Abstract
Chronic psychosocial stress is an important environmental risk factor of psychiatric diseases such as schizophrenia. Social defeat in rodents has been shown to be associated with maladaptive cellular and behavioral consequences including cognitive impairments. Although gene expression changes upon psychosocial stress have been described, a comprehensive transcriptome profiling study at the global level in precisely defined hippocampal subregions which are associated with learning has been lacking. In this study, we exposed adult C57Bl/6N mice for 3 weeks to “resident-intruder” paradigm and combined laser capture microdissection with microarray analyses to identify transcriptomic signatures of chronic psychosocial stress in dentate gyrus and CA3 subregion of the dorsal hippocampus. At the individual transcript level, we detected subregion specific stress responses whereas gene set enrichment analyses (GSEA) identified several common pathways upregulated upon chronic psychosocial stress related to proteasomal function and energy supply. Behavioral profiling revealed stress-associated impairments most prominent in fear memory formation which was prevented by chronic lithium treatment. Thus, we again microdissected the CA3 region and performed global transcriptome analysis to search for molecular signatures altered by lithium treatment in stressed animals. By combining GSEA with unsupervised clustering, we detected pathways that are regulated by stress and lithium in the CA3 region of the hippocampus including proteasomal components, oxidative phosphorylation, and anti-oxidative mechanisms. Our study thus provides insight into hidden molecular phenotypes of chronic psychosocial stress and lithium treatment and proves a beneficial role for lithium treatment as an agent attenuating negative effects of psychosocial stress on cognition.
Key words: chronic psychosocial stress, social defeat, resident-intruder paradigm, gene expression, laser capture microdissection, hippocampus, lithium, cognition, fear memory
Introduction
Chronic psychosocial stress is an important environmental factor contributing to the development of psychiatric diseases (reviewed in ref.1). Bullied victims have increased risk of psychiatric disorders including schizophrenia (reviewed in refs2–6). Moreover, the exposure to traumatic experiences is positively correlated with severity of schizophrenia symptoms such as hallucinations, delusions, and specific forms of cognitive dysfunction7 (reviewed in ref.3). In mice and rats, the resident-intruder paradigm is a translationally validated model of social defeat,8,9 analogous to bullying in everyday life, where the resident represents the dominant individual and the intruder the defeated one.10 Psychosocial stress evokes varying physiological changes in rodents11,12 including behavioral alterations resembling depressive symptoms, eg, anhedonia, reduction of activity, curiosity,13–16 and sensorimotor gating.13,14 With respect to cognition, effects of psychosocial stress seem to depend on the task. Psychosocial stress induces working and spatial memory deficits16–18 and triggers short-time memory and spatial memory impairment in a model of Alzheimer disease.18 Nonetheless, the underlying molecular adaptations remain largely unknown.
Lithium (Li) treatment is beneficial in mood disorders19–21 and neurodegenerative diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis, and Parkinson’s disease.22 Chronic Li treatment has been shown to improve cognition in patients of fragile X syndrome23 and prevents cognitive loss in Alzheimer patients in low doses.24 Corresponding to its actions in humans, Li treatment modulates mood-related behavior in rodents, such as behavioral despair and cognitive judgment bias,25,26 alleviates impaired cognition in a mouse model of fragile X syndrome,27 Alzheimer disease28,29 and improves behavioral performance in a mouse model of traumatic brain injury.30 Moreover, it was suggested that it may counteract some effects of chronic stress regarding spatial memory formation,31 although molecular mechanisms remain elusive.
The molecular mode of action of lithium is unknown. Pleiotropic effects described include GSK3ß inhibition, activation of Wnt signaling and modulation of clock gene expression via the nuclear receptor rev-erbα.28,32,33 Lithium treatment has been shown to increase BDNF expression in the hippocampus34 and reverses stress-induced upregulation of CRE/CREB directed gene transcription.35 The molecular consequences of chronic psychosocial stress are also not well understood although some studies have been performed in rodent models.11,36–39 Given the enormous cellular diversity and complexity of the brain, all of these studies, however, were limited by the absence of a precise regional or cellular resolution. To overcome some of the limitations of previous analyses, we combined precise isolation of subregions of the hippocampus with global transcriptome profiling to identify molecular signatures of chronic psychosocial stress. Moreover, we assessed the effects of chronic Li treatment on the maladaptive behavioral and cognitive consequences of psychosocial stress. Finally, we could identify a common molecular signature of psychosocial stress as well as Li treatment in the CA3 region of the hippocampus correlating with improved cognitive performance.
Materials and Methods
Mice
C57Bl6/J male mice in the age of 7–8 weeks (body weight in average 21.1g ± 0.69 SD) were purchased from Charles River Laboratories and habituated for 1 week to colony room prior experiments. Animals were housed in individual cages in colony room under standard conditions (12h light/dark cycle with 8:00/20:00 lights on/off, at 21±2°C and food and water ad libitum). As residents we used individually housed 1-year-old FVB/N male mice (Charles River Laboratories) due to the fact that this strain is characterized by higher level of offensive behavior in resident-intruder paradigm than C57Bl/6.40 To prevent olfactory habituation to residents, FVB/N colony was housed in another room than C57Bl/6J mice. All experiments with mice were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Treatment
Lithium chloride was dissolved in water (300mg/l) available to mice ad libitum. Treatment was started 1 day before chronic psychosocial stress or control procedure and was continued throughout behavioral testing.
Experimental Design
In the first experiment (figure 1a), we used C57Bl/6J mice exposed to chronic social defeat or to control conditions (n = 5 per group). After 3 weeks of daily social defeat, mice were sacrificed by cervical dislocation and brains were used for laser capture microdissection (LCM) and gene expression analysis.
