Abstract
Findings from multiple lines of research provide evidence of aberrant functional brain connectivity in schizophrenia. By using graph-analytical measures, recent studies indicate that patients with schizophrenia exhibit changes in the organizational principles of whole-brain networks and that these changes relate to cognitive symptoms. However, there has not been a systematic investigation of functional brain network changes in schizophrenia to test the consistency of these changes across multiple studies. A comprehensive literature search was conducted to identify all available functional graph-analytical studies in patients with schizophrenia. Effect size measures were derived from each study and entered in a random-effects meta-analytical model. All models were tested for effects of potential moderator variables as well as for the presence of publication bias. The results of a total of n = 13 functional neuroimaging studies indicated that brain networks in patients with schizophrenia exhibit significant decreases in measures of local organization (g = −0.56, P = .02) and significant decreases in small-worldness (g = −0.65, P = .01) whereas global short communication paths seemed to be preserved (g = 0.26, P = .32). There was no evidence for a publication bias or moderator effects. The present meta- analysis demonstrates significant changes in whole brain network architecture associated with schizophrenia across studies.
Key words: schizophrenia, connectivity, cognition, graph analysis, meta-analysis
Introduction
Multiple studies indicate that schizophrenia is associated with changes in specific brain regions1–4 and functions.5,6 However, it has long been argued that these localized abnormalities cannot account for the manifold clinical and cognitive symptoms experienced by affected patients. Instead, Wernicke7 and Kraepelin8 first suggested that schizophrenia could be best understood as a neuropathology of connections between brain regions. This “dysconnectivity” hypothesis has stimulated a whole field of research,9–11 and numerous neuroimaging studies report evidence for abnormal brain connectivity in patients with schizophrenia across modalities.9,12,13
Functional brain connectivity can be determined by calculating the statistical dependency between neurophysiological signals measured by correlation coefficients or mutual information metrics, as employed in functional magnetic resonance imaging (fMRI) or electroencephalography (EEG) data. Most functional studies of brain connectivity in patients with schizophrenia have focussed on isolated circuits based on hypotheses related to abnormal behavior, such as dopaminergic pathways of fronto-striatal regions,6,14–16 language subnetworks,17 or subnetworks of cognitive control.18 However, a centrally important question is whether the network abnormalities are spatially restricted to such circuits or whether the integrity of brain functioning is globally disrupted.
The recent introduction of quantitative network analysis to the analysis of neuroimaging data19–21 has facilitated research of global aberrant connectivity patterns in schizophrenia.22 This approach represents a methodological framework in which brain connectivity patterns are represented as a network of connections (“edges”) between brain regions (“nodes”). Nodes have been defined as voxels,23 regions of interest24 or brain networks.25 Most importantly, this representation of brain connectivity allows the characterization of the structure or organizational principles of whole brain networks and thus extends beyond the investigation of individual connections or isolated circuits. Moreover, graph-theoretical measures can be used to quantify the degree to which brain networks follow certain principles of organization and how they differ between individual subjects or different patient populations.
Graph-analytical studies of schizophrenia can be understood with reference to the concepts of “global short communication paths” and “local organization.” Networks with highly expressed global short communications paths often show a high degree of integration and thus hold important advantages as they allow information to be transmitted rapidly across different nodes. The degree of integration of brain networks can be quantified by graph-analytical measures like “global efficiency” and “average minimal path length.”26 Another important architecture frequently observed in brain networks is the local organization into functionally independent subsets of regions. Networks with high local organization allow efficient computation on the local level and are resilient to failure of individual nodes. In graph-analysis, this organization can be quantified by measures such as the “clustering coefficient,” “transitivity” or “modularity.”26 Most interestingly, it has also been shown that brain networks follow a so-called “small-world” architecture which is optimized towards a balance of local organization and global integration.24,27,28 Small-worldness organization of brain networks might hold important benefits, such as high degree of global short communication with relatively few connections.29,30
Clinically, patients with schizophrenia exhibit significant changes in their brain network organization as indicated by graph-analytical measures of global short communication paths,31–33 local organization,34–36 and small-worldness.36–43 These domains of investigation have also been linked by the findings indicating that degree of large-scale brain network changes in schizophrenic patients is related to their decline in cognitive functioning.40–43 Also, the relevance of functional network organization to schizophrenia is indicated by studies showing association between graph analytic measures and cognitive functioning in healthy individuals, such as working memory performance44 and the intelligence quotient.45–47
In summary, multiple studies indicate that graph-analysis is a useful tool to describe changes of global network organization of brain connectivity in patients with schizophrenia. However, meta-analytic investigations are required to evaluate the consistency of results across studies as well as the potentially moderating effects of clinical and methodological factors. This is a critical aim because it will inform current theory and drive future research of the pathophysiology of the disorder. In this study we conducted a comprehensive meta-analysis of all available neuroimaging studies using graph-analysis to investigate functional brain network changes in patients with schizophrenia across modalities (ie, EEG and fMRI).
