Abstract
Cognitive training (CT) and aerobic exercise have separately shown promise for improving cognitive deficits in schizophrenia. Aerobic exercise releases brain-derived neurotrophic factor, which promotes synaptic plasticity and neurogenesis. Thus, aerobic exercise provides a neurotrophic platform for neuroplasticity-based CT. The combination of aerobic exercise and CT may yield more robust effects than CT alone, particularly in the initial course of schizophrenia. In a pilot study, 7 patients with a recent onset of schizophrenia were assigned to Cognitive Training & Exercise (CT&E) and 9 to CT alone for a 10-week period. Posit Science programs were used for CT. Neurocognitive training focused on tuning neural circuits related to perceptual processing and verbal learning and memory. Social cognitive training used the same learning principles with social and affective stimuli. Both groups participated in these training sessions 2d/wk, 2h/d. The CT&E group also participated in an aerobic conditioning program for 30 minutes at our clinic 2d/wk and at home 2d/wk. The effect size for improvement in the MATRICS Consensus Cognitive Battery Overall Composite score for CT&E patients relative to CT patients was large. Functional outcome, particularly independent living skills, also tended to improve more in the CT&E than in the CT group. Muscular endurance, cardiovascular fitness, and diastolic blood pressure also showed relative improvement in the CT&E compared to the CT group. These encouraging pilot study findings support the promise of combining CT and aerobic exercise to improve the early course of schizophrenia.
Key words: cognitive remediation, physical exercise, functional outcome, recent-onset schizophrenia
Introduction
Deficits in neurocognition are widely accepted as core features of schizophrenia in symptomatic states and in remission.1–3 Multiple cognitive domains are impacted, such as attention, working memory, and verbal memory, and overall neurocognitive deficits are typically large in magnitude (Cohen’s effect sizes of d = 0.80 to 1.40).4–6 The severity of neurocognitive deficits predicts functional outcome and often serves as a rate-limiting factor for resumption of full work and social functioning.7,8 Recent meta-analyses have also documented large deficits in social cognition, including facial affect recognition (d = −0.71)9 and emotion processing (d = −0.91 and d = −0.85)10 in schizophrenia patients. Impairments in social cognitive functioning serve as a major obstacle, beyond neurocognitive deficits, to improving psychosocial functioning in patients with schizophrenia11,12 and are potential mediators of the relationship between neurocognition and functioning.13 Neurocognitive and social cognitive deficits remain largely untreated, which impedes functional recovery.
Cognitive Remediation to Improve Cognitive Functioning in Schizophrenia
Over the last decade, an increasing number of studies have reported that systematic cognitive training (CT) can produce significant improvements in cognitive functioning in schizophrenia patients. Two meta-analyses indicated that schizophrenia patients participating in CT experience moderate improvements in neurocognition (d = 0.4114 to 0.4315). CT has produced improvements within the domains of verbal memory, problem solving, attention, and cognitive control.16–18 Social cognitive deficits have also been moderately amenable to CT (facial recognition d = 0.67, emotion perception d = 0.47, and higher order social cognition d = 0.46).19 These studies indicate that CT can have broad effects on neurocognition and social cognition in schizophrenia, but the improvements are typically moderate in magnitude, while the cognitive deficits in schizophrenia are large.
Providing CT in early phases of schizophrenia might be expected to produce even larger cognitive benefits and greater transfer to everyday functioning than in people with chronic schizophrenia because the cognitive gains would occur before any further declines in cognitive functioning, before the detrimental impacts of the chronicity of the illness on brain structure and function,20–22 and before patterns of work disability were established. Indeed, Bowie and colleagues23 compared the efficacy of cognitive remediation in schizophrenia patients with early (first 5 years) vs long-term illness and found that improvement in processing speed and executive functions was greater for those patients in the early course of psychosis.
The impact of cognitive remediation in the initial years of schizophrenia has only recently begun to be systematically studied but clear benefits for several neurocognitive domains24–27 and social cognitive functioning28,29 have been demonstrated. While the duration of treatment and type of cognitive remediation have varied, these studies have shown that a substantial impact on cognitive performance and to a lesser extent on functional outcome can be achieved. Yet, despite the promise of early CT intervention, current CT approaches, even in early course patients, appear to remediate only about a third to a half of the cognitive deficit. Further steps to enhance the impact of CT would clearly be helpful.