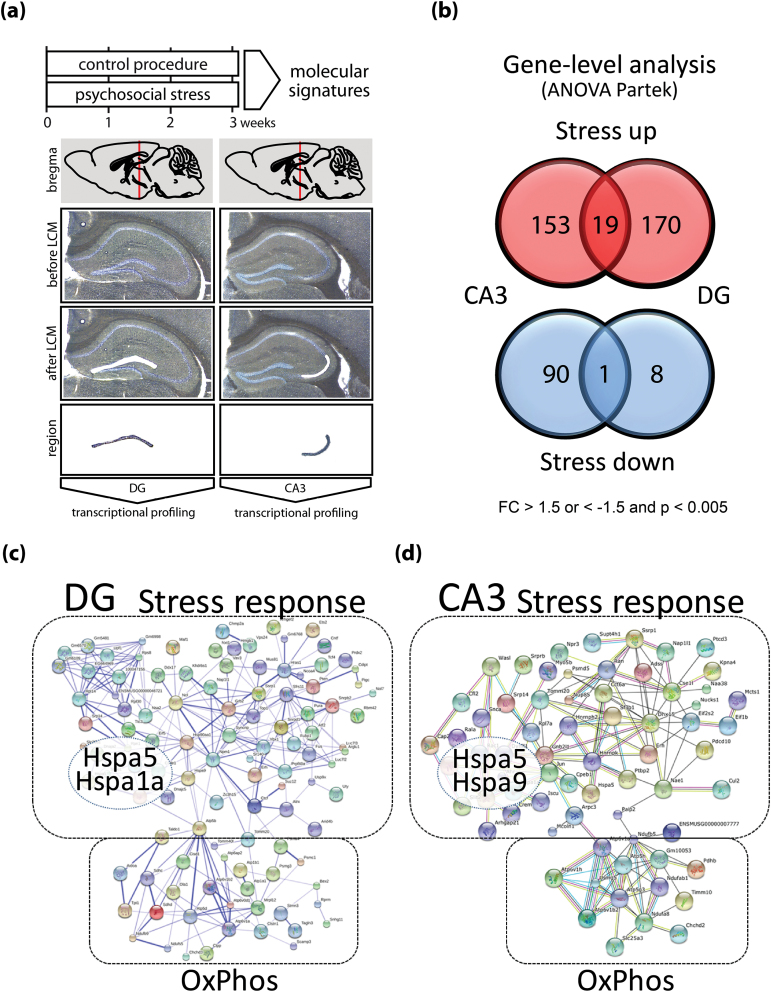
Fig. 1.
Experimental design and transcriptional profiling. (a) C57Bl/6J male mice were exposed to chronic psychosocial stress or control conditions for 3 weeks. Mouse brains were harvested 24h after the last stress or control handling session. Dentate gyrus (DG) and cornum ammonium 3 (CA3) subregions of the hippocampus were isolated by laser capture microdissection (LCM). Isolated RNA from specific regions was subjected to transcriptional profiling using microarrays. (b) Analysis at the individual gene level (performed by ANOVA) revealed that the majority of mRNAs were upregulated upon psychosocial stress in a region specific manner (172 in CA3 and 189 in DG, “stress up”); only 19 were upregulated in both regions. Stress lowered the expression of 91 genes in CA3 and 9 in DG in a nearly strict region specific manner with only 1 co-regulated gene (“stress down”). Cut-off for selection fold-change (FC) >1.5 or FC < −1.5 and P < 0.005. See supplementary tables 2–5 for details. Network analysis of genes identified as upregulated in DG (c) and CA3 (d). Internal connectivity was determined with the String algorithm, the largest cluster is depicted for each region. Both networks separate into 2 distinct highly interconnected sub-cluster termed “stress response” (upper cluster) and “OxPhos” (lower cluster). The “stress response” subcluster contains prototype stress associated hub genes such as heat shock proteins (Hspa1a, Hspa5, Hspa9) as indicated. The OxPhos cluster is comprised of hub genes encoding, eg, members of complex V (ATPase) of the respiratory chain (such as Atp6v1a, Atp6v1b2, Atp1b1) (see also supplementary tables 2 and 4).
In the second experiment, we used C57Bl/6J male mice divided into experimental groups: stressed and control mice with or without supplementary lithium in drinking water (figure 3a). After 3 weeks of corresponding treatment mice were subjected to behavioral tests (see supplementary information and ref.41).
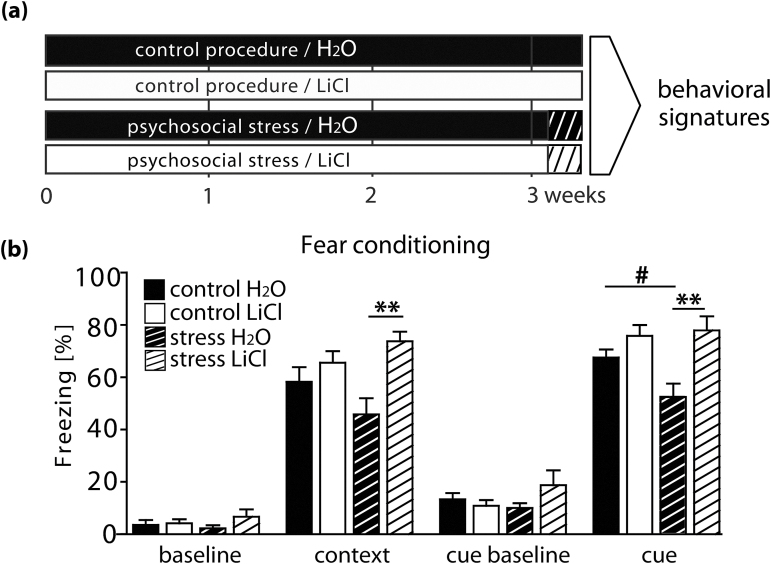
Fig. 3.
Lithium treatment ameliorates maladaptive effects of stress on cognition. (a) Experimental design. C57Bl/6J male mice were subjected to chronic psychosocial stress or the control procedure with or without chronic lithium treatment and analyzed for behavioral signatures. (b) Fear conditioning. Baseline freezing behavior (baseline) was not affected by stress (F 1,46 = 0.11; P = 0.7471, 2-way ANOVA). Lithium treatment did not alter baseline freezing levels (F 1,46 = 1.98; P = 0.1664, 2-way ANOVA). Vehicle treated stressed animals displayed a tendency to perform less freezings in the contextual fear memory test (context; effect of stress F 1,46 = 0.14; P = 0.1749 and interaction of treatment × stress F 1,46 = 3.19; P = 0.0809). Lithium treatment increased freezing rate in the context (effect of treatment F 1,46 = 9.32; P = 0.0038), most prominent in psychosocially stressed mice (P < 0.01, Bonferroni post hoc test). Testing for baseline freezing before presentation of cue (cue baseline) revealed no effects of stress or lithium treatment (F 1,46 = 0.64; P = 0.4274 and F 1,46 = 0.23; P = 0.6314, respectively; 2-way ANOVA). When analyzing vehicle treated animals during cue presentation (cue), stressed animals showed significantly less freezings than controls (P = 0.0225; Mann-Whitney test). A 2-way ANOVA performed on all experimental groups revealed a significant effect of treatment (F 1,46 = 13.61; P = 0.0006) and a close to significant interaction treatment × stress (F 1,46 = 3.53; P = 0.0665; 2-way ANOVA); however, no effect of stress (F 1,46 = 2.03; P = 0.1612). Post hoc Bonferroni comparison showed that stressed animals treated with lithium displayed significantly more freezings than untreated stressed controls (P < 0.01). n = 8–15 per group. # P < 0.05, Mann-Whitney test. **P < 0.01, Bonferroni post-test.