Methods
Search and Selection Strategy
A comprehensive literature search was conducted in the PubMed database to include all published studies until August 01, 2015. Initially studies were screened using the following search term: (“graph analysis” OR “graph-analysis” OR “small-worldness” OR “small worldness” OR “clustering” OR “path length” OR “global efficiency” OR “local efficiency” OR “modularity” OR “assortativity”) AND (“magnetic resonance imaging” OR “MRI” OR “fMRI” OR “resting-state” OR “resting state” OR “EEG” OR “electroencephalography” OR “MEG” OR “magnetic encephalography”) AND (“schizophrenia” OR “schizophreniform” OR “psychosis” OR “psychotic”). To be included in the meta-analysis, studies needed to report graph-analytical measures (small-worldness, clustering coefficient, minimal path length, global efficiency, local efficiency) of brain networks in healthy control subjects and patients with schizophrenia or schizophreniform disorder as diagnosed by the DSM-IV.48 Studies were required to report sufficient statistics to allow the computation of an effect size quantifying group differences. Even though the source of the measured signal is fundamentally different between MRI-based and electrophysiological measures (EEG, MEG), studies of all modalities studies were included as in both cases data is typically modeled under the assumption that what is measured is the same (neural activity).
Data Extraction
The main outcome measure extracted from the individual studies was the standardized mean difference (Hedges’ g 49) describing differences between healthy control subjects and patients with schizophrenia. If data was not available in the published manuscripts to calculate effect sizes of group differences, authors were contacted via email and asked to provide the additional information. If no data was available upon request, data was obtained using the “webplotdigitizer” software (www.webplotdigitizer.org 50). This allowed the computer-guided extraction data in the form of means and measures of dispersion (eg, standard error of mean, confidence intervals) from published figures. This approach has previously been employed successfully by multiple studies.51 Otherwise, if no sufficient data was available to calculate effect sizes, studies were excluded from the meta-analysis. If studies reported multiple comparisons for the same graph-analytical measure (eg, for different thresholds or for different frequency bands in case of EEG studies), effect sizes were averaged across all reported comparisons. If a study reported multiple measures of global short communication paths or local organization, effect sizes for these measures from this study were averaged. In order to avoid bias we excluded samples from the same neuroimaging modality with large overlap (shared n > 20%).
Data Analysis
For every study included in the analysis, Hedges’ g was calculated and entered into a random-effects meta-analytic model.49,52 The summary effect sizes across all studies were computed using a restricted maximum-likelihood estimator.53 As a first step, functional studies were analyzed by combining all modalities. Subsequently, further analyses were employed separately for EEG and fMRI studies, as well as for studies at rest and during task performance. Heterogeneity was assessed via the I 2 value which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.54 I 2 values of 25%, 50%, and 75% can be interpreted as indicating low, moderate, and high heterogeneity respectively.54 A significance level of P < .05 (2-tailed) was used for all analyses. The potential moderating effects of publication year, gender, and the age of subjects was evaluated using meta-regression.49 Publication bias was assessed by visual inspection of funnel plots and by employing Egger’s test for funnel plot asymmetry55 for every separate meta-analysis. All statistical analyses were conducted using the R statistical programming language version 2.10.156 with the package “metafor.”57
Results
The literature search identified n = 161 potential studies. After study selection using the predefined exclusion criteria, n = 8 fMRI studies34–38,58–60 (patients: n = 218, age = 26.7 years; controls: n = 235, age = 26.5 years) and n = 5 EEG studies43,61–64 (patients: n = 137, age=28.2 years; controls: n = 78, age = 27.6 years) were included in the meta-analysis. All studies included samples of patients diagnosed with schizophrenia according to the DSM-IV.
