Abstract
Radioembolization (RE) is an emerging treatment strategy for patients with primary hepatic malignancies and metastatic liver disease. Though RE is primarily performed in the palliative setting, a shift toward the curative setting is seen. Currently, hepatic resection and in selected cases liver transplantation are the only curative options for patients with a hepatic malignancy. Unfortunately, at diagnosis most patients are not eligible for liver surgery due to the imbalance between the necessary liver resection and the remaining liver remnant. However, in borderline resectable cases, tumor volume reduction and/or increasing the future liver remnant can lead to a resectable situation. The combination of selective tumor treatment, the induction of hypertrophy of untreated liver segments, and its favourable toxicity profile make RE an appealing strategy for downstaging. The present review discusses the possibilities for RE in the preoperative setting as a downstaging tool or as a bridge to liver transplantation.
Keywords: Radioembolization, Downstaging, Bridge to transplant, Future liver remnant
Introduction
The incidence of both primary and secondary hepatic malignancies is continuously increasing worldwide [1–3]. At the same time, treatment strategies have changed considerably in the last two decades and continue to evolve. Though treatment strategies vary substantially between primary [most common types: hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)] and secondary liver malignancies and their individual subtypes, less than 30 % can be curatively resected at diagnosis [4–7]. However, the number of patients with resectable disease can be increased, if the individual tumor load is decreased (i.e. downstaging) [8].
Metastatic liver disease is far more common than primary hepatic malignancy, with colorectal malignancy being the most common tumor type. Hepatic resection is considered the only potentially curative option for colorectal cancer liver metastasis (CRLM) with 5- and 10-year survival rates approaching 60 and 25 %, respectively [7, 9, 10]. Ongoing improvements in chemotherapeutic regimens, the addition of monoclonal antibodies and the more liberal attitude towards hepatic resections, have led to a significant increase in the number of hepatic resections [4, 5, 7, 8, 10, 11]. In the series of Adam et al. 13 % of patients with initial unresectable CRLM underwent liver surgery after downstaging with a 5-year disease-free survival (DFS) of 22 % and a 5-year overall survival (OS) of 33 % [5].
The number of hepatic resections is even further increasing since metastases of other primaries are progressively treated with surgery, such as breast carcinoma, melanoma, GIST and neuroendocrine tumors [12, 13]. For example, the reported 5-year survival rates in carefully selected populations are 21–60 % for breast carcinoma and up to 60–80 % for neuroendocrine tumors.
Contrary to metastatic liver disease (apart from selected cases with metastases from neuroendocrine tumors), liver transplantation (LTX) is part of the standard curative treatment arsenal in patients with limited HCC and in rare cases of ICC [14–17]. HCC nearly always develops in patients with known liver disease (mostly cirrhosis due to HBV or HCV) and thus often compromised liver function [14, 18]. LTX has the advantage of treating the underlying liver disease and associated future “de novo” HCC risk, leading to better overall and recurrence-free survival (RFS) than hepatic resection. Patient selection for LTX is strict and generally based on the Milan criteria (a solitary lesion of <5 cm in diameter or up to 3 lesions all <3 cm in diameter and no macroscopic vascular invasion or extrahepatic metastasis) [19]. Adherence to these criteria has resulted in 5-year survival rates of >70 %, slightly worse than survival rates after LTX for non-tumorous conditions (3-year survival of 71 vs 84 % [20]) [14, 15, 19, 21, 22]. Paucity of donor organs has made resection a reasonable alternative for LTX in selected cases (patients with a solitary HCC <5 cm and a Child Pugh A score without portal hypertension) [23, 24]. Furthermore, in patients with an HCC <3 cm ablative techniques, such as radiofrequency ablation, are also a curative option [24].
Negative resection margins are an important prognostic factor for survival in both primary and secondary hepatic malignancies [10, 25, 26]. Rees et al. reported in a cohort of 929 CRLM patients a 5-year survival of 18 % in R1 resections compared to 40 % in R0 resections [10]. Similarly, Spolverato et al. reported an incrementally worsening RFS and OS with decreasing margin width in ICC patients [26]. Several factors have been associated with positive resection margins, such as multiple lesions, bilobar disease, tumor size, major liver resections, vascular reconstruction/invasion and caudate lobe resections [25–27]. Downstaging strategies aim to reduce abovementioned factors, thus hoping to improve OS. Fortunately, preoperative imaging can assess the presence of these factors adequately, facilitating the decisions on downstaging and surgical treatments.
Radioembolization (RE) is an emerging treatment strategy for patients with hepatic malignancy. Hepatic tumors are targeted by the injection of radio-active microspheres into the hepatic or tumor supplying arteries, resulting in selective radiation of these tumors. Currently microspheres with varying radio-isotopes are being tested in clinical trials, however only two types of microspheres are commercially available, both embedded with yttrium-90 (90Y): glass spheres (Theraspheres®, BTG International, London, England) and resin spheres (SIR-Spheres®, Sirtex Medical, North Sydney, Australia). Until now RE is primarily performed as a salvage treatment, yet the qualities of RE (such as selective tumor targeting, the lack of heat-sink effect near the great vessels and the induction of hypertrophy in non-embolized lobes) make its use in the curative setting, as an adjunct to surgery for example, appealing.
In this systematic review, we will discuss the potential of RE in downstaging and as a bridge to liver transplantation.
Downstaging or bridging to LTX in general
Pre-transplant locoregional liver therapies are mainly focused on preventing drop-out from the LTX waiting list in patients with an HCC within the Milan criteria (i.e. bridge to LTX) or on downstaging to meet the Milan criteria (instead of enabling hepatic resection, as is the case in ICC and metastatic disease) [23, 24, 28]. Several studies have shown that drop-out due to tumor progression is rare in the first 3 months after enlisting, but drop-out numbers increase with longer waiting times (up to 57 % after 12 months) and also with increasing lesion size and number [18, 22, 28, 29]. Pre-transplant locoregional liver therapies have shown to reduce the drop-out rates [20, 29, 30] and increase long-term post-transplant patient and graft survival [20]. Currently up to 65 % of patients within the Milan criteria receive bridging therapies, primarily in the form of trans-arterial chemoembolization (TACE) or radiofrequency ablation (RFA) [18, 20, 22, 24]). TACE seems preferable in HCC lesions >3 cm or multifocal HCC, while RFA seems most promising as a bridging strategy in HCC lesions <3 cm [24, 28].
Several studies have addressed the potential of TACE as a downstaging treatment strategy [29–35]. Yao et al. reported successful downstaging to the Milan criteria in 70 %, with a 4-year survival of 92 % [29]. Others reported similar transplantation rates, tumor recurrence rates and survival rates compared to patients within the Milan criteria [30, 33–35]. Tumor recurrence rates were however influenced by tumor progression in the waiting time [33, 35]. Therefore, a waiting time of 3 months from the enlisting for LTX is recommended in order to select HCC’s with less aggressive biological behaviour and prevent recurrences [24].
Most institutions currently reserve LTX for patients with HCC within the Milan criteria, as validated by the United Network for Organ Sharing (UNOS). However, some centers apply expanded criteria, such as the UCSF criteria or up-to-7 criteria.