Microarray Analysis
RNA isolation from brain tissue and probe synthesis were performed as described previously.42 The Gene Set Enrichment Analysis (GSEA) approach43,44 was used to identify coordinated changes in a priori defined sets of functionally grouped genes. See supplementary information for details.
Social Stress Procedure and Behavioral Testing
The resident-intruder paradigm, as a model of social defeat, was described elsewhere for rats15,45 and for mice.12,14 The most of behavioral experiments were performed as described41 (see also supplementary information). Working memory performance was assessed in radial arm water maze (RAWM) following a protocol of ref.46 with some modifications.47
Statistical Analysis of Behavioral Data
Behavioral data were analyzed with the Mann-Whitney test or 2-way ANOVA and Bonferroni post-tests where applicable with a cut-off value P < 0.05. Data are shown as mean ± SEM in figures and text if not otherwise stated. The behavioral data were analyzed using the software GraphPad Prism4 (GraphPad Software).
Results
Gene Expression Signatures of Social Defeat in Subregions of the Hippocampus
To study transcriptomic signatures of chronic psychosocial stress in precisely isolated subregions of the hippocampus, we exposed adult C57Bl/6J male mice to 3 weeks of social defeat in a resident-intruder paradigm (referred to throughout the manuscript as “stress”) or to control procedure (figure 1a). Twenty-four hours after the last stress session, brains were sampled and dentate gyrus (DG) and CA3 neurons were isolated by laser capture microdissection (figure 1b). DG and CA3 are nearly exclusively comprised of granule cells or pyramidal neurons, respectively, thus representing rather homogenous cellular samples. We chose the dorsal part of the hippocampus as it seems to be particularly important for the encoding of memories.48,49
We first evaluated the precision of the sampling procedure (figure 1a) and performance of the microarray results by comparing DG and CA3 expression patterns. We detected 899 and 797 genes to be specific or enriched in DG vs CA3 samples, respectively (supplementary table 1). For selected subregion-specific genes, we corroborated the microarray findings by scanning in situ hybridization (ISH) profiles from the Allen Brain Atlas (http://www.brain-map.org/). For 3 CA3 specific genes (Neurod6, Elavl2, Lpl) and 3 DG specific genes (Dsp, Calb1, DOH4S114) the ISH analysis validated the regional selectivity and thus, the isolation and microarray performance (supplementary figure 1).
We then compared gene expression differences of chronic psychosocial stress in CA3 and DG at the individual transcript level (figure 1b, supplementary tables 2–5). We detected 172 and 189 genes as upregulated (supplementary tables 2 and 3) and 91 and 9 as downregulated (supplementary tables 4 and 5) under stress in DG and CA3, respectively. The hippocampal expression of selected candidates was again evaluated with available ISH data from the Allen Brain Atlas and showed heterogeneous, mainly neuronal expression patterns in the adult mouse brain (supplementary figure 2). The overlap of individual genes to be co-regulated in both regions was considerably low, with 19 co-upregulated and 1 gene co-downregulated, respectively (figure 1b), suggesting a highly region specific transcriptional response program.
Next, we used the String algorithm to identify and visualize potential clusters of functionally grouped genes (see supplementary information for details).50 This analysis revealed 1 large bimodal cluster comprised of 2 connected yet separate subnetworks for stress upregulated genes in CA3 and DG (figure 1c and 1d). Each of the larger subnetwork contains several prototypical stress-response genes such as heat-shock proteins (eg, Hspa5, Hspa1a, Hspa9) and other chaperones (see also supplementary tables 2 and 3) and was thus termed “stress response” (figure 1c and 1d). The smaller subnetworks contains several components of the respiratory chain/oxidative phosphorylation (Atp1a1, Atp6v1a, Atp6v1b, Atp1b1) and was thus termed “OxPhos” (figure 1c and 1d). Notably, all of these genes were among those upregulated upon stress in both regions ( supplementary table 6). Moreover, “oxidative phosphorylation” was the most significantly enriched KEGG pathway (GO_id: mmu00190) in both regions (CA3: FDR corrected p-val 0.01, DG: FDR corrected p-val <0.001) (supplementary table 7).
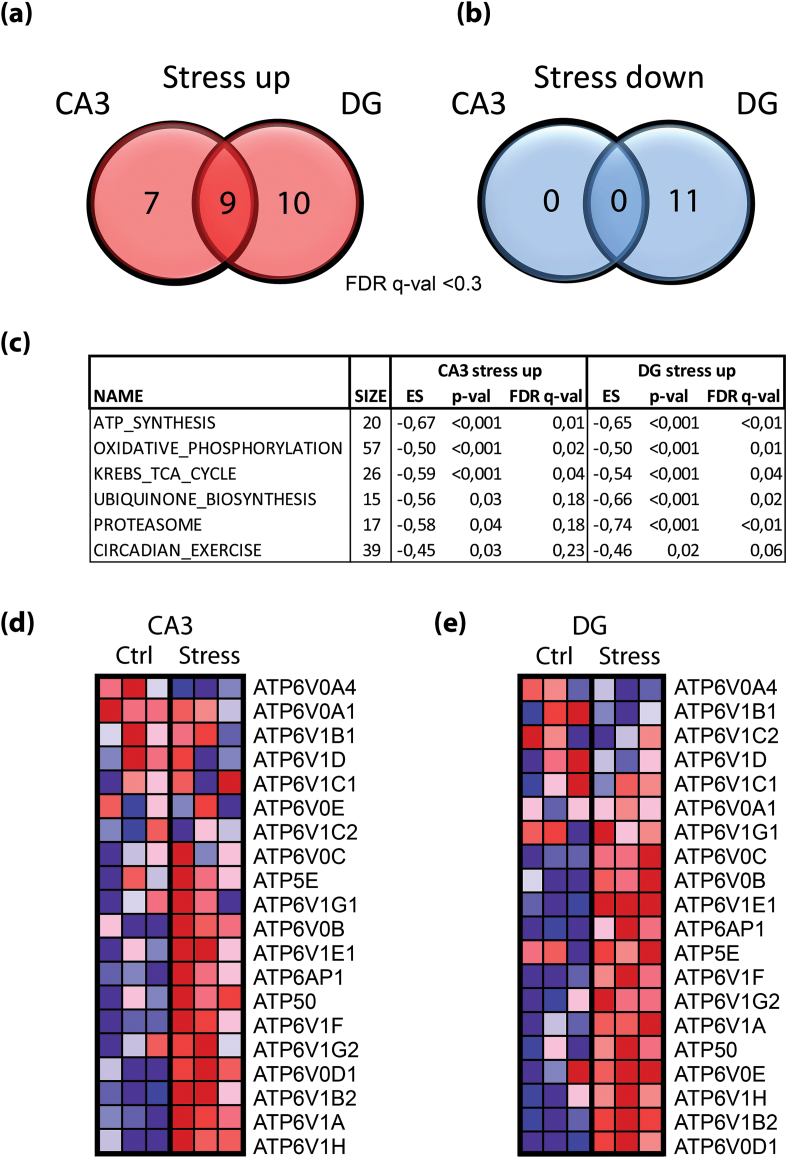
This analysis suggested that the molecular stress response, although highly distinctive at the level of individual genes (figure 1b), appears to share a similar architecture and involved pathways (figure 1c and 1d). To substantiate this finding, we used a knowledge-based approach and applied a gene-set enrichment analysis (GSEA) with a priori defined pathways as references.44 With this approach, highly sensitive analyses of transcriptome data can be performed that allow to detect changes at the pathway level potentially hidden in gene-centric approaches.43 This analysis identified 16 CA3 and 19 DG upregulated pathways of which 9 were co-upregulated in both regions representing 56% and 47% overlap, respectively (figure 2a). No pathway was detected as downregulated in CA3; however 11 were upregulated in DG (figure 2b). Among the top upregulated gene-sets (with FDR q-val <0.3), there were 3 major metabolic pathways (ATP-Synthesis, oxidative phosphorylation, and the TCA cycle) and genes encoding components of the proteasome supporting our assumption of a common stress signature in CA3 and DG (figure 2c). Heatmap clustering of members of the components of complex V of the respiratory chain, the “ATP-synthesis” (F0F1-ATPase) pathway, clearly indicated that the majority of the members are upregulated upon stress (figure 2d), whereas only 3 of those were identified with the statistically more selective gene-by-gene analysis (supplementary table 6). In summary, psychosocial stress causes specific responses as well as common transcriptional adaptions including core metabolic pathways in pyramidal and granular neurons of the CA3 and DG regions of the hippocampus.