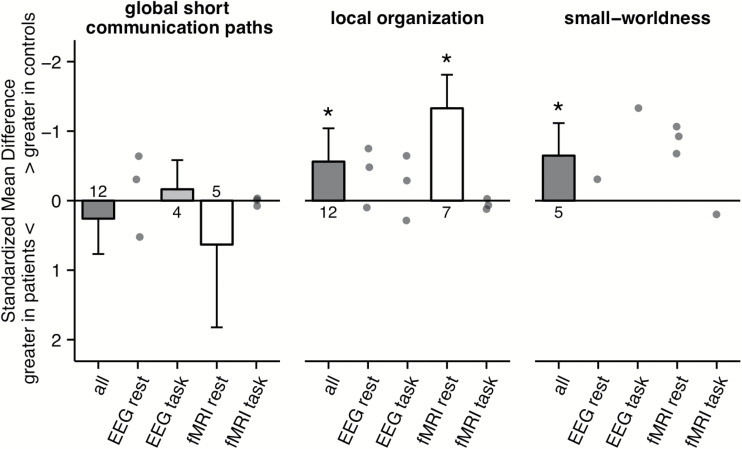
The meta-analysis of all studies reporting measures of networks’ global short communication paths (minimal path length or global efficiency) showed no significant change in patients with schizophrenia (g = 0.26, k = 12 studies, z = 1, P = .32, 95% CI: −0.25 to 0.77; heterogeneity: I 2 = 89.71%, 95% CI: 79.28 to 96.35%, figure 1). There was no effect of potential moderators such as year of publication, age of patients, age of controls or gender ratio (all P > .1). Similarly, there was no significant effect in the subanalysis of EEG studies during task (g = −0.16, k = 4 studies, z = −0.76, P = .45, 95% CI: −0.58 to 0.26; heterogeneity: I 2 = 49.2%, 95% CI: 0 to 96.44%), fMRI studies at rest (g = 0.63, k = 5 studies, z = 1.04, P = .3, 95% CI: −0.56 to 1.82; heterogeneity: I 2 = 94.62%, 95% CI: 84.98 to 99.34%), or fMRI studies during task (Fornito et al38, 2011: g = 0.06, 95% CI: −0.51 to 0.62; He et al59, 2012: g = 0.1, 95% CI: −0.46 to 0.48; Ma et al58, 2012: g = −0.05, 95% CI: −0.57 to 0.48). For the EEG studies at rest 2 studies reported a nonsignificant decrease of minimal path length in patients (Jhung et al61, 2013: g = −0.29, 95% CI: −0.98 to 0.39; Micheloyannis et al62, 2006: g = −0.63, 95% CI: −1.27 to 0.01) whereas 1 study reported a significant increase in patients (Rubinov et al63, 2009: g = 0.53, 95% CI: 0.08 to 0.97).
Fig. 1.
Effect sizes of studies reporting changes in functional brain networks in patients with schizophrenia as indicated by measures of global short communication paths (minimal path length, global efficiency), local organization (clustering coefficient, local efficiency) and small-worldness. Summary effect sizes of meta-analyses are presented for cases with n ≥ 4 using bar plots and individual studies’ effect sizes are plotted as single points. For each meta-analysis the number of available studies is indicated below each bar plot. Negative values on the y-axis represent lower graph-analytical parameters for patients compared to healthy controls. Error-bars represent the upper bound of the 95% CI. *Indicates significance on the level of P < .05.
The meta-analysis of all studies reporting measures of local organization (clustering coefficient or local efficiency) indicated a significant reduction in patients with schizophrenia (g = −0.56, k = 12 studies, z = −2.3, P = .02, 95% CI: −1.04 to −0.08; heterogeneity: I 2 = 88.24%, 95% CI: 76.24 to 95.83%, figure 1) with no evidence for a publication bias (z = 0.04, P = .96). There was no effect of potential moderators such as year of publication, age of patients, age of controls or gender ratio (all P > .1). This effect was also apparent in the subanalysis of fMRI studies at rest (g = −1.33, k = 7 studies, z = −5.39, P < .001, 95% CI: −1.81 to −0.85; heterogeneity: I 2 = 73.43%, 95% CI: 38.63 to 92.92%) with no evidence for a publication bias (z = 1.15, P = .25). For EEG studies at rest 1 study reported a significant decrease in the clustering coefficient in patients (Micheloyannis et al62, 2006: g = −0.74, 95% CI: −1.37 to −0.10) and 2 studies reported a no significant change (Jhung et al61, 2013: g = 0.1, 95% CI: −0.58 to 0.77; Rubinov et al63, 2009: g = −0.46, 95% CI: −0.91 to −0.02). Similarly for EEG studies 1 study reported a decrease in the clustering coefficient in patients (Micheloyannis et al62, 2006: g = −0.64, 95% CI: −1.27 to −0.01) and 2 studies reported no significant change (Shim et al43, 2014: g = −0.3, 95% CI: −0.77 to 0.18; Jhung et al61, 2013: g = 0.28, 95% CI: −0.40 to 0.96). All 3 available studies of fMRI during task indicated no change in measures of local organization in patients (Fornito et al38, 2011: g = 0.14, 95% CI: −0.43 to 0.71; He et al59, 2012: g = −0.02, 95% CI: −0.50 to 0.44; Ma et al58, 2012: g = 0.07, 95% CI: −0.45 to 0.59).
