Abstract
Objective:
Individuals at clinical high risk (CHR) for psychosis demonstrate cognitive impairments that predict later psychotic transition and real-world functioning. Cognitive training has shown benefits in schizophrenia, but has not yet been adequately tested in the CHR population.
Methods:
In this double-blind randomized controlled trial, CHR individuals (N = 83) were given laptop computers and trained at home on 40 hours of auditory processing-based exercises designed to target verbal learning and memory operations, or on computer games (CG). Participants were assessed with neurocognitive tests based on the Measurement and Treatment Research to Improve Cognition in Schizophrenia initiative (MATRICS) battery and rated on symptoms and functioning. Groups were compared before and after training using a mixed-effects model with restricted maximum likelihood estimation, given the high study attrition rate (42%).
Results:
Participants in the targeted cognitive training group showed a significant improvement in Verbal Memory compared to CG participants (effect size = 0.61). Positive and Total symptoms improved in both groups over time.
Conclusions:
CHR individuals showed patterns of training-induced cognitive improvement in verbal memory consistent with prior observations in schizophrenia. This is a particularly vulnerable domain in individuals at-risk for psychosis that predicts later functioning and psychotic transition. Ongoing follow-up of this cohort will assess the durability of training effects in CHR individuals, as well as the potential impact on symptoms and functioning over time. Clinical Trials Number: NCT00655239. URL: https://clinicaltrials.gov/ct2/show/NCT00655239?term=vinogradov&rank=5.
Key words: cognitive remediation, prodrome, schizophrenia
Introduction
The cognitive impairments that characterize schizophrenia are present even prior to full psychosis onset, though to a lesser degree, and appear to have prognostic value.1,2 In a recent meta-analysis, clinical high-risk (CHR) individuals showed highly significant impairments in visual and verbal memory as compared to age-matched healthy subjects, with additional deficits observed in general intelligence, executive function, verbal fluency, attention and working memory, and social cognition.2 Furthermore, verbal fluency, verbal and visual memory, and working memory impairments were more pronounced at baseline in those CHR individuals who later transitioned to full psychosis, compared to those who did not transition.2
The variability in outcomes for CHR patients requires treatments that offer the prospect of high benefit and low risk.3 Behavioral interventions such as computer-based cognitive training exercises, if successful, may help to ameliorate cognitive impairments and improve outcomes, without carrying the risk of unwanted side effects such as the metabolic changes seen with antipsychotic medications. A body of evidence has shown that computerized cognitive training can drive cognitive gains in both chronic and recent-onset schizophrenia patients, but much less is known about the use of this intervention in CHR individuals.4–10 In our work, we have focused on targeted training of the auditory system to improve neural system operations underlying verbal memory, based on a large literature demonstrating functionally important abnormalities in schizophrenia in the earliest stages of auditory processing and throughout the verbal encoding, working memory, and episodic memory system.4,5,11 Interestingly, abnormalities in the language processing network have also been identified in young CHR individuals, and predict the transition to psychosis—suggesting that this neural system may be an important treatment target for this population.12–14
To date, there have been 4 published studies of various forms of cognitive training in the CHR population. The first compared supportive psychotherapy in an unblinded trial of an integrated treatment package of family support, cognitive behavioral therapy, low-dose Risperidone and computerized training across a range of domains using CogPack.15 After 12 months, the active treatment group (N = 51) had a lower transition rate to psychosis (3.2%) than the treatment as usual group (16.9%, N = 57), with continued differences after 24 months.15 While these results were quite promising, effects on cognition were not reported, nor was it possible to assess the relative impact of the individual treatment components in the active condition. Two single-sample pilot studies of cognitive training in CHRs have been conducted. In the first, Rauchensteiner and colleagues16 had 10 individuals complete 10 hours of training with the CogPack software program, training a variety of cognitive domains. The CHR group showed significant improvement in verbal learning and memory from pre- to post-training. In the second study, a combined package of 40 hours of visual cognitive (Lumosity) and social cognitive (SocialVille; PositScience) training was given to 14 CHRs, who demonstrated significant improvement in processing speed and trend-level improvements in visual learning and memory and global cognition17; improvements in processing speed were associated with gains in role functioning. Finally, a small double-blind, randomized controlled trial of the same auditory training program used in the present study (Positscience), delivered for an average of 20 hours, did not demonstrate significant effects on cognition between the active (N = 13) and control (N = 12; standard computer games [CG]) groups.18 However, the very small sample sizes suggest the study was underpowered statistically, and it is also possible that the training may have been “underdosed.” Thus, many open questions remain on the specific cognitive and clinical effects of well-defined computerized cognitive training programs in CHR individuals, when delivered alone as a “neural system treatment” at a sufficient “dose,” as compared to an active control condition, in a double-blind, randomized controlled trial.