Fig. 2.
Pathway-level analysis of transcriptome data. (a, b) Analysis at the pathway level (performed by gene-set enrichment analysis (GSEA) using all Genmap pathways) detected a large fraction of common responses with 9 out of 19 and 28 pathways in CA3 and DG, respectively, upregulated in both regions (a, reddish Venn diagrams). No overlapping downregulated pathways were detected upon social defeat in both regions. In DG, 21 pathways were down regulated at the given cutoff (b, blueish Venn diagrams). Cutoff for selection p-val <0.05 and false discovery rate (FDR) q-val <0.3. (c) Top upregulated pathways in CA3 and DG listing gene-set size, enrichment score (ES), p-value and FDR q-value. Note that the top 4 gene-sets are the core components of the mitochondrial catabolism dedicated to generate ATP (tricarboxylic acid cycle, ubiquinone synthesis, oxidative phosphorylation, and ATP synthesis) indicating higher energy demands upon stress. (d, e) Heatmap of all individual components of the top co-upregulated pathway (ATP synthesis) in CA3 (d) and DG (e). The gene-set comprises 20 components of the final component of the respiratory chain, the mitochondrial ATPase (complex V). Most genes of this pathway display increased gene expression levels upon stress as indicated by rank order and color code (red, upregulated; blue, downregulated; scale according to default settings of the GSEA algorithm).
Behavioral Profiling of Psychosocially Stressed Mice
The central aim of this study was to characterize molecular signatures of psychosocial stress paralleling cognitive impairments and to identify which of these correlate with a beneficial treatment indicating key target mechanisms. Based on available literature51 we speculated that contextual and cued fear conditioning should allow profiling psychosocial stress-induced alterations in hippocampus-dependent cognitive performance in mice. Moreover, we hypothesized that chronic Li treatment could ameliorate cognitive impairments to enable studying the underlying transcriptional changes. Therefore, we exposed cohorts of C57Bl/6J male mice to 3 weeks of chronic psychosocial stress utilizing the resident-intruder paradigm or to a control procedure and exposed both groups to Li solution treatment or water (figure 3a).
In accordance with published observations,52 Li treatment slightly reduced body weight only in stressed animals proving treatment effects and interaction of Li and stress (supplementary figure 3a). Importantly, stressed and control animals drunk a similar volume of water independent of Li supplement (5.826 and 5.595ml of LiCl solution for control and stressed groups, respectively; supplementary figure 3b) corresponding to 11.89mg Li/kg for control and 11.96mg Li/kg for stressed animals. This Li dose results in Li concentration in serum between 0.12 and 0.25mM, which is below the therapeutic concentration in patients.53 We assessed also pain sensitivity to rule out biasing effects of stress or Li on the perception of the foot-shock underlying fear conditioning. As described previously,16,54 neither stress nor Li had a significant impact on pain sensitivity as assessed by the hot plate test (supplementary figure 3c). Additional behavioral tests (supplementary figure 3d) revealed significant impact of psychosocial stress on locomotor activity, exploratory drive, curiosity, and anxiety in line with previous observations12,14,15 and hardly any effects of Li treatment on these parameters (summarized in supplementary figure 4).
Li Treatment Prevents Stress-Dependent Cognitive Impairment
We applied the contextual and cued fear conditioning test to assess hippocampus-dependent memory formation and recall for all treatment groups (figure 3a and 3b). Neither stress nor Li treatment affected baseline freezing behavior prior conditioning (figure 3b). In the contextual memory test we found a close to significance interaction of factors stress and lithium treatment (P = 0.08). Stress significantly reduced freezing rates in the cue paradigm (P < 0.05) (figure 3b). Stress dependent cognitive impairments observed in cue fear memory formation were completely rescued by Li treatment (P < 0.01; figure 3b). Moreover, Li treated mice displayed significantly higher freezing rate in the context (P < 0.05). These changes cannot be contributed to altered pain perception as the pain sensitivity was influenced neither by stress nor by lithium treatment (supplementary figure 3c). We also assessed the effects of Li treatment in a RAWM monitoring spatial learning and working memory46 (supplementary figure 5). Li treatment had no effect in unstressed mice (supplementary figure 5a), but significantly improved cognitive performance in stressed animals (supplementary figure 5b) supporting the findings of the fear conditioning.
