the most effective soil organisms in stabilizing soil structure (Augé, 2004). AMF hyphae grow into the soil matrix to create the skeletal structure that holds primary soil particles together to form soil aggregates (Augé, 2004; Al-Karaki, 2013). AMF also improve soil aggregation by influencing bacterial communities that can improve soil aggregate formation (Rilling, 2004). Furthermore, the dead AMF hyphae produce glomalin which is hydrophobic stable aggregate former (Barea et al., 2002; Simard and Austin, 2010). Hence, AMF increase both soil aggregation and stability. AMF may stabilize soils up to 5 months after their host’s death (Soka and Ritchie, 2014).
Meanwhile, as a result of the significant amount of mycorrhiza derived soil carbon (Rilling, 2004) and improved soil aggregation and stability, AMF increase soil organic matter content and stability (Rilling, 2004; Leifheit et al., 2014). Improved soil aggregation also increases soil water relation. It was observed that a naturally non-mycorrhizal plant planted in mycorrhizal soils tolerated drought more than the ones planted in a non-mycorrhizal soils indicating that AMF hyphae improves water holding capacity of soils (Marschner, 1995).
AMF Improve Plant Nutrition and Nutrients Cycling Index
The most important role of AMF is their role in phosphorous nutrition (Skujins and Allen, 1986). There are also data indicating that AMF can transfer nitrogen from one plant to another (e.g., Requena et al., 2001), increase the utilization of different forms of nitrogen by plants and can also take up nitrogen directly and transfer it to host roots (Govindarajulu et al., 2005). However, there is considerable doubt as to the cost-benefit of AMF in plant N nutrition (Smith and Smith, 2011). Although few data exist, AMF were observed to improve potassium nutrition in plants (Dag et al., 2009; Garcia and Zimmermann, 2014). AMF can also increase the uptake of other macro and micro nutrients by plants (Birhane et al., 2012). Generally, the external mycelium of AMF establishes an underground network that links the different plants and hence sequester carbon, nitrogen, and phosphorous and also allow the transfer of these nutrients among plants (Rodriguez-Echeverria et al., 2007). These important roles of AMF therefore play great role in nutrient cycling where the need for further nutrient inputs is significantly reduced (Gianinazzi et al., 2010; Al-Karaki, 2013).
Arbuscular mycorrhizal fungi not only improve nutrient cycling but also reduce nutrient leaching from the soil (Rodriguez-Echeverria et al., 2007). In a comprehensive assessment done by Bender et al. (2015), it was possible to determine the role AMF have in nutrient cycling and leaching. Accordingly, it was determined that while AMF inoculation increased nutrient uptake by plants it also reduced leaching of dissolved organic N and un-reactive P (Bender et al., 2015).
AMF Increase Plants’ Abiotic Stress Tolerance
It was, several times, demonstrated that AMF can increase plants’ tolerance to drought and salinity (Al-Karaki, 2013). AMF are also known to alleviate heavy metal stress in plants (Leyval et al., 1997; Hildebrandt et al., 2007; Soares and Siqueira, 2008; Amir et al., 2013). By inoculating plants with drought tolerant AMF, up to 42% reduction in plants’ water requirement could be achieved (Gianinazzi et al., 2010). Also, Navarro et al. (2013) found out that, Citrus rootstocks inoculated with AMF showed significantly increased growth than non-inoculated individuals despite the fact that inoculated individuals were irrigated with saline water and the non-inoculated ones got irrigated with non-saline water.
The mechanism by which AMF increase plants’ tolerance to drought, salinity and heavy metal stresses is mainly nutritional (Marschner, 1995; Soares and Siqueira, 2008; Birhane et al., 2012; Al-Karaki, 2013; Navarro et al., 2013). Soares and Siqueira (2008) demonstrated that both P fertilization and AMF inoculation of plants significantly improved plants’ growth on heavy metal polluted soils. Hence, they concluded, AMF increase plants’ heavy metal stress tolerance mainly through P nutrition.
The non-nutritional mechanisms by which AMF increase plants’ tolerance to drought include; hormonal changes, hyphal soil improvement (delayed soil drying), hyphal ability to scavenge water from micro-pores, increased plants’ photosynthetic rate, and accumulation of compatible osmolites (Marschner, 1995; Birhane et al., 2012; Al-Karaki, 2013). Likewise, immobilizing heavy metals in their biomass mainly cell wall, vesicles and in the glomaline is the non-nutritional mechanism by which AMF improve plants’ tolerance to heavy metals stress (Hildebrandt et al., 2007).
The positive AMF effects on plants’ drought tolerance can improve plants’ salinity tolerance as well. Better water intake by plants can effectively dilute salts within the plants’ cells (Larcher, 1995). Other non-nutritional mechanisms by which AMF improve plants’ salinity tolerance include; exclusion of salt from plant cells by accumulating the salt within the fungal hyphae, production of enzymes involved in antioxidant defense, and change in cell wall elasticity and membrane stability (Al-Karaki, 2013).
AMF Increases Plants’ Resistance and Tolerance to Pathogens and Herbivores
There are several published articles showing the role of AMF in increasing plant tolerance against biotic stressors. The meta-analysis of 144 published papers clearly reveals that (Yang et al., 2014). Considering the role AMF have in bioprotection, Gianinazzi et al. (2010) described AMF as ‘health insurance’ of plants. One mechanism by which AMF increase plants’ pathogen tolerance could be the synergistic interaction of AMF have with plant growth promoting rhizobacteria (PGPR). PGPR have a very well documented role in plant pathogen inhibition (Figueiredo et al., 2010). The fact that AMF stimulate the synthesis of plant secondary metabolites (Gianinazzi et al., 2010) may also explain why AMF inhibit herbivory. Plants’ secondary metabolites are known to have role in plants’ defense against herbivores (Larcher, 1995). The other reason by which AMF increase plants’ herbivory tolerance is compensatory growth. A microcosm investigation revealed that mycorrhizal plants did not show a reduction in total above ground biomass despite their leaves being fed by grasshoppers indicating that mycorrhiza helped the plant to compensate in growth after herbivory (Kula et al., 2005).
AMF Increase Tree/Shrub Seedlings Growth, Productivity, Field Survival and Establishment on Degraded Lands
Lekberg and Koide (2005), carried out a meta-analysis based on 290 published experiments to determine the role of AMF on plant growth and productivity. The analysis also determined the effects of three common AMF management methods; inoculation, short fallow, and reduced soil disturbance. The result of the meta-analysis revealed that AMF generally increase individual plant’s growth and productivity. Inoculation and short fallow resulted in significantly positive effects on plants’ growth and productivity (Lekberg and Koide, 2005). A recent meta-analysis on 304 papers also concluded that AMF inoculation increases the growth and productivity of plants grown alone (Lin et al., 2015). A similar result was also reported by Birhane et al. (2014). Huante et al. (2012) also did experiment on six tree species and found out that AMF inoculation has significant effect on seedlings growth and most significantly slow growing tree species. Figure 1 below shows how AMF inoculation can significantly increase tree seedlings growth.
FIGURE 1.
Acacia koa A. Gray grew significantly tall in a low-P soil when inoculated with AM fungus (adopted from Miyasaka et al., 2003).
Tree survival and field establishment is an important factor in the restoration of degraded lands. Hence, AMF are important since they can significantly improve tree seedlings field survival and establishment. Pouyu-Rojas and Siqueira (2000), Habte et al. (2001), Ouahmane et al. (2006), Dag et al. (2009), Kapulnik et al. (2010), Karthikeyan and Krishnakumar (2012), and Manaut et al. (2015) have demonstrated the positive effect AMF have in these regards. Pouyu-Rojas and Siqueira (2000) investigated the AMF effect on seven tree species seedlings survival and establishment on degraded pot soils. They found out that AMF inoculation in the nursery or during transplanting have equally significantly positive effect on trees survival and establishment. Later on Habte et al. (2001) determined the effect AMF nursery inoculation has on field establishment of Acacia koa and accordingly, AMF was shown to improve transplanted tree seedlings growth and establishment by increasing seedlings P nutrition. The role of native AMF inoculation was also demonstrated to have a significant positive effect on the field survival and establishment of Cupressus atlantica Gaussen seedlings on a degraded Moroccan field site (Ouahmane et al., 2006).
