Abstract
Identifying predictors and elucidating the fundamental mechanisms underlying onset of psychosis are critical for the development of targeted preemptive interventions. This article presents a selective review of findings on risk prediction algorithms and potential mechanisms of onset in youth at clinical high-risk for psychosis, focusing principally on recent findings of the North American Prodrome Longitudinal Study (NAPLS). Multivariate models incorporating risk factors from clinical, demographic, neurocognitive, and psychosocial assessments achieve high levels of predictive accuracy when applied to individuals who meet criteria for a prodromal risk syndrome. An individualized risk calculator is available to scale the risk for newly ascertained cases, which could aid in clinical decision making. At risk individuals who convert to psychosis show elevated levels of proinflammatory cytokines, as well as disrupted resting state thalamo-cortical functional connectivity at baseline, compared with those who do not. Further, converters show a steeper rate of gray matter reduction, most prominent in prefrontal cortex, that in turn is predicted by higher levels of inflammatory markers at baseline. Microglia, resident immune cells in the brain, have recently been discovered to influence synaptic plasticity in health and impair plasticity in disease. Processes that modulate microglial activation may represent convergent mechanisms that influence brain dysconnectivity and risk for onset of psychosis and thus may be targetable in developing and testing preventive interventions.
Key words: psychosis, prodrome, prevention
Introduction
Given that currently available pharmacological treatments for schizophrenia are limited and poorly tolerated,1 with most patients continuing to show substantial deficits in social and occupational functioning throughout life, there is considerable interest in exploring preventive approaches to the disorder.2–4 The 3 primary challenges for realizing such a prevention strategy are: (1) developing reliable and efficient means to predict psychosis, so that we can identify populations at greatest risk; (2) elucidating changes at the neural and molecular levels that participate mechanistically in functional decline and onset of full symptoms; and (3) developing and testing interventions targeting the contributing molecular signaling pathways. This article selectively reviews evidence concerning these 3 interrelated aims, focusing primarily on recent findings of the North American Prodrome Longitudinal Study (NAPLS).
Prediction of Psychosis
For the majority of patients with schizophrenia and related disorders, onset of fully psychotic symptoms is preceded by the emergence of subtler changes in belief, thought, and perception that appear to represent attenuated forms of delusions, formal thought disorder, and hallucinations, respectively.5 A young person with an onset or worsening of these features within the past 12 months and who is distressed and treatment seeking is said to have a clinical high-risk (CHR) or prodromal risk syndrome.6 According to a meta-analysis incorporating data from 27 studies comprising a total of 2502 patients, 22% of such cases transitioned to a fully psychotic form of illness by 1 year and 36% by 3 years from initial ascertainment.7 Because most studies employ follow-up periods of 3 years or less, the rate of conversions after this point remains unclear. Nevertheless, most of the conversions occur during the first year following ascertainment, and the conversion rate significantly decelerates thereafter, suggesting that the CHR criteria are sensitive to an imminent risk for onset of full psychosis.8 Among those who convert, about 80% of the diagnostic outcomes are in the schizophrenia spectrum and the remaining 20% are in relation to mood-related and atypical forms of psychosis. Importantly, among the ~64% who do not convert, roughly half have been observed to remit the symptoms that indexed their initial risk status and improve functionally, while the remainder show continuing levels of attenuated psychotic-like symptoms and functional impairment.9,10 It remains unclear whether some of those who remit subsequently revert to a CHR state and if so, whether such reversions are preceded by particular risk factors (eg, major life stressors).
A number of studies have examined combinations of clinical and demographic variables ascertained at baseline assessment to determine whether prediction of psychosis can be enhanced beyond the 20%–35% risk associated with a CHR syndromal status.11 In general, multivariate algorithms requiring particular combinations of symptoms and demographic factors achieve high positive predictive power and specificity (eg, in the 70%–80% range), but low sensitivity (eg, in the 10%–30% range).8 There is consistency among studies in showing (unsurprisingly) that higher levels of the prodromal symptoms at baseline are the best predictors of conversion; nevertheless, the most predictive multivariate profiles vary widely across studies.11 Although it should be noted that few studies have attempted direct replication of each other’s risk algorithms, this pattern hints at the likelihood of substantial heterogeneity among profiles of clinical and demographic risk indicators among those who convert.
