Abstract
Schizophrenia is a psychiatric disorder which has a lifetime prevalence of ~1%. Multiple candidate mechanisms have been proposed in the pathogenesis of schizophrenia. One such mechanism is the involvement of neuroinflammation. Clinical studies, including neuroimaging, peripheral biomarkers and randomized control trials, have suggested the presence of neuroinflammation in schizophrenia. Many studies have also measured markers of neuroinflammation in postmortem brain samples from schizophrenia patients. The objective of this study was to conduct a systematic search of the literature on neuroinflammation in postmortem brains of schizophrenia patients indexed in MEDLINE, Embase and PsycINFO. Databases were searched up until 20th March 2016 for articles published on postmortem brains in schizophrenia evaluating microglia, astrocytes, glia, cytokines, the arachidonic cascade, substance P and other markers of neuroinflammation. Two independent reviewers extracted the data. Out of 5385 articles yielded by the search, 119 articles were identified that measured neuroinflammatory markers in schizophrenic postmortem brains. Glial fibrillary acidic protein expression was elevated, lower or unchanged in 6, 6 and 21 studies, respectively, and similar results were obtained for glial cell densities. On the other hand, microglial markers were increased, lower or unchanged in schizophrenia in 11, 3 and 8 studies, respectively. Results were variable across all other markers, but SERPINA3 and IFITM were consistently increased in 4 and 5 studies, respectively. Despite the variability, some studies evaluating neuroinflammation in postmortem brains in schizophrenia suggest an increase in microglial activity and other markers such as SERPINA3 and IFITM. Variability across studies is partially explained by multiple factors including brain region evaluated, source of the brain, diagnosis, age at time of death, age of onset and the presence of suicide victims in the cohort.
Introduction
Schizophrenia is a psychiatric disorder which affects ~0.5 to 1% of the population in their lifetime.1, 2 Psychosis normally arises in the late teenage years or early adulthood, between 18 and 25 years of age.3 Although the cause underlying this mental illness remains to be elucidated, several biological factors have been proposed, including abnormalities in oligodendrocytes,4, 5 N-methyl-D-aspartate (NMDA) signaling6 and dopaminergic transmission.7
Neuroinflammation has been suggested to be a potential contributor in the pathogenesis of the schizophrenia.8, 9, 10, 11 Classically, the brain is considered to be immunologically privileged due to the blood–brain barrier limiting cell entry.12 Under normal conditions, microglia, the resident immune cells of the brain, are found in a ramified (‘resting') state, surveying the environment. Following injury or the exposure to pro-inflammatory signals such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, ramified microglia can become activated and release pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, IFN-γ or chemokine (c-x-c motif) ligand (CCL) 11.13 Microglia also increase the expression of cyclooxygenase (COX)-2, an enzyme involved in the arachidonic cascade, which can lead to the production of the pro-inflammatory lipid mediator prostaglandin E2.14 Pro-inflammatory cytokines released from microglia, such as IL-1β, can activate astrocytes. In turn, activated astrocytes also have the ability to release pro-inflammatory cytokines and chemokines, such as IL-1β, CCL5 and TNF-α,15 and typically display increased glial fibrillary acidic protein (GFAP) expression.16
Evidence has accumulated supporting a link between inflammation and schizophrenia. Serum or plasma concentrations of pro-inflammatory markers have been investigated in several studies. Two meta-analyses illustrate that IL-6 is consistently elevated in serum and plasma of patients with schizophrenia,17, 18 whereas IL-1β and TNF-α were found to be increased in one meta-analysis,18 but not in the other.17 Genetic studies have also linked polymorphisms in major histocompatibility complex (MHC) regions with risk of schizophrenia.19, 20
Neuroinflammation has also been associated with schizophrenia. Advancements in in vivo PET imaging has enabled imaging of neuroinflammation in schizophrenic patients.21 However, studies imaging the translocator protein 18 kDA (TSPO), a marker of activated microglia, have yielded mixed results. Early studies utilizing the TSPO ligand [11C]PK11195 suggested that schizophrenic patients have higher levels of activated microglia compared with healthy controls.22, 23 More recent studies, using second-generation TSPO ligands, however, had mixed results, with some reporting increased microglia activation in schizophrenia,24 whereas others failed to replicate earlier studies and found no difference between patients and healthy controls.25, 26 The reasons for the disparities between studies are not clear, but likely related to different TSPO ligands used or different samples studied across research groups.
As schizophrenia has been associated with inflammation, attempts have been made to treat symptoms with non-steroidal anti-inflammatory drugs (NSAID) as an add-on therapy to conventional treatments. Although some studies found added benefits of NSAID on symptoms,27, 28, 29 one study did not show any beneficial effects.30 A meta-analysis of five published and three non-published studies found no effect of NSAID on the Positive and Negative Syndrome Scale total scores, but did detect a small yet statistically significant beneficial effect of NSAID add-on therapy for the treatment of positive symptoms.31 Omega-3 polyunsaturated fatty acids (n-3 PUFA), which are also thought to have anti-neuroinflammatory properties,32, 33 have also yielded mixed results in the treatment of schizophrenia. Administration of 3 g per day of n-3 PUFA in combination with 300 mg per day of alpha-lipoic acid for up to 2 years did not decrease the relapse rate of schizophrenic patients.34 An earlier report, however, found that administration of n-3 PUFA was beneficial in reducing the conversion of subthreshold psychosis to a first episode psychotic event in adolescents.35
It is unclear whether neuroinflammation associated with schizophrenia is causing or is a result of the disorder. It has been suggested that microglia activation and cytokine release could lead to neuronal and glial injury,36 resulting in dopaminergic and glutaminergic system dysregulation.37, 38 Neurogenesis and synapse connectivity may also be affected by neuroinflammation.39, 40 Moreover, activation of astrocytes may also cause abnormal production of kynurenic acid and upregulate the expression of glutamate transporters.9, 11, 41
Despite the mixed results in both in vivo imaging and clinical trials, it appears plausible that inflammation may have a role in schizophrenia. Numerous postmortem studies have measured pro-inflammatory markers in patients suffering from schizophrenia. To date, no systematic review of the field has been published on the topic. This article set out to systematically characterize the literature on neuroinflammation as measured in postmortem brains from schizophrenia patients.