Similarly, Kapulnik et al. (2010) determined AMF nursery inoculation effect on seedlings field establishment and growth of Olea europaea L. Meanwhile, they were able to observe that AMF inoculation improved seedlings field performance significantly and most importantly for the first 2.5 years from transplanting. They also observed that AMF effect decreased with increasing seedlings age. Karthikeyan and Krishnakumar (2012) also determined AMF effect on survival and establishment of Eucalyptus tereticornis Sm. on pot soil of highly degraded origin (mine spoils). Meanwhile, they were able to observe that AMF inoculation almost doubled seedling survival and significantly increased establishment. Recently, Manaut et al. (2015) demonstrated that native AMF consortia inoculation of Ceratonia siliqua L. seedlings more than doubled seedlings’ survival and significantly improved seedlings’ height and collar diameter.
AMF Drive Succession and Influence Plant Community Structure
According to Janos (1980), the mycorrhizal fungus status and the fertility of soil influence the occurrence of plant species. It is also hypothesized that AMF are drivers and as well, passengers of plant community succession (Zobel and pik, 2014). Meanwhile, the AMF status of a site determines the composition of a seral plant community, and the composition of that particular seral plant community determines the composition of infective AMF communities which will further influence the composition of the next seral plant community (Janos, 1980; Renker et al., 2004). Thus, if specific compatible relationships between certain AMF and plant taxa are required for mutual symbiont survival, the loss of compatible AMF species or individuals may limit the distribution of a particular plant species (Renker et al., 2004). Plant-soil feedback (plant-AMF feedback) is also an important concept explaining the role AMF have in succession (Kikvidze et al., 2010). Positive feedbacks promote the development of early successional communities and negative feedbacks promote plant species replacement to drive succession (Kikvidze et al., 2010).
Arbuscular mycorrhizal fungi could also potentially influence plant community structure by affecting richness or evenness of coexisting plants (Brundrett and Abbott, 2002). Only some 240 AMF morphospecies have been described forming associations with 80% of terrestrial plants (Lee et al., 2013). This indicates that AMF have no host specificness. Meanwhile, a single mycorrhizal fungus can link different plants together, thus forming mycorrhizal networks (Simard and Austin, 2010; Song et al., 2014). These networks have been shown to facilitate regeneration of new seedlings, alter species interactions, and change the dynamics of plant communities therefore, increasing plant diversity (Simard and Austin, 2010). Sowing seeds of plant species in microcosms that resembled the grassland community of the temperate zone, on soils of AMF inoculated and non-inoculated, Van der Heijden et al. (1998), Vogelsang et al. (2006), and Schnitzer et al. (2011) were able to observe that AMF inoculation improved plant community diversity by mainly increasing plants’ fitness and evenness. AMF may also be important organisms to inhibit invasion by alien species. This could be indirectly by reducing the vacant niche through increased native plants survival, establishment and diversity or can be by direct inhibition of invasives. Janos et al. (2013) reported that the presence of established extraradical mycelium prevented the survival and establishment of seedlings migrating from another ecosystem.
AMF Increase Soil Microbial Diversity and Abundance
Arbuscular mycorrhizal fungi hyphae and root litter are the most abundant carbon source in the soil (Brundrett and Abbott, 2002). Therefore, AMF provide increased supply of energy for soil microbes to flourish. The fact that AMF influence plant communities is also considered to be one of the potential mechanisms by which AMF influence soil microbial communities (Rilling, 2004). Furthermore, AMF hyphal exudates may also stimulate microorganisms present in the mycorrhizal hyphosphere. However, the effect is variable: AMF hyphal exudates may stimulate some microorganisms but still inhibit others (Herman et al., 2012). Hence, AMF may increase the diversity and abundance of microorganisms that are beneficial to plants’ growth and health.
AMF Effects on Plant Community Primary Productivity and Plant Competition
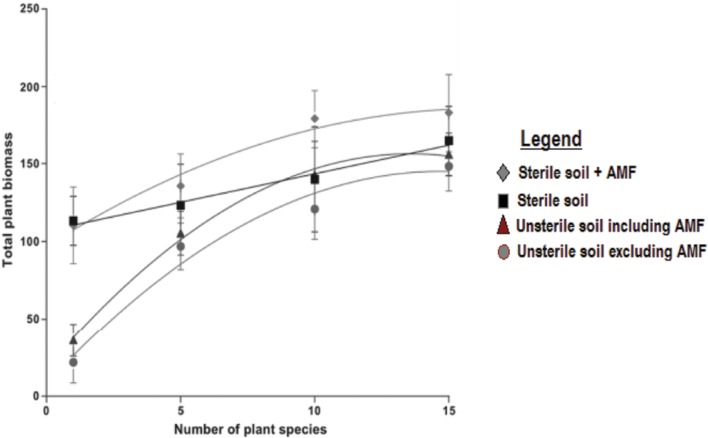
Arbuscular mycorrhizal fungi inoculation increases plant productivity at community level and the effect increases with increase in plant species richness following the common ascending but asymptotic diversity-productivity pattern (Schnitzer et al., 2011). At low plant diversity soil microbes suppress plant productivity since their pathogenic effect increases and as the plant diversity increases, plant productivity can increase up to fivefold (Figure 2; circle and triangle). In the absence of soil microbes plant productivity has a weak positive linear relationship with plant diversity (Figure 2; square).
FIGURE 2.
The role of microbes and AMF in plant diversity-productivity relationship (adopted from Schnitzer et al., 2011).
Sowing seeds of plant species that resembled the grassland community of the temperate zone on AMF inoculated and non-inoculated soils, Van der Heijden et al. (1998) and Vogelsang et al. (2006) were able to determine AMF effect on plant productivity at community level. Accordingly, AMF improved plant community productivity (Van der Heijden et al., 1998; Vogelsang et al., 2006). Furthermore, Van der Heijden et al. (1998) observed that increasing AMF richness resulted in increased productivity while Vogelsang et al. (2006) observed although AMF species richness increased productivity, the effect was not significant compared to single AMF inoculums effect. So, the former observation is Van der Heijden et al. (1999) argued, due to niche complementarity while the latter is Vogelsang et al. (2006) argued, due to sampling effect. The “niche complementarity” theory argues that the presence of many species and functional types results in more complete utilization of resources because different species specialize on different resources, resulting in higher overall productivity while the “sampling effect” theory argues species identity is more important than diversity and asserts that productivity increases with diversity solely due to an increased probability that communities with more species contain a few very productive species that disproportionately contribute to community-wide productivity (Schnitzer et al., 2011).
Despite the fact that the above research observations reveal that AMF increase plant productivity at community level, the recent meta-analysis based on 304 study results, which also cited the above research observation, found out that, at community level, AMF inoculation either has no effect on plant productivity or even has a negative effect (Lin et al., 2015). AMF inoculation increases plant productivity at community level only when experiments were conducted in the green house (Lin et al., 2015). Klironomos et al. (2000) were, in a greenhouse setting, able to determine the AMF effect on plant diversity and that of productivity. Accordingly, it was observed that AMF inoculation increased plant productivity but not for all AMF species. Inoculating Glomus intraradices N. C. Schenck and G. S. Sm. even lowered productivity compared to the non-inoculated plant community (Klironomos et al., 2000).
Similarly, variable AMF effect is observed in plant competition. Plant species competitive ability response to AMF inoculation depends on plants’ functional group, mycorrhizal status, plants’ life history (Scheublin et al., 2007; Lin et al., 2015), and also maybe below ground functional traits of the plant species (Birhane et al., 2014). The meta-analysis conducted by Lin et al. (2015) also concludes, AMF inoculation significantly increases N-fixing forbs, decreases C3 grasses and non-N-fixing forbs and woody plants, and has no effect on C4 grasses competitive ability whether these functional groups compete intra or inter-specifically (Lin et al., 2015). According to Birhane et al. (2014), in a pot experiment, AMF inoculation did not have positive effect on the competitive ability of both Acacia etbaica Schweinf. and Boswellia papyrifera Hochst. seedlings grown together. In other instances, mycorrhizal networks may result in asymmetric competition by favoring strong carbon-donor roots (Weremijewicz and Janos, 2013) or vice-versa (Walder et al., 2012).
AMF Biotechnology for the Restoration of Degraded Lands
Degraded lands have low level of infective AMF and nursery seedlings around degraded sites may less likely be infected with sufficient AMF (e.g., Michelsen, 1992). Therefore, these sites can support the growth of late successional tree species when appropriate AMF inocula are reintroduced. Late successional tree species are obligately mycotrophic and may necessarily require AMF for their survival and fitness (Janos, 1980). More importantly, at the early stages of seedlings growth, mycorrhizal early/mid successional tree/shrub species can be even more AMF dependent than the late successional ones (Kiers et al., 2000). Therefore, AMF inoculation could potentially be considered as an important biotechnological tool in degraded lands restoration.