In the meta-analysis of all studies reporting small-worldness, there was evidence for a significant decrease in patients with schizophrenia compared to healthy controls (g = −0.65, k = 5 studies, z = −2.71, P = .01, 95% CI: −1.12 to −0.18; heterogeneity: I 2 = 68.73%, 95% CI: 15.93 to 95.73%, figure 1) with no evidence for a publication bias (z = 0.22, P = .83). This effect was not affected by potential moderators such as year of publication, age of patients, age of controls or gender ratio (all P > .1). All 3 available studies of fMRI at rest reported a significant decrease of small-worldness in patients (Lynall et al34, 2010: g = −0.91, 95% CI: −1.71 to −0.11; Alexander-Bloch et al37, 2013: g = −0.68, 95% CI: −1.33 to −0.03; Tomasi et al36, 2014: g = −1.07, 95% CI: −1.42 to −0.72). The only study that investigated small-worldness in patients during task did not report a significant decrease (Fornito et al38, 2011: g = 0.19, 95% CI: −0.37 to 0.76). The only available EEG study indicated that small-worldness in patients at rest is similar to healthy controls (Jhung et al61, 2013: g = −0.33, 95% CI: −1.01 to 0.36), whereas during task there seems to be a reduction in small-worldness (Jhung et al61, 2013: g = −1.32, 95% CI: −2.06 to −0.57).
Discussion
Extant literature includes comprehensive reviews of brain functional changes in schizophrenia.22 However, in the present meta-analysis the effect of network changes in schizophrenia are quantified and analyzed with respect to their consistency across studies. Our results suggest a significant reduction in local organization of brain networks in patients with schizophrenia with a moderate effect size (g = −0.56). This effect was robust with respect to the inclusion of potential confounding variables and there was no evidence for a publication bias. Also, schizophrenia was also associated with a lower degree of small-worldness organization with a moderate effect size (g = −0.65). In contrast, measures of global short communication paths indicated no change in functional brain networks of patients. However, it is important to note that the number of available studies investigating brain network architecture in schizophrenia is still small so results need to be interpreted with care.
Local Organization of Brain Networks in Patients With Schizophrenia
In the present meta-analysis 2 measures of network local organization were investigated: the clustering coefficient and local efficiency. A functional network architecture with high local organization allows efficient computation at a local level in functionally specialized regions. At the same time, such networks are also robust to node failure. In case of damage to a local node, the high within-cluster connectivity enables the network to preserve its functionality. High local organization in brain networks has been associated with higher cognitive performance in tasks of attention, memory, executive functions, and psychomotor speed.34,59,65–67 Interestingly, local organization of brain networks in schizophrenic patients might be a predictor of future decline of intelligence scores and increase of psychotic symptoms.68 Also, in patients with schizophrenia reduced local organization is related to more severe psychotic symptoms as measured by Positive and Negative Syndrome Scale (PANSS),60,69 in particular with the cognitive subscales.43 Interestingly, in our analysis the reductions in network local organization were most pronounced in resting-state fMRI studies when compared to those using a task design. One potential hypothesis for this finding is that differences in brain network organization detected in task-based fMRI are affected by individuals’ task performance70 and thus are associated with additional heterogeneity. In summary, there is evidence for a reduction of local organization in brain networks of patients with schizophrenia and multiple reports indicate that reduced network local organization might relate to the cognitive and clinical symptoms43,60,69 seen in patients with schizophrenia.
Global Short Communication Paths in Brain Networks of Patients With Schizophrenia
In the present meta-analysis 2 mathematically related metrics of brain networks’ global short communication paths were investigated: functional minimal path length and global efficiency. Global short communication paths of a network are a critical feature of efficient network architecture as this organization allows the rapid transfer of information across the network. Thus, multiple studies report that individuals with highly expressed global short communications paths also show superior performance in tests of general intelligence45–47 as well as attention and verbal memory.67 Overall, in functional brain networks 2 measures of global short communication paths (global efficiency, minimal path length) indicated no change in patients with schizophrenia. However, some studies indicated that minimal path length is related to the cognitive symptoms in schizophrenic patients43 and structural studies examining changes in anatomical wiring of brain networks in schizophrenia have noted strong reductions in global communication paths.22,41,71,72 Also, the severity of psychotic symptoms correlated with graph analytical measures of brain networks’ integration.58,60,63,69 However, it needs to be noted that the 2 investigated measures (minimal path length, global efficiency) might only partially capture the amount of network integration.73,74 Thus alternative measures of integration might be more sensitive to investigate organizational changes of brain networks in patients with schizophrenia.