Aerobic Exercise to Improve Cognitive Functioning in Schizophrenia
A separate literature suggests that physical exercise can enhance cognitive functioning. Studies in healthy subjects have shown that regular physical exercise can improve cognitive functioning, particularly attention, processing speed and visuospatial learning and memory processes,30–32 similar to the improvements produced by CT. These cognitive findings have paralleled regional increases in brain volume and functioning, primarily in the prefrontal cortex and hippocampus,33–35 after several months of intensive aerobic exercise. The cognitive deficits in patients with schizophrenia, which affect attention, memory, and executive functions, have been correlated with physical inactivity,36 suggesting that increased physical exercise might impact these deficits. Initial research has begun to evaluate the impact of physical activity on brain morphology and cognitive functioning in patients with chronic schizophrenia. Pajonk et al37 found a 12% relative hippocampal volume increase in schizophrenia patients after a 12-week aerobic exercise intervention, in comparison with a 1% decrease in the nonexercising patient group. Increased hippocampal volume in the exercise group was correlated with improved short-term memory performance. However, this improvement in brain morphology as a result of an exercise intervention has not yet been replicated in subsequent studies in chronic schizophrenia38 or first-episode patients.39 Two studies of an aerobic exercise intervention in first-episode schizophrenia patients were able to show feasibility of a 12-week exercise intervention and also improvements in cardiovascular fitness (V02max), but they did not examine cognitive functioning outcomes or putative peripheral blood markers that may mediate cognitive change.40 Thus, the potential of physical exercise to enhance cognitive functioning in schizophrenia has yet to be fully realized.
Exercise-induced structural brain changes have been linked to increased neural growth factors such as brain-derived neurotrophic factor (BDNF), which is highly expressed in the hippocampus, and is essential in facilitating neurogenesis, cell survival, synaptic plasticity, memory and learning.41–43 To date, no studies have explored BDNF levels in response to an exercise intervention in first-episode schizophrenia patients, only in chronic schizophrenia patients. Those studies have shown that increased serum BDNF levels were related to improvements in physical fitness as a result of aerobic exercise intervention.44,45 A convergence of this potential mediator of cognitive improvement is suggested in the finding that chronic schizophrenia patients who engaged in 50 hours of CT showed significant cognitive gains and a significant increase in serum BDNF compared with patients who just played computer games.46 Parallels between the impact of physical exercise and CT raise the possibility that combining these interventions may be a means of enhancing the impact of CT.43
Benefits of Adding Exercise to a CT Program
Given that cognitive deficits in schizophrenia may be improved through either CT or physical exercise interventions but neither alone generally produces large cognitive gains, we have developed an interest in determining whether a combination of Cognitive Training & Exercise (CT&E) results in larger improvement in cognitive deficits. Animal models have shown that hippocampal neuroplasticity occurs in both exercise and environmental enrichment (ie, cognitive stimulation) paradigms; however, these neuroplastic benefits are only apparent when learning is simultaneously occurring with physical exercise.47,48 Fabel et al49 found that combined aerobic exercise and cognitive enrichment for rodents yielded an approximately 30% greater increase in new neurons than either stimulus alone. They proposed that aerobic exercise can “prime” the neurogenic region of the dentate gyrus for increased neurogenesis when the animal is exposed to an additional cognitive stimulus. Further, animal research suggests that exercise and cognitive stimulation provide qualitatively different gains in neuroplasticity. Exercise increases the proliferation and division of neuronal precursor cells in the dentate gyrus of the hippocampus (expansion phase), whereas CT promotes the survival of these cells, indicating the interdependence of these forms of activity.50,51 Indeed, most of the net neurogenic regulation is determined not by the expansion phase but rather by survival of the cells.52 In other words, new neurons created during the course of participation in physical exercise will quickly die off if adequate learning opportunities or novel experiences do not accompany the increased physical activity that could be provided via CT.53,54
Two recent studies in chronic schizophrenia patients have explored neurocognitive outcomes in a combined CT plus exercise intervention.55,56 These studies concluded that the combination of aerobic exercise training and CT led to neurocognitive gains in the domains of processing speed, working memory, executive functioning and verbal and visual memory, compared to a mental relaxation or table soccer control condition. This initial evidence suggests that the combining physical exercise and CT may potentiate their impact on cognitive functioning. Thus, we hypothesized that adding aerobic exercise to CT would substantially enhance the impact of CT on cognitive functioning in first-episode schizophrenia patients, taking advantage of a period in which large cognitive gains may be easier to achieve and have more functional impact than later in the course of schizophrenia.
Methods
Participants
The study participants in this initial pilot study were enrolled in the UCLA Aftercare Research Program57–59 from November 2009, through December 2011. This research was approved by the UCLA IRB and was consistent with international ethical standards. All participants provided written informed consent. Participants were recruited from a variety of Los Angeles psychiatric hospitals and clinics. Study inclusion criteria for entry into the Aftercare Research Program were: (1) a recent onset of psychotic illness, with the beginning of the first major psychotic episode within the last 2 years; (2) a diagnosis by the DSM-IV of schizophrenia, schizoaffective disorder, depressed type, or schizophreniform disorder; (3) 18 to 45 years of age; (4) sufficient acculturation and fluency in the English language to avoid invalidating research measures; and (5) residence within commuting distance of the UCLA Aftercare Research Program. Study exclusion criteria were: (1) a known neurological disorder; (2) significant and habitual drug abuse or alcoholism in the 6 months prior to hospitalization, or psychosis that was accounted for by substance abuse; and (3) premorbid mental retardation. Criteria for first episode psychosis samples vary in the literature; some may refer to onset within 2 years as defining a recent-onset schizophrenia sample. The participants had an age, educational level, and sex distribution typical of individuals with a first episode of psychosis, and a racial and ethnic breakdown that was representative of the Greater Los Angeles area (table 1). Their total 24-item Expanded Brief Psychiatric Rating Scale (BPRS) scores60 are consistent with a period of clinical stability (table 1).