Pursuant to these considerations, in this study, we posed the following question: What are the cognitive and clinical effects, in a sample of adolescent and young adult CHR participants of 40 hours of computerized auditory system training previously studied by us in patients with both chronic and recent-onset schizophrenia? Our primary hypothesis was that CHR subjects in the active treatment group would show greater improvements in verbal memory outcome measures from pre- to post-training as compared to a CG control group, based on our studies of the auditory training software in recent onset and chronic schizophrenia that demonstrated the strongest improvements in the verbal memory domain.4,5 Secondary exploratory analyses addressed potential change in other cognitive domains, as well as in symptoms and functioning.
Methods
Participants
Study participants consisted of 83 adolescents and young adults who presented to our prodromal research clinic in the University of California, San Francisco Psychiatry Department for evaluation of a potential CHR syndrome. CHR subjects were recruited via community clinicians, schools, family members, and self-referred from seeing information on the Internet. They met criteria for 1 of 3 syndromes on the Structured Interview for Prodromal Syndromes (SIPS): (1) Attenuated Positive Symptom Prodromal Syndrome (APS): attenuated positive psychotic symptoms present at least once per week, started or worsened in the past year (unusual thought content/delusional ideas, suspiciousness/persecutory ideas, grandiosity, perceptual abnormalities/distortions, and conceptual disorganization; (2) Brief Intermittent Psychosis Prodromal Syndrome (BIPS): brief and intermittent fully psychotic symptoms that have started recently; (3) Genetic Risk and Deterioration Prodromal Syndrome (GRDS): decline of at least 30% in the past 12 months on the Global Assessment of Function (GAF) scale PLUS either a family history of a psychotic disorder in any first-degree relative or meets criteria for schizotypal personality disorder.19 All subjects had achieved outpatient status for at least 3 months and participants taking psychiatric medications (N = 41) were on a stable dose for at least 1 month prior to participation. Forty-two CHR participants did not take psychiatric medications. All participants met the following additional inclusion/exclusion criteria: (1) Good general physical health; (2) Age 12–30 years; (3) Fluent and proficient in English; (4) IQ ≥ 70; (5) No neurological disorder; (6) No substance dependence in past year or current use that would interfere with training.
Procedures
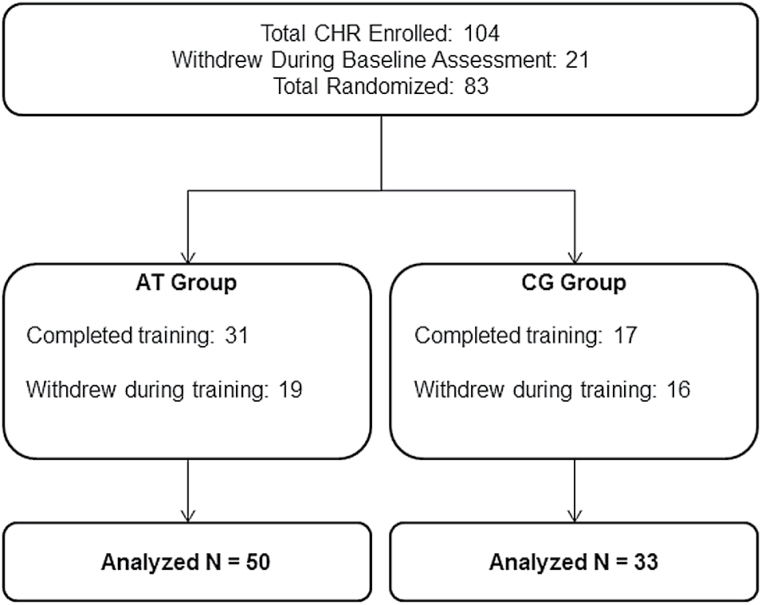
Study procedures were parallel to those used in our cognitive training trial in recent-onset schizophrenia and are repeated here.5 Participants age 18 and older gave written informed consent, while those younger than age 18 provided assent, with written parental/legal guardian consent. Baseline assessments were conducted prior to randomization. CHR subjects were stratified by age, IQ, symptom severity and gender and randomly assigned to auditory training or to the CG control condition (CONSORT diagram in figure 1). Subjects were loaned laptop computers and participated in the intervention at home. Subjects were asked to participate for 40 hours (1h/d, 5d/wk, for 8wk), and then returned for post-training assessments.
Fig. 1.
CONSORT diagram.