Small-Worldness Architecture in Patients With Schizophrenia
In general, functional small-worldness properties have been reported in a multitude of various networks suggesting important advantages associated with this organization.28 Specifically, small-worldness represents an optimized trade-off between local organization into functionally specialized modules and global communication. Deviations from this optimum might result in reduced computational efficiency. While there has only been 1 study that has reported a direct association between reduced small-worldness and cognitive functioning in schizophrenia,59 others report associations of graph-analytical measures with positive and negative symptoms.60,69 Specifically, Wang et al69 report that PANSS positive and negative scores of patients were related to both local and global connectivity measures. Additionally, reduced small-worldness in healthy controls is associated with lower cognitive functioning in domains usually associated with schizophrenia, including memory, attention, executive functioning, and psychomotor speed.34,43,65 Combined, these results suggest that small-worldness may be implicated in the functional deficits and symptoms seen in schizophrenia, although more research is required to support this hypothesis.
Limitations
There are a large number of methodological factors in the analysis of neuroimaging data that most likely have an effect on graph-analytical measures and potentially on the effect size reported in the present meta-analysis. As an example, there are a variety of methods for determining statistical significance of connectivity differences between groups75 or different ranges of thresholds for determining networks (see table 1, “Thresholding”), which can affect the effect size of the findings and thus introduce heterogeneity of results into subsequent meta-analyses. Also, some studies calculated graph-measures that were normalized with respect to random networks generated by randomly rewiring connectivity matrices, while other studies did not implement such normalization what may have also contributed to the heterogeneity in the present meta-analysis. These methodological differences likely reflect the infancy of graph analytical analyses, which is addressed in comprehensive discussion elsewhere (see Fallani et al75 for a review and Fornito et al76 for detailed discussion).
Table 1.
Overview of All Samples Included in the Meta-analysis
| Study | Year | Modality | n | Age | Males (n) | n | Age | Males (n) | Condition | Reported Graph- Analytical Measures | Statistical Dependency Measure | Connectivity Matrix | Thresholding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Micheloyannis et al62 | 2006 | EEG | 20 | 32.4 | 15 | 20 | 27.4 | 15 | Rest | Clustering coefficient, minimal path length | Synchronization likelihood | Binary | Absolute |
| Rubinov et al63 | 2009 | EEG | 40 | 19.6 | 26 | 40 | 19.7 | 26 | Rest | Clustering coefficient, minimal path length | Nonlinear interdependence | Weighted | Relative (10%–20%) |
| Fogelson et al64 | 2013 | EEG | 20 | 35.5 | 18 | 20 | 36.2 | 18 | Task | Minimal path length | Synchronization likelihood | Binary | Relative (8%–15%) |
| Jhung et al61 | 2013 | EEG | 23 | 19.6 | 5 | 13 | 20.2 | 5 | Task & rest | Clustering coefficient, minimal path length, small-worldness | Synchronization likelihood | Binary | Relative |
| Shim et al43 | 2014 | EEG | 34 | 33.9 | 14 | 34 | 34.7 | 20 | Task | Clustering coefficient, minimal path length | Phase locking value | Weighted | None |
| Lynall et al34 | 2010 | fMRI | 12 | 10.0 | 33 | 15 | 14.0 | 33 | Rest | Average clustering, small-worldness, global efficiency | Wavelet correlation | Binary | Relative (37%–50%) |
| Alexander-Bloch et al35 | 2010 | fMRI | 13 | 18.0 | 5 | 19 | 19.0 | 9 | Rest | Clustering coefficient, global efficiency, local efficiency | Wavelet correlation | Binary | Relative (30%–50%) |
| Yu et al60 | 2011 | fMRI | 19 | 36.5 | 15 | 19 | 33.9 | 9 | Rest | Clustering coefficient, minimal path length, global efficiency, local efficiency | Partial correlation, z-transformed | Binary | Relative (35%–43%) |
| Fornito et al38 | 2011 | fMRI | 23 | 20.5 | 14 | 25 | 22.1 | 13 | Task | Clustering coefficient, minimal path length, small-worldness, global efficiency, local efficiendy | Beta series correlation | Binary | Relative (5%–45%) |
| Ma et al58 | 2012 | fMRI | 28 | 37.8 | 23 | 28 | 32.7 | 19 | Task & rest | Clustering coefficient, minimal path length | Normalized mutual information | Binary | Absolute |
| He et al59 | 2012 | fMRI | 35 | 34.3 | 26 | 35 | 34.6 | 27 | Task | Clustering coefficient, minimal path length, global efficiency, local efficiency | Partial correlation, z-transformed | Binary | Relative (19%–34%) |
| Alexander-Bloch et al37 | 2013 | fMRI | 19 | 18.7 | 9 | 20 | 19.4 | 10 | Rest | Clustering coefficient, small-worldness | Wavelet correlation | Binary | Relative (1%–10%) |
| Tomasi et al36 | 2014 | fMRI | 69 | 38.0 | 55 | 74 | 36.0 | 51 | Rest | Clustering coefficient, minimal path length, small-worldness | Correlation | Binary | Relative (40%–65%) |
Note: EEG, electroencephalography; fMRI, functional magnetic resonance imaging.