The NAPLS consortium has pursued the development of an individualized risk prediction tool in collaboration with Prof. Michael Kattan at the Cleveland Clinic, who has pioneered these techniques for disease prognosis in various areas of somatic medicine.12–15 Limiting the scope to a pool of potential clinical, demographic, and cognitive predictors that could be easily implemented in community settings, data from the second phase of NAPLS were used to build a risk calculator, which was then instantiated in a web-based tool that can generate a conversion risk estimate for new cases.16 The variables that carried the greatest weight in the prediction algorithm were: greater severity of unusual thought content and suspiciousness, slower processing speed, poorer verbal list learning performance, and a decline in social functioning in the year prior to baseline assessment (the NAPLS2 on-line risk calculator will be made public in a future publication). This risk calculator could help to facilitate a stepped care approach, whereby less invasive interventions may be applied at lower risk levels.
Biological assays in CHR cases are less confounded with exposure to antipsychotic treatments and other secondary factors than in patients with established schizophrenia. Some promising leads on the use of biological assays to improve prediction among CHR cases have emerged using empirically based discovery approaches, including machine learning algorithms for gray matter variations in structural brain images17,18 and so-called “greedy” regression algorithms for proteomic/metabolic plasma parameters.19 For example, in a pilot study of plasma samples on a subsample from the NAPLS study, an aggregate index (z-transformed) of 15 analytes (selected from 117) was able to distinguish subsequent converters from nonconverters and HC subjects, with high predictive ability (area under receiver operating curve of 0.9 [on a scale of 0.5 to 1.0]).19 The most strongly predictive analytes are involved in immunomodulation, hypothalamic-pituitary-adrenal axis (HPA) function, and oxidative stress, supporting the hypothesis that dysregulation of these systems is prominent in CHR cases at greatest risk for psychosis. The ultimate value of such algorithms awaits crucial validation tests in independent datasets.
Mechanisms of Onset of Psychosis
Current models of psychosis emphasize disruptions in integrated synaptic activity and neuronal connectivity.20,21 If these factors (generally or within particular brain networks) contribute to psychotic symptoms, they should worsen as symptoms worsen in the ramp-up to full psychosis. Most studies of dysconnectivity in schizophrenia are based on diagnosed patients,22–24 and do not address the timing of onset or course of dysconnectivity in relation to the onset of symptoms. Given that the causes and consequences of psychosis are generally confounded in studies of diagnosed patients25 and that some of the contributing factors are likely to change in the transition to psychosis,26 studies tracking potential biomarkers using a prospective, longitudinal design can be very informative.