Table 1.
Sample Characteristics at Study Entry for Recent-Onset Schizophrenia Patients
| Cognitive Training (n = 9) | Cognitive Training & Exercise (n = 7) | Combined (n = 16) | ||||
|---|---|---|---|---|---|---|
| Mean age at program entry, years (SD) | 23.5 (5.4) | 21.8 (3.8) | 22.7 (4.7) | |||
| Mean education, years (SD) | 12.4 (0.7) | 12.9 (1.2) | 12.6 (0.8) | |||
| Mean months from psychosis onset to program entry (SD) | 11.2 (6.3) | 6.7 (6.5) | 9.2 (6.6) | |||
| Mean months from psychosis onset to baseline test (SD) | 24.8 (5.4) | 21.8 (3.8) | 23.5 (4.9) | |||
| Mean total 24-item BPRS | 44.4 (11.1) | 37.0 (8.9) | 41.2 (10.6) | |||
| Sex | 89% Male | 57% Male | 73% Male | |||
| Race | Caucasian | 33% | Caucasian | 29% | Caucasian | 31% |
| Asian | 11% | Asian | 14% | Asian | 13% | |
| Pacific Islander | 0% | Pacific Islander | 0% | Pacific Islander | 0% | |
| Native American | 11% | Native American | 0% | Native American | 6% | |
| African American | 33% | African American | 14% | African American | 25% | |
| Mixed | 11% | Mixed | 43% | Mixed | 25% | |
| Ethnicity | 67% Hispanic | 57% Hispanic | 63% Hispanic | |||
| Diagnosis | Schizophrenia | 56% | Schizophrenia | 57% | Schizophrenia | 56% |
| Schizophreniform | 22% | Schizophreniform | 43% | Schizophreniform | 31% | |
| Schizoaffective | 22% | Schizoaffective | 0% | Schizoaffective | 13% | |
| Handedness | 89% Right | 86% Right | 88% Right | |||
Note: BPRS, Brief Psychiatric Rating Scale.
Interventions
All patients received treatment with a second-generation antipsychotic medication, regular visits with the treating psychiatrist, and individual case management, in addition to the interventions described below. We compared outcomes for a patient group that was provided CT with those for a patient group provided CT&E. Participants were selected from UCLA Aftercare Research Program patients so that the groups would be comparable in their demographic and clinical characteristics. Given that this initial feasibility pilot study involved only a small number of participants, this group matching yielded better comparability of key background characteristics than randomization was likely to achieve. The 2 groups did not differ significantly from each other on key demographic, illness duration, or symptom severity variables, as shown in table 1. All of the patients were on second-generation antipsychotic medication (11 oral risperidone, 3 long-acting injectable risperidone, 2 aripiprazole, 2 with supplemental quetiapine), 5 were on low dosages of antiparkinsonian medication, and 3 were on antidepressants, with distributions not differing significantly between the 2 treatment groups.
Cognitive Training
Both the CT and CT&E groups participated in the same systematic CT program. We selected Posit Science programs for CT because they are designed to accentuate the human brain’s capacity to establish and maintain new neural connections as a result of systematic learning exercises that capitalize on the brain’s capacity for neuroplasticity.61 The emphasis on neuroplasticity interfaces well with the impact of aerobic exercise on neurogenesis and synaptic plasticity. Posit Science programs have been demonstrated to improve cognition in schizophrenia27,62 and allow a coordinated sequence of neurocognitive and social cognitive exercises designed to enhance basic discrimination and processing skills and then generalize to more complex stimuli. The advantages of the Posit Science programs include: (1) a strong behavioral neuroscience base guiding program development,63–65 (2) fully automated functionality that maintains an optimal difficulty level for learning (80% correct response level), and (3) Internet-based software that enables users to monitor real-time progress and daily training through personalized feedback. The CT was administered at the clinic, with the first 5 weeks focused on neurocognitive training and the second 5 weeks focused on social cognitive training. Patients attended the clinic twice a week and completed 4h/wk of computerized CT. Thus, each patient was provided 20 hours of neurocognitive training and 20 hours of social cognitive training.
Neurocognitive Training.