Common Molecular Signatures of Psychosocial Stress and Li Treatment
So far, we characterized stress-associated molecular signatures in the DG and CA3 region as well as cognitive impairments in hippocampus dependent tasks (figures 1–3). Moreover, stress-induced cognitive impairments were substantially mitigated by Li treatment (figure 3). We next asked if and which molecular signatures might potentially be regulated both by psychosocial stress and Li treatment in the CA3 region of the dorsal hippocampus (figure 4a), as a region important for learning and memory.48,49
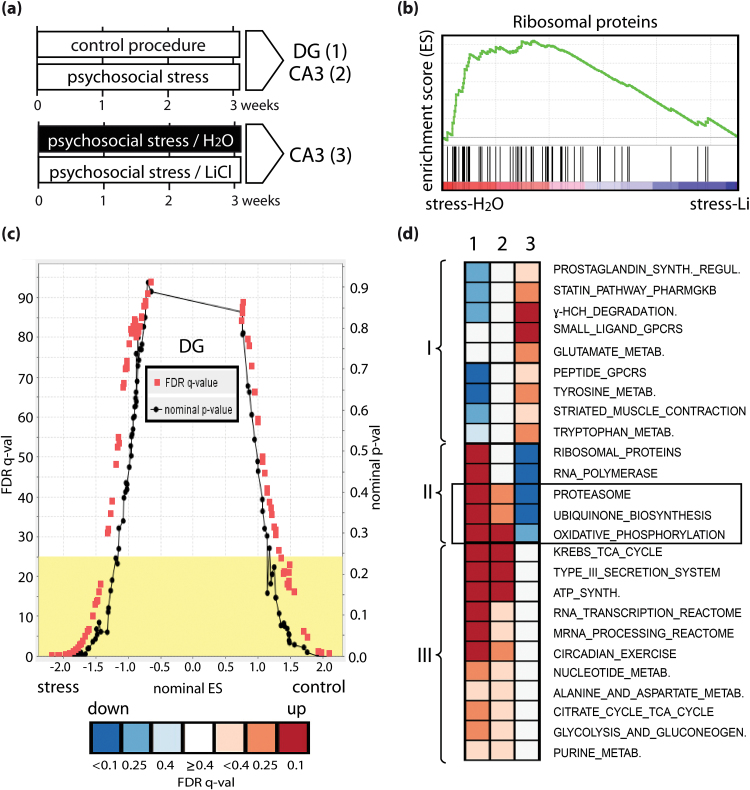
Fig. 4.
Psychosocial stress deregulates multiple pathways implicated in cellular metabolism with a substantial fraction being modulated by lithium treatment. (a) Experimental design. Mouse brains were dissected 24h after the last stress session or control procedure. Gene expression alterations were analyzed in microdissected subregions of the dentate gyrus (DG) and CA3 region of the hippocampus without treatment (upper panel, samples 1 and 2) and without or with lithium treatment under stress conditions in CA3 (sample 3). (b, c) Characteristics of gene set enrichment analysis (GSEA) and visualization of results obtained from pairwise comparisons of DG and CA3 control vs stress conditions (samples 1 and 2) and CA3 no lithium/stress vs lithium/stress treatments (sample 3). (b) Representative enrichment plot depicting the course of the enrichment score (green line) calculated from the rank-order positions of members of the ribosomal gene set in CA3 upon psychosocial stress and vehicle vs Li treatment. Left (down) or right (up) shifted clustering indicates deregulated expression of pathway components. (c) Representative p-val/FDR q-val vs ES plot (DG stress vs control) depicting the distribution of gene-sets at different significance levels along the ES score (cut-off > 1.0; < −1.0). Yellow box marks default cutoff by the GSEA algorithm (FDR q-val < 0.25). (d) Heat map visualization of significantly deregulated pathways selected by hierarchical cluster analysis. We identified 3 pathway clusters (I, II, III) differentially regulated by psychosocial stress and Li treatment: (I) downregulated in DG stress and upregulated in CA3 stress upon Li treatment, (II) upregulated in DG and/or CA3 stress and downregulated in CA3 stress upon Li treatment (boxed: proteasome, ubiquinone synthesis and oxidative phosphorylation pathways representing a common regulatory node), and (III) upregulated in DG and/or CA3 stress and unchanged in CA3 stress upon Li treatment.
By applying the identical stringency cut-off for the individual gene-level analysis (P < 0.005, fold-change > 1.5 < −1.5), we detected only 22 genes that were regulated by Li treatment in stressed animals (20 up, 2 down upon Li) (supplementary figure 6a, supplementary table 12). Only one gene, Gdap1, had been detected already in the CA3 response profiles as stress-downregulated gene (supplementary table 12). Notably, GDAP1 (gangliosyde induced differentiation associated protein 1) is localized to the outer mitochondrion membrane and has been associated with peripheral neuropathies of the Charcot-Marie-Tooth (CMT) 4A subtype.55 Inspecting in situ hybridization data from the Allen Brain Atlas revealed an expression pattern mainly confined to neurons of the cortex and hippocampus including the CA3 region (supplementary figure 6b). Using the String algorithm, we searched for interaction partners of GDAP1 that might reveal insight into functional relationships. This analysis revealed 2 associated functionally grouped genes, one associated with the synthesis and metabolism of the antioxidant glutathione, the other linking several axonal and myelin associated risk genes of neuropathies (supplementary figure 6c).
We next applied the more sensitive GSEA approach to further identify commonalities of psychosocial stress and Li treatment at the level of gene expression signatures (figure 4b and 4c). By using a rank-order statistics, the GSEA algorithm detected robustly shifts in functionally grouped genes as exemplified by the coordinated down-regulation of ribosomal proteins upon Li treatment (figure 4b). We also plotted the enrichment score vs the FDR q-value and nominal p-value of all stress vs control regulated pathways in DG to depict deregulated pathways (figure 4c). To identify common signatures of stress and Li treatment, we used the stringent FDR q-value to perform an unsupervised hierarchical clustering comparing stress associated gene-sets from DG and CA3 (supplementary tables 8, 9, and 11) and those obtained under Li-stress conditions in CA3 (supplementary tables 14 and 15) depicted as a heatmap (figure 4d). The combined GSEA/clustering approach identified a common “core” set of pathways that are regulated under stress in DG and CA3 and upon Li treatment in CA3 comprised of the “proteasome,” “ubiquinone,” and “oxidative phosphorylation’ pathways (figure 4d). Notably, all of these were upregulated under stress in DG and CA3 and downregulated in CA3 upon stress when Li treatment was performed and was highly efficient in ameliorating hippocampus-dependent learning impairments (figure 3b). Our findings suggest that this cluster indicates a core set of “protective” mechanisms of Li actions in the hippocampus.