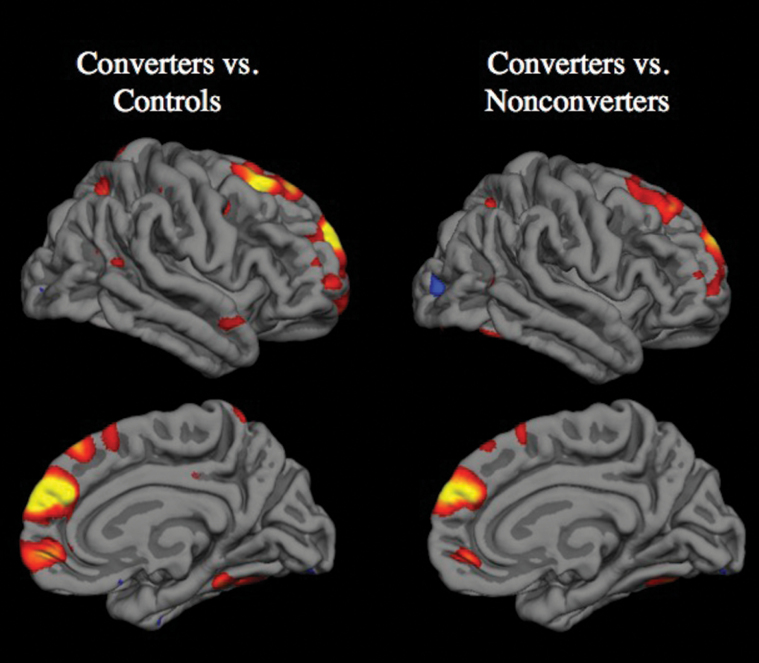
Several such prospective longitudinal neuroimaging studies have now been published, encompassing multiple independent samples of CHR cases.27–32 All of these studies found that CHR cases who converted to psychosis showed a steeper rate of cortical gray matter loss compared with nonconverters. Among the studies that also included a healthy comparison group, the rate of gray matter decline was also greater in converters compared with age- and gender-matched controls. The regions showing significantly greater rates of gray matter decline differ somewhat across the studies using whole-brain approaches, with 1 finding differential change only in prefrontal cortex,29 and the others finding differential change in prefrontal as well as temporal cortical regions.27,28,32 This variation is not surprising given that most studies included relatively small sample sizes (Ns of 8 to 12 converters), employed varying interscan intervals (1–2 y), and did not correct for multiple comparisons voxel-wise throughout the brain. However, a recent study by the NAPLS consortium, with the largest CHR group reported to date (N = 274, including 35 converters), showed that converters experienced a steeper rate of gray matter loss in right superior frontal, middle frontal, and medial orbitofrontal cortex, even when a stringent multiple correction method was applied at the whole-brain level (figure 1).33 If the statistical threshold was relaxed, accelerated cortical thinning was also observed in superior temporal cortex, parietal cortex, and parahippocampal gyrus. Taken together, these findings are remarkably consistent in demonstrating a steeper rate of cortical gray matter decline among CHR cases who convert to psychosis compared with those who do not and with healthy comparison subjects.
Fig. 1.
Cortical surface maps from the NAPLS2 study showing regions in which converters to psychosis had significantly greater progressive loss of gray matter thickness compared with controls and nonconverters after correction for multiple comparisions using the false discovery rate.33
Given that antipsychotic drugs are associated with gray matter decline in animal models34 and in patients with schizophrenia,35,36 including first-episode patients,37 antipsychotic drug use represents the most plausible competing explanation for differential gray matter loss among CHR cases who convert to psychosis. Keeping in mind that these factors cannot be controlled in patients for ethical and practical reasons, several lines of evidence argue against this perspective. First, in the NAPLS longitudinal neuroimaging study, CHR converters to psychosis who had not been exposed to antipsychotics during the interscan interview showed significantly greater thinning of prefrontal cortex than CHR nonconverters (regardless of medication status) and healthy controls.33 Second, a recent meta-analysis demonstrated that antipsychotic naïve first-episode schizophrenia patients have less cortical gray matter than healthy comparison subjects,38 indicating that at least some amount of gray matter reduction in schizophrenia is independent of antipsychotic drug exposure. Third, longitudinal imaging studies of twins discordant for schizophrenia and other genetically informative samples have observed that a steeper rate of gray matter decline is associated with a genetic diathesis to schizophrenia.39,40
If the accelerated gray matter loss associated with psychosis onset is not a secondary phenomenon, then it could be related to factors that participate in the pathophysiology of schizophrenia and related disorders, such as neuroinflammation.41 Notably, in the NAPLS longitudinal MRI study, prefrontal gray matter decline was predicted by baseline levels of proinflammatory cytokines in plasma.33 Inflammatory markers are elevated in postmortem neural tissue from patients with schizophrenia,42–45 and these same markers are associated with microglial-mediated synaptic pruning and dendritic retraction in animal models,46,47 thus providing a potential mechanism for the reduced neuropil seen in patients.20,48,49 Although prenatal neuroinflammatory processes could “program” for vulnerability,50 subsequent exposure to stress, infection, autoimmune processes, and/or synaptic pruning during adolescent brain development represent influences more proximal to psychosis onset.20,41,50,51 Also supporting this interpretation, microglia are activated by peripheral blood cytokines,52 and in vivo PET studies find greater activated microglia in schizophrenia.53,54