Another limitation is that exposure to antipsychotic medication is a potentially important moderator of global brain network changes in schizophrenia, but this was not addressed in this meta-analysis. Antipsychotics have been shown to affect results in neuroimaging studies of schizophrenia in different modalitiew.1,6,63,77 However, most graph-analytical structural78 and functional34,43 neuroimaging studies report no effect of antipsychotic medication. Additionally, Zhang et al32 report changes in structural network organization in drug-naïve patients. As such, it is unlikely that the results of the present study could only be explained by the effect of antipsychotic medication. Unfortunately, most studies included in the present analysis did not report a quantitative measure of current or previous antipsychotic medication (eg, chlorpromazine equivalent measures) that could have been investigated. This represents a methodological shortcoming of the current graph-analytical literature of schizophrenia that needs to be addressed in future research.
Finally, it needs to be noted that some studies report reduced overall connectivity in patients with schizophrenia.9 Typically, reduced overall connectivity in brain networks is associated with reductions in measures of local organization. However, few of the studies included in the present analysis reported a measure of overall connectivity strength, which precluded any analysis of this potential moderator variable. Future studies should address the interplay of overall connectivity changes and specific alterations of network architecture in patients with schizophrenia.
Future Directions
A central question for future investigations is whether differences in functional brain network architecture represent vulnerability traits and are already present in subjects at genetic79 or clinical80 risk for schizophrenia, or whether these differences are more related to the current clinical symptoms of patients. Also, more studies are needed to investigate the relationship between task- and rest-associated network organization. As such, future studies could investigate the longitudinal course and stability of the differences found here. Additionally, it has been suggested that structural and functional brain connectivity can be altered by meditation,81,82 pharmacological treatment,83 or physical exercise84 that reduce symptoms and improve outcomes. Given the sensitivity of measures of brain functional connectivity to treatment interventions, as well as their role for cognition in psychiatric disorders, future studies need to investigate the potential of graph-analytical parameters to monitor improvements in cognition following interventions85 in addition to their use as biomarkers for classification of psychiatric disorders.86
Funding
This work was funded by the EU project IN-SENS FP7-PEOPLE-2013 ITN (607616).
Acknowledgments
J.K., L.K-I., C.C., V.D.C., D.B.D., M.P.vdH., N.K., and B.M. declare no conflicts of interest. P.F. has been an honorary speaker for AstraZeneca, Bristol Myers Squibb, Eli Lilly, Essex, GE Healthcare, GlaxoSmithKline, Janssen Cilag, Lundbeck, Otsuka, Pfizer, Servier and Takeda. During the past 5 years, but not presently, P.F. has been a member of the advisory boards of Janssen-Cilag, AstraZeneca, Eli Lilly, and Lundbeck.
References
- 1. Kambeitz J, Kambeitz-Ilankovic L, Leucht S, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015;40:1742–1751. doi:10.1038/npp.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan RCK, Di X, McAlonan GM, Gong Q-Y. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. doi:10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–113. doi:10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 4. Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–1138. doi:10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi:10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry. 2012;69:776–786. doi:10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wernicke C. Grundrisse Der Psychiatrie. Leipzig, Germany: Thieme; 1906. [Google Scholar]
- 8. Kraepelin E. Dementia Praecox. Cambridge University Press; 1987. http://psycnet.apa.org/psycinfo/1987-97125-001 Accessed August 12, 2015. [Google Scholar]
- 9. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Psychol Med. 2013;43:2547-2562. doi:10.1017/S003329171300024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anticevic A, Cole MW, Repovs G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. doi:10.3389/fpsyt.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci N Y N. 1995;3:89–97. [PubMed] [Google Scholar]
