Abstract
Purpose
To investigate the clinical and morphological characteristics in relation to risk of bifurcation intracranial aneurysm rupture.
Materials and Methods
Data from 202 consecutive patients with 219 bifurcation aneurysms (129 ruptured and 90 unruptured) managed at the authors' facility between August 2011 and July 2014 were retrospectively reviewed. Based on their clinical records and CT angiographic findings, the ability of risk factors to predict aneurysm rupture was assessed using statistical methods.
Results
Age, hypertension, diabetes mellitus, and cerebral atherosclerosis were negatively correlated with aneurysm rupture. Aneurysms located in the middle cerebral artery, daughter artery ratio, lateral angle ratio (LA ratio), and neck width were negatively correlated with rupture. Aneurysms located in the anterior communicating artery, irregularity, with daughter sac, depth, width, maximum size, aspect ratio (AR), depth-to-width ratio, and bottleneck factor were significantly and positively correlated with rupture. Binary logistic regression model revealed that irregular shape [odds ratio (OR) 6.598] and AR (OR 3.507) strongly increased the risk of bifurcation aneurysm rupture, while age (OR 0.434), cerebral atherosclerosis (OR 0.125), neck width (OR 0.771), and LA ratio (OR 0.267) were negatively correlated with rupture (p<0.05). Receiver operating characteristic analysis revealed the threshold values of AR and LA ratio to be 1.18 and 1.50, respectively.
Conclusion
Age (≥60 yr), cerebral atherosclerosis, and aneurysms with a larger neck width and larger LA ratio are protective factors against bifurcation aneurysm rupture. An aneurysm with an irregular shape and an increased AR reflect the greater likelihood of a rupture.
Keywords: Intracranial aneurysm, rupture, risk factors, subarachnoid hemorrhage, computed tomography, angiography
INTRODUCTION
Ruptured intracranial aneurysms (RIAs) are the most common cause of subarachnoid hemorrhage (SAH), causing high mortality and morbidity rates up to 40–50% and 10–20%, respectively.1 However, only 1% of all intracranial aneurysms (IAs) actually rupture,2 and the surgical and endovascular treatments for unruptured intracranial aneurysms (UIAs) are not without risk.3 Thus, the ability to predict the rupture risk for UIAs would be of enormous clinical value.
IAs usually occur at Willis' or arterial bifurcations, and many cases of SAH arise from bifurcation aneurysms. The International Study of Unruptured Intracranial Aneurysms (ISUIA) reported that treatment decisions regarding UIAs are to be based mainly on the size and location of the aneurysm.4 A recent study suggested that the risk factors of UIAs are related to a difference between sidewall- and bifurcation-type aneurysms,5 although they did not provide comprehensive results on the clinical parameters associated with the rupture of aneurysms. For this reason, the purpose of this study was to identify the relationships between personal factors and image characteristics and the rupture of bifurcation IAs.
MATERIALS AND METHODS
Patients
All protocols were reviewed and approved by the local institutional ethics committee. The clinical data for the study were extracted from the hospital medical records, including age, gender, history of hypertension, heart disease, diabetes mellitus, cerebral atherosclerosis, alcohol consumption, cigarette smoking, and history of SAH. The patients' records/information were anonymized and de-identified prior to analysis, and the requirement for informed consent was waived.
From August 2011 and July 2014, a total of 246 consecutive patients with bifurcation aneurysms underwent computer tomography angiography (CTA) examinations. We included patients with bifurcation aneurysms that were managed with both treatment (coiling or clipping) and observation. Among these, 44 patients with mycotic, traumatic, extradural, or fusiform aneurysms, aneurysms with poor image quality, and aneurysms with a maximum diameter <1.8 mm (too small to measure accurately) were not eligible for this study. Thus, 202 patients (16 patients with multiple aneurysms) with 219 bifurcation aneurysms (129 ruptured and 90 unruptured aneurysms) were finally included. In cases with multiple aneurysms, the ruptured aneurysm was determined based on the location of the hemorrhage on CT, angiographic, or operative findings.