Discussion
In this study, we examined the effects of chronic lithium treatment in mice exposed to chronic psychosocial stress at the behavioral and molecular level. We aimed at identifying transcriptional profiles caused by psychosocial stress in subregions of the hippocampus which are known to be involved in cognitive processing. Moreover, we applied sophisticated bioinformatic analyses to characterize molecular signatures of Li treatment that correlate with the amelioration of stress-associated cognitive impairments. Our final goal was to identify key cellular mechanisms which are treatment-accessible, and operate in the hippocampus.
Psychosocial Stress Induces Treatment Responsive Cognitive Impairments
In our study, chronic psychosocial stress-increased anxiety, and diminished physical activity and exploratory behavior, which is in line with earlier observations.13–16,56 At the cognitive level, chronic psychosocial stress reduced fear memory in contrast to some previously published reports11,16,56 which, however, employed different stress paradigms. In addition, upon social defeat we did not observe an impairment in RAWM paradigm, assessing spatial working memory.46 In contrast, Yu et al16 reported that social defeat induced impairments in spatial working memory monitored, however, by a T-maze whereas spatial reference learning and memory formation remained intact as assessed with the Morris water maze.11,16 It has been observed previously, that stress can induce diverse effects on cognitive functions depending not only on the respective stress paradigms, intensity and duration but also on the applied learning task and intervals between stress exposure and cognitive phenotyping which may explain differences between superficially similar reports.57 Nonetheless, the fear memory impairment induced by social defeat that we observed in this study was efficiently prevented by a chronic Li treatment. Furthermore, we could show that Li improved the performance in the RAWM in stressed but not control animals. Similarly to these findings, Li treatment alleviated spatial processing impairments in Fmr1 27 and APP null mutant mice28,29 supporting our results. In summary, our findings showed that psychosocial stress induces treatment responsive impairments of hippocampus dependent cognitive processing that allowed us to study associated molecular mechanisms.
Assessing Transcriptional Signatures with High Regional Precision
There are some studies on transcriptional alterations upon psychosocial stress in rodent models11,36,38,39,58 which, however, targeted different brain regions and lacked a precise, cell type specific resolution. In our study, we have chosen dorsal hippocampus as a region which is stress vulnerable and important for encoding of memories.48,49,59 The hippocampus is anatomically and functionally subdivided into different subregions including the CA fields and the DG which are mainly comprised of glutamatergic pyramidal and granule neurons, respectively, and are molecularly highly divergent.60 The comparison of CA3 and DG specific gene expression profiles from our study strongly supported these findings and validated our technical approach. The DG has been associated as a target region involved in adaptive processes underlying depression and stress.59 In the future, our approach could be extended to other regions or cell-types of hippocampus, eg, ventral part due to its proposed role in modulating dopamine functions in schizophrenia.61 Moreover, although technically challenging, profiling of isolated parvalbumin-positive interneurons of the hippocampus might as well represent an interesting option for future applications given the implication of these cells in chronic stress and schizophrenia.62–64
When analyzing physiological and subtle pathophysiological conditions at the molecular level, such as chronic psychosocial stress and treatment effects, in such a diversified structure like the hippocampus, subregion specific resolution of isolation is essential. In consequence, a meaningful interpretation of the molecular responses requires precise sampling strategies to avoid biases caused by heterogeneous cellular samples.42,60 Thus, we are convinced that our approach combining highly precise sampling procedures using LCM joined with microarray analysis and sophisticated bioinformatic tools is optimally suited to obtain the critical gain of resolution and sensitivity in detecting relevant changes in gene expression upon social defeat and Li treatment. Another strategy could involve isolation of precisely defined cell types. For that, a combined approach of LCM with immunohistochemical staining or FACS enrichment could be applied.65–67 Alternatively, given the high effort of isolating pools of individual cells with LCM, methods based on cell sorting could be advantageous for these applications.
Pathway-Level Signatures of Li Treatment Counteract the Molecular Effects of Psychosocial Stress
The expression analyses at the level of single genes (“gene centric” approach) identified highly regional-specific cluster of hundreds of stress-associated genes. This was somehow expected due to the high level of molecular divergence between CA3 pyramidal and DG granular neurons.60 Interestingly, upon social defeat, network analysis revealed a deregulation of a prominent cluster including several heat shock proteins in DG and CA3 subfields of hippocampus. This response was more evident in DG which is in accordance with previous observations.62
After Li treatment, we failed, however, to identify significant alterations at the level of individual genes. This could be attributed to a low response at the single gene level and/or masking effects of molecular adaptations in response to Li treatment. Nonetheless, we hypothesized that the efficient rescue of the stress mediated cognitive impairments by Li should be accompanied by corresponding molecular changes, possibly in a wider extent. Therefore, we performed highly sensitive analyses using the “pathway-level” GSEA approach which allowed us to identify common molecular signatures of stress and Li treatment in the CA3 subregion of the hippocampus. Most prominently, gene-sets encoding proteasomal components and mitochondrial genes related to the electron transport chain (oxidative phosphorylation and complex II/ubiquinone biosynthesis) represented a common signature of stress and Li response in both CA3 and in the DG. Notably, proteasome, mitochondrial, and neuronal deficits have also been detected in frontal cortex homogenates at the level of reduced protein and RNA expression in laser captured and microdissected DG granule neurons from postmortem samples of schizophrenia patients.68–70 Still, it may be possible that the findings from human studies on postmortem samples potentially represent age-dependent secondary compensatory mechanisms. Altar et al.68 mentioned that it remains unclear whether hippocampal CA3 neurons show similar reduction in gene expression as described for DG granular cells. If so, the authors speculated, that drugs increasing expression of these gene sets may better address at least some of the core deficits of schizophrenia, like cognition and attention deficits. This assumption is challenged by our study. Although we detected a similar set of core pathways, the molecular changes in CA3 are showing opposite directions compared to those in DG.