Materials and methods
We performed a systematic search for literature indexed in MEDLINE, Embase and PsycINFO up to 20th March 2016. Full search criteria can be found in the Supplementary Materials. Only peer-reviewed primary research articles were considered as eligible studies. References of yielded articles were searched for possible eligible articles that were missed by the search.
Once duplicate articles were removed, studies were screened based on title and abstract for several components including studies which were on schizophrenia and (1) carried out with postmortem brain samples, (2) measured neuroinflammatory markers and (3) were compared with matched psychiatrically and neurologically healthy controls. Studies evaluating markers of astroglia, microglia, gliosis, cytokines, arachidonic acid cascade and substance P were included (for full search terms, see Supplementary Materials). Other markers were considered if the authors referred to their implication in neuroinflammation. Although not always stated by the authors as a microglial marker, MHC (also know as human leukocyte antigen, HLA) complex I and II were both considered as possible microglial markers as both have been shown to be elevated in microglia.42 Untargeted approaches, such as microarray and shotgun proteomics, were also excluded unless targeted approaches were used to confirm the results. Viruses and infection were not considered for this review and were excluded. Reviews were searched for relevant articles, but themselves were excluded from the results. Finally, non-English papers and conference abstracts were also excluded.
Articles were evaluated and data were extracted onto an electronic data extraction form by MOT. Extractions were confirmed by a second independent reviewer (KEH). From eligible studies, number of subjects, sex, race, duration of illness, onset of illness, postmortem interval, freezer time, death from suicide, substance abuse, medication, RNA quality and brain pH were extracted as background information. Unless specifically stated, suicide was not assumed as cause of death. Study design information, such as neuroinflammatory markers measured, measuring techniques and in which brain regions the measurements were made were all extracted, along with comparative results between schizophrenia and healthy controls. Thus, all results discussed below are relative to controls unless otherwise stated.
Results
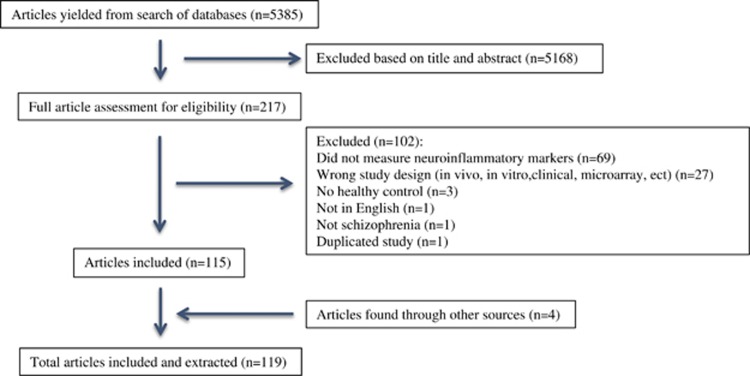
Following removal of duplicates, the search yielded 5385 unique results. A total of 5168 articles were excluded based on either title or abstract. The remaining 217 articles were fully screened for potential inclusion. Out of those remaining 217 articles, only 115 articles met the inclusion criteria. Four more articles were found in the reference section of papers yielded from the search (Figure 1).
Figure 1.
Systematic search results.
Astroglia
Our search yielded a total of 42 studies which assessed astrocytes in postmortem brain in schizophrenia (Table 1).
Table 1. Astrocytes in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Altshuler et al.48 | SFNC | scz 9 ctr 14 | scz 5/4 ctr 8/6 | scz 45 ctr 47 | scz 3 | Basolateral nucleus of the amygdala | IHC | GFAP | ↔ |
| Arnold et al.45 | Prospective study | scz 7* scz+d 14# ctr 12 | scz 3/4 scz+d 6/8 ctr 5/7 | scz 74 scz+d 82 ctr 75 | NA | EC*, SB#, CA3*, CA1*, DG*, MFC*, OFC#, VC* | IHC | GFAP#, VIM* | ↔*↑# |
| Arnold et al.46 | Prospective study | scz 23 ctr 14 | scz 8/15 ctr 6/8 | scz 80 ctr 75 | NA | EC (BA 28) CA1 HPC, SB MFC (BA9 and 46), OFC (BA11), CL (BA17) | IHC | GFAP | ↔ |
| Barley et al.74 | SFNC | Varies across brain regions | Varies across brain regions | Varies across brain regions | NA | AVN, PU, IC, MTN | PCR | GFAP, ALDH1 | ↑ |
| Beasley et al.59 | NYSPIBC | scz 15 ctr 13 | scz 9/6 ctr 10/3 | scz 54 ctr 51 | None | Anterior limb of internal capsule | ELISA | GFAP | ↔ |