Participants were contacted 1–2 times per week by telephone to discuss progress. Phone surveys regarding participation were completed (N = 50) after the first 10 hours of training to assess participants’ experience with the training and factors that might affect adherence. Coaching was provided if a participant indicated difficulty in completing the recommended number of h/wk (eg, goal-setting; discussion of scheduling; setting an alarm and using reminders). At a “check-in” in-person appointment after every 10 sessions completed, the same coaching was provided and participants were paid $5 for each completed hour, $20 for every 10 sessions, and $30 after 40 hours, as well as $20 per assessment appointment. Participants were asked to complete 20–40 hours of training; mean intervention time was 21.5 hours (SD = 16.3) across both groups (table 1). While in the trial, participants received treatment by outside providers or clinic personnel not involved in the study (psychoeducation, psychotherapy, medications as clinically indicated). Demographic characteristics are presented in table 1.
Table 1.
Baseline Characteristics of the Sample Groups
| CHR AT (N = 50) | CHR CG (N = 33) | Test Statistic | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | t | df | P | |
| Male/femalea | 26 (52%)/ 24 | 16 (49%)/ 17 | 0.10a | 1 | .75 |
| Age (range 12–30) | 17.76 (3.06) | 18.73 (4.60) | −1.15 | 81 | .25 |
| Education | 11.42 (2.49) | 11.55 (2.69) | −0.22 | 81 | .83 |
| WASI IQ | 107.23 (14.9) | 108.94 (15.26) | −0.49 | 77 | .62 |
| SOPS total | 38.41 (12.28) | 34.70 (11.40) | 1.38 | 80 | .17 |
| Positive symptoms | 10.54 (3.38) | 9.36 (4.44) | 1.37 | 81 | .18 |
| Negative symptoms | 13.08 (6.82) | 11.67 (6.13) | 0.96 | 80 | .34 |
| Disorganized symptoms | 6.46 (3.68) | 5.61 (3.54) | 1.05 | 81 | .30 |
| General symptoms | 8.20 (4.20) | 8.06 (3.92) | 0.15 | 81 | .88 |
| Strauss carpenter | 9.52 (2.31) | 9.73 (1.77) | −0.46 | 79 | .65 |
| GFS: role | 5.94 (2.23) | 6.12 (1.63) | −0.41 | 79 | .69 |
| GFS: social | 6.00 (1.61) | 6.15 (1.09) | −0.47 | 79 | .64 |
| GAF | 47.96 (9.48) | 46.61 (9.83) | 0.63 | 81 | .53 |
| Hours of training | 21.80 (15.68) | 21.12 (18.62) | 0.17 | 81 | .86 |
Note: CHR, clinical high risk; AT, auditory training; CG, computer games; WASI, Wechsler Abbreviated Scale of Intelligence; GFS, Global Functioning Scale; SOPS, Scale of Prodromal Symptoms; GAF, Global Assessment of Function.
aPearson Chi-square statistic.
Cognitive Training Intervention and CG Control Condition
The targeted auditory training program (AT) was provided by Posit Science Corporation and has been described in detail previously.4 It consists of computerized exercises designed to improve speed and accuracy of auditory information processing while engaging auditory and verbal working memory. This training approach is based on evidence that schizophrenia is characterized by widespread disturbances in fronto-temporal neural systems subserving auditory processing and verbal memory.20,21 Exercises continuously adjust difficulty level to maintain an 80%–85% correct performance rate in order to engage the user in a dense reward schedule and drive successful learning. Correct trials are rewarded with points and animations. In each session, a participant works with 4 of 6 exercises for 15 minutes per exercise. Compliance is monitored by electronic data upload.
The CG control condition allows for maintenance of a double-blind trial design and controls for the effects of computer exposure, contact with research personnel, monetary payments, and nonspecific engagement of attention, executive functions, and motivation. Subjects in this condition rotated through a series of 16 different commercially available games (supplementary table 1) for the same number of hours as training subjects, playing 4–5 games on any given day.
Assessment Procedures
All assessment staff were blind to group assignment. Cognitive assessment staff were trained and monitored on manualized assessment procedures by one of the authors (M.F.) to ensure consistency. Clinical assessment staff were trained and observed by other authors (R.L., B.S., D.A.S.). Subject eligibility was determined in regular reliability rounds. Inter-rater reliability was calculated from staff ratings of training tapes, with an average intra-class correlation of 0.83 for symptom ratings and an average kappa value of 0.95 for diagnostic agreement.