Computer tomography angiography
IAs were evaluated with CTA using a 64-slice CT machine (GE LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA) and a dedicated workstation (Advantage Windows 4.5). A total of 80 mL of non-ionic contrast medium (Visipaque 320; GE Healthcare) was injected into the antecubital vein at a rate of 4–4.5 mL/s. Next, the images were obtained with a slice thickness of 0.625 mm and no overlap, and were transferred to the GE Advantix workstation to generate three-dimensional (3D) volume rendering (VR).
Image analysis
Bifurcation aneurysms were defined as lesions originating from major bifurcations,5 including the anterior cerebral artery (ACA), anterior communicating artery (ACoA), posterior communicating artery (PCoA), internal carotid artery (ICA), middle cerebral artery (MCA), and top of the basilar artery (TOBA). PCoA aneurysms were classified as bifurcations depending on the size of the PCoA relative to the ICA diameter (more than one-fifth the diameter).5 Based on the neck location, the neck types were classified into two types:6,7,8 Type C, the neck was located along the extension of the midline axis of the parent artery, and Type D, the neck deviated from the midline axis of the parent artery (Fig. 1 and Fig. 2).
Fig. 1. The image of an aneurysm is classified as a classical neck type (Type C). The larger DA is defined as DA A, and the other DA is defined as DA B. LA mean diameter of the vessel measure at the LA1 and LA2, and the other vessel is measured as LB; the DA ratio is defined as LA/LB. The angle between the parent artery and DA A is defined as angle A, and the angle between the parent artery and DA B is defined as angle B; the LA ratio is defined as angle A/angle B. DA, daughter artery; LA ratio, lateral angle ratio; LA, diameter of DA A; LB, diameter of DA B.
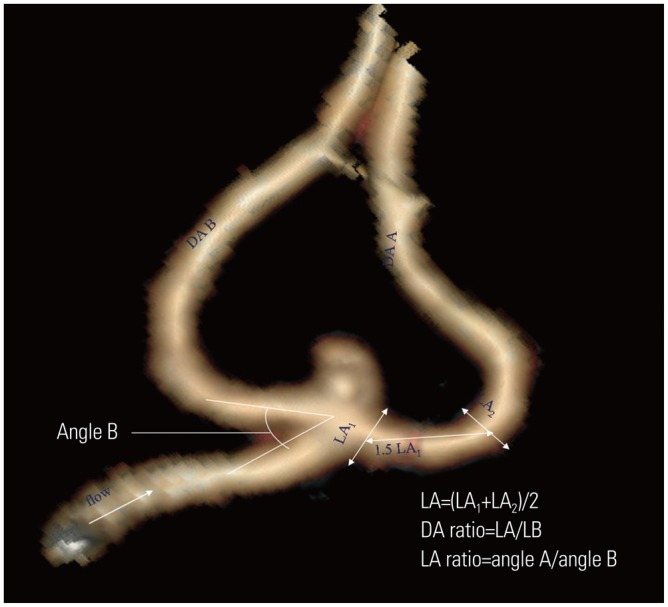
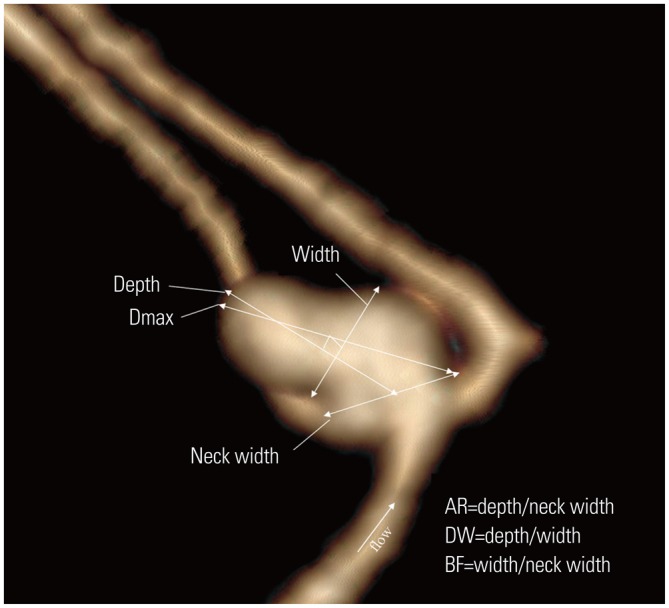
Fig. 2. The image of an aneurysm is classified as a deviated neck type (Type D). The image illustrates the method of dimension measurements: neck width, depth (the longest diameter between the neck and dome), width (the maximum distance vertical to height) and maximum size (Dmax, the largest measurement in terms of maximum dome diameter or width). Aspect ratio (AR) is calculated as depth divided by neck width, depth-to-width ratio (DW) is calculated as depth divided by width, and the bottleneck factor (BF) is calculated as width divided by neck width.