The components of the Posit Science Brain Fitness Program (BFP) selected for this study included 6 computerized exercises aimed at improving the fundamental neurocognitive processes of auditory discrimination, speed of processing, working memory, verbal memory, and verbal reasoning. The BFP’s auditory exercises start with basic processes involving auditory discrimination and progress to more complex processes involving memory processing and reasoning. Thus, “Sound Sweeps” involves indicating whether brief auditory pitch changes are going up or down. In “Sound Replay” participants learn to reconstruct sequences of syllables. “Tell Us Apart,” “Match It!,” and “Story Teller” are additional exercises of intermediate cognitive complexity. “Listen and Do” is a higher-level auditory task in which participants listen to a list of verbal instructions and then complete the steps, with the number of critical components and complexity level increasing over trials.
Social Cognitive Training.
The Posit Science program called Targeted Affect Remediation Application (TARA; updated later as SocialVille) is a web-based series of engaging exercises that use human faces, video presentations, and social games designed to improve cognitive processes underlying social and emotional interactions. TARA starts with bottom-up exercises that are aimed at remediating fundamental impairments in social perception, processing of emotion, and interpretation of social information. The facial recognition and memory exercises are designed to improve a patient’s ability to discriminate and remember human faces. The “Emotion Perception” exercises are designed to improve the discrimination and memory of auditory and visual displays of emotions. “Social Cue Perception” exercises focus on improving a patient’s ability to judge social cues and infer emotions and intentions from contextual information and nonverbal gestures.
Aerobic Exercise Program
In addition to the CT sessions, the CT&E group participated in an aerobic exercise program. Our aerobic conditioning program consisted of a series of commercially available exercise videos approved by a certified personal trainer familiar with the cognitive and physical health issues of first-episode schizophrenia patients. We chose workout videos which included instructors leading calisthenics (eg, lunges, squats, pushups) and simple movement sequences at several levels of intensity, without resistance training. A different video was used each week. For ease of dissemination, we avoided use of specialized exercise equipment. Because antipsychotics can disrupt body temperature regulation, exercise was completed in an indoor temperature-controlled area when the outdoor temperature was more than 85°F. The exercise dosage was 150min/wk, over 4 days a week, and includes 2 separate exercise sessions at the clinic (45-minute duration) and 2 at home (30-minute duration).66 Clinic sessions were in groups led by a trained staff member. The intensity during the exercise sessions was individually calibrated at 60 to 80% of aerobic capacity using the gold standard heart rate calculation Karvonen formula [Target Heart Rate = ((max HR − resting HR) × %Intensity) + resting HR]. Participants wore individually programmed heart rate monitors (Polar RS 100) during exercise sessions at the clinic and at home to ensure that they exercised at the prescribed intensity. Exercise intensity was increased over time as needed to maintain intensity within individualized heart rate zones. The heart rate monitor recorded the number of minutes each person spent in their target heart rate zone.
Each participant earned $5 for every homework session completed to address the particular motivational issues that can be related to exercising at home. In addition, an incentive reward program capitalized on in-clinic peer-to-peer interaction and friendly competition. Points were rewarded for staying in the target heart rate zone, for perceived effort exerted during the in-clinic exercise sessions, for meeting weekly fitness goals, and for completing the homework exercise sessions. Every other month, the first place point leader received a $10 reward and the second place winner received a $5 reward. When needed, clinic staff made phone calls to patients between clinic sessions to remind them to complete their home exercises.
Bridging Group
The Bridging Group occurred for 1h/wk, separately for the CT and CT&E groups, and provided an opportunity for patients to learn strategies for directly applying the CT to their daily lives. Discussions emphasized how the computerized training exercises could help them achieve work and school goals, as well as improve social interactions. For example, computerized training to improve one’s ability to recall a sequence of instructions through chunking of information can help a person better remember an employer’s directions and instructions. In the CT&E group, there were also discussions about the benefits of aerobic exercise on brain functioning and cognition.
Assessments
The assessment batteries were administered at baseline and immediately following the completion of the CT or CT&E interventions.
Cognition
The MATRICS Consensus Cognitive Battery (MCCB),67 with its age and gender-corrected T scores for 7 domains and an Overall Composite score, was used to assess cognitive functioning.
Functional Outcome
The Role Functioning Scale (RFS)68 was used to measure functional outcome. It consists of four 7-point rating scales that assess degree of self-sufficiency in managing everyday tasks, work productivity, family relationships, and social relationships. Ratings were completed by the individual case managers who interacted weekly with patients and regularly with family members and participated in weekly quality assurance meetings led by J.V.
Body Composition and Fitness Testing
We measured weight and computed Body Mass Index and the waist-to-hip ratio as body composition indices. We administered the YMCA Fitness Assessment protocol69 and used the 3-minute step test, the modified sit-and-reach test, and the half sit-up test to operationalize cardiorespiratory endurance, flexibility, and muscular strength and endurance, respectively.