Symptoms were assessed with the Scale of Prodromal Symptoms (SOPS) and functioning was assessed with the Global Functioning: Role and Social Scales (each containing a single clinician-rated item ranging from 1 to 10 developed for the late adolescent/young adult early psychosis population).22 An abbreviated battery of MATRICS-recommended measures23 was administered (table 2). The Tower Test from the Delis-Kaplan Executive Function System (D-KEFS)24 was used in place of NAB Mazes. For participants aged 12–21, raw scores were converted to z-scores using a set of healthy control normative data (N = 188) collected and stratified by the following age ranges, with a minimum of 20 participants in each age range: 12–13, 14–15, 16–17, 18–19, and 20–21. For participants over 21 years of age, raw scores were converted to z-scores using age-appropriate normative data provided in testing manuals, and age-appropriate, published normative data for Trails A25 and Category Fluency.26 All primary outcome measures were distinct and independent from tasks practiced during training. Alternate forms of the HVLT-R and BVMT-R were administered and counterbalanced at baseline and post-training.
Table 2.
Group Comparisons of Cognition, Symptoms and Functioning Before and After Intervention
| Outcome Measuresa | CHR AT (N = 50) | CHR CG (N = 33) | Time | Condition × Time | Condition × Time Effect Size | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | Post Mean (SD) | Baseline Mean (SD) | Post Mean (SD) | F | P | F | P | d | 95% CI | |
| Global cognition | −0.44 (0.80) | −0.11 (0.78) | −0.55 (0.66) | −0.038 (0.86) | 15.32 | <.001b | 1.24 | .30 | 0.23 | −0.22–0.67 |
| Speed of processing | −0.22 (1.10) | −0.04 (1.13) | −0.42 (0.85) | −0.30 (1.21) | 1.92 | .17 | 0.56 | .57 | 0.06 | −0.38–0.50 |
| Working memory | −0.46 (0.88) | −0.05 (0.99) | −0.54 (0.90) | −0.01 (1.03) | 29.67 | <.001b | 0.29 | .75 | −0.14 | −0.57–0.31 |
| Verbal learning | −0.39 (1.13) | −0.29 (1.48) | −0.79 (1.58) | −0.95 (1.61) | 0.04 | .85 | 1.83 | .17 | 0.20 | −0.25–0.64 |
| Verbal memory | −0.54 (1.28) | −0.11 (1.41) | −0.47 (1.29) | −0.81 (1.55) | 0.07 | .79 | 3.41 | .04 | 0.61 | 0.15–1.05 |
| Visual learning | −0.74 (1.22) | −0.25 (1.41) | −0.62 (1.04) | 0.08 (1.49) | 12.27 | .001b | 0.51 | .60 | −0.18 | −0.62–0.26 |
| Visual memory | −1.10 (1.66) | −0.51 (1.91) | −1.11 (1.57) | −0.93 (2.07) | 2.45 | .12 | 0.46 | .63 | 0.25 | −0.19–0.69 |
| Problem solving | 0.03 (0.82) | 0.52 (0.99) | 0.03 (0.81) | 0.22 (1.03) | 9.09 | .004 | 1.06 | .35 | 0.37 | −0.08–0.81 |
| SOPS total | 38.24 (12.24) | 33.90 (16.40) | 34.60 (11.71) | 25.49 (17.23) | 13.23 | .001b | 2.50 | .09 | 0.40 | −0.05–0.84 |
| Positive symptoms | 10.56 (3.41) | 8.75 (5.16) | 9.27 (4.47) | 6.63 (5.40) | 17.12 | <.001b | 1.72 | .19 | 0.22 | −0.23–0.66 |
| Negative symptoms | 13.09 (6.82) | 11.03 (8.13) | 11.71 (6.21) | 9.38 (8.50) | 5.80 | .02 | 0.57 | .57 | 0.05 | −0.39–0.49 |
| Disorganized symptoms | 6.46 (3.68) | 6.11 (4.45) | 5.59 (3.58) | 3.38 (4.65) | 7.84 | .007 | 3.64 | .03 | 0.53 | 0.08–0.97 |
| General symptoms | 8.20 (4.20) | 7.83 (5.37) | 7.98 (3.95) | 6.02 (5.63) | 3.33 | .07 | 1.11 | .33 | 0.39 | −0.06–0.83 |
| Global functioning role | 5.96 (2.23) | 6.06 (2.55) | 6.12 (1.63) | 6.29 (2.64) | 0.22 | .64 | 0.09 | .92 | −0.02 | −0.46–0.42 |
| Global functioning social | 6.02 (1.61) | 6.51 (1.77) | 6.15 (1.09) | 6.25 (1.78) | 3.05 | .09 | 0.66 | .52 | 0.29 | −0.16–0.73 |
| GAF | 47.96 (9.48) | 51.8 (12.59) | 46.61 (9.83) | 51.44 (13.21) | 8.56 | .005 | 0.19 | .83 | −0.10 | −0.54–0.34 |
Note: D-KEFS, Delis-Kaplan Executive Function System.
aCognitive measures were transformed to z-scores using normative data of healthy samples. Global Cognition (average z-score across all measures); Speed of Processing (Trail Making Test Part A; Category Fluency Animal Naming; Symbol Coding); Working Memory (Letter-Number Span; WMS-III Spatial Span); Verbal Learning and Verbal Memory (HVLT-R Immediate and Delayed Recall); Visual Learning and Visual Memory (BVMT-R Immediate and Delayed Recall); Problem Solving (D-KEFS Tower Test). Symptoms (SOPS) and functional outcome measures (Global Functioning Role and Social, Global Assessment of Functioning) are clinician ratings.
bStatistically significant after Bonferroni correction for multiple comparisons.