The 3D VR images were manually measured independently by two neuro-radiologists. The mean values from two radiologists were used for analysis. Controversial cases were resolved through discussion. The diameters of the two daughter arteries of the bifurcation were measured: the larger daughter artery (DA) was defined as DA A, and the smaller one defined as DA B. Next, the DA ratio (diameter of DA A/diameter of DA B) was calculated.6,7,8 There are two angles on the lateral side of the bifurcation that are called lateral angles (LAs).6,7,8 The angle between the parent artery and DA A was defined as angle A, and another angle between the parent artery and DA B was defined as angle B; the LA ratio (angle A/angle B) was then calculated (Fig. 1).
The following four dimensions were measured in the plane parallel to the blood flow of the parent arteries: aneurysm depth, width, neck width,5 and maximum size (Dmax).9,10 The aspect ratio (AR), the depth-to-width ratio (DW), and the bottleneck factor (BF) were then calculated (Fig. 2).5,10,11,12 The aneurysm shapes were classified as simple lobed or irregular, and an aneurysm with lobular or daughter sacs was classified as irregular.5
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA, version 17.0). In the current study, rupture events were assessed as a dependent variable in the model. Independent t-test and chisquared test were used to compare the means for continuous data and categorical data, respectively. All variables with a p value less than 0.2 were entered into a logistic regression model. The odds ratio (OR) and 95% confidence interval (CI) were also calculated. Binary logistic regression was performed separately, using forward stepwise regression to control for those features that achieved univariate statistical significance (p<0.05). Receiver operating characteristic (ROC) curve analysis was also performed to determine the sensitivity and specificity of the area under the curve values.
RESULTS
Of the 202 patients, 119 (58.9%) were women and 83 (41.1%) were men. The mean age of all of the patients was 58.2±11.91 years (range, 21–84 years), 56.7±12.01 years among the males (range, 21–84 years) and 59.3±11.77 years among the females (range, 31–84 years). Because the mean patient age was 58.2 years, 60 years was chosen to dichotomize the sample. Chisquared test were used to compare means for the patient characteristics (Table 1). Independent t-test and chi-squared test were used to compare the means for the morphological features of aneurysms (Table 2). To determine the risk factors predisposing the patients to aneurysm rupture, 17 independent variables (p≤0.2) were entered into a univariate logistic regression model (Table 3). Of these variables, 16 independent variables were found to be significant predictors of aneurysm rupture (p≤0.05). Next, these variables were entered into a forward stepwise binary logistic regression model (Table 4). Among these variables, the binary logistic regression model showed that aneurysms with an irregular shape (OR 6.598) and AR (OR 3.507) strongly increased the risk of aneurysm rupture. By contrast, age (OR 0.434), cerebral atherosclerosis (OR 0.125), neck width (OR 0.771), and the LA ratio (OR 0.267) were associated with a decreased risk of aneurysm rupture.
Table 1. Patient Characteristics with Ruptured and Unruptured Aneurysms.
| Factors | Patient groups | p value | |
|---|---|---|---|
| Unruptured | Ruptured | ||
| Patients No. | 73 (36.1%) | 129 (63.9%) | |
| Female | 40 (54.8%) | 79 (61.2%) | 0.371 |
| Age (≥60 yr) | 24 (32.9%) | 63 (48.8%) | 0.028 |
| Hypertension | 37 (50.7%) | 45 (34.9%) | 0.028 |
| Heart disease | 4 (5.5%) | 3 (2.3%) | 0.437 |
| Diabetes mellitus | 8 (11.0%) | 2 (1.6%) | 0.009 |
| Cerebral atherosclerosis | 30 (41.1%) | 8 (6.2%) | 0.000 |
| Alcohol history | 16 (21.9%) | 33 (25.6%) | 0.560 |
| Cigarette smoking | 16 (21.9%) | 34 (26.4%) | 0.483 |
| Bleeding history | 5 (6.9%) | 3 (2.3%) | 0.8 |
| Multiple aneurysms | 5 (6.9%) | 11 (8.5%) | 0.671 |
Table 2. The Morphological Features of Aneurysms.