Though accelerated decline in cortical gray matter among UHR cases who convert to psychosis may be one of the leading indicators of processes underlying the development of psychosis, theoretically it seems likely that changes in synaptic signaling and functional connectivity are the more proximal mechanisms underlying symptom expression. Key systems involve thalamo-cortical loops55 through which most neural computations flow. Complementing structural diffusion tractography studies56,57 and nonhuman primate anatomical studies,58 resting state (rs) fMRI has revealed the functional architecture of thalamo-cortical systems in humans,59 showing that the thalamus is organized into parallel pathways that form segregated information routes with the neocortex. This property makes thalamus a possible “lens” onto distributed large-scale disruptions in the brain that may underlie the development of schizophrenia.60,61 A recent rs-fMRI study of CHR cases in the NAPLS consortium observed a pattern highly consistent with effects reported in chronic psychosis.61,62 Specifically, CHR converters to psychosis showed increased thalamic connectivity with sensorimotor cortices, but reduced thalamic connectivity with PFC, anterior cingulate cortex, and cerebellum.63 However, a critical gap in knowledge remains; namely, whether disrupted functional connectivity progresses prior to onset of psychosis and in association with accelerated gray matter decline. Answering this question requires multiple assessments prior to onset, enabling application of time-lagged and growth-curve analytic methods capable of establishing temporal precedence and mediation among multiple cascading influences.64–67
Prevention of Psychosis
A small number of controlled prevention trials in CHR cases have appeared. Collectively, the results support the view that any targeted intervention, whether biological or psychological in approach, is associated with better outcomes than less targeted control conditions.68 Results of 2 small trials with antipsychotic drugs do not support a prophylactic effect on conversion risk beyond the period of active treatment.69,70 In general, the use of such medicines in individuals who are below the threshold of full psychosis is not recommended. Intriguing results have been obtained in an initial trial of omega-3 fatty acid supplementation71; this finding awaits confirmation by independent studies. Psychosocial interventions such as cognitive behavior therapy and family-focused psychoeducation may be beneficial in deflecting the course of illness severity and chronicity72,73; however, it remains unclear whether such approaches can prevent onset of illness.
Progress on elucidating mechanisms of onset of psychosis could yield novel or repurposed compounds with more than palliative efficacy; ie, capable of preventing or mitigating the changes in brain structure and function underlying functional decline and onset of full symptoms.4 Such compounds, combined with psychosocial interventions, could also help to redirect a young person otherwise predisposed to schizophrenia towards a trajectory of social engagement, educational completion, and independent living. Linking this line of argument to the material reviewed above, a question of major importance is whether increasing neuroinflammation precedes and predicts changes in cortical gray matter and functional connectivity prior to onset. The answer is critical; if yes, a prevention trial with anti-inflammatory agents would be strongly indicated, but if no, clearly not. In another possible cascade, dysregulated NMDA-dependent synaptic plasticity may be a proximal cause of the accelerated gray matter decline and disrupted functional connectivity seen in those who convert to psychosis (via pruning of overabundant weak synapses).21,51,74–79 Other risk factors (eg, elevated HPA activity20,41,50) would then influence psychosis risk via disrupted plasticity, with neuroinflammation (microglial activation) representing a secondary signal (ie, as in a clean-up operation). In this case, targeting NMDA-dependent plasticity mechanisms would be indicated over other potential strategies. Alternatively, we may find evidence of feed-forward mediation of psychosis risk primarily through microglia/inflammatory processes in some patients and plasticity-related processes in others, in which case, biomarkers may be useful for selecting interventions targeted to particular mechanisms for different individual patients. In either case, cognitive training has potential as a component of a prevention strategy, as harnessing learning-induced neuroplasticity to strengthen weak neural networks might reduce neuroinflammatory processes and help to correct this unbalanced cascade.80
Summary and Conclusion
In summary, the CHR paradigm appears to be useful for elucidating predictors and mechanisms of onset of psychosis and for the development and testing of preventive interventions. Challenges to be addressed in the next phase of research using this paradigm include deciphering heterogeneity of risk profiles and outcomes, clarifying temporal precedence and mediation among multiple cascading pathophysiological influences, and developing interventions that target the molecular and cellular mechanisms underlying reduced brain connectivity and psychosis.