Arbuscular mycorrhizal fungi show no host specificity to forge symbiotic relationship with plants and are very ubiquitous, found almost in every soil (Abbott and Robson, 1991; Brundrett and Abbott, 2002; Barea et al., 2011; Al-Karaki, 2013). Hence, many researchers argue that AMF inoculation is likely to be valuable in only few conditions such as mine fields where indigenous AMF inoculum is surely little or none available (Brundrett and Abbott, 2002). Koide and Mosse (2004) suggested instead of going for AMF inoculation it would be quite economical and appropriate to focus on managing the indigenous AMF population of a site. According to Renker et al. (2004), inoculation is an important but the last option. However, contrary to having several dispersal agents such as; wind, water, rodents, birds, worms, and ants (Brundrett and Abbott, 2002), AMF were observed to have poor dispersal. Accordingly, Hailemariam et al. (2013) were able to observe that within a single piece of farm land, soil AMF status and infectiveness can vary in short distances indicating poor dispersal. Similarly, Friese and Allen (1991), also indicated that AMF have poor dispersal. Therefore, to overcome the dispersal limitation of AMF, inoculation may be a worthily intervention.
Meanwhile, AMF inoculation has proved to be effective under wide range of soil conditions (Janos, 1980; Brundrett and Abbott, 2002) including on soils with good AMF abundance (e.g., Banerjee et al., 2013). Positive AMF effect is not ensured by the presence of abundant indigenous AMF but by both abundance (quantity) and efficiency (quality) of indigenous fungal populations (Onguene and Kuyper, 2005). Veiga et al. (2011) also demonstrated that AMF inoculation suppressed weeds and, interestingly enough, hypothesized that AMF inoculation could suppress ruderal plants which are known to invade degraded sites (Veiga et al., 2011). This is particularly important in ecological restoration since ruderal plants could invade degraded lands and compete with tree/shrub seedlings planted.
If the importance of AMF inoculation in the restoration of degraded lands is agreed, the next question to ask will be; what kind of inocula should be prepared? AMF show wide range of functional diversity (Johnson et al., 1997; Klironomos, 2003; Smith et al., 2011) and their effect is within the mutualism-parasitism continuum (Johnson et al., 1997). Likewise, Hoeksema et al. (2010) summarized that certain plants functional groups viz. non-N-fixing forbs and woody plants and C4 grasses show more positive responses to AMF inoculation. Klironomos (2003) also demonstrated that exotic-native AMF strain-host or vice-versa combination results in highly parasitic interaction. Therefore, deciding on the type of inoculum to prepare is a very important step. Based on the currently available data, the use of native inocula should be preferred to the use of exotic inocula. Early seral AMF should be used when seedlings are inoculated for restoration, even for late seral tree species (Allen et al., 2003). Late seral AMF have big spores and demand much carbon and hence, seedlings may not benefit from them. Instead, seedlings benefit from early successional AMF which are usually having small spores and smaller carbon demand (Allen et al., 2003). Likewise, the use of inocula from grasslands is promoted. AMF abundance in grasslands can be more than tenfold than that of in the forestlands and AMF from grasslands do have significantly high inoculation effect (Fischer et al., 1994). That was why Onguene and Kuyper (2005) applied fresh grassland whole-soil inoculum on various soils and three tree species seedlings. According to the result Onguene and Kuyper (2005) obtained, although early successional grassland inoculum had positive effect for most of the cases (80%), the fact that it is an inoculum from grassland resulted in significantly negative effect on Terminalia superba Engl. and Diels seedlings grown on agricultural and early successional forest soils. Hence, Onguene and Kuyper (2005) concluded; allochthonous AM inocula may not be always effective. Hence, the use of planting site adapted AM inocula may be recommended. The other reason for the observed negative effect may also be related to host plant’s fungi preference (Onguene and Kuyper, 2005). There are data to demonstrate that inocula from conspecific source show better affinity to the host plants’ root (e.g., Kiers et al., 2000). Similarly, there are data to show that plant species even that do co-occur may prefer to associate with distinct AM fungi communities (e.g., Wubet et al., 2006; Davison et al., 2011). There are also data to show that distinctively different AMF communities colonize seedlings’ and adults’ roots of a single tree species (e.g., Wubet et al., 2009). Therefore, one has to ask; does inoculating seedlings with AM inocula from seedlings’ rhizosphere or adults’ deliver better positive effect? Kiers et al. (2000) have found out that although conspecific inocula from adults had better affinity to inoculated seedlings, the effect on their growth was mostly relatively small showing that, inocula even from conspecific adults, may not be suited for seedlings inoculation.
Selecting few of the dominant planting site adapted AMF species, multiplying them and applying as inocula may not be also a very good idea specially when there are established AMF in the planting site. Increasing the density of few of the dominant AMF species and applying as inocula had resulted in negative effects on plant growth by disrupting indigenous AMF community structure and thereby creating competition among AMF to ultimately result in inoculum failure (Janoušková et al., 2013). Therefore, in areas with low levels of indigenous AMF abundance, multiplying all not only the dominant AMF species and applying all may be the best option.
The AMF richness in AM inocula is considered to improve inocula effectiveness. Plant response is substantially lower when inoculated with single AMF species and the response keeps increasing from multiple fungal species to whole-soil inoculums (Hoeksema et al., 2010). Likewise, Barea et al. (2011) compiling long years of experience in AMF research recommend the use of autochthonous foundation shrub inoculated with autochthonous AMF consortia inoculums to best restore degraded lands of the Mediterranean. The shrub not only acts as a foundation species but also serves as a resource island for AMF (Barea et al., 2011). However, not all ecologists agree by the application of AMF species rich inocula; some argue that better results due to inocula with better AMF species richness is due to sampling effect and selecting single effective AMF species should get the attention of restoration ecologists. Sampling effect is discussed earlier.
The other challenge associated with AMF biotechnology is related with inocula production for large-scale application. This is due mainly to the obligate nature of AMF. Meanwhile, AMF cannot be cultured axenically (Azcón-Aguilar et al., 1999; Fortin et al., 2005) and host plant based AMF multiplication is mandatory. These host plant based conventional inocula production methods (substrate based pot culturing and substrate free methods of hydroponics and aeroponics techniques) are costly and large scale production of AMF inocula may hardly be possible. Effective monexenic in vitro culturing of AMF has been made possible few decades ago (Bécard and Fortin, 1988) and in India, using this method, large-scale industrial production of biologically clean AMF inocula was possible (Adholeya et al., 2005). Readers are directed to read Adholeya et al. (2005) and Cranenbrouck et al. (2005) to grasp the potential and the technique of monoxenic in vitro AMF culture production for large-scale application. Readers are also directed to read Azcón-Aguilar et al. (1999) to get proper definitions of axenic and monoxenic cultures.
However, until now, monexenic in vitro culturing is not widely practiced. This is due mainly to the fact that; (1) undesired contamination is hardly avoidable and the technique is technology and skill demanding (Bago and Cano, 2005), (2) there are ethical and legal concerns, and (3) it is rather very hard to identify each genotype (even morphotype) hence, most if not all, AMF are not readily culturable (Fortin et al., 2005). AMF momoxenic in vitro culturing uses transformed [using Rhizobium rhizogenes (Riker et al.) Young et al.] hairy roots as host owing to the fact that these hairy roots are better suited than the non-transformed hairy roots since they grow on hormone free media and without developing shoots and leaves (Puri and Adholeya, 2013). Meanwhile, AMF monoxenic culture as it is practiced now could potentially be challenged with biosafety related issues.
Due to the lack of cheap and easy AMF inocula production for large scale application, managing the in situ AMF is sometimes considered to be an effective AMF biotechnology for the restoration of degraded lands. The meta-analysis by Lekberg and Koide (2005) showed that short fallow could be as good as inoculation to improve plants growth and productivity. It was shown that an obligately arbuscular mycorrhizal pioneer nurse shrub Lavandula stoechas L. improved the field survival and establishment of Cupressus atlantica Gaussen seedlings by increasing, among others, in situ infective AMF abundance (Duponnois et al., 2011). Kumar et al. (2010) also compared different plant composition effects on in situ management of AMF on a degraded coal mine spoil. Accordingly, they demonstrated that using cover crops mainly grasses and N-fixing shrubs in the plant composition, significantly enhanced AMF abundance, diversity and infectiveness. Hence, AMF can be manipulated by fallowing or/and by designing the plant species composition to ultimately result in increased AMF abundance which intern facilitates restoration. However, some investigations indicated that grass cover can significantly suppress individual tree/shrub seedlings-saplings growth (Riginos, 2009) or may have variable seasonal effects (Good et al., 2014). Therefore, investigation on cover plant management options to effectively manage AMF and facilitate tree/shrub seedlings growth can be an important research topic.