Search strategy
A PubMed literature search was performed on 11 November 2015 to identify all articles related to the use of RE in downstaging of liver malignancy or as a bridge to LTX. Search terms used to identify these articles were ‘radioembolization’, ‘downstaging’, ‘hepatectomy’, ‘bridge to transplant’, ‘liver remnant’ and their synonyms. This search yielded 148 articles. The following exclusion criteria were applied: animal studies, reviews, metaananalyses, conference abstracts, consensus statements and protocol publications, and languages other than English or German. After application of these exclusion criteria 42 articles were screened on full-text. Another 13 articles were excluded [no data on the downstaging success rate (n = 11) or only data after combined multimodality liver therapy (n = 3)]. The remaining 21 original studies and 7 case reports were included in this review [6, 36–60]. Cross-referencing of their references yielded 15 relevant additional publications [61–75].
Downstaging with RE
Several prospective and retrospective studies in patients with intermediate HCC (not eligible for resection or LTX) have shown multiple advantages of RE over TACE. RE was associated with a longer overall survival, longer time to progression, faster time to radiological response, shorter hospitalization, less postembolization syndrome and a smaller number of treatment sessions, while having a similar toxicity profile [51, 64, 69, 76]. In case of RE with lobar delivery, another possible advantage is treatment of non-detected HCC nodules, as these are reported in 36 % of the liver explants [32]. These advantages make RE an attractive option for downstaging and as a bridging therapy.
A few small studies and case reports have reported on RE in this context (Table 1) [6, 36, 40, 45, 47–51, 57, 58, 73]. The largest study compared TACE and RE in a non-randomized cohort study of 86 patients with UNOS stage T3 HCC [51]. Downstaging to UNOS T2 HCC occurred in 58 % after RE and in 31 % after TACE (p = 0.02). This resulted in LTX in 21 % after RE and 26 % after TACE, whereas 42 and 23 %, respectively were downstaged to RFA. OS was better after RE than after TACE (3-year survival of 59 vs 19 %; p = 0.008), as was 1-year RFS after LTX (89 vs 73 %). Others report similar downstaging success rates of 29–50 % [6, 40, 57]. Kulik et al. performed a pilot study to assess the benefit of adding Sorafenib to RE in patients awaiting LTX [48]. Tumor size reduction was comparable in both groups, but biliary complications and LTX rejection were only encountered in the RE + Sorafenib group. Based on the limited available evidence, applying RE as a tool for downstaging or bridging to LTX in HCC seems feasible. However, RCTs are mandatory to further investigate its potential.
Table 1.
Overview of response rates and downstaging success rates with 90Y-RE in patients with HCC
| Author | Year | N | mRECIST (%) | WHO (%) | EASL (%) | Downstaging success rate | Median time to response/downstaging | Resection or RFA | LTX | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | CR | PR | CR | PR | % | Months (range) | %e | %e | |||
| Kulika [49] | 2006 | 34 | – | – | – | 50 | – | – | 67 | 4 (1.9–16.3) | 34 | 23 |
| Heckman [43] | 2008 | 16 | – | – | – | – | – | – | 13 | – | – | 100f |
| Lewandowskia [51] | 2009 | 43 | – | – | 0 | 61 | 47 | 39 | 58 | 3.1 (1.8–8.7) | 42 | 21 |
| Ibrahima [45] | 2012 | 8 | – | – | 13 | 63 | 37 | 50 | 50 | – | – | 37 |
| Iñarrairaegui [6] | 2012 | 21 | – | – | – | – | – | – | 29 | – | 19 | 10 |
| Tohme [57] | 2013 | 20 | 37 | 19 | – | – | – | – | 33 | – | – | 100f |
| Donahueb [108] | 2013 | 12 | – | – | 0 | 50 | 25 | 42 | 8 | (1.4–11.3) | – | 50 |
| Vouche [75] | 2014 | 102 | 47 | 39 | – | – | – | – | – | – | – | 32 |
| Ettorrec [40] | 2014 | 22 | – | – | – | – | – | – | 50 | – | 5 | 45 |
| Kulikd [48] | 2014 | 20 | – | – | – | – | – | – | – | – | 5 | 85 |
| Abdelfattah [36] | 2015 | 9 | – | – | – | – | – | – | 100 | – | – | 100f |
aOverlapping patientpopulations
bProspective study on 90Y-RE in patients with a transjugular intrahepatic portosystemic shunt (TIPS)
cCorrespondence to editor
dProspective randomized pilot study comparing 90Y-RE + Sorafenib with 90Y-RE alone
ePercentage of the total population
f100 % LTX is inherent to retrospective patient study design
In case of intrahepatic cholangiocarcinoma (ICC), downstaging is less often reported. Apart from few case reports, two institutions have reported their experiences with 90Y-RE in downstaging (Table 2) [53, 63, 65, 67, 70]. Rayar et al. reported successful downstaging to resection with RE and chemotherapy in eight patients with initially unresectable ICC [70]. R0 resections were achieved in all patients, with a median of six resected segments [70]. Mouli et al. reported successful downstaging to resection in 5/46 patients with unresectable ICC and successful LTX in one patient [53].
Table 2.
Overview of response rates and downstaging success rates with 90Y-RE in patients with ICC
| Authora | Year | N | RECIST (%) | WHO (%) | EASL (%) | Downstaging success rate | Median time to response/downstagingb | Resection | LTX | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | CR | PR | CR | PR | % | Months (range) | %c | %c | |||
| Ibrahim [67] | 2008 | 24 | 0 | 27 | – | – | 9 | 77 | 8 | – | 4 | 4 |
| Mouli [53] | 2013 | 46 | – | – | 0 | 25 | 9 | 64 | 13 | 3.6 | 11 | 2 |
| Rayar [70] | 2015 | 10 | – | – | – | – | – | – | 80 | 7.6 (3.4–16.7) | – | 0 |
| Edeline [63] | 2015 | 24 | 0 | 25 | – | – | – | – | 46 | – | 46 | 0 |
aThe patientpopulations of Ibrahim and Mouli (partially) overlap, as well as the populations of Rayar and Edeline
bRayar et al.: median time from start chemotherapy to resection in a study on chemotherapy and 90Y-RE as first-line treatment for ICC
cPercentage of the total population
Similar to HCC and ICC, the evidence for downstaging of metastatic liver disease with RE is limited, with a few case reports/case series (<5 patients) and one small clinical study (Table 3) [37, 38, 42, 46, 52, 58, 59, 71]. Justinger et al. reported on 13 CRLM patients with marginally resectable disease, who were treated with resin microspheres for intended downstaging [46]. Hepatic resection was performed in 11/13 patients after a median of 57 days (range 39–153) following RE; combined with ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) in 7/11 and with PVE in 1/11.
Table 3.