Funding
National Institutes of Health (MH081902 to T.D.C.).
Acknowledgments
T.D.C. is a consultant to the Los Angeles County Department of Mental Health and to Boehringer Ingelheim Pharmaceuticals and is a co-inventor (with the other NAPLS investigators) on a pending patent for a blood-based predictive biomarker for psychosis. Many of the ideas expressed in this manuscript were developed in discussions with the NAPLS investigators, including Diana Perkins, Daniel Mathalon, Elaine Walker, Scott Woods, Robert Heinssen, Thomas McGlashan, Jean Addington, Larry Seidman, Kristin Cadenhead, Barbara Cornblatt, Carrie Bearden, and Ming Tsuang. The material in this article was presented at the Kraepelin Symposium in Munich, Germany on September 27, 2014.
References
- 1. Lieberman JA, Stroup TS, McEvoy JP, et al. ; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. [DOI] [PubMed] [Google Scholar]
- 2. Insel TR. The arrival of preemptive psychiatry. Early Interv Psychiatry. 2007;1:5–6. [DOI] [PubMed] [Google Scholar]
- 3. Insel TR, Sahakian BJ. Drug research: a plan for mental illness. Nature. 2012;483:269. [DOI] [PubMed] [Google Scholar]
- 4. Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. [DOI] [PubMed] [Google Scholar]
- 6. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 7. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 8. Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Addington J, Cornblatt B, Cadenhead K, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schlosser DA, Jacobson S, Chen Q, et al. Recovery from an at-risk state: clinical and functional outcomes of putatively prodromal youth who do not develop psychosis. Schizophr Bull. 2012;38:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kattan MW, Yu C, Stephenson AJ, Sartor O, Tombal B. Clinicians versus nomogram: predicting future technetium-99m bone scan positivity in patients with rising prostate-specific antigen after radical prostatectomy for prostate cancer. Urology. 2013;81:956–961. [DOI] [PubMed] [Google Scholar]
- 13. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. [DOI] [PubMed] [Google Scholar]
- 14. Pfeiffer RM, Park Y, Kreimer AR, et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med. 2013;10:e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Specht MC, Kattan MW, Gonen M, Fey J, Van Zee KJ. Predicting nonsentinel node status after positive sentinel lymph biopsy for breast cancer: clinicians versus nomogram. Ann Surg Oncol. 2005;12:654–659. [DOI] [PubMed] [Google Scholar]
- 16. Cannon TD. The development and implementation of a psychosis risk prediction algorithm. Presentation at the Annual Meeting of the Society of Biological Psychiatry, New York, NY, April 2014. [Google Scholar]
- 17. Koutsouleris N, Riecher-Rossler A, Meisenzahl EM, et al. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr Bull. 2015;41:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perkins DO, Jeffries CD, Addington J, et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull. 2015;41:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zalesky A, Fornito A, Egan GF, Pantelis C, Bullmore ET. The relationship between regional and inter-regional functional connectivity deficits in schizophrenia. Hum Brain Mapp. 2012;33:2535–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cannon TD, Mednick SA, Parnas J. Antecedents of predominantly negative- and predominantly positive-symptom schizophrenia in a high-risk population. Arch Gen Psychiatry. 1990;47:622–632. [DOI] [PubMed] [Google Scholar]
- 26. Cannon TD. Neurodevelopment and the transition from schizophrenia prodrome to schizophrenia: research imperatives. Biol Psychiatry. 2008;64:737–738. [DOI] [PubMed] [Google Scholar]
- 27. Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. [DOI] [PubMed] [Google Scholar]
- 28. Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. [DOI] [PubMed] [Google Scholar]
- 29. Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. [DOI] [PubMed] [Google Scholar]
- 32. Ziermans TB, Schothorst PF, Schnack HG, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. [DOI] [PubMed] [Google Scholar]
- 35. Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychol Med. 2009;39:1763–1777. [DOI] [PubMed] [Google Scholar]