| Casanova et al.80 | NC | scz 6 ctr 7 | scz 4/2 ctr 4/3 | scz 39 ctr 61 | NA | DG, PP | Holzer's Technique | Astrocytes | ↔ |
| Catts et al.75 | NSWTRC | scz 37 ctr 37 | scz 24/13 ctr 30/7 | scz 51 ctr 51 | scz 8 | DLPFC (BA46) | PCR, IHC, WB | GFAP | ↔ |
| Damadzic et al.54 | (1) CBDBNIMH (2) SFNC | Study (1) scz 7 ctr 8 study (2) scz 14 ctr 15 | Study (1) scz 3/4 ctr 3/5 study (2) scz 9/5 ctr 9/6 | Study 1 scz 49 ctr 47 study 2 scz 46 ctr 48 | Study 1 scz 3 ctr 1 study 2 scz 3 | EC | IHC | GFAP | ↔ |
| Dean et al.62 | NA | scz 20 ctr 20 | scz 13/7 ctr 13/7 | scz 56 ctr 56 | NA | BA9, 10, 40, 46 | WB, PCR | S100b, GFAP | ↔ |
| Falkai et al.49 | DBC | scz 33 ctr 26 | scz 14/19 ctr 13/13 | scz 54 ctr 53 | scz 4 | PMC, SB, EC, IH, SVZ3V | IHC | GFAP | ↔ |
| Falke et al.50 | Prospective study | scz 12 ctr 11 | scz 3/9 ctr 7/4 | Scz 81 ctr 78 | NA | MTN, CT | IHC | GFAP | ↔ |
| Fatemi et al.60 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | lateral CB | WB | GFAP | ↔ |
| Feresten et al.65 | SMRIAC | scz 35 ctr 35 | scz 26/9 ctr 26/9 | scz 43 ctr 44 | scz 7 | DLPFC (BA9) | WB | GFAP#, VIM*, ALDH1L1*, EAAT* | ↔* ↑# |
| Hercher et al.55 | SMRIAC | scz 20 ctr 20 | scz 13/7 ctr 14/6 | scz 45 ctr 45 | scz 4 | DLPFC (BA9) | IHC | GFAP | ↔ |
| Hwang et al.77 | SFNC, SMRIAC | scz 33 ctr 34 | scz 23/10 ctr 23/11 | scz 44 ctr 46 | NA | HPC | PCR, IHC | APOL1, ADORA2A | ↑ |
| Karson et al.61 | DCMEO | scz 25 ctr 28 | scz 22/3 ctr 22/6 | scz 34 ctr 35 | scz 16 ctr 8 | FC, TC, OC, CB, TH, pons | WB | GFAP | ↔ |
| Karson et al.63 | NA | scz 14 ctr 12 | scz 13/1 ctr 11/1 | scz 65 ctr 67 | NA | PFC (BA10) | WB, northern blot | GFAP | ↔ |
| Katsel et al.58 | NA | scz 18 ctr 21 | scz 10/8 ctr 10/11 | scz 78 ctr 77 | scz 0 | Cingulate cortex (BA24/32) | PCR | GFAP #, S100b*, VIM#, EAAT2*, ALDH1L1#, AQP4*, DIO*, GS*, THBS4*, GL# | ↓*↔# (deep layer only) |
| Kolomeets et al.83 | ADMPH | scz 19 ctr 16 | scz 11/8 ctr 11/5 | scz 54 ctr 56 | NA | HPC | Electron microscope | Astrocytes | ↔ |
| Markova et al.72 | NA | scz 12 ctr 10 | NA | scz 62 ctr NA | NA | Olfactory tubercle | IHC | GFAP | ↑ |
| Pakkenberg82 | NA | scz 12 ctr 12 | scz 8/4 ctr 66 | scz 63 ctr 62 | NA | MTN*, AG#, NAS*, PL# | Nissl | Astrocytes | ↓*↔# |
| Pantazopoulos et al.47 | HBTRC | scz 11 ctr 15 | scz 7/4 ctr 10/5 | scz 62 ctr 66 | scz 1 | AG, EC | IHC | GFAP | ↔ |
| Perrone-Bizzozero et al.64 | HBTRC | scz 17 ctr 18 | scz 17/0 ctr 18/0 | scz 44 ctr 48 | scz 4 ctr 2 | VC (BA17,20) PFC (BA9,10) | WB | GFAP | ↔ |
| Radewicz et al.53 | Prospective study | scz 12 ctr 11 | NA | scz 80 ctr 72 | NA | DLPFC (BA9), ACC (BA24), superior TC (BA22) | IHC | GFAP | ↔ |
| Rajkowska et al.70 | CCCO | scz 9 ctr 15 | scz 2/7 ctr 10/5 | scz 47 ctr 47 | scz 3 | DLPFC (BA9) | IHC | GFAP | ↑ (layer V only) |
| Rao et al.73 | HBTRC | scz 10 ctr 10 | scz 6/4 ctr 7/3 | scz 59 ctr 49 | NA | FC (BA10) | IHC, PCR, WB | GFAP | ↑ |
| Roberts et al.43 | VIBR | scz 5 ctr 7 | scz 1/4 ctr 4/3 | scz 39 ctr 51 | NA | TL, PC, PU, CT, AG, HPC, TH | IHC | GFAP | ↔ |
| Roberts et al.44 | Runwell series 1 brain collection | scz 18 ctr 12 | scz 14/4 ctr 9/3 | scz 69. ctr 55 | NA | TL | IHC | GFAP | ↔ |
| Schmitt et al.81 | DBC | scz 10 ctr 10 | scz 5/5 ctr 5/5 | scz 55 ctr 50 | scz 1 | CA1,2/3,4, SB | Cresyl violet | Astrocytes | ↔ |
| Steffek et al.66 | MSMC, BVAMC | scz 23 ctr 27 | scz 16/7 ctr 14/13 | scz 72 ctr 79 | None | DLPFC#, VC#, ACC*, HPC#, temporal gyrus# | WB | GFAP | ↓*↔# |
| Steiner et al.79 | MBC | p scz 9* r scz 9# ctr 16 | p scz 5/4 r scz 4/5 ctr 7/9 | p scz 56 r scz 54 ctr 56 | p scz 3 r scz 2 | ACC*, DLPFC#, OFC #, sTC#, HPC#, MTN# | IHC | S100b | ↑*↔# |
| Steiner et al.78 | WMSH, HUIN | scz 9 ctr 7 | scz 5/4 ctr 5/2 | scz 68 ctr 65 | None | CO | WB, MS | S100b | ↓ |
| Stevens et al.51 | VIBR | scz 5 ctr 7 | NA | NA | NA | CT, PVN | IHC | GFAP | ↔ |