Overview of response rates and downstaging success rates with 90Y-RE in patients with metastatic disease
| Author | Year | N | RECIST (%) | WHO (%) | EASL (%) | Downstaging success rate | Median time to response/downstaging | Resection | LTX | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | CR | PR | CR | PR | % | Months (range) | %d | %d | |||
| Whitney [59] | 2011 | 44 | – | –a | – | – | – | – | 9 | – | 9 | 0 |
| Vouche [58] | 2013 | 8 | – | – | – | – | – | – | 13 | – | 13 | 0 |
| Moirb [52] | 2015 | 44 | 0 | 10 | – | – | – | – | 16 | 4.0 (2.3–10.9) | 16 | 0 |
| Justinger [46] | 2015 | 13 | – | – | – | – | – | – | 85 | 1.8 (1.3–4.9) | 85 | 0 |
| Henryc [44] | 2015 | 9 | – | – | – | – | – | – | 29 | 3.8 (1.8–8.3) | 100 | 0 |
aAll surgical candidates (n = 4) had PR according to RECIST
bMixed population (CRLM 14, 8 HCC, 5 NET, 4 other); 4/14 CRLM underwent surgery
cRetrospective series with a mixed population of secondary liver malignancies (CRLM 4, 3 NET, 1 gastrointestinal stromal tumor and 1 cervical carcinoma)
dPercentage of the total population
The role of RE in downstaging prior to ablation therapy has also been investigated [49, 66]. Hoffman et al. reported the results of RFA in patients with extensive hepatic metastases three months after RE. Downstaging to a tumor size suitable for RFA (<3 cm) was achieved in 12 % of patients (5/44) [66]. Kulik et al. reported successful downstaging of HCC lesions (to <3 cm) by means of RE in 32 % of patients (11/34) [49].
Future liver remnant (FLR)
Liver failure after hepatectomy, i.e. an insufficient liver remnant, currently is the major cause of postoperative mortality, especially after major resections and in patients with liver parenchymal disease (mainly cirrhosis) [77, 78]. In a large series by Cescon et al. (n = 1500) the incidence of transient liver failure was 4.1 %, while liver failure related mortality occurred in 1.7 % [77].
On the other hand, lethal liver failure after RE, i.e. lethal radioembolization-induced liver disease (REILD), occurs in up to 5 % of patients in large series with CTCAE grade 3 bilirubin toxicities in up to 19 % [79–81]. Consequently, RE is likely to result in some compromise of the FLR function. Yet, in the abovementioned studies, complications after RE downstaging and surgery were scarcely reported [6, 40, 46, 48, 57, 59]. However, Henry et al. reported a considerably higher 30-day mortality in patients who were treated with RE before resection than those who were not (33 vs 3 %) [44]. Liver failure related mortality after RE and resection was reported in one case [70]. Others report complication rates comparable to hepatic surgery without prior RE [46, 48, 57].
In the current guidelines, the thresholds for an adequate FLR are based on volumetric measurements. A FLR is considered sufficient if it comprises >20 % [of the initial total liver volume (TLV)] in non-exposed livers, >30 % after heavy chemotherapeutic pretreatment and >40 % in cirrhotic livers [4, 82]. Extensive resections are often in conflict with an adequate FLR. Portal vein embolization (PVE), portal vein ligation (PVL) and in situ liver splitting techniques (e.g. ALPPS) are commonly applied to overcome this problem and increase the FLR [83]. A FLR increase of 44–69 % is reported within 3–8 weeks after PVE of the right liver lobe, with an increase of the relative FLR volume [=FLR volume/(total liver volume − tumor volume)] up to 47 % [84–86]. After 12 months FLR increases of 83 % are reported [87]. Remarkably, FLR increase is more pronounced in small FLR and, as can be expected, less pronounced in cirrhotic livers [83, 85, 88]. The downside of PVE/PVL is the induction of tumor growth in the embolized and non-embolized lobes [84, 86, 88, 89], thus counteracting the downstaging strategy. This tumor increase can be up to 21 % in the treated lobe [88]. Furthermore, a considerable number of patients will become definitely ineligible for surgery due to the development of new lesions in the designated FLR post-PVE (up to 9 %) [84, 86, 89].
Hypertrophy of the untreated lobe(s) is a well-known by-effect of RE [6, 46, 58, 68, 72]. Hypertrophy after RE develops at a slower pace and to a lesser extent than in case of PVE/PVL with FLR increases of ca. 23 % within 1–3 months after treatment (Table 4). Even so, hypertrophy continues with FLR increases of 31–34 and 40–45 % after 6 and 12 months, respectively [56, 58]. Also, contrary to PVE, RE does have a coinciding anti-tumoral effect in the treated lobe [41]. This will allow for a longer interval to surgery and thus time to develop hypertrophy (Fig. 1). The inherent benefit of the prolonged waiting period is the possibility to assess previously undetected contralateral metastases or synchronous HCC, since the occurrence of tumor progression in the non-treated lobe after RE is comparable to PVE (Table 4).
Table 4.
Induction of hypertrophy after 90Y-RE
| Author | Year | Patients | Follow up period | Volume measurement | Degree of hypertrophy contralateral lobea | Degree of atrophy treated lobea | Response assessment | Response treated lobe | Response non-treated lobe |
|---|---|---|---|---|---|---|---|---|---|
| Jakobs [68] | 2008 | 32 | 139 days | CT/MRI | 21 % | 9 % | – | – | |
| Gaba [65] | 2009 | 20 | 3 months | CT/MRI | 40 % | 52 % | EASL | CR 40 % PR 50 % SD 10 % |
NA |
| Ahmadzadehfar [37] | 2013 | 24 | 44–66 days | MRI | 47 % | 8 % | PERCIST and RECIST | CR 8 % PR 74 % SD 8 % PD 8 % |
50 % PD |
| Edeline [39] | 2013 | 34 | 3 months | CT | 29 % | 23 %b | mRECIST | CR 30 % PR 33 % SD 30 % PD 7 % |
NA |
| Vouche [58] | 2013 | 83 | 1 month 1–3 months 3–6 months >9 months |
CT/MRI | 7 % 24 % 35 % 45 % |
2 % 4 % 21 % 32 % |
– | 20 % new lesions | |
| Garlipp [41] | 2014 | 35 141b |
46 days 33 daysb |
MRI | 29 % 62 %b |
8 % 12 %b |
RECIST | CR 4 % PR 19 % SD 73 % PD 4 % |
8 % new lesions 56 % lesion growth |
| Teo [73] | 2014 | 17 | 5,7 months | CT | 34 % | 22 % | RECIST | CR 12 % PR 29 % SD 35 % PD 24 % |
NA |
| Theysohn [56] | 2014 | 45 | 1 month 3 months 6 months 9 months 12 months |
CT | 7 % 23 % 31 % 36 % 40 % |
5 % 23 % 34 % 41 % 45 % |
– | – |
NA not applicable
aDegree of hypertrophy: (volume non-treated lobe posttreatment−volume non-treated lobe at baseline)/volume non-treated lobe at baseline. Degree of atrophy is calculated likewise. Median volume changes are reported in the study of Gaba and Vouche. The others report mean volume changes
bMatched pair analysis of RE vs PVE; PVE data are marked
Fig. 1.