| Factors | Patient groups | p value | |
|---|---|---|---|
| Unruptured | Ruptured | ||
| No. of aneurysm | 90 (41.1%) | 129 (58.9%) | |
| Location | |||
| ACoA | 21 (23.3%) | 57 (44.2%) | 0.000 |
| ACA | 1 (1.1%) | 3 (2.3%) | 0.883 |
| MCA | 30 (33.3%) | 23 (17.8%) | 0.008 |
| PComa | 31 (34.4%) | 38 (29.5%) | 0.434 |
| ICA | 6 (6.7%) | 3 (2.3%) | 0.213 |
| PCC | 1 (1.1%) | 5 (3.9%) | 0.416 |
| Irregular shape | 28 (31.1%) | 91 (70.5%) | 0.000 |
| Daughter sac | 18 (20.0%) | 65 (50.4%) | 0.000 |
| Type C | 45 (50%) | 63 (48.8%) | 0.866 |
| DA ratio | 1.70±0.75 | 1.56±0.61 | 0.138 |
| LA ratio | 1.51±0.85 | 1.34±0.60 | 0.104 |
| Depth | 4.64±2.83 | 6.08±2.51 | 0.000 |
| Width | 4.65±2.40 | 5.27±2.53 | 0.068 |
| Neck width | 4.55±1.90 | 4.08±1.51 | 0.044 |
| Maximum diameter | 5.72±2.93 | 7.20±2.76 | 0.000 |
| Aspect ratio | 1.03±0.45 | 1.60±0.70 | 0.000 |
| Depth/width ratio | 1.00±0.31 | 1.24±0.40 | 0.000 |
| Bottleneck factor | 1.02±0.30 | 1.33±0.52 | 0.000 |
ACoA, anterior communicating artery; ACA, anterior cerebral artery; MCA, middle cerebral artery; PComa, posterior communicating artery; ICA, internal carotid artery; PCC, posterior cerebral circulation; DA ratio, daughter artery ratio; LA ratio, lateral angle ratio.
Table 3. Univariate Logistic Regression Model for Prediction of Aneurysm Rupture.
| Characteristic | Odds ratio | p value | 95% CI | β |
|---|---|---|---|---|
| Age (≥60 yr) | 0.300 | 0.000 | 0.165-0.547 | -1.203 |
| Hypertension | 0.521 | 0.029 | 0.291-0.935 | -0.652 |
| Diabetes mellitus | 0.128 | 0.011 | 0.026-0.620 | -2.056 |
| Cerebral atherosclerosis | 0.095 | 0.000 | 0.040-0.223 | -2.356 |
| Location | ||||
| ACoA | 6.810 | 0.000 | 3.385-13.698 | 1.918 |
| MCA | 0.798 | 0.477 | 0.427-1.488 | -0.226 |
| Irregular shape | 4.854 | 0.000 | 2.717-8.671 | 1.580 |
| Daughter sac | 3.679 | 0.000 | 1.994-6.790 | 1.303 |
| DA ratio | 0.836 | 0.046 | 0.701-0.997 | -0.179 |
| LA ratio | 0.650 | 0.000 | 0.546-0.773 | -0.431 |
| Depth | 1.125 | 0.000 | 1.077-1.174 | 0.118 |
| Width | 1.074 | 0.001 | 1.028-1.122 | 0.071 |
| Neck width | 0.808 | 0.000 | 0.759-0.860 | -0.214 |
| Maximum diameter | 1.132 | 0.046 | 1.086-1.180 | 0.124 |
| Aspect ratio | 5.658 | 0.000 | 4.234-7.560 | 1.733 |
| Depth/width ratio | 3.225 | 0.000 | 2.223-4.679 | 1.171 |
| Bottleneck factor | 7.191 | 0.000 | 4.998-10.347 | 1.973 |
CI, confidence interval; ACoA, anterior communicating artery; MCA, middle cerebral artery; DA ratio, daughter artery ratio; LA ratio, lateral angle ratio.