| Tkachev et al.57 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | PFC (BA9) | PCR | GFAP | ↔ |
| Toro et al.71 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 4 | PFC* (BA9,32,46) OFC# (BA11/47) | IAR | GFAP | ↑*↓# |
| Uranova et al.84 | MHRC | scz 26 ctr 26 | scz 11/15 ctr 21/5 | scz 53 ctr 52 | NA | PFC (BA10) and VC (BA17) | Electron microscopy | Astrocytic end feet | ↑ (except for VC of non p scz) |
| Williams et al.67 | CC | scz 10 ctr 19 | scz 5/5 ctr 11/8 | scz 58 ctr 66 | scz 1 | Subgenual cingulate cortex*, CO | IHC | GFAP | ↓ (only in layer I*) |
| Williams et al.52 | CC | scz 13 ctr 16 | Scz 6/7 ctr 4/12 | scz 57 ctr 55 | scz 2 | Nucleus basalis | IHC | GFAP | ↔ |
| Williams et al.69 | CC | scz 12 ctr 13 | scz 7/4 ctr 9/4 | scz 60 ctr 52 | scz 2 | Substantia nigra | IHC | GFAP | ↓ |
| Williams et al.68 | CC | scz 10 ctr 19 | scz 5/5 ctr 11/8 | scz 58 ctr 66 | scz 1 | Subgenual cingulate cortex | IHC | GFAP | ↓ |
| Webster et al.56 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | DLPFC, HPC | IHC | Phosphorylated GFAP | ↔ (except DLPFC blood vessel labeling) |
| Webster et al.76 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 4 | ACC (BA24) | Riboprobe and in situ hybridization | GFAP | ↓ (white matter only) |
Abbreviations: ACC, anterior cingulate cortex; ADMPH, Anatomical Department of Moscow Psychiatric Hospital; ADORA2A, adenosine A2A receptor; AG, amygdala; ALDH, aldehyde dehydrogenase; APOL, apolipoprotein; AQP, aquaporin; AVN, anteroventral nucleus; BA, Brodmann area; BVAMC, Bronx Veterans Administration Medical Center; CA, cornu ammonis; CB, cerebellum; CBDBNIMH, Clinical Brain Disorder Branch at the National Institute of Mental Health; CC, Corsellis Collection; CL, calcarine cortex; CO, corpus callosum; CT; caudate; ctr, control; CCCO, Cuyahoga Country Coroner's Office; d, dementia; DBC; Dusseldorf Brain Collection, DCMEO, District of Columbia Medical Examiner's Office; DG, dentate gyrus; DIO, diodinase; DLFPC, dorsolateral prefrontal cortex; EAAT, excitatory amino-acid transporter; EC, entorhinal cortex; ELISA, enzyme-linked immunoadsorbent assay; FC, frontal cortex; GFAP, glial fibrillary acidic protein; GL, phosphate-activated glutaminase; GS, glutamine synthase; HBTRC, Harvard Brain Tissue Resource Centre; HPC, hippocampus; HUIN, Heidelberg University Institute of Neuropatholagy; IAR, immunoautoradiography; IC, internal capsule; IH, inferior horn; IHC, immunohistochemistry; MBC, Magdeburg Brain Collection; MHRC, Mental Health Research Centre; MFC, midfrontal cortex; MS, mass spectrometry; MSMC, Mount Sinai Medical Centre; MTN, mediodorsal thalamic nucleus; NA, not available; NC, Neuman Collection; NSWTRC, New South Wales Tissue Resource Centre; NYSPIBC, New York State Psychiatric Institute Brain Collection; OC, occipital cortex; OFC, orbitofrontal cortex; PC, parietal cortex; PCR, polymerase chain reaction; PFC, prefrontal cortex; PMC, premotor cortex; PP, perforant path; PU, putamen; PVN, paraventricular nucleus; SB, subiculum scz, schizophrenia; scz (p), paranoid schizophrenia; scz (r), residual schizophrenia; SFNC, Stanley Foundation Neuropathology Consortium; SMRIAC, Stanley Medical Research Institute Array Collection; ST, striatum; SVZ, subventricular zone; TC, temporal cortex; TH, thalamus; THBS, thrombospondin; TL, Temporal lobe; VBBN, Victorian Brain Bank Network; VC, visual cortex; VIBR; Vogt Institute of Brain Research; VIM, vimentin; WB, western blot; WMSH, Wiesloch Mental State Hospital.
*, # indicate which variables results are representing.
Of those 42 studies, 33 studies evaluated potential differences in astrocytes in schizophrenia by measuring GFAP expression or immunoreactive distribution. Out of the 33 studies evaluating GFAP expression, 21 did not detect any schizophrenia-associated changes, 6 studies reported a decrease in GFAP expression, whereas 6 studies reported increased expression.