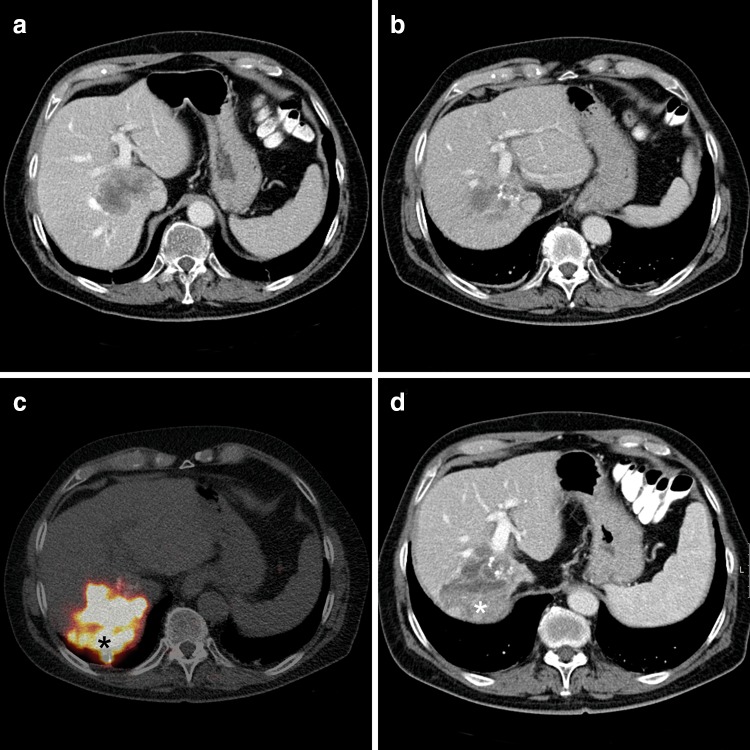
Induction of hypertrophy after 2 RE-treatments in a patient with CRLM. a CT scan prior to the first treatment with a CRLM located centrally in the right hemiliver, also involving the caudate lobe. b Three months after a whole liver treatment a decrease in the lesion size is seen. Segment 2–3 have hypertrophied (degree of hypertrophy: 16 %). c 90Y-PET/CT after a second selective treatment with glass microspheres (8 months after the first RE treatment): an intense accumulation of 90Y is seen in the lesion (*). d CT scan 2 months after the second treatment. The lesion in the right hemiliver has further decreased in size. A wedge-shaped hypodense area surrounds the lesion, consistent with radiation changes of the surrounding parenchyma (corresponding to the normal parenchyma with intense 90Y uptake on c (*). The hypertrophy of segment 2–3 has increased (degree of hypertrophy: 25 %). Also, segment 4 has hypertrophied (degree of hypertrophy: 20 %)
Theoretically, the degree of hypertrophy induction might vary with regard to the used microsphere. The lower activity per microsphere of resin spheres (50 vs 2500 Bq/sphere in glass microspheres) results in a higher amount of injected particles (i.e. embolic load), suggestive of more flow redirection. Edeline et al. compared both types of microspheres without finding a significant difference in the maximal degree of hypertrophy, though the number of treatments with resin microspheres was very low (n = 4) [39]. In the light of downstaging with RE, further investigation of these differences is required.
Assessment of the FLR function
Liver function encompasses multiple subfunctions, such as synthetic, excretory and detoxicifying functions. Several tests are used to assess total liver or FLR–function, though none are able to weigh the entire spectrum of different liver functions. Some tests are based on biochemical and clinical findings, such as the Child-Pugh score and MELD score. Others are based on the liver uptake of one substance, for example the indocyanine green clearance (ICG) test and the galactose elimination capacity.
Currently, non-invasive preoperative assessment of liver function, using nuclear imaging techniques (hepatobiliary scintigraphy) is gaining ground. Two liver-specific radiopharmaceuticals are commonly used: 99mTc- galactosylneoglycoalbumin (99mTc-GSA) (not available in Europe and the United States) and 99mTc-iminodiacetic acid (99mTc-IDA) [90, 91]. 99mTc-GSA is endocytosed and degraded by hepatocytes after binding to the asiologlycoprotein receptor. 99mTc-IDA is processed by hepatocytes by the same organic anion-transporting polypeptides (OATP 1B1 and 1B3) and multidrug resistance protein (MPR2) as bilirubin and ICG [92, 93]. Thus, hepatic uptake of IDA analogs is influenced by hyperbilirubinemia, while 99mTc-GSA uptake generally is not [90, 94]. 99mTc mebrofenin is the most used IDA analog, because it has the strongest resistance to displacement by bilirubin and the highest hepatic extraction fraction.
Several hepatobiliary scintigraphy (HBS) studies have shown that there is a decreased hepatic uptake in patients with parenchymal disease and that there is little to no correlation between hepatic uptake and liver volume (especially in compromised livers) [78, 82, 93–96]. Additionally, as reported by Bennink et al., a strong association exists between the preoperatively determined FLR function and the actual liver remnant function 1 day after surgery as measured by HBS (r = 0.95, p < 0.001) [95]. Also, in a study by Dinant et al. HBS was reported to be more accurate in the prediction of postoperative liver failure than CT volumetry [78]. Their results indicated that a safe resection was possible in patients with a FLR uptake of >2.5 %/min/m2 of body surface area (with a 3 % chance of liver failure development above this uptake value).
However, in current practice FLR sufficiency is often still based on volumetric measurements; even when (the extent of) underlying parenchymal disease is not known preoperatively. Apart from the inadequate quantification of the function of the FLR parenchyma by volumetry, regional differences are not accounted for. Inhomogeneous liver function distribution is quite common, especially in cirrhotic livers, in case of a hilar cholangiocarcinoma and after PVE [82, 91]. In contrast to CT and MRI volumetry, HBS can image regional and segmental differences in liver function, especially when combined with SPECT/CT [82, 97]. Another advantage of HBS SPECT/CT is the better delineation of the separate segments and thus the FLR, when compared to the planar HBS 2-dimensional images [82]. Interestingly, few authors have reported on HBS after PVE [94, 97, 98], with consistent results of a larger increase in FLR function than in FLR volume in both normal and compromised livers. This faster functional increase argues for a shorter interval between PVE (or RE) and surgery, even when volumetric hypertrophy is not yet up to par.
Up to date, only one report of HBS imaging after RE has been published. Bennink et al. reported on 2 cases with multifocal HCC undergoing HBS (with 99mTc-mebrofenin) both prior to and 6 weeks after RE [61]. After RE both patients had a reduced total liver function [reduced body surface area corrected 99mTc mebrofenin uptake rate (cMUR)] due to an uptake decrease in the treated lobe(s). One patient underwent two whole liver treatments in 6 months, resulting in a reduction in cMURtotal liver from 7.4 to 6.1 %/min after the first treatment and from 4.8 to 2.2 %/min after the second treatment. This patient was thereafter diagnosed with REILD.
Discussion
Based on the available evidence RE seems a promising addition to the currently applied downstaging and bridging strategies. The combination of the anti-tumoral effect and simultaneous hypertrophy induction of the non-embolized segments may have clear advantages over preoperative PVE or in situ splitting techniques in terms of tumor control and morbidity.