Planned Analyses
We performed an intent-to-treat analysis on all randomized subjects (N = 83), regardless of hours of intervention (figure 1). All variables were screened and normally distributed after Winsorizing of outlying values (±2.5 SD from the mean).
Pearson’s chi-square was used to test for group differences in attrition rate and Spearman correlations indexed the relationship between phone survey items and hours of training completed. The CHR CG and AT groups were compared on change in cognitive measures, symptoms, and functional outcome ratings using a linear mixed-effects model with group and time as fixed factors. Model parameters were estimated using restricted maximum likelihood. This allowed us to retain all available data for analysis. Effect sizes (Cohen’s d) were computed using the mean change scores of the CHR AT and CG groups (post-training minus baseline) and the baseline pooled standard deviations. All measures are listed in table 2. We applied the conservative Bonferroni correction for multiple comparisons to our secondary analyses, given the large number of dependent variables.
Results
Attrition
After randomization, 19 out of 50 (38%) CHR AT subjects withdrew from the study compared to 16 of 33 (49%) CHR CG subjects, a nonsignificant difference, X 2 (1, N = 83) = .90, P = .34. There were no significant differences in demographic variables, cognition, symptom severity, or functioning between those who completed the study and those who dropped out (supplementary table 2). These variables were also unrelated to the number of hours completed. However, hours of training completed were significantly predicted by participants’ phone survey ratings of whether the training was convenient to do at home (r = .53, P < .001) and easy to do 5 days per week (r = .45, P = .0001), 60 minutes per day (r = .44, P = .001); these correlations were statistically significant after Bonferroni correction for multiple comparisons. Hours completed were also predicted by whether the participants thought they were making progress (r = .38; P = .008), whether they rated the training as interesting (r = .34, P = .017), fun (r = .28, P = .046), and that it was a high priority compared to other activities (r = .31, P = .028), although these correlations did not survive Bonferroni correction. Hours completed were unrelated to whether the participant thought the training was easy, the laptop was easy to use, that the training would improve their thinking and memory, help them do well at school or work, or whether someone helped remind them to do the training.
The Effects of Cognitive Training vs CG in CHR Subjects
At baseline, there were no differences between the CHR AT and CHR CG groups in cognition, symptom severity, functioning, medication use or dosage, or in hours of training completed (table 1 and supplementary table 3). There were significant main effects of time for global cognition, working memory, visual learning, and problem solving (table 2). The main effect of time for problem solving was no longer statistically significant after correction for multiple comparisons.
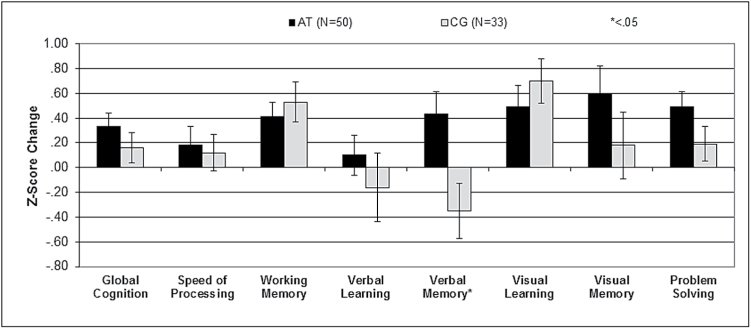
Verbal memory showed a significant condition-by-time interaction (table 2 and figure 2). Post hoc analyses of verbal memory revealed significant improvement in the AT participants (F = 5.46, P = .03), and a nonsignificant decrease in the CG group (F = 2.36, P = .14). Effect sizes of the change between groups were in the small range for global cognition, verbal learning, visual memory, and problem solving, and in the medium range for verbal memory, although the 95% confidence interval was outside zero only for verbal memory (table 2).
Fig. 2.
Cognitive change scores by group.