Chronic psychosocial stress increased while Li treatment reduced gene expression levels of the corresponding pathways. Thereby, Li counteracted stress-induced molecular alterations which, directly or indirectly, led to cognitive impairments. Nonetheless, our study together with other observations68–70 support the assumption that mitochondrial and proteasomal pathways are indicators of psychosocial stress in the hippocampus and that the corresponding gene-sets serve as biomarker of treatment responses by Li, a drug applied clinically to treat bipolar disorder. The precise molecular mode of action of lithium is unknown but pleiotropic effects targeting glycogen synthase kinase-3 (GSK-3) and Wnt/beta catenin signaling, BDNF, and molecular clock genes were reported.28,32–34 Broadly acting mechanisms have been described such as an reducing the increased cerebral protein synthesis levels in Fmr1 null mice.71 Moreover, Li likely operates via rapid proteasomal degradation of the orphan nuclear receptor Rev-erbα, a negative component of the circadian clock that coordinates metabolic and circadian pathways,72 and is involved in learning and memory processes.73 Thus, the critical pathways detected in our study may converge back on transcriptional control systems including circadian clock and cognitive modulators.
Taken together, our findings prove a beneficial role for lithium treatment as a preventive therapy attenuating negative effects of psychosocial stress on cognition, rebalancing the molecular pathways disturbed by stress.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Deutsche Forschungsgemeinschaft (German Research Foundation, DFG) KFO241 grants (RO 4076/1-1 and RO 4076/3-1 to M.J.R. and M.M.B.); DFG Research Center Molecular Physiology of the Brain, CMPB-C1 “Stress, cannabinoids and psychiatric comorbidity” to U.H.-R.
Supplementary Material
Acknowledgments
P.F. has been an honorary speaker for AstraZeneca, Bristol Myers Squibb, Eli Lilly, Essex, GE Healthcare, GlaxoSmithKline, Janssen Cilag, Lundbeck, Otsuka, Pfizer, Servier, and Takeda. During the past 5 years, but not presently, P.F. has been a member of the advisory boards of Janssen-Cilag, AstraZeneca, Eli Lilly, and Lundbeck. The other authors declare no financial interests. Authors’ contributions: M.M.B. and M.J.R. designed the study, M.M.B. performed experiments, S.P.W. and M.J.R. performed bioinformatical analyses, U.H.R., P.F., and M.J.R. contributed conceptually and provided funding for the study, M.M.B. and M.J.R. wrote the manuscript.
References
- 1. Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007;65:209–237. doi:10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2. Dobry Y, Braquehais MD, Sher L. Bullying, psychiatric pathology and suicidal behavior. Int J Adolesc Med Health. 2013;25 295–299. doi:10.1515/ijamh-2013-0065. [DOI] [PubMed] [Google Scholar]
- 3. Holtzman CW, Trotman HD, Goulding SM, et al. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. doi:10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stilo SA, Murray RM. The epidemiology of schizophrenia: replacing dogma with knowledge. Dialogues Clin Neurosci. 2010;12:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468:203–212. doi:10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 6. van Os J, Kapur S. Schizophrenia. Lancet Lond Engl. 2009;374:635–645. doi:10.1016/S0140-6736(09)60995–8. [DOI] [PubMed] [Google Scholar]
- 7. Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2009;76:273–286. doi:10.1016/j.schres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 8. Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- 9. Sakai RR, Tamashiro KLK. Chapter 1.6 Social hierarchy and stress. In: Steckler T, Kalin NH, Reul JMHM, eds. Techniques in the Behavioral and Neural Sciences. Elsevier; 2005: 113–132. http://www.sciencedirect.com/science/article/pii/S0921070905800525 Accessed October 13, 2015. [Google Scholar]
- 10. Björkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. [DOI] [PubMed] [Google Scholar]
- 11. Azzinnari D, Sigrist H, Staehli S, et al. Mouse social stress induces increased fear conditioning, helplessness and fatigue to physical challenge together with markers of altered immune and dopamine function. Neuropharmacology. 2014;85:328–341. doi:10.1016/j.neuropharm.2014.05.039. [DOI] [PubMed] [Google Scholar]
- 12. Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi:10.1016/j.psyneuen. 2003.08.003. [DOI] [PubMed] [Google Scholar]
- 13. Adamcio B, Havemann-Reinecke U, Ehrenreich H. Chronic psychosocial stress in the absence of social support induces pathological pre-pulse inhibition in mice. Behav Brain Res. 2009;204:246–249. doi:10.1016/j.bbr.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 14. Brzózka MM, Fischer A, Falkai P, Havemann-Reinecke U. Acute treatment with cannabinoid receptor agonist WIN55212.2 improves prepulse inhibition in psychosocially stressed mice. Behav Brain Res. 2011;218:280–287. doi:10.1016/j.bbr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 15. Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi:10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16. Yu T, Guo M, Garza J, et al. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol. 2011;14:303–317. doi:10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterlemann V, Rammes G, Wolf M, et al. Chronic social stress during adolescence induces cognitive impairment in aged mice. Hippocampus. 2010;20:540–549. doi:10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- 18. Tran TT, Srivareerat M, Alkadhi KA. Chronic psychosocial stress triggers cognitive impairment in a novel at-risk model of Alzheimer’s disease. Neurobiol Dis. 2010;37:756–763. doi:10.1016/j.nbd.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 19. Can A, Schulze TG, Gould TD. Molecular actions and clinical pharmacogenetics of lithium therapy. Pharmacol Biochem Behav. 2014;123:3–16. doi:10.1016/j.pbb.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–755. doi:10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- 21. Poolsup P, Li Wan Po A, de Oliveira IR. Systematic overview of lithium treatment in acute mania. J Clin Pharm Ther. 2000;25:139–156. [DOI] [PubMed] [Google Scholar]
- 22. Forlenza OV, De-Paula VJR, Diniz BSO. Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci. 2014;5:443–450. doi:10.1021/cn5000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berry-Kravis E, Sumis A, Hervey C, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi:10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- 24. Nunes MA, Viel TA, Buck HS. Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer’s disease. Curr Alzheimer Res. 2013;10:104–107. [DOI] [PubMed] [Google Scholar]
- 25. Pan JQ, Lewis MC, Ketterman JK, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2011;36:1397–1411. doi:10.1038/npp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rygula R, Golebiowska J, Kregiel J, Holuj M, Popik P. Acute administration of lithium, but not valproate, modulates cognitive judgment bias in rats. Psychopharmacology (Berl). 2015;232:2149–2156. doi:10.1007/s00213-014-3847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. King MK, Jope RS. Lithium treatment alleviates impaired cognition in a mouse model of fragile X syndrome. Genes Brain Behav. 2013;12:723–731. doi:10.1111/gbb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One. 2010;5:e14382. doi:10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15:272–285, 228. doi:10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- 30. Yoneyama M, Shiba T, Hasebe S, et al. Lithium promotes neuronal repair and ameliorates depression-like behavior following trimethyltin-induced neuronal loss in the dentate gyrus. PLoS One. 2014;9:e87953. doi:10.1371/journal.pone.0087953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vasconcellos AP, Tabajara AS, Ferrari C, Rocha E, Dalmaz C. Effect of chronic stress on spatial memory in rats is attenuated by lithium treatment. Physiol Behav. 2003;79:143–149. [DOI] [PubMed] [Google Scholar]