However, RE has an important downside. Radiation damage to the non-tumorous parenchyma will compromise the liver function (Fig. 2), with the inherent risk of REILD development and a decrease in regenerative capacity (as illustrated by the report of Bennink et al.) [61]. One of the most important risk factors for REILD is the absorbed dose or administered dose per target volume [80, 99]. Unfortunately, dose distribution in RE is non-uniform [100], thus difficult to predict, even if the tumor-to-non-tumor ratio is taken into account at activity calculation (partition model) [101]. In case of unilobar treatments with a sufficient FLR these uncertainties in dose–response relationships will be less important. However, in whole liver delivery (e.g. bilobar disease) the risk of REILD is higher [72, 80, 99].
Fig. 2.
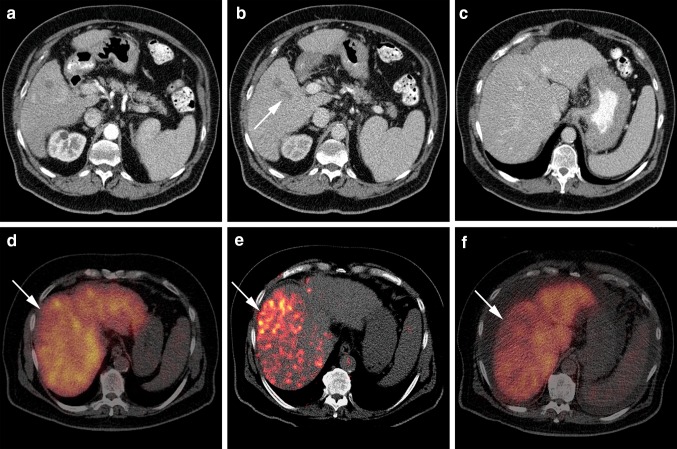
Decrease in 99mTc-mebrofenin uptake after right lobar 90Y-RE treatment. a A solitary, hypervascular lesion is present in segment 5 with wash-out (arrow) on the later obtained portal venous phase (b), consistent with an HCC. c The liver has a cirrhotic appearance (note the nodular surface). No lesions are seen elsewhere in the liver. d Hepatobiliary scintigraphy before RE-treatment shows a fairly homogeneous uptake of 99mTc-mebrofenin (cMUR: 3.0 %/min). e 90Y-PET/CT one day after right lobar treatment. 90Y has heterogeneously distributed in the right lobe with a higher dose in segment 4 and 8 (arrow in d, e and f). f Hepatobiliary scintigraphy 3 months after treatment. The uptake of 99mTc-mebrofenin is decreased in segment 4 and 8, corresponding to the area of higher 90Y deposit on the 90Y-PET/CT
Hypertrophy induction after RE is less pronounced and slower than after PVE [41]. On the other hand, the coinciding anti-tumoral effect of RE allows for more time for the FLR to hypertrophy. And if FLR hypertrophy is insufficient after RE, subsequent PVE/PVL can be considered [46, 62]. Another option might be combining transarterial and transportal RE. Toskich et al. recently reported on transportal RE of 2 HCC lesions (segment VII and VIII) in a patient not amendable for transarterial RE after repeated TACE, resulting in complete devascularization of the lesion in segment VIII [74].
Follow-up imaging plays a key role in determining the success of downstaging and the subsequent surgical planning. Furthermore, in TACE and RFA series tumor response prior to LTX seems to be associated with tumor recurrence [29, 31, 35, 102]. In the study by Tohme et al. histopathologic analysis showed complete tumor necrosis in 5/20 patients after RE, of whom 80 % had complete remission on imaging [57]. In contrary, Vouche et al. reported that only 50 % of patients with complete response (mRECIST) had complete tumor necrosis at histopathology (similar to previously reported TACE series [29, 32]) [75]. However, all explants showed 90–100 % necrosis after RE, with significantly more complete necrosis if the dose exceeded 190 Gy [75]. This discordance of pathology and imaging—regardless of the applied imaging criteria—illustrates the need for improvement, especially if TACE and RE are to be used in the curative setting.
In current practice MRI with hepatobiliary contrast agents (Gd-EOB-DTPA or Gd-BOPTA) is routinely used in the work-up for surgery and RE. In the future MRI with Gd-EOB might also be used to assess liver function [91]. Gd-EOB is processed by hepatocytes in the same way as ICG and 99mTc mebrofenin [103]. Thus, the possible benefits of MRI with Gd-EOB as a liver-function test are obvious. MRI does not use ionizing radiation and has an excellent spatial resolution, resulting in an easier regional liver function assessment (compared to HBS) with the additional benefit of simultaneous assessment of the tumor status. A few studies reported decreased enhancement of irradiated segment(s) or lobes in the hepatobiliary phase (20 min after Gd-EOB-DTPA or 120 min after Gd-BOPTA injection) after external beam radiotherapy or brachytherapy [104, 105]. Seidensticker et al. correlated these findings with histopathology [105]. In 11/14 biopsies signs of radiation damage were present; all receiving >20 Gy and showing no enhancement 2 h after Gd-BOPTA injection. Another study assessed the reduction in enhancement in the hepatobiliary phase after RE [106]. After 60 days an evident reduction in enhancement was seen in the treated lobes with normalization of the enhancement after 4 months in most cases, suggestive of liver regeneration (i.e. a tolerable dose to the non-tumorous parenchyma). However, in some cases the enhancement of the treated lobes did not recover, indicative of permanent damage. These observations could be of value to estimate the regenerative capacity of treated lobes in case of repeated RE or post-RE surgery. Even though the parenchymal changes on MRI after RE are evident, the use of MRI with Gd-EOB-DTPA as a liver function test is not yet established and still requires further development and validation to be a clinically acceptable method [91, 107].
Conclusion
The results of RE as a downstaging tool or bridge to LTX are encouraging. However, a better understanding of the dose–response relationships is imperative to prevent both insufficient tumor response and liver failure, especially in bilobar treatments and patients with a compromised liver function. An accurate measurement of the FLR function is essential to determine the feasibility of a safe resection (with HBS or in the future possibly with MRI).
Compliances with ethical standards
Conflict of interest
MGEH Lam is a consultant for Sirtex, BTG and Bayer Healthcare. All other authors have no conflict of interest.
Human and animal studies
This article does not contain any studies with human or animal subjects performed by the any of the authors.