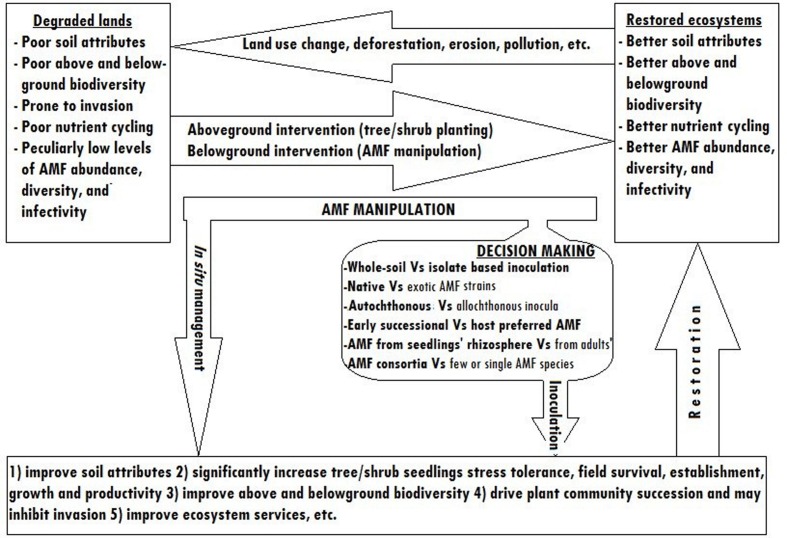
Nowadays, substrate free inocula preparation methods and in vitro production on excised plant roots are being intensively researched to make AMF inoculation less costly (Ijdo et al., 2011). The pot culture inocula preparation method, although it is labor intensive and costly, can be a source of employment especially in developing countries. Therefore, pot culture based AMF biotechnology will remain to be a feasible way of degraded lands restoration in most parts of the world. Figure 3 shows the simplified schematic model of degraded lands restoration using AMF.
FIGURE 3.
Simplified schematic model showing degraded lands restoration through AMF manipulation.
Conclusion
This review paper has compiled facts regarding the AMF role in the above and belowground ecosystem processes relevant to ecological restoration. Accordingly, it is possible to conclude that AMF; have a well documented positive role in nutrient cycling and improved soil attributes. AMF also improve plants’ tolerance to biotic and abiotic stresses, and significantly increase tree/shrub seedlings survival, establishment and growth. AMF play pivotal role in plant community succession and may directly or indirectly prevent invasion by alien plant species. At plant community level, AMF increase both above and below ground biodiversity but their effect on primary productivity maybe low. The AMF effect on plant competition is also variable and mostly negative. Available data as of yet, indicate that there are very few outfield experiments done on AMF effects on tree/shrubs seedlings survival and establishment. This review was not also able to clearly trace a research result showing the AMF effect on the competitive ability of tree seedlings planted with annual and perennial grass and/or herbaceous weeds. Based on the currently available data, however, it can be concluded that AMF inoculation can significantly increase the success of degraded lands restoration and for better results reducing competitors and seedlings density (increased seedling spacing) is recommended.
Based on the data reviewed in this article, we recommend for future AMF effect researches to give emphasis to outfield experiments. The AMF effect on the competitive ability of tree seedlings compared with annual and perennial herbaceous weeds should be investigated. Data reviewed here showed that almost all research observations conducted on AMF effect at community level are on microcosms of grasslands; and mainly temperate grasslands. Future researches should focus on forest communities of both the temperate and tropics. For an effective large scale application of AMF inocula biotechnology, pot based inocula multiplication will remain to be significantly cost ineffective. Therefore, investigating and researching on cost effective multiplication methods of substrate free and in vitro culture and/or optimization of the effects of low-cost fresh AMF inoculation techniques like using grassland top soil or managing AMF in situ using several cover crops including grasses need further attention in the future. Optimization of monoxenic in vitro AMF culture products and using non-transformed hairy root organ could also be an important research area until axenic in vitro AMF culturing is ultimately made possible.
Author Contributions
FA did all the data gathering and write-up. TB and EB considerably contributed intellectually by providing comments and guidance at every milestone of the manuscript development. All authors approved publication of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Marc Ducousso and Dr. Laila Pamela Partida-Martinez for their valuable and enriching comments. Reviewers of the first draft are also acknowledged.
Footnotes
References
- Abbott L. K., Robson A. D. (1991). Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 35 121–150. 10.1016/0167-8809(91)90048-3 [DOI] [Google Scholar]
- Adholeya A., Tiwari P., Singh R. (2005). “Large scale inoculum production of arbuscular mycorrhizal fungi on root organs and inoculation strategies,” in Soil Biology In Vitro Culture of Mycorrhizae Vol. 4 eds Declerck S., Strullu D. G., Fortin A. (Berlin: Springer-Verlag; ), 315–338. [Google Scholar]
- Aerts R., Honnay O. (2011). Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 11:29 10.1186/1472-6785-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander I., Ahmad N., See L. S. (1992). The role of mycorrhizas in the regeneration of some Malaysian forest trees. Philos. Trans. Biol. Sci. 335 379–388. 10.1098/rstb.1992.0029 [DOI] [Google Scholar]
- Al-Karaki G. N. (2013). “The role of mycorrhiza in the reclamation of degraded lands in arid environments,” in Developments in Soil Classification, Land Use Planning and Policy Implications: Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources, eds Shahid S. A., Taha F. K., Abdelfattah M. A. (Dordrecht: Springer Science+Business Media; ), 823–836. [Google Scholar]
- Allen E. B., Allen M. F., Egerton-Warburton L., Corkidi L., Gomez-Pompa A. (2003). Impacts of early and late-seral mycorrhizae during restoration in seasonal tropical forest, Mexico. Ecol. Appl. 13 1701–1717. 10.1890/02-5309 [DOI] [Google Scholar]
- Amir H., Lagrange A., Hassaïne N., Cavaloc Y. (2013). Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23 585–595. 10.1007/s00572-013-0499-6 [DOI] [PubMed] [Google Scholar]
- Angelini C., Altieri A. H., Silliman B. R., Bertness M. D. (2011). Interactions among foundation species and their consequences for community brganization, biodiversity, and conservation. BioScience 61 782–789. 10.1525/bio.2011.61.10.8 [DOI] [Google Scholar]
- Antunes P. M., Schneider K., Hillis D., Klironomos J. N. (2007). Can the arbuscular mycorrhizal fungus Glomus intraradices actively mobilize P from rock phosphates? Pedobiologia 51 281–286. 10.1016/j.pedobi.2007.04.007 [DOI] [Google Scholar]
- Aradottir A. L., Hagen D. (2013). Ecological restoration: approaches and impacts on vegetation, soils and society. Advan. Agron. 120 173–222. 10.1016/b978-0-12-407686-0.00003-8 [DOI] [Google Scholar]
- Aronson J., Fled C., Le Floc’h E., Ode C., Pontanier R. (1993). Restoration and rehabilitation of degraded ecosystems in arid and semi-arid lands: a view from the south. Restor. Ecol. 1 8–17. 10.1111/j.1526-100X.1993.tb00004.x [DOI] [Google Scholar]
- Augé R. M. (2004). Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 84 373–381. 10.4141/S04-002 [DOI] [Google Scholar]
- Azcón-Aguilar C., Bago B., Barea J. M. (1999). “Saprophytic growth of arbuscular mycorrhizal fungi,” in Mycorrhiza: Structure, Function, Molecular Biology and Biotechnology, eds Varma A., Hock B. (New York, NY: Springer; ), 391–407. [Google Scholar]
- Bago B., Cano C. (2005). “,” in Soil Biology In Vitro Culture of Mycorrhizae Vol. 4 eds Declerck S., Strullu D. G., Fortin A. (Berlin: Springer-Verlag; ), 111–138. [Google Scholar]
- Bai Z. G., Dent D. L., Olsson L., Schaepman M. E. (2008). Soil Use Manag. 24 223–234. 10.1111/j.1475-2743.2008.00169.x [DOI] [Google Scholar]
- Banerjee K., Gadani M. H., Srivastava K. K., Verma N., Jasrai Y. T., Jain N. K. (2013). Screening of efficient arbuscular mycorrhizal fungi for Azadirachta indica under nursery condition: a step towards afforestation of semi-arid region of Western India. Braz. J. Microbiol. 44 587–593. 10.1590/S1517-83822013005000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea J. M., Azcon R., Azcón-Aguilar C. (2002). Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek 81 343–351. 10.1023/A:1020588701325 [DOI] [PubMed] [Google Scholar]