Table 4. Binary Logistic Regression Model for Prediction of Aneurysm Rupture.
| Characteristics | Odds ratio | p value | 95% CI | β |
|---|---|---|---|---|
| Age | 0.434 | 0.042 | 0.194-0.971 | -0.835 |
| Cerebral atherosclerosis | 0.125 | 0.000 | 0.041-0.382 | -2.081 |
| Irregular shape | 6.598 | 0.000 | 2.563-16.985 | 1.887 |
| Neck width | 0.771 | 0.048 | 0.596-0.998 | -0.260 |
| Aspect ratio | 3.507 | 0.004 | 1.494-8.234 | 1.255 |
| LA ratio | 0.267 | 0.000 | 0.149-0.477 | -1.320 |
CI, confidence interval; LA ratio, lateral angle ratio.
To identify optimal thresholds for RIAs, ROC analysis (excluding the outliers) was performed for AR, neck width, and LA ratio. The resulting curves are shown in Fig. 3,Fig. 4, and Fig. 5. The AUC, threshold value, and sensitivity and specificity values were calculated for a range of thresholds (Table 5). Our data showed that the threshold value of the neck width was 4.35 mm, although the AUC value was small (0.566, p=0.097). The threshold values of the AR and LA ratio were 1.18 and 1.50, respectively, and the corresponding AUC values were 0.781 and 0.622, respectively.
Fig. 3. The area under the receiver operating characteristic curve for the neck width is 0.566 (95% confidence interval, 0.490–0.642). The cut point for the neck width is 4.35 mm, the sensitivity is 67.4%, and the specificity is 40%.
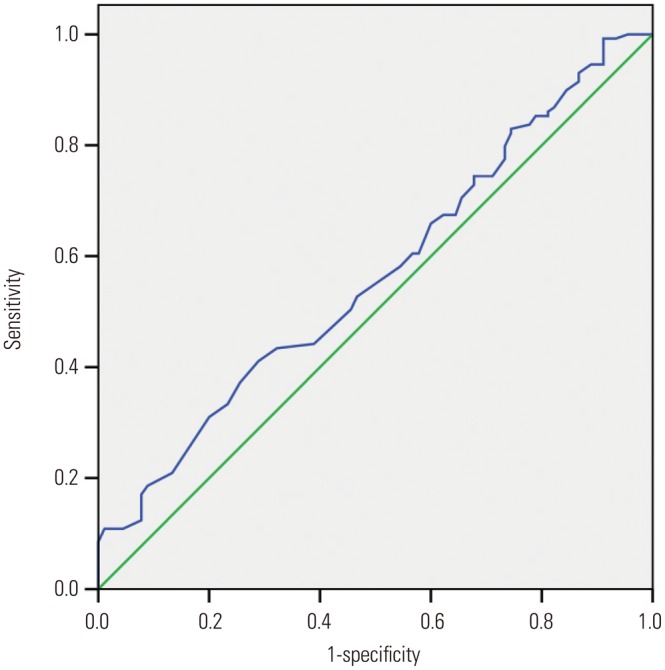
Fig. 4. The area under the receiver operating characteristic curve for the aspect ratio is 0.781 (95% confidence interval, 0.719–0.844). The cut point for the aspect ratio is 1.18, the sensitivity is 70.5%, and the specificity is 74.4%.
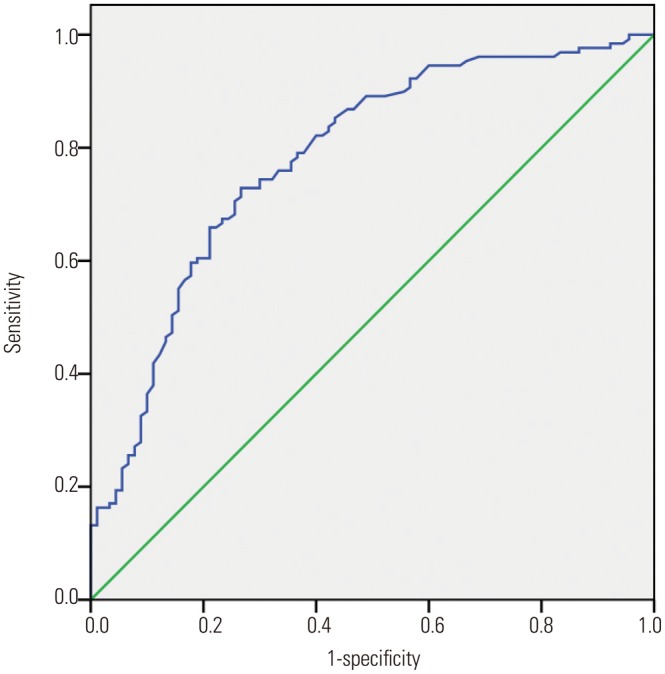
Fig. 5. The area under the receiver operating characteristic curve for the lateral angle ratio is 0.622 (95% confidence interval, 0.543–0.711). The cut point for the lateral angle ratio is 1.50, the sensitivity is 73.6%, and the specificity is 48.9%.