- 12. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi:10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kühn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. 2013;39:358–365. doi:10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi:10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204:420–429. doi:10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- 16. Tost H, Meyer-Lindenberg A, Klein S, et al. D2 antidopaminergic modulation of frontal lobe function in healthy human subjects. Biol Psychiatry. 2006;60:1196–1205. doi:10.1016/j.biopsych.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 17. Benetti S, Pettersson-Yeo W, Allen P, et al. Auditory verbal hallucinations and brain dysconnectivity in the perisylvian language network: a multimodal investigation. Schizophr Bull. 2015;41:192–200. doi:10.1093/schbul/sbt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter CS, Minzenberg M, West R, Macdonald A. CNTRICS imaging biomarker selections: executive control paradigms. Schizophr Bull. 2012;38:34–42. doi:10.1093/schbul/sbr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi:10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 20. Fornito A, Zalesky A, Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. doi:10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 21. Fornito A, Zalesky A, Breakspear M. Graph analysis of the human connectome: promise, progress, and pitfalls. NeuroImage. 2013;80:426–444. doi:10.1016/j.neuroimage.2013.04.087. [DOI] [PubMed] [Google Scholar]
- 22. Van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. doi:10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 23. Van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. NeuroImage. 2008;43:528–539. doi:10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24. Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci Off J Soc Neurosci. 2006;26:63–72. doi:10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calhoun VD, Eichele T, Adalı T, Allen EA. Decomposing the brain: components and modes, networks and nodes. Trends Cogn Sci. 2012;16:255–256. doi:10.1016/j.tics.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi:10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27. Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi:10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- 28. Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393:440–442. doi:10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 29. Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22:340–347. doi:10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latora V, Marchiori M. Economic small-world behavior in weighted networks. Eur Phys J B-Condens Matter Complex Syst. 2003;32:249–263. [Google Scholar]
- 31. Yan H, Tian L, Wang Q, et al. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci Bull. 2015;31:275–287. doi:10.1007/s12264-014-1518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang R, Wei Q, Kang Z, et al. Disrupted brain anatomical connectivity in medication-naïve patients with first-episode schizophrenia. Brain Struct Funct. 2015;220:1145–1159. doi:10.1007/s00429-014-0706-z. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Lin L, Lin C-P, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–118. doi:10.1016/j.schres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 34. Lynall M-E, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci Off J Soc Neurosci. 2010;30:9477–9487. doi:10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander-Bloch AF, Gogtay N, Meunier D, et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. doi:10.3389/fnsys.2010.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomasi D, Volkow ND. Mapping small-world properties through development in the human brain: disruption in schizophrenia. PloS One. 2014;9:e96176. doi:10.1371/journal.pone.0096176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alexander-Bloch AF, Vértes PE, Stidd R, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex N Y N 1991. 2013;23:127–138. doi:10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi:10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ottet M-C, Schaer M, Debbané M, Cammoun L, Thiran J-P, Eliez S. Graph theory reveals dysconnected hubs in 22q11DS and altered nodal efficiency in patients with hallucinations. Front Hum Neurosci. 2013;7:402. doi:10.3389/fnhum.2013.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheffield JM, Repovs G, Harms MP, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi:10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zalesky A, Fornito A, Seal ML, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi:10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li X, Xia S, Bertisch HC, Branch CA, Delisi LE. Unique topology of language processing brain network: a systems-level biomarker of schizophrenia. Schizophr Res. 2012;141:128–136. doi:10.1016/j.schres.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shim M, Kim D-W, Lee S-H, Im C-H. Disruptions in small-world cortical functional connectivity network during an auditory oddball paradigm task in patients with schizophrenia. Schizophr Res. 2014;156:197–203. doi:10.1016/j.schres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 44. Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci U S A. 2009;106:11747–11752. doi:10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5:e1000395. doi:10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci Off J Soc Neurosci. 2009;29:7619–7624. doi:10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pamplona GSP, Santos Neto GS, Rosset SRE, Rogers BP, Salmon CEG. Analyzing the association between functional connectivity of the brain and intellectual performance. Front Hum Neurosci. 2015;9:61. doi:10.3389/fnhum.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. American Psychiatric Association. DSM-IV-TR–Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 49. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 50. WebPlotDigitizer. http://arohatgi.info/WebPlotDigitizer/ Accessed January 1, 2013.