- Barea J. M., Palenzuela J., Cornejo P., Sánchez-Castro I., Navarro-Fernández C., Lopéz- García A., et al. (2011). Ecological and functional roles of mycorrhizas in semi-arid ecosystems of Southeast Spain. J. Arid Environ. 75 1292–1301. 10.1016/j.jaridenv.2011.06.001 [DOI] [Google Scholar]
- Bécard G., Fortin J. A. (1988). Early events of vesicular-arbuscular mycorrhiza formation in RiT-DNA transformed roots. New Phytol. 108 211–218. 10.1111/j.1469-8137.1988.tb03698.x [DOI] [PubMed] [Google Scholar]
- Bender S. F., Conen F., Van der Heijden M. G. A. (2015). Mycorrhizal effects on nutrient cycling, nutrient leaching and N2O production in experimental grassland. Soil Biol. Biochem. 80 283–292. 10.1016/j.soilbio.2014.10.016 [DOI] [Google Scholar]
- Bever J. D., Richardson S. C., Lawrence B. M., Holmes J., Watson M. (2009). Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 12 13–21. 10.1111/j.1461-0248.2008.01254.x [DOI] [PubMed] [Google Scholar]
- Birhane E., Kuyper T. W., Sterck F. J., Bongers F. (2010). Arbuscular mycorrhizal associations in Boswellia papyrifera (frankincense-tree) dominated dry deciduous woodlands of Northern Ethiopia. For. Ecol. Manag. 260 2160–2169. 10.1016/j.foreco.2010.09.010 [DOI] [Google Scholar]
- Birhane E., Kuyper T. W., Sterck F. J., Gebrehiwot K., Bongers F. (2015). Arbuscular mycorrhiza and water and nutrient supply differently impact seedling performance of acquisitive and conservative dry woodland species. Plant Ecol. Divr. 8 1–13. 10.1080/17550874.2014.992488 [DOI] [Google Scholar]
- Birhane E., Sterck F. J., Bongers F., Kuyper T. W. (2014). Arbuscular mycorrhizal impacts on competitive interactions between Acacia etbaica and Boswellia papyrifera seedlings under drought stress. J. Plant Ecol. 1 298–308. 10.1093/jpe/rtt031 [DOI] [Google Scholar]
- Birhane E., Sterck F. J., Fetene M., Bongers F., Kuyper T. W. (2012). Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 169 895–904. 10.1007/s00442-012-2258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett M. C. (2009). Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320 37–77. 10.1007/s11104-008-9877-9 [DOI] [Google Scholar]
- Brundrett M. C., Abbott L. K. (2002). “Arbuscula mycorrhiza in plant communities,” in Plant Conservation and Biodiversity, eds Sivasithamparam K., Dixon K. W., Barrett R. L. (Dordrecht: Kluwer Academic Publishers; ), 151–193. [Google Scholar]
- Brundrett M. C., Bougher N., Dell B., Grove T., Malajczuk N. (1996). Working with Mycorrhizas in Forestry and Agriculture, ACIAR Monograph 32. Canberra: Australian Centre for International Agricultural Research. [Google Scholar]
- Cardozo-Junior F. M., Carneiro R. F. V., Goto B. T., Bezerra A. A. C., Araújo A. S. F., Nunes L. A. P. L. (2012). Arbuscular mycorrhizal fungi in degraded lands in Northeast Brazil. Afr. J. Microbiol. Res. 6 7198–7205. 10.5897/AJMR12.1132 [DOI] [Google Scholar]
- CBD (2010). Aichi Biodiversity Targets. Available at: http://www.cbd.int/sp/targets/ [Google Scholar]
- Chagnon P.-L., Bradley R. L., Maherali H., Klironomos J. N. (2013). A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 18 484–491. 10.1016/j.tplants.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Choi Y. D. (2004). Theories for ecological restoration in changing environment: toward ‘futuristic’ restoration. Ecol. Res. 19 75–81. 10.1111/j.1440-1703.2003.00594.x [DOI] [Google Scholar]
- Cortines E., Valcarcel R. (2009). Influence of pioneer-species combinations on restoration of disturbed ecosystems in the Atlantic Forest, Rio de Janeiro, Brazil. Rev. Arvore 33 927–936. 10.1590/S0100-67622009000500015 [DOI] [Google Scholar]
- Cranenbrouck S., Voets L., Bivort C., Renard L., Strullu D.-G., Declerck S. (2005). “Methodologies for in vitro cultivation of arbuscular mycorrhizal fungi with root organs,” in Soil Biology In Vitro Culture of Mycorrhizae Vol. 4 eds Declerck S., Strullu D. G., Fortin A. (Berlin: Springer-Verlag; ), 341–375. [Google Scholar]
- Dag A., Yermiyahu U., Ben-Gal A., Zipori I., Kapulnik Y. (2009). Nursery and post-transplant field response of olive trees to arbuscular mycorrhizal fungi in an arid region. Crop Pasture Sci. 60 427–433. 10.1071/CP08143 [DOI] [Google Scholar]
- Davison J., Öpik M., Daniell T. J., Moora M., Zobel M. (2011). Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microb. Ecol. 78 103–115. 10.1111/j.1574-6941.2011.01103.x [DOI] [PubMed] [Google Scholar]
- Duponnois R., Ouahmane L., Kane A., Thioulouse J., Hafidi M., Boumezzough A., et al. (2011). Nurse shrubs increased the early growth of Cupressus seedlings by enhancing belowground mutualism and soil microbial activity. Soil Biol. Biochem. 43 2160–2168. 10.1016/j.soilbio.2011.06.020 [DOI] [Google Scholar]
- Egler F. E. (1954). Vegetation science concepts. I. Initial floristic composition, a factor in old-field vegetation development. Vegetatio 4 412–417. 10.1007/BF00275587 [DOI] [Google Scholar]
- Figueiredo M. D. B., Seldin L., de Araujo F. F., Mariano R. D. R. (2010). “Plant growth promoting rhizobacteria: fundamentals and applications,” in Plant Growth and Health Promoting Bacteria. Microbiology Monographs, ed. Maheshwari D. K. (Berlin: Springer-Verlag; ), 21–43. [Google Scholar]
- File A. L., Klironomos J. N., Maherali H., Dudley S. A. (2012). Plant kin recognition enhances abundance of symbiotic microbial partner. PLoS ONE 7:e45648 10.1371/journal.pone.0045648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay R. D. (2008). Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J. Exp. Bot. 59 1115–1126. 10.1093/jxb/ern059 [DOI] [PubMed] [Google Scholar]
- Fischer C. R., Janos D. P., Perry D. A., Liberman R. G., Sollins P. (1994). Mycorrhiza inoculum potentials in tropical secondary succession. Biotropica 26 369–377. 10.2307/2389230 [DOI] [Google Scholar]
- Fortin J. A., Declerck S., Strullu D.-G. (2005). “In vitro culture of mycorrhizas,” in Soil Biology In Vitro Culture of Mycorrhizae Vol. 4 eds Declerck S., Strullu D. G., Fortin A. (Berlin: Springer-Verlag; ), 3–14. [Google Scholar]
- Friese C. F., Allen M. F. (1991). The spread of VA Mycorrhizal fungal hyphae in the soil: inoculum types and external hyphae architecture. Mycologia 83 409–418. 10.2307/3760351 [DOI] [Google Scholar]
- Garcia K., Zimmermann S. D. (2014). The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 5:337 10.3389/fpls.2014.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S., Gollotte A., Binet M.-N., van Tuinen D., Redecker D., Wipf D. (2010). Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20 519–530. 10.1007/s00572-010-0333-3 [DOI] [PubMed] [Google Scholar]
- Gleason H. A. (1927). Further views on the succession-concept. Ecology 8 229–326. 10.2307/1929332 [DOI] [Google Scholar]
- Gómez-Aparicio L. (2009). The role of plant interactions in the restoration of degraded ecosystems: a meta-analysis across life-forms and ecosystems. J. Ecol. 97 1202–1214. 10.1111/j.1365-2745.2009.01573.x [DOI] [Google Scholar]
- Good M. K., Clarke P. J., Price J. N., Reid N. (2014). Seasonality and facilitation drive tree establishment in a semi-arid floodplain savanna. Oecologia 175 261–271. 10.1007/s00442-014-2886-x [DOI] [PubMed] [Google Scholar]
- Gotzenberger L., de Bello F., Brathen K. A., Davison J., Dubuis A., Guisan A., et al. (2011). Ecological assembly rules in plant communities-approaches, patterns and prospects. Biol. Rev. 87 111–127. 10.1111/j.1469-185X.2011.00187.x [DOI] [PubMed] [Google Scholar]