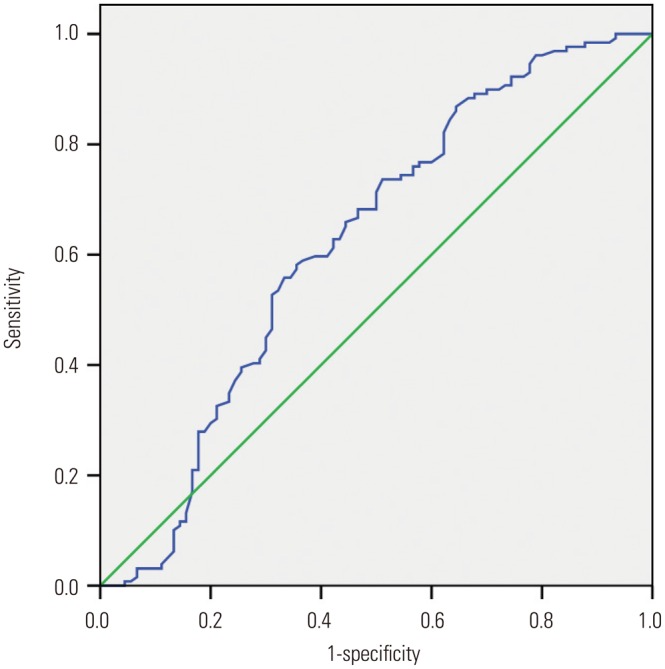
Table 5. Area Under the Curve for Neck Width, Aspect Ratio, and Lateral Angle Ratio.
| Characteristics | Area | Threshold value (mm) | p value | Sen (%) | Spe (%) | 95% CI |
|---|---|---|---|---|---|---|
| Neck width | 0.566 | 4.35 | 0.097 | 67.4 | 40 | 0.490-0.642 |
| Aspect ratio | 0.781 | 1.18 | 0.000 | 70.5 | 74.4 | 0.719-0.844 |
| LA ratio | 0.622 | 1.50 | 0.002 | 73.6 | 48.9 | 0.543-0.711 |
CI, confidence interval; LA ratio, lateral angle ratio.
DISCUSSION
As an increasing number of UIAs are detected incidentally with the increased use of imaging, the treatment of asymptomatic UIAs continues to be debated. However, surgical and endovascular treatments for asymptomatic aneurysms are not always safe. In recent reports, many studies on UIAs have focused on rupture risk factors; however, results differ for different countries or regions,1,2,4,5,6,7,8,9,11,12,13,14 and few papers have described the patterns of IAs in Chinese populations.
Clinical characteristics
In the current study, we revealed that patient characteristics including age and cerebral atherosclerosis are associated with a decreased risk of aneurysm rupture. It is well known that atherosclerotic or calcified walls will lower the risk of IA rupture.14 With increased age, the risk of cerebral atherosclerosis will be higher. Popular factors, such as smoking, alcohol consumption, and hypertension, were not significantly associated with the risk of RIAs. The reasons for these findings may possibly be that most patients in this study had healthy lifestyles and mild to moderate hypertension.