Symptom and Functional Outcome Measures
There was a significant main effect of time on the SOPS total score and the positive, negative, and disorganized symptom subscales, with both groups showing improved symptoms over time (table 2). There was a significant condition by time interaction in the disorganized symptoms subscale, with the CG group showing a greater decrease relative to the AT group. There was a significant main effect of time on the GAF, with both groups showing improvement in functioning over time (table 2). Only the main effect of time for SOPS Total and Positive symptoms remained statistically significant after correction for multiple comparisons.
Discussion
Summary of Findings
In a double-blind controlled study aiming to deliver 40 hours of targeted auditory system training vs CG performed on laptops at home in a sample of 83 CHR individuals, using an intent-to-treat analysis we found a significant group-by-time effect in verbal memory. This effect was driven by a significant improvement in verbal memory performance in the active treatment subjects, similar to the pattern we have reported previously in 2 independent samples of patients with schizophrenia.4,5 Also consistent with our prior studies of established psychosis, small positive effect sizes were observed in the domains of global cognition (d = 0.23) and problem solving (d = 0.37), although these results did not reach statistical significance. The lack of significance may be due to the large attrition rate, which may have also contributed to the lack of significant differences between cognitive training and control game groups in these domains.
Although the decline in verbal memory performance in the CG group is not statistically significant in this study, a significant decline was shown in our studies in recent-onset and chronic schizophrenia.4,5 We have previously suggested that intensive practice with visually-focused CG may reduce the capacity for verbal processing/verbal memory in an impaired neural system, a finding that, if replicated, may have important longer-term functional consequences for this population.5 A WHO survey in 2010 found that 55% of adolescent males and 20% of females reported computer gaming more than 2 hours daily; presumably CHR individuals fall within this general range.27 The findings on the cognitive effects of casual video games in healthy young adults are very mixed, and while some of them do appear to drive gains in visual processing and visual attentional control, some studies have shown that they are associated with changes in cognitive strategies (eg, Baniqued et al28; see Shams et al29 for a review). Furthermore, surprisingly few studies have examined the effects of video game exposure on verbal memory in either healthy or cognitively impaired individuals. For individuals with impaired or vulnerable neural systems, the impact of exercising certain networks or functions (such as those involved in visual perception and attention, visual working memory, and visuo-motor processing) may have compensatory consequences on other neural systems.30 In other studies of CHRs, diminished verbal memory performance is related to concurrent social functioning, and also predicts later role functioning and transition to full psychosis.2,31 Therefore, a treatment that directly improves this domain and/or that may pre-empt decline in verbal memory systems is of particular long-term clinical importance for CHR individuals.
Effects of Cognitive Training on Symptoms and Functioning
Similar to other CHR studies, and similar to what we reported in recent-onset schizophrenia participants, positive and total symptoms improved across both subject groups over the study duration.5,32–34 Individuals often enter research protocols subsequent to their first CHR diagnosis, and in our study, were not restricted from seeking other psychosocial or pharmacological treatments (supplementary table 3). In patients with established illness, symptom and functional improvement appears to emerge over the longer-term (eg, 6-month follow-up).35 People with recent-onset schizophrenia who complete the same auditory processing cognitive training show improvement in positive symptoms at 6-month follow-up related to cognitive change during training, suggesting a longer-term clinical salutory effect that occurs in response to improvement in cognition (Loewy et al, in preparation). This potential relationship has not yet been investigated in the CHR population.
Study Limitations
The most significant limitation of this study is the high attrition rate (42%). This is greater than the dropout rates in our studies of the same software in chronic and recent-onset schizophrenia patients (15% and 28%, respectively), suggesting specificity to the younger age group or individuals in the CHR state. This finding is consistent with the high attrition rates found in other studies that involve CHR individuals. For example, drop-out rates of 61% were reported in a pilot study of cognitive training,18 45% attrition in an antipsychotic trial,36 and 42% dropout in the active treatment arm of a trial using antipsychotic medications and cognitive behavioral therapy.37
Although it is unlikely that participants dropped out of our study at random, attrition was unrelated to any baseline measures. The high attrition rate may be due to: (1) CHRs are more active in social, educational and occupational pursuits, with less time available for cognitive training; (2) CHRs are not as impaired as persons with established psychosis clinically, functionally and cognitively, therefore reducing their motivation for this intervention; (3) Younger individuals are very familiar with graphically advanced computer and mobile games, and may find the study software and control games less appealing and engaging than entertainment software; and (4) CHRs show greater impairments in reward anticipation than individuals with schizophrenia, related to depressed mood.38 Interestingly, the strongest predictors of completing more hours in our study were focused on being able to meet the intensity requirements of the training. Much like other behavioral interventions that require active engagement and participation multiple days per week, such as physical exercise for obesity, these activities may be difficult to sustain without a high degree of support. Yet, effective interventions are critical for this vulnerable population. Therefore, supports similar to those employed in improving physical exercise participation may be helpful, such as setting rewards for short-term goals, including behavioral neuroeconomics approaches,39 structured participation in groups rather than relying solely on at-home practice, and adapting effective training approaches to be more enjoyable for this age group.