- 32. Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2005;30:1223–1237. doi:10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 33. Osland TM, Fernø J, Håvik B, et al. Lithium differentially affects clock gene expression in serum-shocked NIH-3T3 cells. J Psychopharmacol Oxf Engl. 2011;25:924–933. doi:10.1177/0269881110379508. [DOI] [PubMed] [Google Scholar]
- 34. Fukumoto F, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl). 2001;158:100–106. doi:10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- 35. Böer U, Cierny I, Krause D, et al. Chronic lithium salt treatment reduces CRE/CREB-directed gene transcription and reverses its upregulation by chronic psychosocial stress in transgenic reporter gene mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:2407–2415. doi:10.1038/sj.npp.1301640. [DOI] [PubMed] [Google Scholar]
- 36. Abumaria N, Rygula R, Havemann-Reinecke U, et al. Identification of genes regulated by chronic social stress in the rat dorsal raphe nucleus. Cell Mol Neurobiol. 2006;26:145–162. doi:10.1007/s10571-006-9024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alfonso J, Pollevick GD, Van Der Hart MG, Flügge G, Fuchs E, Frasch AC. Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. Eur J Neurosci. 2004;19:659–666. [DOI] [PubMed] [Google Scholar]
- 38. Feldker DEM, Morsink MC, Veenema AH, et al. The effect of chronic exposure to highly aggressive mice on hippocampal gene expression of non-aggressive subordinates. Brain Res. 2006;1089:10–20. doi:10.1016/j.brainres.2006.02.110. [DOI] [PubMed] [Google Scholar]
- 39. Kabbaj M, Evans S, Watson SJ, Akil H. The search for the neurobiological basis of vulnerability to drug abuse: using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47(suppl 1):111–122. doi:10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 40. Mineur YS, Crusio WE. Behavioral and neuroanatomical characterization of FVB/N inbred mice. Brain Res Bull. 2002;57:41–47. [DOI] [PubMed] [Google Scholar]
- 41. Brzózka MM, Radyushkin K, Wichert SP, Ehrenreich H, Rossner MJ. Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the brain. Biol Psychiatry. 2010;68:33–40. doi:10.1016/j.biopsych.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 42. Rossner MJ, Hirrlinger J, Wichert SP, et al. Global transcriptome analysis of genetically identified neurons in the adult cortex. J Neurosci Off J Soc Neurosci. 2006;26:9956–9966. doi:10.1523/JNEUROSCI.0468-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mootha VK, Lindgren CM, Eriksson K-F, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi:10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 44. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi:10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tornatzky W, Miczek KA. Behavioral and autonomic responses to intermittent social stress: differential protection by clonidine and metoprolol. Psychopharmacology (Berl). 1994;116:346–356. [DOI] [PubMed] [Google Scholar]
- 46. Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi:10.1038/nprot. 2006.275. [DOI] [PubMed] [Google Scholar]
- 47. Baier PC, Brzózka MM, Shahmoradi A, et al. Mice lacking the circadian modulators SHARP1 and SHARP2 display altered sleep and mixed state endophenotypes of psychiatric disorders. PLoS One. 2014;9:e110310. doi:10.1371/journal.pone.0110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi:10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi:10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0. CO;2–7. [DOI] [PubMed] [Google Scholar]
- 50. Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi:10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 51. Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. [DOI] [PubMed] [Google Scholar]
- 52. Silva R, Mesquita AR, Bessa J, et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–669. doi:10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 53. Nciri R, Allagui M, Vincent C, Murat JC, Croute F, El Feki A. The effects of subchronic lithium administration in male Wistar mice on some biochemical parameters. Hum Exp Toxicol. 2009;28:641–646. doi:10.1177/0960327109106486. [DOI] [PubMed] [Google Scholar]
- 54. Kovacsics CE, Gould TD. Shock-induced aggression in mice is modified by lithium. Pharmacol Biochem Behav. 2010;94:380–386. doi:10.1016/j.pbb.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 55. Pedrola L, Espert A, Wu X, Claramunt R, Shy ME, Palau F. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet. 2005;14:1087–1094. doi:10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- 56. Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl). 2010;211:69–77. doi:10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- 57. Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi:10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stankiewicz AM, Goscik J, Swiergiel AH, et al. Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci. 2014;15:130. doi:10.1186/s12868-014-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malykhin NV, Coupland NJ. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015. doi:10.1016/j.neuroscience.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 60. Thompson CL, Pathak SD, Jeromin A, et al. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi:10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 61. Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32:507–513. doi:10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Filipović D, Zlatković J, Gass P, Inta D. The differential effects of acute vs. chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. doi:10.1016/j.neuroscience.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 63. Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. doi:10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu W, Zhang M, Czéh B, Flügge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35:1693–1707. doi:10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gurok U, Loebbert RW, Meyer AH, et al. Laser capture microdissection and microarray analysis of dividing neural progenitor cells from the adult rat hippocampus. Eur J Neurosci. 2007;26:1079–1090. doi:10.1111/j.1460-9568.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 66. Khodosevich K, Inta D, Seeburg PH, Monyer H. Gene expression analysis of in vivo fluorescent cells. PLoS One. 2007;2:e1151. doi:10.1371/journal.pone.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Saxena A, Wagatsuma A, Noro Y, et al. Trehalose-enhanced isolation of neuronal sub-types from adult mouse brain. BioTechniques. 2012;52:381–385. doi:10.2144/0000113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Altar CA, Jurata LW, Charles V, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi:10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 69. Nascimento JM, Martins-de-Souza D. The proteome of schizophrenia. npj Schizophr. 2015;1:14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697, 643. doi:10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- 71. Liu Z-H, Huang T, Smith CB. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile X syndrome. Neurobiol Dis. 2011;45:1145–1152. doi:10.1016/j.nbd.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi:10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 73. Jager J, O’Brien WT, Manlove J, et al. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor Rev-erbα. Mol Endocrinol Baltim Md. 2014;28:490–498. doi:10.1210/me.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.