Morphological characteristics
At the same time, we showed that irregular aneurysms and AR strongly increased the risk of aneurysm rupture; neck width and LA ratio lowered the risk of aneurysm rupture. Aneurysm wall irregularity has been associated with a higher rupture risk:15 the reason may be that an irregular shape leads to instability of the blood flow pattern. Traditionally, aneurysm size, including the height, width, and maximum size, has been reported to be an unquestionable factor regarding rupture risk. It is generally believed that larger aneurysms are more prone to rupture than smaller ones. The ISUIA showed a near-zero rupture risk for aneurysms <10 mm in diameter.16 However, smaller aneurysms may also rupture. In fact, in 94.4% of ACoAs and 87.5% of PCoAs, the RIA size was less than 10 mm,17 a finding that was concordant with our data and explained why size is not a rupture risk factor. Neck width has also been reported in many studies, most of which showed no significantly statistical difference.15,18 Our data indicated that the mean neck width for unruptured and ruptured aneurysms was 4.55 mm and 4.08 mm, respectively, and there was a slight inverse correlation with aneurysm rupture (p=0.048), possibly indicating that small necks induce small inflow jets and small concentrated impaction zones. The AR has been studied widely, and was shown to correlate with IA rupture.11,19,20 Different studies have varying threshold values of the AR. Our data showed that the threshold value of the AR was 1.18, a value that was significantly smaller than that for most of Caucasian and Japanese populations,20,21 but concordant with that reported by Dhar, et al.22
This study focused on aneurysms located in bifurcating arteries. Of all aneurysms, ACoA is the most common site and ACoA aneurysms account for the largest percentage of ruptured aneurysms. Although, the ISUIA showed that location is correlated with aneurysm rupture4 in multivariate logistic regression, we found no differences in relation to location. Recent studies have indicated that location is not associated with increased rupture risk after a long period of follow-up,12,23,24,25 which is consistent with our study. Comparing the results of previous literature,6,7,8 we investigated neck position, DA ratio, and LA ratio. In contrast, our results showed that neck type and DA ratio do not show significant differences between RIAs and UIAs, and that LA ratio significantly lowers the risk of aneurysm rupture. The reasons might be that our cases numbered more than those in the previous studies, and they studied single location. Not with standing, the present data need to be confirmed in further studies.
Limitations of the study
Our study has some limitations. First, it was a retrospective analysis of patients at a single institution. Second, the shape or size of the RIAs might have changed owing to the rupture. Third, we did not perform a long-term follow up examination, and the UIAs may be ruptured at some point in the future. Fourth, we focused on bifurcation aneurysms, not including sidewall aneurysms, and did not study the relationships between multiple aneurysms. We will further strengthen our study in the future.
In conclusion, selecting a unique risk factor for predictive aneurysm rupture remains difficult. From clinical records and CTA findings from patients with bifurcation aneurysms, we found that advanced age, cerebral atherosclerosis, a large neck width, and a large LA ratio were protective factors against bifurcation aneurysm rupture; bifurcation aneurysm with an irregular shape and a high AR were more prone to rupture.
ACKNOWLEDGEMENTS
The authors thank American Journal Experts (AJE) for assisting in the preparation of this paper.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Ujiie H, Sato K, Onda H, Oikawa A, Kagawa M, Takakura K, et al. Clinical analysis of incidentally discovered unruptured aneurysms. Stroke. 1993;24:1850–1856. doi: 10.1161/01.str.24.12.1850. [DOI] [PubMed] [Google Scholar]
- 3.Jang EW, Kim YB, Chung J, Suh SH, Hong CK, Joo JY. Clinical risk factors affecting procedure-related major neurological complications in unruptured intracranial aneurysms. Yonsei Med J. 2015;56:987–992. doi: 10.3349/ymj.2015.56.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 5.Baharoglu MI, Lauric A, Gao BL, Malek AM. Identification of a dichotomy in morphological predictors of rupture status between sidewall- and bifurcation-type intracranial aneurysms. J Neurosurg. 2012;116:871–881. doi: 10.3171/2011.11.JNS11311. [DOI] [PubMed] [Google Scholar]