Several other factors may have reduced our power to detect between-group differences in other cognitive outcome measures. Our participants represent a wide age range, covering a period in which cognitive abilities, particularly executive functioning, are still developing. Another complicating factor in CHR studies is the high degree of clinical and cognitive heterogeneity. Only a subset of these individuals are likely to develop nonaffective psychosis—others will develop affective psychoses, non-psychotic affective disorders, anxiety disorders and some will remit entirely.40 This high variability in baseline neural system functioning across a sample of CHR subjects thus will generate a highly variable pattern of response to cognitive treatments.
Finally, the mean IQ of our CHR participants was in the average range, which may limit the generalizability of our results. CHR samples tend to show a small deficit in overall IQ compared to their healthy peers.2 The higher IQ of our participants may be due to recruitment at a university medical center within San Francisco, which attracted participants from a relatively high socioeconomic range. However, despite their average IQ, our sample demonstrated deficits in the specific and expected cognitive domains, consistent with what has been reported for other CHR samples.2
Future Directions
This study adds to a growing body of research literature on preventive approaches to psychotic illness, with a focus on identifying effective methods to improve the cognitive dysfunction that characterizes the CHR state and that contributes to poor outcomes. Participants in our study are currently being followed for up to 24 months post-training to evaluate the duration of treatment effects. Based on our current study alone, we cannot recommend use of the auditory processing software in regular clinical practice, but hope that additional studies are able to replicate and expand our results. Future work should: (1) Use advances in the cognitive neuroscience of the CHR syndrome to identify whether auditory processing circuits or other neural system treatment targets are likely to be of the highest benefit in terms of preventing or mitigating psychosis onset and improving outcome; (2) Use these neuroscientifically-derived treatment targets to inform the design and implementation of cognitive training strategies; (3) Tackle the difficulty of engaging and retaining this clinical population in cognitive training treatments, which may be improved by the use of more engaging software and/or behavioral neuroeconomics strategies39; (4) Perform dose-response studies to establish the minimum hours and intensity of training necessary to generate clinically significant gains in verbal memory and other key cognitive outcomes; (5) Improve the prediction of psychosis in order to define a more homogeneous group at highest risk for psychosis as the target group for treatment; (6) Embed cognitive training within evidence-based psychosocial treatments in order to ensure maximum generalization and real-world benefit for patients; (7) Include a control group with no intervention (wait list control) for comparison of cognitive changes that may occur naturally in CHR individuals over time; and (8) Perform double blind RCTs with an adequate sample size that generates sufficient statistical power to test predictors of treatment response, taking into account the high attrition rates that characterize this group of patients. A large-scale trial with an initial sample size of 126 has recently been proposed.41 A substantial sample size would also allow for testing of response predictors to inform a precision medicine approach in which specific training approaches could be selected for specific patients. This is, of course, the ideal future of CHR treatment.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the National Institutes of Health (grant number MH081051).
Supplementary Material
Acknowledgments
The cognitive training software used in this study was supplied to the first author free of charge by Posit Science. S.V. is a site PI on an SBIR grant to Brain Plasticity Inc (now a division of Posit Science), a company with a commercial interest in the cognitive training software used in this study. B.B. is a post-doctoral research fellow at Posit Science. None of the other authors have any financial interest in Brain Plasticity Inc or Posit Science. D.H.M. is a consultant to Boehringer Ingelheim. S.V. serves on an advisory board for Forum pharmaceuticals. All authors declare no other conflicts of interest. We would like to thank Dr Kevin Delucchi for statistical consultation, Felix Amirfathi and Sarah Corey for their assistance with data management, Connie Ludwig for her supervision of assessors, Jennifer Arjona for assistance with study management, and the First Hope Program and Contra Costa County Behavioral Health Services for their partnership in participant recruitment.