References
- 1.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray FJA, Grey N, et al. Global cancer transitions according to the human development index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 3.Mosadeghi S, Liu B, Bhuket T, Wong RJ. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the U.S.: an updated analysis of the 2000–2011 surveillance, epidemiology, and end results registry. Hepatol Res. 2015 doi: 10.1111/hepr.12605. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13(10):1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 5.Adam RDV, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–658. doi: 10.1097/01.sla.0000141198.92114.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iñarrairaegui MPF, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38(7):594–601. doi: 10.1016/j.ejso.2012.02.189. [DOI] [PubMed] [Google Scholar]
- 7.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones RP, Hamann S, Malik HZ, Fenwick SW, Poston GJ, Folprecht G. Defined criteria for resectability improves rates of secondary resection after systemic therapy for liver limited metastatic colorectal cancer. Eur J Cancer. 2014;50(9):1590–1601. doi: 10.1016/j.ejca.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257(6):1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 11.Thomasset SC, Dennison AR, Metcalfe MS, Steward WP, Garcea G. Changing trends in the presentation of colorectal liver metastases in a single hepatobiliary tertiary referral centre over fourteen years. Eur J Surg Oncol. 2013;39(11):1243–1247. doi: 10.1016/j.ejso.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Neri F, Ercolani G, Di Gioia P, Del Gaudio M, Pinna AD. Liver metastases from non-gastrointestinal non-neuroendocrine tumours: review of the literature. Updates Surg. 2015;67(3):223–233. doi: 10.1007/s13304-015-0315-2. [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke TR, Tekkis P, Yeung S, Fawcett J, Lynch S, Strong R, et al. Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann Surg Oncol. 2008;15(1):207–218. doi: 10.1245/s10434-007-9649-4. [DOI] [PubMed] [Google Scholar]
- 14.Adam R, McMaster P, O’Grady JG, Castaing D, Klempnauer JL, Jamieson N, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9(12):1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Prasad MA, Kulik LM. The role of bridge therapy prior to orthotopic liver transplantation. J Natl Compr Canc Netw. 2014;12(8):1183–1190. doi: 10.6004/jnccn.2014.0113. [DOI] [PubMed] [Google Scholar]
- 16.Sapisochin G, Fernandez de Sevilla E, Echeverri J, Charco R. Liver transplantation for cholangiocarcinoma: current status and new insights. World J Hepatol. 2015;7(22):2396–2403. doi: 10.4254/wjh.v7.i22.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sher LS, Levi DM, Wecsler JS, Lo M, Petrovic LM, Groshen S, et al. Liver transplantation for metastatic neuroendocrine tumors: outcomes and prognostic variables. J Surg Oncol. 2015;112(2):125–132. doi: 10.1002/jso.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9(7):684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferro VRE, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 20.Freeman RB, Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant. 2008;8(4 Pt 2):958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transplant. 2011;17(Suppl 2):S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 22.Schlansky B, Chen Y, Scott DL, Austin D, Naugler WE. Waiting time predicts survival after liver transplantation for hepatocellular carcinoma: a cohort study using the United Network for Organ Sharing registry. Liver Transplant. 2014;20(9):1045–1056. doi: 10.1002/lt.23917. [DOI] [PubMed] [Google Scholar]
- 23.Padhya KTMJ, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29(3):285–292. doi: 10.1097/MOG.0b013e32835ff1cf. [DOI] [PubMed] [Google Scholar]
- 24.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transplant. 2010;16(3):262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 25.Cady BJR, Steele GD, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg. 1998;227(4):566–571. doi: 10.1097/00000658-199804000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, et al. The impact of surgical margin status on long-term outcome after resection for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2015;22(12):4020–4028. doi: 10.1245/s10434-015-4472-9. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Shimada H, Yamada M, Shimizu T, Ueda M, Matsuo K, et al. Clinical features and surgical outcome of hepatic caudate lobe metastases from colorectal cancer. Anticancer Res. 2006;26(2B):1447–1453. [PubMed] [Google Scholar]
- 28.Bhoori S, Sposito C, Germini A, Coppa J, Mazzaferro V. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int. 2010;23(7):712–722. doi: 10.1111/j.1432-2277.2010.01111.x. [DOI] [PubMed] [Google Scholar]
- 29.Yao FY, Kerlan RK, Jr, Hirose R, Davern TJ, 3rd, Bass NM, Feng S, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48(3):819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cillo U, Vitale A, Grigoletto F, Gringeri E, D’Amico F, Valmasoni M, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7(4):972–981. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 31.Lesurtel M, Mullhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006;6(11):2644–2650. doi: 10.1111/j.1600-6143.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 32.Maluf D, Fisher RA, Maroney T, Cotterell A, Fulcher A, Tisnado J, et al. Non-resective ablation and liver transplantation in patients with cirrhosis and hepatocellular carcinoma (HCC): safety and efficacy. Am J Transplant. 2003;3(3):312–317. doi: 10.1034/j.1600-6143.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 33.Otto G, Herber S, Heise M, Lohse AW, Monch C, Bittinger F, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 34.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8(12):2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 35.Seehofer D, Nebrig M, Denecke T, Kroencke T, Weichert W, Stockmann M, et al. Impact of neoadjuvant transarterial chemoembolization on tumor recurrence and patient survival after liver transplantation for hepatocellular carcinoma: a retrospective analysis. Clin Transplant. 2012;26(5):764–774. doi: 10.1111/j.1399-0012.2012.01609.x. [DOI] [PubMed] [Google Scholar]
- 36.Abdelfattah MR, Al-Sebayel M, Broering D, Alsuhaibani H. Radioembolization using yttrium-90 microspheres as bridging and downstaging treatment for unresectable hepatocellular carcinoma before liver transplantation: initial single-center experience. Transplant Proc. 2015;47(2):408–411. doi: 10.1016/j.transproceed.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Ahmadzadehfar HMC, Ezziddin S, et al. Hepatic volume changes induced by radioembolization with 90Y resin microspheres. A single-centre study. Eur J Nucl Med Mol Imaging. 2013;40(1):80–90. doi: 10.1007/s00259-012-2253-2. [DOI] [PubMed] [Google Scholar]
- 38.Chua TC, Bester L, Akther J, Morris DL. Successful right hepatectomy after four treatments of yttrium-90 microspheres (SIR-Spheres) and concomitant FOLFOX as bridging therapy to resection of colorectal liver metastases. Anticancer Res. 2010;30(7):3005–3007. [PubMed] [Google Scholar]
- 39.Edeline JLL, Boudjema K, et al. Volumetric changes after (90)y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol. 2013;20(8):2518–2525. doi: 10.1245/s10434-013-2906-9. [DOI] [PubMed] [Google Scholar]