The first study to evaluate GFAP was published in 1986 by Robert et al.43 In their study of the temporal cortex of 5 schizophrenic patients, immunohistochemical analysis found no differences in GFAP staining in schizophrenia brains compared with healthy controls,43 and was confirmed in a subsequent study with a larger cohort.44 Similarly, many quantitative immunohistochemical studies found no differences in GFAP cell density in several other brain regions including the hippocampus,45, 46 amygdala,47, 48 subiculum,45, 49 mediodorsal thalamus,50 caudate,50, 51 periventricular nucleus,51 nucleus basalis,52 premotor cortex,49 dorsolateral prefrontal cortex,53 midfrontal cortex,45, 46 orbitofrontal cortex,45, 46 entorhinal cortex,45, 46, 47, 49, 54 visual cortex,45 calcarine cortex46 and anterior cingulate cortex.53 When compared with Alzheimer's and Huntington's disease patients, schizophrenic patients had lower GFAP-labeled cells.43, 45, 46 However, schizophrenic patients presenting with dementia had significantly higher GFAP cell density than schizophrenic patients without dementia in multiple brain regions including hippocampus, entorhinal cortex and orbitofrontal cortex.45 GFAP was also reported to be correlated with age.54 Although Hercher et al.55 also found no differences in GFAP cell density in the dorsolateral prefrontal cortex in schizophrenia, they did find a decrease in GFAP fraction area and increased clustering. Phosphorylated GFAP was investigated in one immunohistochemical study. In a cohort of 15 patients, no difference in phosphorylated GFAP was observed between schizophrenic brains and those of healthy controls in the hippocampus.56 The authors did note, however, a decrease in phosphorylated GFAP-labeled cells in the dorsolateral prefrontal cortex next to blood vessels.56
Similar to the immunohistochemical studies mentioned above, multiple studies reported no increases in GFAP expression measured by other methods. No increase in GFAP mRNA expression was detected in the prefrontal57 and cingulate cortices58 of schizophrenics. Beasley et al.59 found no differences in GFAP in the anterior limb of internal capsule of schizophrenia compared with healthy controls as measured by enzyme-linked immunosorbent assay. Western blot analysis, similarly, found no increase in GFAP protein concentration in the cerebellum,60, 61 frontal cortex,61, 62 prefrontal cortex,63, 64, 65 visual cortex,64 occipital cortex,61 temporal cortex,61 parietal cortex,62 thalamus61 and pons61 of schizophrenic patients. Another study evaluating GFAP protein expression by western blot of various brain regions of 23 schizophrenics, including the dorsolateral prefrontal cortex, visual cortex, anterior cingulate cortex, hippocampus and temporal gyrus, failed to detect any changes in GFAP protein expression in schizophrenia, except for a significant decrease in the anterior cingulate cortex.66
A few studies have detected differences in GFAP protein expression. Williams et al.67 reported a decrease in GFAP cell density in the subgenual cingulate cortex and the corpus callosum in both the gray and white matter in a cohort of 10 schizophrenic patients compared to healthy controls. More specifically, another study found a decrease in number of fibrillary astrocytes in the subgenual anterior cingulate cortex.68 The authors, however, found no differences in gemistocytic astrocytes.68 In a separate study, the same group also found a decrease in GFAP cell density in the substantia nigra.69 Falkai et al.49 reported a decrease in GFAP cell density in the left inferior horn in men, whereas no effect was observed in women. On the other hand, Rajkowska et al.70 reported, in a cohort of 9 schizophrenic brains, an increase in GFAP cell density in layer V of the dorsolateral prefrontal cortex, whereas GFAP labeling area was reduced by 32%. These changes were layer specific, as no differences were detected in layer III and IV.70 This is slightly different from what Toro et al.71 observed, where an increase in GFAP, as measured by autoradiography, were observed in layers II, III and IV of the prefrontal cortex in schizophrenia. Importantly, this increase in GFAP was correlated with antipsychotic use. A decrease in GFAP in the orbitofrontal cortex was also observed.71 The authors proposed that the increase in prefrontal cortex was due to medication use whereas the decrease in the orbitofrontal cortex was due to the disease.71 Markova et al.72 reported increased GFAP positive cell area and reduced anisotropy, indicating gliosis, in the olfactory tubercle in schizophrenia. This is in agreement with another study where GFAP-labeled cells had changed in morphology in the prefrontal cortex of schizophrenics, being more stained and stunted, whereas also having a 2.4-fold increase in protein concentration and 30% increase in mRNA expression.73 Other studies have also shown that GFAP mRNA expression changes in schizophrenia. Barley et al.74 found that schizophrenic patients had increased GFAP mRNA expression in the putamen and mediodorsal thalamic nuclei. Like Toro et al., increases in GFAP expression were correlated with duration of neuroleptic treatment.74 Although Catts et al.75 found no changes in GFAP mRNA expression in the dorsolateral prefrontal cortex between schizophrenic patients and healthy controls, a difference was observed in schizophrenia patients when they were stratified based on the presence of other neuroinflammatory markers including serpin peptidase inhibitor (SERPIN) A3, IL-1β, IL-6 and IL-8. Individuals with elevated neuroinflammation had a larger proportion of hypertrophic astrocytes compared with low neuroinflammation subjects.75 On the other hand, GFAP mRNA, as measured by riboprobe was decreased in the white matter of the anterior cingulate cortex.76 This effect, however, was not seen in the gray matter.76
Other astrocytic markers have also been measured in postmortem brain specimen of patients with schizophrenia. Hwang et al.77 showed increases in apolipoprotein 1 and adenosine A2A receptor mRNA expression, markers of perivascular astrocytes and implicated in inflammatory responses, in the hippocampus in schizophrenia. Similarly, along with increases in GFAP, schizophrenia was associated with increases in aldehyde dehydrogenase (ALDH)1 mRNA in several brain regions including the putamen, anteroventral nucleus, internal capsule and mediodorsal thalamic nucleus.74 In contrast, two other studies found no association between schizophrenia and ALDH1L1 mRNA measured in the deep layer of the cingulate cortex58 and protein concentration in the dorsolateral prefrontal cortex.65 Similar results were observed for GFAP and other astrocytic markers including vimentin,58, 65 excitatory amino-acid transporter (EAAT)165 and phosphate-activated glutaminase.58 Katsel et al.,58 however, did find several other astrocytic markers, including S100b and EAAT2 mRNA to be downregulated in the cingulate cortex in schizophrenia. Differences in expression of various astrocytic markers may point to different types of astrocytes being affected in schizophrenia.58 S100b has been measured in a few other studies with mixed results. While one study found decreases in S100b protein measured by western blot analysis in the corpus callosum,78 another found no effect in several brain regions including Brodmann area (BA) 9, 10, 40 and 46.62 When separating paranoid schizophrenia from residual schizophrenia, one study found an increase in S100b-positive cells in paranoid schizophrenia compared with both residual schizophrenia and healthy controls in the dorsolateral prefrontal cortex.79 No effect was seen, however, in the white matter, as well as other brain regions such as hippocampus, mediodorsal thalamus, anterior cingulate cortex, superior temporal cortex and orbitofrontal cortex.79
Astrocytes have also been identified in postmortem brains by microscopic analysis with other staining techniques. Casanova et al.80 found no differences in astrocytes identified using Holzer's technique between the hippocampus of six schizophrenia patients and seven healthy controls. Similar to other studies comparing schizophrenic brains to those with Alzheimer's disease,45, 46 Alzheimer's disease brains had more astrocytes compared to both the schizophrenia and control groups.80 Similarly, stereological counting of Nissl stained astrocytes showed no differences in cell counts in the hippocampus,81 basolateral nucleus of the amygdala82 and pallidum.82 However, a significant decrease in astrocytes was measured in both the nucleus accumbens and mediodorsal thalamic nucleus.82
Changes in astrocytes in schizophrenia have also been investigated by electron microscopy.83 In a cohort of 19 schizophrenia patients, astrocyte morphology was unchanged in the hippocampus compared with healthy controls.83 However, when patients were separated based on age, increased astrocytes were observed in patients younger than 50 years old, but this effect was lost in older patients. On the other hand, astrocytic end feet were increased in both paranoid and non-paranoid schizophrenia in the prefrontal cortex,84 however, this effect was not present in the visual cortex in non-paranoid schizophrenics.84
Microglia
From our search, a total of 22 articles reported on microglial markers in postmortem schizophrenic brains (Table 2). Out of these 22 studies, 11 studies reported an increase in microglial markers in postmortem brains, whereas 8 studies found no effect and 3 studies found a decrease in microglial markers.