The 3-minute step test requires a participant to step up and down on a 30.5-cm high bench at the rate of 96 beats (24 steps) per minute for a total of 3 minutes. The test is scored as the recovery heart rate in beats per minute over 1 minute, taken while seated beginning 5 seconds after the participant’s final step, with a lower heart rate representing better cardiovascular fitness. We used the Acuflex I (Novel Products, Inc) sit-and-reach box to administer the modified sit-and-reach test to measure flexibility. The half sit-up test score is the number of properly executed repetitions in 1 minute.
Serum BDNF
Assays of a key neurotrophic growth factor, BDNF, were included as an exploratory measure to better understand a potential biological mechanism by which physical exercise and CT improve cognitive functioning. Serum processing and assays took place at UCLA Clinical and Translational Research Center (CTRC). ELISA immunoassay kits were obtained through R&D Systems (http://www.rndsystems.com/Products/DBD00).
Results
Because the current report is for a small pilot study, we will evaluate effect sizes rather than statistical significance, as the sample sizes are too small to have adequate power for statistical significance for anything smaller than very large effects. As recommended by Cohen70 for Group × Time interactions, Cohen’s f is used as the index of effect size. Compared to the more familiar Cohen’s d values for effect size, Cohen’s f values are half as large when there are only 2 groups (eg, Cohen’s d = 0.80 is comparable to Cohen’s f = 0.40).
Adherence to Treatment
Attendance at the CT sessions was highly consistent, with both groups attending over 90% of these in-clinic sessions. For the exercise groups at the clinic, we achieved 95% attendance. Adherence with the at-home exercise component was almost as high (92%).
Cognition
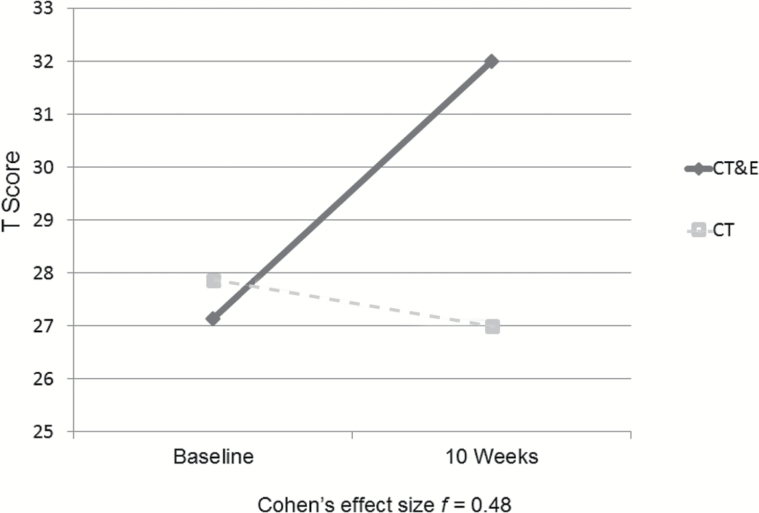
The CPT-IP program did not store results for 2 patients in the CT condition, so the Attention/Vigilance domain and the Overall Composite score were available for 7 CT patients. For the MCCB Overall Composite score, Group × Time (pre- vs post-test) interaction results suggest that the CT&E patients (n = 7) improve notably more than the CT patients (n = 7; see figure 1). The Cohen’s f for this Group × Time interaction is 0.48, a large effect (f = 0.40 is large70). The individual cognitive domains with largest differential gains for CT&E compared to CT alone were Social Cognition (f = 0.65), Working Memory (f = 0.50), Speed of Processing (f = 0.38), and Attention/Vigilance (f = 0.33).
Fig. 1.
Cognitive training & exercise (CT&E) might enhance impact of cognitive training on global cognition (MCCB overall composite score; n = 14).
Functional Outcome
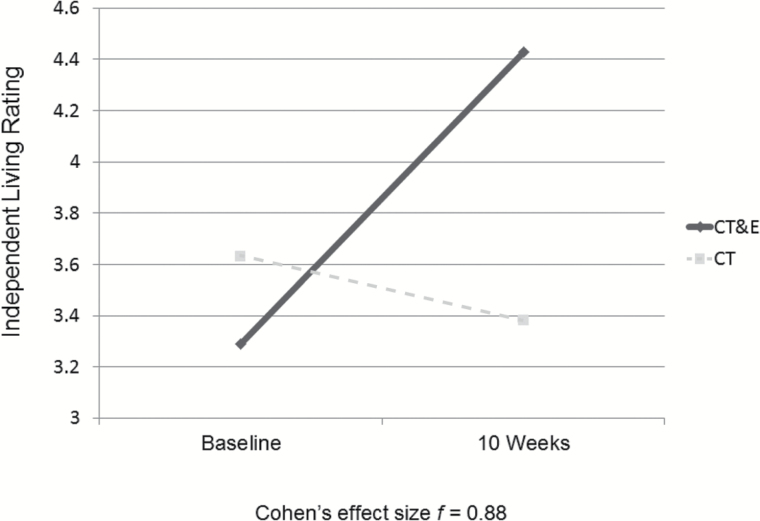
The Role Functioning Scale differentiates 4 domains, Independent Living, Working Productivity, Family Network Relationships, and Immediate Social Network Relationships.68 The Group × Time interactions indicate that the CT&E group tended to improve more than the CT alone group in Independent Living (Cohen’s f = 0.88; see figure 2), Family Network Relationships (f = 0.43), and Working Productivity (f = 0.18), but not in Immediate Social Network Relationships (f = 0.21 favoring CT).