- 40.Ettorre GM, Laurenzi A, Vennarecci G. Downstaging Hepatocellular Carcinoma with Yttrium-90 radioembolization: resection or transplantation? Eur J Surg Oncol. 2014;40(6):789–790. doi: 10.1016/j.ejso.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Garlipp B dBT, Damm R, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization cis substantial but less than after portal vein embolization. Hepatology. 2013. doi:10.1002/hep.26947 [DOI] [PubMed]
- 42.Gulec SA, Pennington K, Hall M, Fong Y. Preoperative Y-90 microsphere selective internal radiation treatment for tumor downsizing and future liver remnant recruitment: a novel approach to improving the safety of major hepatic resections. World J Surg Oncol. 2009;7:6. doi: 10.1186/1477-7819-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckman JT, Marsh JW, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15(11):3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 44.Henry LR, Hostetter RB, Ressler B, Bowser I, Yan M, Vaghefi H, et al. Liver resection for metastatic disease after y90 radioembolization: a case series with long-term follow-up. Ann Surg Oncol. 2015;22(2):467–474. doi: 10.1245/s10434-014-4012-z. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim SMKL, Baker T, et al. Treating and downstaging hepatocellular carcinoma in the caudate lobe with yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2012;35(5):1094–1101. doi: 10.1007/s00270-011-0292-x. [DOI] [PubMed] [Google Scholar]
- 46.Justinger C, Kouladouros K, Gartner D, Tatsch K, Reimer P, Rudiger T, et al. Liver resection after selective internal radiotherapy (SIRT): proof of concept, initial survival, and safety. J Surg Oncol. 2015;112(4):436–442. doi: 10.1002/jso.24000. [DOI] [PubMed] [Google Scholar]
- 47.Khalaf H, Alsuhaibani H, Al-Sugair A, Al-Mana H, Al-Mutawa A, Al-Kadhi Y, et al. Use of yttrium-90 microsphere radioembolization of hepatocellular carcinoma as downstaging and bridge before liver transplantation: a case report. Transplant Proc. 2010;42(3):994–998. doi: 10.1016/j.transproceed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Kulik L, Vouche M, Koppe S, Lewandowski RJ, Mulcahy MF, Ganger D, et al. Prospective randomized pilot study of Y90 ± sorafenib as bridge to transplantation in hepatocellular carcinoma. J Hepatol. 2014;61(2):309–317. doi: 10.1016/j.jhep.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 49.Kulik LMAB, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94(7):572. doi: 10.1002/jso.20609. [DOI] [PubMed] [Google Scholar]
- 50.Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240(2):299–305. doi: 10.1097/01.sla.0000133123.11932.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewandowski RJKL, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 52.Moir JA, Burns J, Barnes J, Colgan F, White SA, Littler P, et al. Selective internal radiation therapy for liver malignancies. Br J Surg. 2015;102(12):1533–1540. doi: 10.1002/bjs.9924. [DOI] [PubMed] [Google Scholar]
- 53.Mouli S, Memon K, Baker T, Benson AB, 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24(8):1227–1234. doi: 10.1016/j.jvir.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Servajean C, Gilabert M, Piana G, Monges G, Delpero JR, Brenot I, et al. One case of intrahepatic cholangiocarcinoma amenable to resection after radioembolization. World J Gastroenterol. 2014;20(17):5131–5134. doi: 10.3748/wjg.v20.i17.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperling J, Justinger C, Schuld J, Ziemann C, Seidel R, Kollmar O. Intrahepatic cholangiocarcinoma in a transplant liver–selective internal radiation therapy followed by right hemihepatectomy: report of a case. World J Surg Oncol. 2014;12:198. doi: 10.1186/1477-7819-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theysohn JM, Ertle J, Muller S, Schlaak JF, Nensa F, Sipilae S, et al. Hepatic volume changes after lobar selective internal radiation therapy (SIRT) of hepatocellular carcinoma. Clin Radiol. 2014;69(2):172–178. doi: 10.1016/j.crad.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Tohme SSD, Chen HW, et al. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24(11):1632–1638. doi: 10.1016/j.jvir.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Vouche MLR, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59(5):1029–1036. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whitney R, Tatum C, Hahl M, Ellis S, Scoggins CR, McMasters K, et al. Safety of hepatic resection in metastatic disease to the liver after yttrium-90 therapy. J Surg Res. 2011;166(2):236–240. doi: 10.1016/j.jss.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 60.Kulik LM, Mulcahy MF, Hunter RD, Nemcek AA, Jr, Abecassis MM, Salem R. Use of yttrium-90 microspheres (TheraSphere) in a patient with unresectable hepatocellular carcinoma leading to liver transplantation: a case report. Liver Transplant. 2005;11(9):1127–1131. doi: 10.1002/lt.20514. [DOI] [PubMed] [Google Scholar]
- 61.Bennink RJ, Cieslak KP, van Delden OM, van Lienden KP, Klumpen HJ, Jansen PL, et al. Monitoring of total and regional liver function after SIRT. Front Oncol. 2014;4:152. doi: 10.3389/fonc.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouazza F, Poncelet A, Garcia CA, Delatte P, Engelhom JL, Galdon MG, et al. Radioembolisation and portal vein embolization before resection of large hepatocellular carcinoma. World J Gastroenterol. 2015;21(32):9666–9670. doi: 10.3748/wjg.v21.i32.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edeline J, Du FL, Rayar M, Rolland Y, Beuzit L, Boudjema K, et al. Glass microspheres 90Y selective internal radiation therapy and chemotherapy as first-line treatment of intrahepatic cholangiocarcinoma. Clin Nucl Med. 2015;40(11):851–855. doi: 10.1097/RLU.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 64.El Fouly A, Ertle J, El Dorry A, Shaker MK, Dechene A, Abdella H, et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35(2):627–635. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- 65.Gaba RCLR, Kulik LM. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16(6):1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann RT, Jakobs TF, Kubisch CH, Stemmler HJ, Trumm C, Tatsch K, et al. Radiofrequency ablation after selective internal radiation therapy with Yttrium90 microspheres in metastatic liver disease-Is it feasible? Eur J Radiol. 2010;74(1):199–205. doi: 10.1016/j.ejrad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113(8):2119–2128. doi: 10.1002/cncr.23818. [DOI] [PubMed] [Google Scholar]
- 68.Jakobs TFSS, Atassi B, et al. Fibrosis, portal hypertension, and hepatic volume changes induced by intra-arterial radiotherapy with 90yttrium microspheres. Dig Dis Sci. 2008;53(9):2556–2563. doi: 10.1007/s10620-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 69.Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22(9):3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 71.Saxena A, Meteling B, Kapoor J, Golani S, Morris DL, Bester L. Is yttrium-90 radioembolization a viable treatment option for unresectable, chemorefractory colorectal cancer liver metastases? A large single-center experience of 302 patients. Ann Surg Oncol. 2015;22(3):794–802. doi: 10.1245/s10434-014-4164-x. [DOI] [PubMed] [Google Scholar]
- 72.Seidensticker R, Seidensticker M, Damm R, Mohnike K, Schutte K, Malfertheiner P, et al. Hepatic toxicity after radioembolization of the liver using (90)Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35(5):1109–1118. doi: 10.1007/s00270-011-0295-7. [DOI] [PubMed] [Google Scholar]
- 73.Teo JY, Goh BK, Cheah FK, Allen JC, Lo RH, Ng DC, et al. Underlying liver disease influences volumetric changes in the spared hemiliver after selective internal radiation therapy with 90Y in patients with hepatocellular carcinoma. J Dig Dis. 2014;15(8):444–450. doi: 10.1111/1751-2980.12162. [DOI] [PubMed] [Google Scholar]