Table 2. Microglia in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Arnold et al.46 | Prospective study | scz 23 ctr 14 | scz 8/15 ctr 6/8 | scz 80 ctr 75 | NA | EC (BA 28) CA1, SB, MFC (BA9 and 46), OFC (BA11), CL (BA17) | IHC | CD68 | ↔ |
| Bayer et al.85 | INUBMC, INMD | scz 14 ctr 13 | scz 3/11 ctr 8/5 | scz 64 ctr 58 | NA | FC, HPC | IHC | HLA-DR | ↑ |
| Busse et al.88 | MBC | scz (p) 10* scz (r) 7 ctr 11 | scz (p) 5/5 scz (r) 4/3 ctr 6/5 | scz (p) 50 scz (r) 56 ctr 56 | scz 5# | HPC | IHC | HLA-DR | ↑*# |
| Comte et al.95 | SFNC | Scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | SVZ | IHC | MHC II | ↔ |
| Connor et al.97 | HBTRC | scz 22 ctr 45 | scz 9/13 ctr 24/21 | scz 68 ctr 70 | NA | ACC (BA24), DLPFC | IHC | Iba1 | ↔ |
| Durrenberger et al.98 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 5/5 | scz 66 ctr 61 | NA | Temporal lobe (BA22) | PCR | HLA-DRA, HLA-DRB4 | ↓ |
| Falke et al.50 | Prospective study | scz 12 ctr 11 | scz 3/9 ctr 7/4 | scz 81 ctr 78 | NA | MTN, CT | IHC | CD68 | ↔ |
| Fillman et al.86 | NSWTRC | scz 37 ctr 37 | scz 24/13 ctr 30/7 | scz 53 ctr 51 | NA | DLPFC (BA46) | WB, IHC | HLA-DR/DP/DQ | ↑ |
| Foster et al.90 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | DLPFC (BA9) | ELISA, IHC | Calprotectin* CD68# | ↑*↔# |
| Gos et al.99 | MBC | scz 13 ctr 12 | scz 7/6 ctr 6/6 | scz 51 ctr 49 | scz 2 | CA1*,2,3, DG | IHC | HLA-DR#, quinolinic acid* | ↓*↔# |
| Hercher et al.55 | SMRIAC | scz 20 ctr 20 | scz 13/7 ctr 14/6 | scz 45 ctr 45.3 | scz 4 | DLPFC (BA9) | IHC | Iba1 | ↔ |
| Kano et al.100 | SMRIAC | scz 35 ctr 35 | scz 26/9 ctr 26/9 | scz 43 ctr 44 | scz 7 | DLFPC*, OFC# | WB | MHC I | ↓*↔# |
| Nakatani et al.96 | VIFM | scz 7 ctr 7 | scz 3/4 ctr 3/4 | scz 61 ctr 61 | scz 1 | DLPFC (BA46), PC (BA40) | PCR | HLA-DRA | ↔ |
| Radewicz et al.53 | Prospective study | scz 12 ctr 11 | NA | scz 80 ctr 72 | NA | DLPFC (BA9)*, ACC (BA24)#, superior TC (BA22)* | IHC | HLA-DR | ↑*↔# |
| Rao et al.73 | HBTRC | scz 10 ctr 10 | scz 6/4 ctr 7/3 | scz 59 ctr 49 | NA | FC (BA10) | IHC, PCR, WB | HLA-DR, CD11b | ↑ |
| Saetre et al.93 | SFNC, HBTRC, MBB | scz 55 ctr 55 | NA | scz 58 ctr 56 | NA | FC (BA8 and 9), superior frontal gyrus | PCR | HLA-A | ↔ |
| Schmitt et al.94 | BBPDGU | 10 per group | scz 5/5 ctr 8/2 | scz 66 ctr 61 | NA | TC (BA22) | PCR | HLA-DRB3, HLA-DPA1 | ↔ |
| Sinkus et al.101 | SRCBB | scz 42 ctr 47 | scz 28/14 ctr 33/14 | scz 51 ctr 53 | scz 0 ctr 1 | HPC | PCR | HLA-A#, HLA-B* | ↑*↔# |
| Steiner et al.91 | MBC | scz 16* ctr 16 | scz 8/8 ctr 8/8 | scz 55 ctr 58 | scz 2# | HPC, ACC, DLPFC, MTN | IHC | HLA-DR | ↔*↑# |
| Steiner et al.92 | MBC | scz 16* ctr 10 | scz 7/9 ctr 5/5 | scz 54 ctr 55 | scz 6# | HPC*, DLPFC#, ACC#, MTN# | IHC | HLA-DR | ↔*↑# |
| Wierzba-Bobrowicz et al.89 | NA | scz 12 ctr 7 | scz 0/12 ctr 0/6 | scz 59 ctr 56 | NA | Frontal lobe, cingulate gyrus (BA24) | IHC | HLA-DP/DQ/DR | ↑ |
| Wierzba-Bobrowicz et al.87 | NA | scz 9 ctr 6 | NA | scz 56 ctr 56 | NA | Gyrus temporal inferior (BA20), gyrus cinguli (BA24) | IHC | HLA-DP/DQ/DR | ↑ |
Abbreviations: ACC, anterior cingulate cortex; BA, Brodmann area; BBPDGU, Brain Bank for Psychiatric Diseases at the Gottingen University; CA, cornu ammonis; CD, cluster of differentiation; CL, calcarine cortex; CT, caudate; ctr, control; DG, dentate gyrus; DLFPC, dorsolateral prefrontal cortex; EC, entorhinal cortex; ELISA; enzyme-linked immunoadsorbent assay; FC, frontal cortex; HBTRC, Harvard Brain Tissue Resource Centre; HLA, Human Leukocyte Antigen; HPC, hippocampus; IHC, immunohistochemistry; Iba, ionized calcium-binding adaptor molecule; INMD, Institute for Nervous and Mental Diseases; INUBMC, Institute of Neuropathology, University of Bonn Medical Centre; MBB, Maudsley Brain Bank; MBC, Magdeburg Brain Collection; MHC, major histocompatibility complex; MFC, midfrontal cortex; MTN, mediodorsal thalamic nucleus; NA, not available; NSWTRC, New South Wales Tissue Resource Centre; OFC, orbitofrontal cortex; PC, parietal cortex; PCR, polymerase chain reaction; SB, subiculum; scz, schizophrenia; scz (p), paranoid schizophrenia, scz (r). residual schizophrenia; SFNC, Stanley Foundation Neuropathology Consortium; SMRIAC, Stanley Medical Research Institute Array Collection; SRCBB, Schizophrenia Research Center Brain Bank; SVZ, subventricular zone; TC, temporal cortex; VIFM, Victorian Institute of Forensic Medicine; WB, western blot.
*, # indicate which variables results are representing.