- Govindarajulu M., Pfeffer P. E., Jin H., Abubaker J., Douds D. D., Allen J. W., et al. (2005). Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nat. Lett. 435 819–823. 10.1038/nature03610 [DOI] [PubMed] [Google Scholar]
- Gutjahr C. (2014). Phytohormone signaling in arbuscular mycorrhiza development. Curr. Opin. Plant Biol. 20 26–34. 10.1016/j.pbi.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Habte M. (1989). Impact of simulated erosion on the abundance and activity of indigenous vesicular-arbuscular mycorrhizal endophytes in an Oxisol. Biol. Fer. Soils 7 164–167. 10.1007/BF00292576 [DOI] [Google Scholar]
- Habte M., Miyasaka S. C., Matsuyama D. T. (2001). “Arbuscular mycorrhiza fungi improve early forest-tree establishment,” in Plant Nutrition: Food Security and Sustainability of Agroecosystems Through Basic and Applied Research, eds Horst W. J., Schenk M. K., Bürkert A., Claassen N., Flessa H., Frommer W. B., et al. (Dordrecht: Kluwer Academic Publishers; ), 644–645. [Google Scholar]
- Hailemariam M., Birhane E., Asfaw Z., Zewdie S. (2013). Arbuscular mycorrhizal association of indigenous agroforestry tree species and their infective potential with maize in the rift valley, Ethiopia. Agrofor. Sys. 87 1–14. 10.1007/s10457-013-9634-9 [DOI] [Google Scholar]
- Harris J. A., Hobbs R. J., Higgs E., Aronson J. (2006). Ecological restoration and global climate change. Restor. Ecol. 14 170–176. 10.1111/j.1526-100X.2006.00136.x [DOI] [Google Scholar]
- Hartnett D. C., Wilson G. W. T. (1999). Mycorrhiza influence plant community structure and diversity in tall grass Prairie. Ecology 80 1187–1195. 10.1890/0012-9658(1999)080[1187:MIPCSA]2.0.CO;2 [DOI] [Google Scholar]
- Heneghan L., Miller S. P., Baer S., Callaham M. A., Montgomery J., Pavao-Zuckerman M., et al. (2008). Integrating soil ecological knowledge into restoration management. Restor. Ecol. 16 608–617. 10.1111/j.1526-100X.2008.00477.x [DOI] [Google Scholar]
- Herman D. J., Firestone M. K., Nuccio E., Hodge A. (2012). Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 80 236–247. 10.1111/j.1574-6941.2011.01292.x [DOI] [PubMed] [Google Scholar]
- Higgs E., Falk D. A., Guerrini A., Hall M., Harris J., Hobbs R. H., et al. (2014). The changing role of history in restoration ecology. Front. Ecol. Environ. 12:499–506. 10.1890/110267 [DOI] [Google Scholar]
- Hildebrandt U., Regvar M., Bothe H. (2007). Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 68 139–146. 10.1016/j.phytochem.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Hobbs R. J., Jentsch A., Temperton V. M. (2007). “Restoration as a process of assembly and succession mediated by disturbance,” in Linking Restoration and Ecological Succession, eds Walker L. R., Walker J., Hobbs R. J. (New York, NY: Springer; ), 150–167. [Google Scholar]
- Hoeksema J. D., Chaudhary V. B., Gehring C. A., Johnson N. C., Karst J., Koide R. T., et al. (2010). A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13 267–407. 10.1111/j.1461-0248.2009.01430.x [DOI] [PubMed] [Google Scholar]
- Holl K. D., Zahawi R. A., Cole R. J., Ostertag R., Cordell S. (2010). Planting seedlings in tree islands versus plantations as a large-scale tropical forest restoration strategy. Restor. Ecol. 19 470–479. 10.1111/j.1526-100X.2010.00674.x [DOI] [Google Scholar]
- Hrynkiewicz K., Baum C. (2011). “The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils,” in Environmental Protection Strategies for Sustainable Development. Strategies for Sustainability, eds Malik A., Grohmann E. (New York, NY: Springer Science+Business; ), 35–64. [Google Scholar]
- Huante P., Ceccon E., Orozco-Segovia A., Sánchez-Coronado M. E., Acosta I., Rincón E. (2012). The role of arbuscular mycorrhizal fungi on the early stage restoration of seasonally dry tropical rain forest in Chamela, Mexico. Rev. Árvore Viçosa-MG 36 279–289. 10.1590/S0100-67622012000200009 [DOI] [Google Scholar]
- Ijdo M., Cranenbrouck S., Declerck S. (2011). Methods for large-scale production of AM fungi: past, present, and future. Mycorrhiza 21 1–16. 10.1007/s00572-010-0337-z [DOI] [PubMed] [Google Scholar]
- Jacobs D. F., Oliet J. A., Aronson J., Bolte A., Bullock J. M., Donoso P. J., et al. (2015). Restoring forests: what constitutes success in the twenty-first century? New For. 46 601–614. 10.1007/s11056-015-9513-5 [DOI] [Google Scholar]
- Janos D. P. (1980). Mycorrhizae influence tropical succession. Biotropica 12 56–64. 10.1007/s00572-012-0464-9 [DOI] [Google Scholar]
- Janos D. P., Scott J., Aristiza’bal C., Bowman D. M. J. S. (2013). Arbuscular-mycorrhizal networks inhibit Eucalyptus tetrodonta seedlings in rain forest soil microcosms. PLoS ONE 8:e57716 10.1371/journal.pone.0057716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoušková M., Krak K., Wagg C., Štorchová H., Caklová P., Vosátkaa M. (2013). Effects of inoculum additions in the presence of a preestablished arbuscular mycorrhizal fungal community. Appl. Environ. Microbiol. 79 6507–6515. 10.1128/AEM.02135-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper D. A., Abbott L. K., Robson A. D. (1989). Hyphae of a vesicular-arbuscular mycorrhizal fungus maintain infectivity in dry soil, except when the soil is disturbed. New Phytol. 112 101–107. 10.1111/j.1469-8137.1989.tb00314.x [DOI] [Google Scholar]
- Jeffries P., Craven-Griffiths A., Barea J. M., Levy Y., Dodd J. C. (2002). “Application of arbuscular mycorrhizal fungi in the revegetation of desertified Mediterranean ecosystems,” in Mycorrhizal Technology in Agriculture, eds Gianinazzi S., Schuepp H., Barea J. M., Haselwandter K. (Basel: Birkhauser verlag; ), 151–174. [Google Scholar]
- Johnson N. C., Graham J. H., Smith F. A. (1997). Functioning of mycorrhizal associations along the mutualism parasitism continuum. New Phytol. 135 575–586. 10.1046/j.1469-8137.1997.00729.x [DOI] [Google Scholar]
- Kapulnik Y., Tsror L., Zipori I., Hazanovsky M., Wininger S., Dag A. (2010). Effect of AMF application on growth, productivity and susceptibility to Verticillium wilt of olives grown under desert conditions. Symbiosis 52 103–111. 10.1007/s13199-010-0085-z [DOI] [Google Scholar]
- Karthikeyan A., Krishnakumar N. (2012). Reforestation of bauxite mine spoils with Eucalyptus tereticornis Sm. seedlings inoculated with arbuscular mycorrhizal fungi. Ann. For. Res. 55 207–216. [Google Scholar]
- Kiers E. T., Lovelock C. E., Krueger E. L., Herre E. A. (2000). Differential effects of tropical arbuscular mycorrhizal fungal inocula on root colonization and tree seedling growth: implications for tropical forest diversity. Ecol. Lett. 3 106–113. 10.1046/j.1461-0248.2000.00126.x [DOI] [Google Scholar]
- Kikvidze Z., Armas C., Fukuda K., Martínez-García L. B., Miyata M., Oda-Tanaka A., et al. (2010). The role of arbuscular mycorrhizae in primary succession: differences and similarities across habitats. Web Ecol. 10 50–57. 10.5194/we-10-50-2010 [DOI] [Google Scholar]
- Kikvidze Z., Brooker R. W., Butterfield B. J., Callaway R. M., Cavieres L. A., Cook B. J., et al. (2015). The effects of foundation species on community assembly: a global study on alpine cushion plant communities. Ecology 96 2064–2069. 10.1890/14-2443.1 [DOI] [PubMed] [Google Scholar]
- Klironomos J. N. (2003). Variation in plant response to native and exotic mycorrhizal fungi. Ecology 84 2292–2301. 10.1890/02-0413 [DOI] [Google Scholar]
- Klironomos J. N., McCune J., Hart M., Neville J. (2000). The influence of arbuscular mycorrhiza on the relationship between plant diversity and productivity. Ecol. Lett. 3 137–141. 10.1046/j.1461-0248.2000.00131.x [DOI] [Google Scholar]