Fig. 2.
Cognitive training & exercise (CT&E) might enhance impact of cognitive training on independent living skills (n = 15).
Body Composition and Fitness Testing
A potential benefit of adding aerobic conditioning to CT is that such physical exercise may help counteract some physical health risks associated with schizophrenia and antipsychotic medication.71 Cardiovascular fitness (measured by lower recovery heart rate in the 3-min step test) tended to improve more in the CT&E group than in the CT group (mean change: CT&E: −10.9 bpm, SD = 11.5, CT: −1.6 bpm, SD = 9.8; Cohen’s f = 0.47). Muscular endurance (measured by the 1-min half sit-up YMCA Fitness Test) tended to show greater improvement in the CT&E group compared to the CT group (mean change: CT&E: 2.4 reps, SD = 9.0, CT: −1.6 reps, SD = 9.4; Cohen’s f = 0.23). In addition, diastolic blood pressure showed a tendency toward greater differential improvement in the CT&E group (mean change: CT&E: −2.1 mmHG, SD = 8.3, CT: +2.0 mmHG, SD = 6.1, Cohen’s f = 0.31).
The amount of exercise used in this pilot study did not result in weight loss (mean change: CT&E: 3.0 lbs, SD = 7.6; CT: −1.1 lbs, SD = 11.6). Similarly, BMI was not reduced (mean change: CT&E: 0.51, SD = 1.31; CT: −0.38, SD = 2.02) and waist-to-hip ratio reduced only very slightly in the CT&E group (mean change: CT&E: −0.02, SD = 0.09; CT: +0.02, SD = 0.06).
Serum BDNF
In an exploratory analysis, we drew blood samples from 4 CT&E patients at baseline and again at the follow-up time point. Initial analyses suggest that serum BDNF concentrations increased from baseline to follow-up in the CT&E group (mean change: 4220 pg/mL, SD = 4110).
Discussion
After only 10 weeks of training, the enhancement of cognitive functioning achieved by combining aerobic conditioning and CT, as compared to completing the same CT alone, was very encouraging. The improvements cut across several cognitive domains. Some of the estimated effect sizes for differential gains in CT&E compared to CT alone were large. Estimated effect sizes with such a small sample per group are of course highly unstable and need to be confirmed with larger samples, but we do find these initial tendencies promising.
The lack of cognitive improvement, on average, with CT alone was unexpected and reflects diversity in cognitive performance changes in this group. With larger samples, we would anticipate that both groups would improve but the CT&E group would show larger gains. We believe that the Independent Living rating of the Role Functioning Scale is sensitive to initial cognitive changes because it reflects self-care skills and everyday household functioning that occur in the patient’s existing environment. Working Productivity reflects work/school resumption that is likely to require a longer duration for change, as will the establishment of a broader social network outside of the family. Our initial feasibility study data are consistent with this anticipated sensitivity to immediate change.
Exercise adherence in our structured exercise program (95% in clinic and 92% at home) was notably higher than in published studies that included at-home exercise assignments (35%72 and 36.4%73). Thus, our focus on the first-episode phase of illness, patient engagement, and our incentive systems appeared to result in high adherence to these treatments. We observed that our first-episode patients typically viewed physical exercise as a normal, non-stigmatizing, low-risk activity and were engaged by the group format of exercise sessions at the clinic, which likely increased adherence.
The exercise intensity and adherence were likely contributors to our encouraging results. Outcomes for exercise programs in multiple populations have generally been positive, as long as the exercise intervention was of adequate intensity and duration. A recent meta-analysis of the effects of physical activity in people with mental illness indicated reductions in symptoms of schizophrenia and depressive symptoms, and improvements in anthropometric measures, aerobic capacity, and quality of life.74 Interventions that are purely psychoeducational, that do not feature structured group exercise, or that do not involve a sufficiently intense exercise regimen have been unable to demonstrate significant physical health changes in patients.75 Exercise intervention studies demonstrating significantly improved cognitive outcomes in older adults typically incorporate sustained aerobic exercise at a minimum duration of 30min/session and a total of about 150min/wk, using individualized aerobic targets set at least 50%–80% of the heart rate reserve.