Bayer et al.85 found that 3 of 14 schizophrenic patients had positive HLA-antigen D-related (DR) staining, MHC class II molecules involved in antigen presentation, whereas control subjects showed no staining in the hippocampus and frontal cortex. This is in agreement with two subsequent studies, where HLA-DR was increased in the prefrontal cortex,86 dorsolateral prefrontal cortex,53 superior temporal gyrus,53 inferior temporal gyrus87 and frontal lobe in schizophrenia.87 No changes, however, were seen in the cingulate cortex.53 This increase in HLA-DR labeling in the hippocampus appears to be more pronounced in paranoid schizophrenics, as this group has increased HLA-DR compared with both control and residual schizophrenics, although only significantly different from residual schizophrenics.88 Immunohistochemistry revealed differences in morphology of HLA-DR-labeled cells in schizophrenia, presenting a stunted and stronger labeling phenotype in the frontal cortex.73 It also has been reported that although patients show stronger HLA-DR labeling in the anterior cingulate cortex, microglia appear to be degenerating.89 Calprotectin, a member of the S100 family, co-expressed with microglial marker CD68 and was increased twofold in the dorsolateral prefrontal cortex in schizophrenic patients compared with healthy controls.90
Not all studies found significant differences in microglia density. Steiner et al.91 found no differences in HLA-DR protein in various brain regions between schizophrenia and healthy controls, but did note that the two individuals who committed suicide in their cohort did show more HLA-DR labeling. A follow-up study by the same group found a similar lack of effect of diagnosis, but that suicide was accompanied with higher HLA-DR-positive cells.92 In a microarray analysis, an increase in HLA-A, MHC I molecules, mRNA expression in the frontal cortex and superior frontal gyrus was observed between schizophrenia and healthy controls in the frontal cortex.93 This effect, however, was not statistically significant when mRNA expression was confirmed by qPCR.93 Schmitt et al.94 observed, in a microarray analysis of the temporal cortex of 10 schizophrenic patients and controls, lower mRNA expression of HLA-DRB3 and HLA-DPA1, subunits of HLA-DR, in schizophrenia. Similar to Saetre and colleagues, however, this effect was once again lost when analyzed by qPCR.94 Similarly, MHC II-positive cells were also unchanged in the subventricular zone in schizophrenia compared to healthy controls.95 Nakatani et al.96 also found no differences in HLA-DRA mRNA expression in the dorsolateral prefrontal cortex in schizophrenia, despite seeing a difference between control and bipolar disorder. Other microglial markers are also unchanged in schizophrenia. For example, ionized calcium-binding adapter molecule (Iba)1 as measured by immunohistochemistry showed no differences in microglial density in the cingulate cortex or dorsolateral prefrontal cortex.55, 97 Two prospective studies following patients who developed schizophrenia found no change in CD68 protein in the caudate nucleus,50 mediodorsal nucleus of the thalamus,50 hippocampus,46 and entorhinal46 and calcarine46 cortices in schizophrenic patients.
Similar decreases in HLA-DRA and HLA-DRB4 mRNA expression were observed in the temporal lobe.98 Despite not seeing changes in HLA-DR-positive cells, a separate study found microglial production of quinolinic acid was reduced in the hippocampus, and more specifically in the cornu ammonis (CA)1, of schizophrenic patients.99 MHC I protein concentration was lower in the dorsolateral prefrontal cortex in a non-smoking schizophrenic population, whereas no differences were seen in the orbitofrontal cortex.100 This effect was not seen in a smoking population.100 Systemic inflammation, however, appears to have a role in potential differences between patients with schizophrenia and healthy controls. In one study, schizophrenic patients with no systemic inflammation showed no differences as compared with healthy controls, but schizophrenics displaying systemic inflammation had lower HLA-A mRNA expression compared with psychiatrically healthy controls with systemic inflammation.101 However, when that same cohort was divided into smokers and non-smokers, regardless of systemic inflammation, HLA-B mRNA expression was increased in schizophrenic patients.101 The authors did report that HLA-A appeared to co-localize with glutaminergic neurons.101
Undifferentiated glial cells
Multiple studies have evaluated glial cells in schizophrenia without the use of cell type-specific markers. Some studies separated the types of glial cells (that is, astrocytes, oligodendrocytes, microglia), as discussed previously. However, many studies using Nissl staining, evaluated the effect of schizophrenia on glial cells without differentiating between cell types. In total, 34 studies evaluated glial cells in schizophrenia, where 25 studies reported no difference, 7 studies found a decrease and 2 found an increase in glial cell densities (Table 3).