- Koide R. T., Mosse B. (2004). A history of research on arbuscular mycorrhiza. Mycorrhiza 14 145–163. 10.1007/s00572-004-0307-4 [DOI] [PubMed] [Google Scholar]
- Kula A. A. R., Hartnett D. C., Wilson G. W. T. (2005). Effects of mycorrhizal symbiosis on tall grass prairie plant–herbivore interactions. Ecol. Lett. 8 61–69. 10.1111/j.1461-0248.2004.00690.x [DOI] [Google Scholar]
- Kumar A., Raghuwanshi R., Upadhyay R. S. (2010). Arbuscular mycorrhizal technology in reclamation and revegetation of coal mine spoils under various revegetation models. Engineering 2 683–689. 10.4236/eng.2010.29088 [DOI] [Google Scholar]
- Lamb D., Erskine P. D., Parrotta J. D. (2005). Restoration of degraded tropical forest landscapes. Science 310 1628–1632. 10.1126/science.1111773 [DOI] [PubMed] [Google Scholar]
- Larcher W. (1995). Physiological Plant Ecology. Berlin: Springer-Verlag. [Google Scholar]
- Lee E.-H., Eo J.-K., Ka K.-H., Eom A.-H. (2013). Diversity of arbuscular mycorrhizal fungi and their roles in ecosystems. Mycobiology 41 121–125. 10.5941/MYCO.2013.41.3.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifheit E. F., Verbruggen E., Rillig M. C. (2014). Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol. Biochem. 81 323–328. 10.1016/j.soilbio.2014.12.003 [DOI] [Google Scholar]
- Lekberg Y., Koide R. T. (2005). Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 168 189–204. 10.1111/j.1469-8137.2005.01490.x [DOI] [PubMed] [Google Scholar]
- Lerat S., Lapointe L., Gutjahr S., Piche Y., Vierheilig H. (2003). Carbon partitioning in a split-root system of arbuscular mycorrhizal plants is fungal and plant species dependent. New Phytol. 157 589–595. 10.1046/j.1469-8137.2003.00691.x [DOI] [PubMed] [Google Scholar]
- Leyval C., Turnau K., Haselwandter K. (1997). Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza 7 139–153. 10.1007/s005720050174 [DOI] [Google Scholar]
- Lin G., McCormack M. L., Guo D. (2015). Arbuscular mycorrhizal fungal effects on plant competition and community structure. J. Ecol. 103 1224–1232. 10.1111/1365-2745.12429 [DOI] [Google Scholar]
- Mack R. N., Simberloff D., Lonsdale W. M., Evans H., Clout M., Bazzaz F. A. (2000). Biotic invasions: causes, epidemiology, global consequences and control. Ecol. Appl. 10 689–710. 10.2307/2641039 [DOI] [Google Scholar]
- Maherali H., Klironomos J. N. (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316 1746–1748. 10.1126/science.1143082 [DOI] [PubMed] [Google Scholar]
- Manaut N., Sanguin H., Ouahmane L., Bressan M., Thioulouse J., Baudoin E., et al. (2015). Potentialities of ecological engineering strategy based on native arbuscular mycorrhizal community for improving afforestation programs with carob trees in degraded environments. Ecol. Eng. 79 113–119. 10.1016/j.ecoleng.2015.03.007 [DOI] [Google Scholar]
- Marschner H. (1995). Mineral Nutrition of Higher Plants, 2nd Edn London: Academic Press. [Google Scholar]
- Michelsen A. (1992). Mycorrhiza and root nodulation in tree seedlings from five nurseries in Ethiopia and Somalia. For. Ecol. Manag. 48 335–344. 10.1016/0378-1127(92)90154-2 [DOI] [Google Scholar]
- Miyasaka S. C., Habte M., Friday J. B., Johnson E. V. (2003). Manual on Arbuscular Mycorrhizal Fungus Production and Inoculation Techniques. Honolulu: CTAHR, University of Hawaii. [Google Scholar]
- Navarro J. M., Perez-Tornero O., Morte A. (2013). Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the rootstock salt tolerance. J. Plant Physiol. 171 76–85. 10.1016/j.jplph.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Odum E. P. (1969). The strategy of ecosystem development. Science 164 262–270. 10.1126/science.164.3877.262 [DOI] [PubMed] [Google Scholar]
- Onguene N. A., Kuyper T. W. (2005). Growth response of three native timber species to soils with different arbuscular mycorrhizal inoculums potentials in South Cameroon Indigenous inoculum and effect of addition of grass inoculums. For. Ecol. Manag. 210 283–290. 10.1016/j.foreco.2005.02.038 [DOI] [Google Scholar]
- Osoria N. W., Habte M. (2001). Synergetic influence of an arbuscular mycorrhizal fungus and P solubilizing fungus on growth and P uptake of Leucaena leucocephala in an oxisol. Arid Land Res. Manag. 1115 263–274. 10.1080/15324980152119810 [DOI] [Google Scholar]
- Ouahmane L., Hafidi M., Thioulouse J., Ducousso M., Kisa M., Prin Y., et al. (2006). Improvement of Cupressus atlantica Gaussen growth by inoculation with native arbuscular mycorrhizal fungi. J. Appl. Microbiol. 103 683–690. 10.1111/j.1365-2672.2007.03296.x [DOI] [PubMed] [Google Scholar]
- Padilla F. M., Ortega R., Sánchez J., Pugnaire F. I. (2009). Rethinking species selection for restoration of arid shrublands. Basic Appl. Ecol. 10 640–647. 10.1016/j.baae.2009.03.003 [DOI] [Google Scholar]
- Palmer M. A., Ambrose R. F., Poff N. L. (1997). Ecological theory and community restoration ecology. Restor. Ecol. 5 291–300. 10.1046/j.1526-100X.1997.00543.x [DOI] [Google Scholar]
- Parniske M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Microbiology 6 763–775. 10.1038/nrmicro1987 [DOI] [PubMed] [Google Scholar]
- Perry D. A., Molina R., Amaranthus M. P. (1987). Mycorrhizae, mycorhizosphere, and reforestation: current knowledge and research need. Can. J. For. Res. 17 929–940. [Google Scholar]
- Pouyu-Rojas E., Siqueira J. O. (2000). Arbuscular mycorrhizal and soil fertilization on post-transplant development of out plants of seven forest species. Pesqui. Agropecu. Bras. 35 103–114. 10.1590/S0100-204X2000000100013 [DOI] [Google Scholar]
- Puri A., Adholeya A. (2013). A new system using Solanum tuberosum for the co-cultivation of Glomus intraradices and its potential for mass producing spores of arbuscular mycorrhizal fungi. Symbiosis 59 87–97. 10.1007/s13199-012-0213-z [DOI] [Google Scholar]
- Quoreshi A. M. (2008). “The use of mycorrhizal biotechnology in restoration of disturbed ecosystem,” in Mycorrhizae: Sustainable Agriculture and Forestry, eds Siddiqui Z. A., Akhatar M. S., Futai K. (New York, NY: Springer Science + Business Media B.V; ), 303–320. [Google Scholar]
- Redecker D., Raab P. A. (2006). Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98 885–895. 10.3852/mycologia.98.6.885 [DOI] [PubMed] [Google Scholar]
- Renker C., Zobel M., Öpik M., Allen M. F., Allen E. B., Vosátka M., et al. (2004). “Structure, dynamics, and restoration of plant communities: do arbuscular mycorrhizae matter?,” in Assembly Rules and Restoration Ecology : Bridging the Gap Between Theory and Practice, eds Temperton V. M., Hobbs R. J., Nuttle T., Halle S. (Washington, DC: Island Press; ), 189–229. [Google Scholar]
- Requena N., Perez-Solis E., Azcón-Aguilar C., Jeffries P., Barea J. M. (2001). Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl. Environ. Microbiol. 67 495–498. 10.1128/AEM.67.2.495-498.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riginos C. (2009). Grass competition suppresses savanna tree growth across multiple demographic stages. Ecology 90 335–340. 10.1890/08-0462.1 [DOI] [PubMed] [Google Scholar]
- Rilling M. C. (2004). Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 7 740–754. 10.1111/j.1461-0248.2004.00620.x [DOI] [Google Scholar]
- Rodriguez-Echeverria S., Costa S. R., Freitas H. (2007). “Biodiversity and interactions in the rhizosphere: effects on ecosystem functioning,” in Functional Plant Ecology, 2nd Edn, eds Pugnaire F. I., Valladares F. (New York: CRC Press; ), 581–600. [Google Scholar]