- 51. Wright FA, Sullivan PF, Brooks AI, et al. Heritability and genomics of gene expression in peripheral blood. Nat Genet. 2014;46:430–437. doi:10.1038/ng.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. 1998;3:486. [Google Scholar]
- 53. Raudenbusch S. Analysing effect sizes: random effects models. In: Cooper H, Hedges LV, Valentine JC, eds. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. New York, NY: Russell Sage Foundation; 2009: 295–315. [Google Scholar]
- 54. Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 57. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 58. Ma S, Calhoun VD, Eichele T, Du W, Adalı T. Modulations of functional connectivity in the healthy and schizophrenia groups during task and rest. NeuroImage. 2012;62:1694–1704. doi:10.1016/j.neuroimage.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. He H, Sui J, Yu Q, et al. Altered small-world brain networks in schizophrenia patients during working memory performance. PloS One. 2012;7:e38195. doi:10.1371/journal.pone.0038195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu Q, Sui J, Rachakonda S, et al. Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PloS One. 2011;6:e25423. doi:10.1371/journal.pone.0025423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jhung K, Cho S-H, Jang J-H, et al. Small-world networks in individuals at ultra-high risk for psychosis and first-episode schizophrenia during a working memory task. Neurosci Lett. 2013;535:35–39. doi:10.1016/j.neulet.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 62. Micheloyannis S, Pachou E, Stam CJ, et al. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–66. doi:10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 63. Rubinov M, Knock SA, Stam CJ, et al. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30:403–416. doi:10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fogelson N, Li L, Li Y, Fernandez-Del-Olmo M, Santos-Garcia D, Peled A. Functional connectivity abnormalities during contextual processing in schizophrenia and in Parkinson’s disease. Brain Cogn. 2013;82:243–253. doi:10.1016/j.bandc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 65. Douw L, Schoonheim MM, Landi D, et al. Cognition is related to resting-state small-world network topology: an magnetoencephalographic study. Neuroscience. 2011;175:169–177. doi:10.1016/j.neuroscience.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 66. Reijmer YD, Leemans A, Caeyenberghs K, Heringa SM, Koek HL, Biessels GJ; Utrecht Vascular Cognitive Impairment Study Group Disruption of cerebral networks and cognitive impairment in Alzheimer disease. Neurology. 2013;80:1370–1377. doi:10.1212/WNL.0b013e31828c2ee5. [DOI] [PubMed] [Google Scholar]
- 67. Bosma I, Reijneveld JC, Klein M, et al. Disturbed functional brain networks and neurocognitive function in low-grade glioma patients: a graph theoretical analysis of resting-state MEG. Nonlinear Biomed Phys. 2009;3:9. doi:10.1186/1753-4631-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Collin G, de Nijs J, Hulshoff Pol HE, Cahn W, van den Heuvel MP. Connectome organization is related to longitudinal changes in general functioning, symptoms and IQ in chronic schizophrenia [published online ahead of print April 02, 2015]. Schizophr Res. doi:10.1016/j.schres.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 69. Wang Q, Su T-P, Zhou Y, et al. Anatomical insights into disrupted small-world networks in schizophrenia. NeuroImage. 2012;59:1085–1093. doi:10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 70. Tan H-Y, Sust S, Buckholtz JW, et al. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi:10.1176/appi.ajp.163.11.1969. [DOI] [PubMed] [Google Scholar]
- 71. Griffa A, Baumann PS, Ferrari C, et al. Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp. 2015;36:354–366. doi:10.1002/hbm.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van den Heuvel MP, Sporns O, Collin G, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi:10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 73. Goñi J, van den Heuvel MP, Avena-Koenigsberger A, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A. 2014;111:833–838. doi:10.1073/pnas.1315529111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. De Reus MA, van den Heuvel MP. Simulated rich club lesioning in brain networks: a scaffold for communication and integration? Front Hum Neurosci. 2014;8:647. doi:10.3389/fnhum.2014.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fallani FDV, Richiardi J, Chavez M, Achard S. Graph analysis of functional brain networks: practical issues in translational neuroscience. Philos Trans R Soc B Biol Sci. 2014;369:20130521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62:2296–2314. doi:10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 77. Ansell BRE, Dwyer DB, Wood SJ, et al. Divergent effects of first-generation and second-generation antipsychotics on cortical thickness in first-episode psychosis. Psychol Med. 2015;45:515–527. doi:10.1017/S0033291714001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926. doi:10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP. Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull. 2014;40:438–448. doi:10.1093/schbul/sbt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lord L-D, Allen P, Expert P, et al. Characterization of the anterior cingulate’s role in the at-risk mental state using graph theory. NeuroImage. 2011;56:1531–1539. doi:10.1016/j.neuroimage.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 81. Jao T, Li C-W, Vértes PE, et al. Large-scale functional brain network reorganization during Taoist meditation [published online ahead of print October 06, 2015]. Brain Connect. doi:10.1089/brain.2014.0318. [DOI] [PubMed] [Google Scholar]
- 82. Gard T, Taquet M, Dixit R, et al. Fluid intelligence and brain functional organization in aging yoga and meditation practitioners. Front Aging Neurosci. 2014;6:76. doi:10.3389/fnagi.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shin D-J, Jung WH, He Y, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:606–614. doi:10.1016/j.biopsych.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 84. Foster PP. Role of physical and mental training in brain network configuration. Front Aging Neurosci. 2015;7:117. doi:10.3389/fnagi.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Taya F, Sun Y, Babiloni F, Thakor N, Bezerianos A. Brain enhancement through cognitive training: a new insight from brain connectome. Front Syst Neurosci. 2015;9:44. doi:10.3389/fnsys.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fekete T, Wilf M, Rubin D, Edelman S, Malach R, Mujica-Parodi LR. Combining classification with fMRI-derived complex network measures for potential neurodiagnostics. PloS One. 2013;8:e62867. doi:10.1371/journal.pone.0062867. [DOI] [PMC free article] [PubMed] [Google Scholar]