Combining CT and physical exercise interventions may allow the potential positive impact of aerobic exercise to be realized and may enhance the pace and magnitude of cognitive improvement that has been demonstrated for CT alone. As McGurk et al14 and Wykes et al15 have noted, combining CT with other rehabilitation efforts often enhances the effect of the CT. Physical exercise is likely to have an even more specific impact on CT results due to its influence on neurogenesis and synaptic plasticity. Targeting individuals in the initial phase of schizophrenia may also maximize cognitive gains and offer opportunities for greater transfer to improved functional outcome.
This initial pilot study is limited by its very small sample size, its brief duration of treatment, and the fact that the BDNF measures were completed for feasibility purposes on only a subsample. This initial report focuses on the theoretical conception and demonstrating intervention feasibility rather than on anything more than suggestive directions in results. A randomized controlled trial with a larger sample of first-episode patients and a 6-month treatment period is underway at UCLA to further evaluate these effects.
Funding
This research was supported by National Institute of Mental Health (NIMH) Center grant P50 MH066286 and research grants R01 MH037705 and R34 MH102529.
Acknowledgments
We thank the UCLA Aftercare Research Program patients for their willing participation and the staff for their consistent dedication to improving treatments for the initial phase of schizophrenia. K.H.N. is an officer of MATRICS Assessment, Inc, but receives no financial compensation, has research grants from Posit Science and Janssen Scientific Affairs that support other research, and has been a consultant to Janssen, Otsuka, and Takeda. J.V. has been a paid consultant to Posit Science, Lumosity, and Boehringer Ingelheim and has a research grant from Posit Science. S.V. has been a paid consultant to Posit Science and is a site investigator on an SBIR grant to Posit Science, Inc. K.L.S. has been a consultant to Otsuka. S.C.M. and D.G-D. report no conflicts of interest.
References
- 1. Gold JM, Green MF. Neurocognition in Schizophrenia. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. Vol 8 Baltimore, MD: Lippincott, Williams & Wilkins; 2005:1436–1448. [Google Scholar]
- 2. Braff D. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. [DOI] [PubMed] [Google Scholar]
- 3. Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. [DOI] [PubMed] [Google Scholar]
- 4. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 5. Aleman A, Hijman R, de Haan EHF, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. [DOI] [PubMed] [Google Scholar]
- 6. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 7. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 8. Nuechterlein KH, Subotnik KL, Green MF, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37:S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan RCK, Li H, Cheung EFC, Gong Q-Y. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–390. [DOI] [PubMed] [Google Scholar]
- 10. Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fett AKJ, Viechtbauer W, Dominguez MG, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. [DOI] [PubMed] [Google Scholar]
- 12. Horan WP, Green MF, Degroot M, et al. Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull. 2012;38:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. [DOI] [PubMed] [Google Scholar]
- 14. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wykes T, Huddy H, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 16. Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Brit J Psychiatry. 2007;190:421–427. [DOI] [PubMed] [Google Scholar]
- 17. Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGurk S, Mueser K, DeRosa T, Wolfe R. Work, recovery, and comorbidity in schizophrenia. Schizophr Bull. 2009;35:319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartzokis G. Schizophrenia: breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. [DOI] [PubMed] [Google Scholar]
- 21. Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53:412–421. [DOI] [PubMed] [Google Scholar]
- 22. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowie CR, Grossman M, Gupta M, Oyewumi LK, Harvey PD. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early Interv Psychiatry. 2014;8:32–38. [DOI] [PubMed] [Google Scholar]
- 24. Fisher M, Loewy R, Hardy K, Schlosser D, Vinogradov S. Cognitive interventions targeting brain plasticity in the prodromal and early phases of schizophrenia. Annu Rev Clin Psychol. 2013;9:435–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94:221–230. [DOI] [PubMed] [Google Scholar]
- 26. Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fisher M, Loewy R, Carter C, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2015;41:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nahum M, Fisher M, Loewy R, et al. A novel, online social cognitive training program for young adults with schizophrenia: a pilot study. Schizophr Res Cogn. 2014;1:e11–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ussorio D, Giusti L, Wittekind CE, et al. Metacognitive training for young subjects (MCT young version) in the early stages of psychosis: is the duration of untreated psychosis a limiting factor? Psychol Psychother. 2016;89:50–65. [DOI] [PubMed] [Google Scholar]
- 30. Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Rev Neurosci. 2008;9:58–65. [DOI] [PubMed] [Google Scholar]
- 31. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults a meta-analytic study. Psychological Sci. 2003;14:125–130. [DOI] [PubMed] [Google Scholar]