- 74.Toskich BB, Tabriz DM, Zendejas I, Cabrera R, Geller B. Transportal radioembolization as salvage hepatocellular carcinoma therapy to maintain liver transplant candidacy. J Vasc Interv Radiol. 2015;26(10):1479–1483. doi: 10.1016/j.jvir.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 75.Vouche MHA, Ward TJ, et al. Unresectable solitary HCC not amenable to RFA: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014 doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 76.Salem RLR, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cescon M, Vetrone G, Grazi GL, Ramacciato G, Ercolani G, Ravaioli M, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg. 2009;249(6):995–1002. doi: 10.1097/SLA.0b013e3181a63c74. [DOI] [PubMed] [Google Scholar]
- 78.Dinant S, de Graaf W, Verwer BJ, Bennink RJ, van Lienden KP, Gouma DJ, et al. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48(5):685–692. doi: 10.2967/jnumed.106.038430. [DOI] [PubMed] [Google Scholar]
- 79.Bester L, Feitelson S, Milner B, Chua TC, Morris DL. Impact of prior hepatectomy on the safety and efficacy of radioembolization with yttrium-90 microspheres for patients with unresectable liver tumors. Am J Clin Oncol. 2014;37(5):454–460. doi: 10.1097/COC.0b013e31827deea1. [DOI] [PubMed] [Google Scholar]
- 80.Kennedy ASMP, Dezarn WA, et al. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74(5):1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Lam MG, Louie JD, Iagaru AH, Goris ML, Sze DY. Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36(5):1320–1328. doi: 10.1007/s00270-013-0547-9. [DOI] [PubMed] [Google Scholar]
- 82.de Graaf W, van Lienden K, Dinant S, et al. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14(2):369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denys APJ, Bize P, et al. Portal vein embolization: what do we know? Cardiovasc Intervent Radiol. 2012;35(5):999–1008. doi: 10.1007/s00270-011-0300-1. [DOI] [PubMed] [Google Scholar]
- 84.de Baere TTC, Deschamps F, et al. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17(8):2081–2089. doi: 10.1245/s10434-010-0979-2. [DOI] [PubMed] [Google Scholar]
- 85.Farges OBJ, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg Oncol. 2003;237(2):208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simoneau E, Aljiffry M, Salman A, Abualhassan N, Cabrera T, Valenti D, et al. Portal vein embolization stimulates tumour growth in patients with colorectal cancer liver metastases. HPB (Oxford). 2012;14(7):461–468. doi: 10.1111/j.1477-2574.2012.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Correa DSL, Jarnagin WR, et al. Kinetics of liver volume changes in the first year after portal vein embolization. Arch Surg. 2010;145(4):351–354. doi: 10.1001/archsurg.2010.42. [DOI] [PubMed] [Google Scholar]
- 88.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34(2):267–272. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]
- 89.Al-Sharif E, Simoneau E, Hassanain M. Portal vein embolization effect on colorectal cancer liver metastasis progression: lessons learned. World J Clin Oncol. 2015;6(5):142–146. doi: 10.5306/wjco.v6.i5.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Graaf W, Bennink RJ, Vetelainen R, van Gulik TM. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J Nucl Med. 2010;51(5):742–752. doi: 10.2967/jnumed.109.069435. [DOI] [PubMed] [Google Scholar]
- 91.Geisel D, Ludemann L, Hamm B, Denecke T. Imaging-based liver function tests-past. Present and Future. Rofo. 2015;187(10):863–871. doi: 10.1055/s-0035-1553306. [DOI] [PubMed] [Google Scholar]
- 92.Krishnamurthy GTKS. Nuclear hepatology: a textbook of hepatobiliary diseases. New York: Springer; 2000. [Google Scholar]
- 93.Nanashima AYH, Shibasaki S, et al. Relationship between indocyanine green test and technetium-99 m galactosyl serum albumin scintigraphy in patients scheduled for hepatectomy: clinical evaluation and patient outcome. Hepatol Res. 2004;28:184–190. doi: 10.1016/j.hepres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Hirai IKW, Fuse A, et al. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99 mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506. doi: 10.1067/msy.2003.138. [DOI] [PubMed] [Google Scholar]
- 95.Bennink RJDS, Erdogan D, et al. Preoperative assessment of postoperative remnant liver function using hepatobiliary sintigraphy. J Nucl Med. 2004;45(6):965–971. [PubMed] [Google Scholar]
- 96.Kono Y, Kariya S, Komemushi A, Nakatani M, Yoshida RY, Suzuki S, et al. Comparison of Tc-99m GSA scintigraphy and CT volumetry for evaluation in portal vein embolization. Minim Invasive Ther Allied Technol. 2014;23(4):241–246. doi: 10.3109/13645706.2014.897955. [DOI] [PubMed] [Google Scholar]
- 97.de Graaf W, van Lienden K, van den Esschert JW, et al. Increase in future remnant function after preoperative portal vein embolization. Br J Surg. 2011;98:825–834. doi: 10.1002/bjs.7456. [DOI] [PubMed] [Google Scholar]
- 98.Nishiyama YYY, Hino I, et al. 99mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous tranhepatic portal embolization. Nucl Med Commun. 2003;24:809–817. doi: 10.1097/00006231-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Gil-Alzugaray BCA, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57(3):1078–1087. doi: 10.1002/hep.26191. [DOI] [PubMed] [Google Scholar]
- 100.Burton MA, Gray BN, Klemp PF, Kelleher DK, Hardy N. Selective internal radiation therapy: distribution of radiation in the liver. Eur J Cancer Clin Oncol. 1989;25(10):1487–1491. doi: 10.1016/0277-5379(89)90109-0. [DOI] [PubMed] [Google Scholar]
- 101.Wondergem MSM, Elschot M, et al. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54(8):1294–1301. doi: 10.2967/jnumed.112.117614. [DOI] [PubMed] [Google Scholar]
- 102.De Giorgio M, Vezzoli S, Cohen E, Armellini E, Luca MG, Verga G, et al. Prediction of progression-free survival in patients presenting with hepatocellular carcinoma within the Milan criteria. Liver Transplant. 2010;16(4):503–512. doi: 10.1002/lt.22039. [DOI] [PubMed] [Google Scholar]
- 103.Van Beers BE, Pastor CM, Hussain HK. Primovist, eovist: what to expect? J Hepatol. 2012;57(2):421–429. doi: 10.1016/j.jhep.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 104.Okamoto D, Nishie A, Asayama Y, Tajima T, Ishigami K, Kakihara D, et al. Gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced MR finding of radiation-induced hepatic injury: relationship to absorbed dose and time course after irradiation. Magn Reson Imaging. 2014;32(6):660–664. doi: 10.1016/j.mri.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 105.Seidensticker M, Burak M, Kalinski T, Garlipp B, Koelble K, Wust P, et al. Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol. 2015;38(1):213–221. doi: 10.1007/s00270-014-0872-7. [DOI] [PubMed] [Google Scholar]
- 106.Powerski MJ, Scheurig-Munkler C, Hamm B, Gebauer B. Impaired hepatic Gd-EOB-DTPA enhancement after radioembolisation of liver malignancies. J Med Imaging Radiat Oncol. 2014;58(4):472–480. doi: 10.1111/1754-9485.12187. [DOI] [PubMed] [Google Scholar]
- 107.Bae KE, Kim SY, Lee SS, Kim KW, Won HJ, Shin YM, et al. Assessment of hepatic function with Gd-EOB-DTPA-enhanced hepatic MRI. Dig Dis. 2012;30(6):617–622. doi: 10.1159/000343092. [DOI] [PubMed] [Google Scholar]
- 108.Donahue LA, Kulik L, Baker T, Ganger DR, Gupta R, Memon K, et al. Yttrium-90 radioembolization for the treatment of unresectable hepatocellular carcinoma in patients with transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 2013;24(1):74–80. doi: 10.1016/j.jvir.2012.09.030. [DOI] [PubMed] [Google Scholar]