- Ruiz-Jaen M. C., Aide T. M. (2005). Restoration success: how is it being measured? Restor. Ecol. 13 569–577. 10.1111/j.1526-100X.2005.00072.x [DOI] [Google Scholar]
- Saharan B. S., Nehra V. (2011). Plant growth promoting rhizobacteria: a critical review. Life Sci. Med. Res. 21 1–30. [Google Scholar]
- Sanon A., Ndoye F., Baudoin E., Prin Y., Galiana A., Duponnois R. (2010). “Management of the mycorrhizal soil infectivity to improve reforestation programs’ achievements in Sahelian ecosystems,” in Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology Vol. 2 ed. Méndez-Vilas A. (Badajoz: FORMATEX Research Center; ), 230–238. [Google Scholar]
- Scheublin T. R., van Logtestijn R. S. P., van der Heijden M. G. A. (2007). Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J. Ecol. 95 631–638. 10.1111/j.1365-2745.2007.01244.x [DOI] [Google Scholar]
- Schnitzer S. A., Klironomos J. N., Lambers J. H. R., Kinkel L. L., Reich P. B., Xiao K., et al. (2011). Soil microbes drive the classic plant diversity–productivity pattern. Ecology 92 296–303. 10.1890/10-0773.1 [DOI] [PubMed] [Google Scholar]
- Schnoor T. K., Lekberg Y., Rosendahl S., Olsson P. A. (2011). Mechanical soil disturbance as a determinant of arbuscular mycorrhizal fungal communities in semi-natural grassland. Mycorrhiza 21 211–220. 10.1007/s00572-010-0325-3 [DOI] [PubMed] [Google Scholar]
- Schüßler A., Martin H., Cohen D., Fitz M., Wipf D. (2007). Arbuscular mycorrhiza: studies on the Geosiphon symbiosis lead to the characterization of the first Glomeromycotan sugar transporter. Plant Signal. Behav. 2 431–434. 10.4161/psb.2.5.4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SER (2004). The SER International Primer on Ecological Restoration. Society for Ecological Restoration International Science & Policy Working Group Version 2 Number 1. Available online: http://www.ser.org/resources/resources-detail-view/ser-international-primer-on-ecological-restoration [Google Scholar]
- Simard S. W., Austin M. E. (2010). “The role of mycorrhizas in forest soil stability with climate change,” in Climate Change and Variability, ed. Simard S. W. (Rijeka: InTech Open Access; ), 275–302. [Google Scholar]
- Skujins J., Allen M. F. (1986). Use of mycorhiza for land rehabilitation. MIRCEN J. Microbiol. Biotechnol. 2 161–176. 10.1007/BF00937191 [DOI] [Google Scholar]
- Smith S. E., Jakobsen I., Grønlund M., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156 1050–1057. 10.1104/pp.111.174581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. E., Smith F. A. (2011). Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystems scales. Annu. Rev. Plant Biol. 63 227–250. 10.1146/annurev-arplant-042110-103846 [DOI] [PubMed] [Google Scholar]
- Soares C. R. F. S., Siqueira J. O. (2008). Mycorrhiza and phosphate protection of tropical grass species against heavy metal toxicity in multi-contaminated soil. Biol. Fert. Soils 44 833–841. 10.1007/s00374-007-0265-z [DOI] [Google Scholar]
- Soka G., Ritchie M. (2014). Arbuscular mycorrhizal symbiosis and ecosystem processes: prospects for future research in tropical soils. Open J. Ecol. 4 11–22. 10.4236/oje.2014.41002 [DOI] [Google Scholar]
- Song Y. Y., Ye M., Li C., He X., Zhu-Salzman K., Wang R. L., et al. (2014). Hijacking common mycorrhizal networks for herbivore-induced defence signal transfer between tomato plants. Sci. Rep. 4 1–8. 10.1038/srep03915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T. N., Remy W., Hass H., Kerp H. (1995). Fossil arbuscular mycorrhizae from the early Devonian. Mycologia 87 560–573. 10.2307/3760776 [DOI] [Google Scholar]
- Thomas E., Jalonen R., Loo J., Boshier D., Gallo L., Cavers S., et al. (2014). Genetic considerations in ecosystem restoration using native tree Species. For. Ecol. Manag. 333 66–75. 10.1016/j.foreco.2014.07.015 [DOI] [Google Scholar]
- Timonen S., Marschner P. (2006). “Mycorrhizosphere concept,” in Soil Biology: Microbial Activity in the Rhizosphere Vol. 7 eds Mukerji K. G., Manoharachary C., Singh J. (Berlin: Springer-Verlag; ), 155–172. [Google Scholar]
- UN (2015). Transforming Our World: The 2030 Agenda for Sustainable Development. Available at: https://sustainabledevelopment.un.org/post2015/transformingourworld [Google Scholar]
- Van der Heijden M. G. A., Klironomos J. N., Ursic M., Moutoglis P., Streitwolf-Engel R., Boller T., et al. (1998). Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396 69–72. 10.1038/23932 [DOI] [Google Scholar]
- Van der Heijden M. G. A., Klironomos J. N., Ursic M., Moutoglis P., Streitwolf-Engel R., Boller T., et al. (1999). “Sampling effect,” a problem in biodiversity manipulation? A reply to David A. Wardle. Oikos 87 408–410. 10.2307/3546758 [DOI] [Google Scholar]
- Veiga R. S. L., Jansa J., Frossard E., van der Heijden M. G. A. (2011). Can arbuscular mycorrhizal fungi reduce the growth of agricultural weeds? PLoS ONE 6:e27825 10.1371/journal.pone.0027825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelsang K. M., Reynolds H. L., Bever J. D. (2006). Mycorrhizal fungal identity and richness determine the diversity and productivity of a tall grass prairie system. New Phytol. 172 554–562. 10.1111/j.1469-8137.2006.01854.x [DOI] [PubMed] [Google Scholar]
- Walder F., Niemann H., Natarajan M., Lehmann M. F., Boller T., Wiemken A. (2012). Mycorrhizal networks: common goods of plants shared under unequal terms of trade. Plant Physiol. 159 789–797. 10.1104/pp.112.195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Qiu Y. L. (2006). Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16 299–363. 10.1007/s00572-005-0033-6 [DOI] [PubMed] [Google Scholar]
- Weremijewicz J., Janos D. P. (2013). Common mycorrhizal networks amplify size inequality in Andropogon gerardii monocultures. New Phytol. 198 203–213. 10.1111/nph.12125 [DOI] [PubMed] [Google Scholar]
- World Resource Institute (2015). What is Degraded Land? Available at: http://www.wri.org/faq/what-degraded-land [Google Scholar]
- Wubet T., Kottke I., Teketay D., Oberwinkler F. (2009). Arbuscular mycorrhizal fungal community structures differ between co-occurring tree species of dry Afromontane tropical forest, and their seedlings exhibit potential to trap isolates suited for reforestation. Mycol. Prog. 8 317–328. 10.1007/s11557-009-0602-8 [DOI] [Google Scholar]
- Wubet T., Weiß M., Kottke I., Oberwinkler F. (2006). Two threatened coexisting indigenous conifer species in the dry afromontane forests of Ethiopia are associated with distinct arbuscular mycorrhizal communities. Can. J. Bot. 84 1617–1627. 10.1139/b06-121 [DOI] [Google Scholar]
- Yang H., Dai Y., Wang X., Zhang Q., Zhu L., Bian X. (2014). Meta-analysis of interactions between arbuscular mycorrhizal fungi and biotic stressors of plants. Sci. World J. 2014 1–7. 10.1155/2014/746506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. P., Chase J. M., Huddleston R. T. (2001). Community succession and assembly: comparing, contrasting and combining paradigms in the context of ecological restoration. Ecol. Restor. 19 5–18. [Google Scholar]
- Young T. P., Petersen D. A., Clary J. J. (2005). The ecology of restoration: historical links, emerging issues and unexplored realms. Ecol. Lett. 8 662–673. 10.1111/j.1461-0248.2005.00764.x [DOI] [Google Scholar]
- Zedler J. B. (2005). Ecological restoration: guidance from theory. San Francisco Estuar. Watershed Sci. 3 1–31. 10.15447/sfews.2005v3iss2art4 [DOI] [Google Scholar]
- Zobel M., pik M. (2014). Plant and arbuscular mycorrhizal fungal (AMF) communities – which drives which? J. Veg. Sci. 25 1133–1140. 10.1111/jvs.12191 [DOI] [Google Scholar]