- 32. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985). 2006;101:1237–1242. [DOI] [PubMed] [Google Scholar]
- 34. Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front Hum Neurosci. 2011;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee EH, Hui CL, Chang WC, et al. Impact of physical activity on functioning of patients with first-episode psychosis—A 6 months prospective longitudinal study. Schizophr Res. 2013;150:538–541. [DOI] [PubMed] [Google Scholar]
- 37. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143. [DOI] [PubMed] [Google Scholar]
- 38. Scheewe TW, van Haren NE, Sarkisyan G, et al. Exercise therapy, cardiorespiratory fitness and their effect on brain volumes: a randomised controlled trial in patients with schizophrenia and healthy controls. Eur Neuropsychopharmacol. 2013;23:675–685. [DOI] [PubMed] [Google Scholar]
- 39. Rosenbaum S, Lagopoulos J, Curtis J, et al. Aerobic exercise intervention in young people with schizophrenia spectrum disorders; improved fitness with no change in hippocampal volume. Psychiatry Res. 2015;232:200–201. [DOI] [PubMed] [Google Scholar]
- 40. Abdel-Baki A, Brazzini-Poisson V, Marois F, Letendre E, Karelis AD. Effects of aerobic interval training on metabolic complications and cardiorespiratory fitness in young adults with psychotic disorders: a pilot study. Schizophr Res. 2013;149:112–115. [DOI] [PubMed] [Google Scholar]
- 41. Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poo M-M. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. [DOI] [PubMed] [Google Scholar]
- 43. Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. [DOI] [PubMed] [Google Scholar]
- 44. Kim HJ, Song BK, So B, Lee O, Song W, Kim Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatry Res. 2014;220:792–796. [DOI] [PubMed] [Google Scholar]
- 45. Kimhy D, Vakhrusheva J, Bartels MN, et al. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr Bull. 2015;41:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas AG, Dennis A, Bandettini PA, Johansen-Berg H. The effects of aerobic activity on brain structure. Front Psychol. 2012;3:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Langdon KD, Corbett D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair. 2012;26:523–532. [DOI] [PubMed] [Google Scholar]
- 49. Fabel K, Wolf SA, Ehninger D, Babu H, Leal-Galicia P, Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. [DOI] [PubMed] [Google Scholar]
- 51. Kronenberg G, Reuter K, Steiner B, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. [DOI] [PubMed] [Google Scholar]
- 52. Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2006;103:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kempermann G, Fabel K, Ehninger D, et al. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Malchow B, Keller K, Hasan A, et al. Effects of endurance training combined with cognitive remediation on everyday functioning, symptoms, and cognition in multiepisode schizophrenia patients. Schizophr Bull. 2015;41:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oertel-Knöchel V, Mehler P, Thiel C, et al. Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2014;264:589–604. [DOI] [PubMed] [Google Scholar]
- 57. Nuechterlein KH, Dawson ME, Gitlin MJ, et al. Developmental processes in schizophrenic disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. [DOI] [PubMed] [Google Scholar]
- 58. Nuechterlein KH, Subotnik KL, Turner LR, Ventura J, Becker DR, Drake RE. Individual Placement and Support for individuals with recent-onset schizophrenia: integrating supported education and supported employment. Psychiatr Rehabil J. 2008;31:340–349. [DOI] [PubMed] [Google Scholar]
- 59. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Appendix 1: Brief Psychiatric Rating Scale (BPRS) Expanded Version (4.0): Scales, anchor points, and administration manual. Int J Method Psychiat Res. 1993;3:227–243. [Google Scholar]
- 61. Adcock R, Dale C, Fisher M, et al. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahncke H, Bronstone A, Merzenich M. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. [DOI] [PubMed] [Google Scholar]
- 64. Mahncke H, Connor B, Appelman J, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103:12523–12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Merzenich MM. Cortical plasticity: from synapse to maps. Annual Rev Neurosci. 1998;21:149–186. [DOI] [PubMed] [Google Scholar]
- 66. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. [DOI] [PubMed] [Google Scholar]
- 67. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 68. Goodman SH, Sewell DR, Cooley EL. Assessing levels of adaptive functioning: the role functioning scale. Community Ment Health J. 1993;29:119–131. [DOI] [PubMed] [Google Scholar]
- 69. YMCA. YMCA Fitness Testing and Assessment Manual. 4th ed. Champaign, IL: Human Kinetics Pub; 2000. [Google Scholar]
- 70. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 71. DE Hert M, Schreurs V, Vancampfort D, VAN Winkel R. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marzolini S, Jensen B, Melville P. Feasibility and effects of a group-based resistance and aerobic exercise program for individuals with severe schizophrenia: a multidisciplinary approach. Mental Health Phys Activity. 2009;2:29–36. [Google Scholar]
- 73. Kwon JS, Choi JS, Bahk WM, et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: a 12-week randomized controlled clinical trial. J Clin Psychiatry. 2006;67:547–553. [DOI] [PubMed] [Google Scholar]
- 74. Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:964–974. [DOI] [PubMed] [Google Scholar]
- 75. Firth J, Cotter J, Elliott R, French P, Yung AR. A systematic review and meta-analysis of exercise interventions in schizophrenia patients. Psychol Med. 2015;45:1343–1361. [DOI] [PubMed] [Google Scholar]