- 37. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McIntosh AM, Owens DC, Moorhead WJ, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol Psychiatry. 2011;69:953–958. [DOI] [PubMed] [Google Scholar]
- 40. Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE. Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry. 2008;65:1259–1268. [DOI] [PubMed] [Google Scholar]
- 41. Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res. 2013;147:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. [DOI] [PubMed] [Google Scholar]
- 44. Fung SJ, Joshi D, Fillman SG, Weickert CS. High white matter neuron density with elevated cortical cytokine expression in schizophrenia. Biol Psychiatry. 2014;75:e5–e7. [DOI] [PubMed] [Google Scholar]
- 45. Catts VS, Wong J, Fillman SG, Fung SJ, Weickert CS. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. The Aust NZJ Psychiatry. 2014;48:722–734. [DOI] [PubMed] [Google Scholar]
- 46. Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M. Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol. 2011;256:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. [DOI] [PubMed] [Google Scholar]
- 49. Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 50. Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. [DOI] [PubMed] [Google Scholar]
- 51. McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. [DOI] [PubMed] [Google Scholar]
- 52. Perry VH, Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 54. van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative ®-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. [DOI] [PubMed] [Google Scholar]
- 55. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 56. Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. [DOI] [PubMed] [Google Scholar]
- 57. Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. [DOI] [PubMed] [Google Scholar]
- 58. Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. [DOI] [PubMed] [Google Scholar]
- 59. Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Anticevic A, Haut K, Murray JD, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015; 72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ferrer E, McArdle JJ, Shaywitz BA, Holahan JM, Marchione K, Shaywitz SE. Longitudinal models of developmental dynamics between reading and cognition from childhood to adolescence. Dev Psychol. 2007;43:1460–1473. [DOI] [PubMed] [Google Scholar]
- 65. Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev Psychol. 2003;39:535–550. [DOI] [PubMed] [Google Scholar]
- 66. McArdle JJ, Hamgami F, Jones K, et al. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. J Gerontol B Psychol Sci Soc Sci. 2004;59:P294–P304. [DOI] [PubMed] [Google Scholar]
- 67. Brandmaier AM, von Oertzen T, McArdle JJ, Lindenberger U. Structural equation model trees. Psychol Methods. 2013;18:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Preti A, Cella M. Randomized-controlled trials in people at ultra high risk of psychosis: a review of treatment effectiveness. Schizophr Res. 2010;123:30–36. [DOI] [PubMed] [Google Scholar]
- 69. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. [DOI] [PubMed] [Google Scholar]
- 70. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. [DOI] [PubMed] [Google Scholar]
- 71. Amminger GP, Schäfer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. [DOI] [PubMed] [Google Scholar]
- 72. Miklowitz DJ, O’Brien MP, Schlosser DA, et al. Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. J Am Acad Child Adolesc Psychiatry. 2014;53:848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. [DOI] [PubMed] [Google Scholar]
- 75. Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. [DOI] [PubMed] [Google Scholar]
- 76. Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. [DOI] [PubMed] [Google Scholar]
- 77. Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68 [DOI] [PubMed] [Google Scholar]
- 78. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tessier CR, Broadie K. Activity-dependent modulation of neural circuit synaptic connectivity. Front Mol Neurosci. 2009;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fisher M, Loewy R, Hardy K, Schlosser D, Vinogradov S. Cognitive interventions targeting brain plasticity in the prodromal and early phases of schizophrenia. Annu Rev Clin Psychol. 2013;9:435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]