References
- 1. Keefe RSE. The longitudinal course of cognitive impairment in schizophrenia: an examination of data from premorbid through posttreatment phases of illness. J Clin Psychiatry. 2014;75:8–13. [DOI] [PubMed] [Google Scholar]
- 2. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. [DOI] [PubMed] [Google Scholar]
- 3. McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70:1206–1212. [DOI] [PubMed] [Google Scholar]
- 4. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher M, Loewy R, Carter C, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2014;41:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 7. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee RSC, Redoblado-Hodge MA, Naismith SL, Hermens DF, Porter MA, Hickie IB. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychol Med. 2013;43:1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlati S, De Peri L, Deste G, Fusar-Poli P, Vita A. Cognitive remediation in the early course of schizophrenia: a critical review. Curr Pharm Des. 2012;18:534–541. [DOI] [PubMed] [Google Scholar]
- 10. Bowie CR, Grossman M, Gupta M, Oyewumi LK, Harvey PD. Cognitive remediation in schizophrenia: efficacy and effectiveness in patients with early versus long-term course of illness. Early Interv Psychiatry. 2014;8:32–38. [DOI] [PubMed] [Google Scholar]
- 11. Adcock RA, Dale C, Fisher M, et al. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Francis AN, Seidman LJ, Jabbar GA, et al. Alterations in brain structures underlying language function in young adults at high familial risk for schizophrenia. Schizophr Res. 2012;141:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sabb FW, van Erp TGM, Hardt ME, et al. Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res. 2010;116:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200:22–29. [DOI] [PubMed] [Google Scholar]
- 16. Rauchensteiner S, Kawohl W, Ozgurdal S, et al. Test-performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiatry Res. 2011;185:334–339. [DOI] [PubMed] [Google Scholar]
- 17. Hooker CI, Carol EE, Eisenstein TJ, et al. A pilot study of cognitive training in clinical high risk for psychosis: initial evidence of cognitive benefit. Schizophr Res. 2014;157:314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piskulic D, Barbato M, Liu L, Addington J. Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Res. 2015;225:93–98. [DOI] [PubMed] [Google Scholar]
- 19. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 20. Ragland JD, Gur RC, Raz J, et al. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kasai K, Nakagome K, Itoh K, et al. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am J Psychiatry. 2002;159:546–553. [DOI] [PubMed] [Google Scholar]
- 22. Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 24. Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10:301–303. [DOI] [PubMed] [Google Scholar]
- 25. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 2006: 1235. [Google Scholar]
- 26. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 27. Brooks F, Magnusson J, Klemera E, Spencer N, Morgan A. HBSC England National Report: Health Behaviour in School-aged Children (HBSC). Hertfordshire, UK: World Health Organization Collaborative Cross National Study; 2011. http://uhra.herts.ac.uk/handle/2299/9386 Accessed August 21, 2015. [Google Scholar]
- 28. Baniqued PL, Kranz MB, Voss MW, et al. Cognitive training with casual video games: points to consider. Front Psychol [Internet]. 2014;4:1–19 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3882717/ Accessed August 20, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shams TA, Foussias G, Zawadzki JA, et al. The effects of video games on cognition and brain structure: potential implications for neuropsychiatric disorders. Curr Psychiatry Rep. 2015;17:609. [DOI] [PubMed] [Google Scholar]
- 30. Bernstein LE, Eberhardt SP, Auer ET., Jr Audiovisual spoken word training can promote or impede auditory-only perceptual learning: prelingually deafened adults with late-acquired cochlear implants versus normal hearing adults. Front Psychol. 2014;5:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer EC, Carrión RE, Cornblatt BA, et al. The relationship of neurocognition and negative symptoms to social and role functioning over time in individuals at clinical high risk in the first phase of the North American Prodrome Longitudinal Study. Schizophr Bull. 2014;40:1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Addington J, Liu L, Buchy L, et al. North American Prodrome Longitudinal Study (NAPLS 2): the Prodromal Symptoms. J Nerv Ment Dis. 2015;203:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee TY, Kim SN, Correll CU, et al. Symptomatic and functional remission of subjects at clinical high risk for psychosis: a 2-year naturalistic observational study. Schizophr Res. 2014;156:266–271. [DOI] [PubMed] [Google Scholar]
- 34. Simon AE, Borgwardt S, Riecher-Rössler A, Velthorst E, de Haan L, Fusar-Poli P. Moving beyond transition outcomes: meta-analysis of remission rates in individuals at high clinical risk for psychosis. Psychiatry Res. 2013;209:266–272. [DOI] [PubMed] [Google Scholar]
- 35. Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. [DOI] [PubMed] [Google Scholar]
- 37. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. [DOI] [PubMed] [Google Scholar]
- 38. Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 2014;158:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haff N, Patel MS, Lim R, et al. The role of behavioral economic incentive design and demographic characteristics in financial incentive-based approaches to changing health behaviors: a meta-analysis. Am J Health Promot. 2015;29:314–323. [DOI] [PubMed] [Google Scholar]
- 40. Fusar-Poli P, Bechdolf A, Taylor MJ, et al. At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophr Bull. 2013;39:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glenthøj LB, Fagerlund B, Randers L, et al. The FOCUS trial: cognitive remediation plus standard treatment versus standard treatment for patients at ultra-high risk for psychosis: study protocol for a randomised controlled trial. Trials. 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.