- 6.Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008;62:602–609. doi: 10.1227/01.NEU.0000311347.35583.0C. [DOI] [PubMed] [Google Scholar]
- 7.Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. Evaluation of relation among aneurysmal neck, parent artery, and daughter arteries in middle cerebral artery aneurysms, by three-dimensional digital subtraction angiography. Neurosurg Rev. 2005;28:196–200. doi: 10.1007/s10143-005-0379-4. [DOI] [PubMed] [Google Scholar]
- 8.Sadatomo T, Yuki K, Migita K, Taniguchi E, Kodama Y, Kurisu K. The characteristics of the anterior communicating artery aneurysm complex by three-dimensional digital subtraction angiography. Neurosurg Rev. 2006;29:201–207. doi: 10.1007/s10143-006-0025-9. [DOI] [PubMed] [Google Scholar]
- 9.Rahman M, Ogilvy CS, Zipfel GJ, Derdeyn CP, Siddiqui AH, Bulsara KR, et al. Unruptured cerebral aneurysms do not shrink when they rupture: multicenter collaborative aneurysm study group. Neurosurgery. 2011;68:155–160. doi: 10.1227/NEU.0b013e3181ff357c. [DOI] [PubMed] [Google Scholar]
- 10.Lim YC, Kim CH, Kim YB, Joo JY, Shin YS, Chung J. Incidence and risk factors for rebleeding during cerebral angiography for ruptured intracranial aneurysms. Yonsei Med J. 2015;56:403–409. doi: 10.3349/ymj.2015.56.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu CW, Kwon OK, Koh JS, Kim EJ. Analysis of aneurysm rupture in relation to the geometric indices: aspect ratio, volume, and volume-to-neck ratio. Neuroradiology. 2011;53:883–889. doi: 10.1007/s00234-010-0804-4. [DOI] [PubMed] [Google Scholar]
- 12.You SH, Kong DS, Kim JS, Jeon P, Kim KH, Roh HK, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry. 2010;81:479–484. doi: 10.1136/jnnp.2008.169573. [DOI] [PubMed] [Google Scholar]
- 13.Aarhus M, Helland CA, Wester K. Differences in anatomical distribution, gender, and sidedness between ruptured and unruptured intracranial aneurysms in a defined patient population. Acta Neurochir (Wien) 2009;151:1569–1574. doi: 10.1007/s00701-009-0316-3. [DOI] [PubMed] [Google Scholar]
- 14.Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155–164. doi: 10.1016/j.surneu.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Lall RR, Eddleman CS, Bendok BR, Batjer HH. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26:E2. doi: 10.3171/2009.2.FOCUS0921. [DOI] [PubMed] [Google Scholar]
- 16.Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention.International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 17.Bacigaluppi S, Piccinelli M, Antiga L, Veneziani A, Passerini T, Rampini P, et al. Factors affecting formation and rupture of intracranial saccular aneurysms. Neurosurg Rev. 2014;37:1–14. doi: 10.1007/s10143-013-0501-y. [DOI] [PubMed] [Google Scholar]
- 18.Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61:716–722. doi: 10.1227/01.NEU.0000298899.77097.BF. [DOI] [PubMed] [Google Scholar]
- 19.Beck J, Rohde S, el Beltagy M, Zimmermann M, Berkefeld J, Seifert V, et al. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir (Wien) 2003;145:861–865. doi: 10.1007/s00701-003-0124-0. [DOI] [PubMed] [Google Scholar]
- 20.Ujiie H, Tamano Y, Sasaki K, Hori T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. 2001;48:495–502. doi: 10.1097/00006123-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Nader-Sepahi A, Casimiro M, Sen J, Kitchen ND. Is aspect ratio a reliable predictor of intracranial aneurysm rupture? Neurosurgery. 2004;54:1343–1347. doi: 10.1227/01.neu.0000124482.03676.8b. [DOI] [PubMed] [Google Scholar]
- 22.Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui AH, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. 2008;63:185–196. doi: 10.1227/01.NEU.0000316847.64140.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2008;108:1052–1060. doi: 10.3171/JNS/2008/108/5/1052. [DOI] [PubMed] [Google Scholar]
- 24.Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269:258–265. doi: 10.1148/radiol.13121188. [DOI] [PubMed] [Google Scholar]
- 25.Mehan WA, Jr, Romero JM, Hirsch JA, Sabbag DJ, Gonzalez RG, Heit JJ, et al. Unruptured intracranial aneurysms conservatively followed with serial CT angiography: could morphology and growth predict rupture? J Neurointerv Surg. 2014;6:761–766. doi: 10.1136/neurintsurg-2013-010944. [DOI] [PubMed] [Google Scholar]