Table 3. Undifferentiated glial cells and postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Beasley et al.129 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | Planum temporal | Cresyl violet | Glia | ↔ |
| Beasley et al.107 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | Planum temporal | Cresyl violet | Glia | ↓ |
| Beckmann and Lauer133 | WBC | scz 9 ctr 9 | scz 9/0 ctr 9/0 | scz 55 ctr 52 | scz 1 | ST, PU, NAS, CT | Gallocyanin | Glia | ↔ |
| Benes et al.110 | HBTRC | scz 10 ctr 10 | NA | scz 60 ctr 66 | scz 1 | PFC (BA10)#, motor cortex (BA4)*, cingulate cortex (BA24)# | Cresyl violet | Glia | ↓* (only layer III) ↔# |
| Benes et al.125 | HBTRC | scz 9 scz+md 9 ctr 12 | NA | scz 53 scz+md 49 ctr 59 | NA | PFC (BA10), ACC (BA24) | Cresyl violet | Glia | ↔ |
| Benes et al.123 | HBTRC | scz 11 ctr 12 | scz 7/4 ctr 7/5 | scz 52 ctr 58 | scz 5 | ACC (BA24) | Cresyl violet | Glia | ↔ |
| Bezchlibnyk et al.112 | SFNC | scz 13 ctr 15 | scz 8/5 ctr 9/6 | scz 47 ctr 48 | NA | AG | Nissl | Glia | ↔ |
| Bogerts et al.130 | VIBR | scz 6 ctr 9 | scz 2/6 ctr 5/4 | scz 51 scz 43 | NA | SN | Cresyl violet | Glia | ↔ (reduction in size) |
| Brauch et al.106 | SNFC | scz 13 ctr 14 | NA | scz 46 ctr 47 | NA | TC | Cresyl violet | Glia | ↓ |
| Bruton et al.103 | NA | scz 48 ctr 56 | NA | NA | NA | FC, PC, TC | Holzer's Technique | Glia | ↑ |
| Chana et al.124 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 7 | ACC (BA24) | Cresyl violet | Glia | ↔ |
| Chana et al.121 | SFNC | scz 14 ctr 15 | NA | NA | NA | MTN | NA | Glia | ↔ |
| Cotter et al.109 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 7 | ACC | Cresyl violet | Glia | ↔ |
| Cotter et al.108 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 7 | DLFPC (BA9, 46) | Cresyl violet | Glia | ↓ (only layer V) |
| Cotter et al.122 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 44 ctr 48 | scz 4 | Heschl's gyrus (BA41) (layer 3 and 5) | Cresyl violet | Glia | ↔ |
| Cotter et al.127 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 7 | OFC | Cresyl violet | Glia | ↔ |
| Crow et al.134 | NA | scz 22 ctr 26 | NA | NA | NA | Temporal horn | Holzer's Technique, IHC | Glia, diazepam binding inhibitor-like | ↔ |
| Cullen et al.120 | NA | scz 10 ctr 10 | scz 6/4 ctr 6/4 | scz 60 ctr 60 | NA | Frontal gyrus (BA9) | Cresyl violet | Glia | ↔ |
| Di Rosa et al.119 | NA | scz 11 ctr 13 | scz 6/5 ctr 7/6 | scz 66 ctr 68 | scz 1 | Fusiform gyrus | Cresyl violet | Glia | ↔ |
| Falkai and Bogerts104 | VIBR | scz 13 ctr 11 | scz 2/11 ctr 7/4 | scz 43 ctr 43 | scz 1 | CA1#, 3*, 4,* PSB,* SB# | Nissl | Glia | ↓*↔# |
| Falkai et al.118 | VIBR | scz 13 ctr 11 | scz 11/2 ctr 7/4 | scz 43 ctr 43 | NA | EC | Nissl | Glia | ↔ |
| Hoistad et al.132 | NA | scz 13 ctr 13 | scz 13/0 ctr 13/0 | scz 52 ctr 52 | scz 3 | ACC (BA24) | Gallocyanin | Glia | ↔ |
| Jonsson et al.128 | NA | scz 4 ctr 8 | scz 4/0 ctr 8/0 | scz 82 ctr 77 | NA | HPC | Cresyl violet | Glia | ↔ |
| Kurumaji et al.111 | NA | scz 13 ctr 10 | scz 8/5 ctr 7/3 | scz 60 ctr 67 | NA | PFC#, TC#, OC*, PC*, PU*, CT#, SN#, PL#, TH# | Receptor binding assay | [3H] PK11195 binding (gliosis) | ↓*↔# |
| Nasrallah et al.135 | NIMH | escz 11 lscz 7 ctr 11 | Na | escz 66 lscz 73 ctr 64 | NA | CO | Hematoxylin-eosin stain | Glia | ↔ |
| Ongur et al.117 | SFNC | scz 11 ctr 11 | scz 7/3 ctr 7/4 | scz 40 ctr 39 | scz 4 | sg24 | Nissl | Glia | ↔ |
| Pennington et al.126 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 46 ctr 48 | scz 4 | Insular cortex | Cresyl violet | Glia | ↔ |
| Rajkowska et al.115 | HTBRC, NIMH, UZ | scz 9 ctr 10 | scz 7/2 ctr 6/4 | scz 41 ctr 44 | scz 5 | PFC (BA9), OC (BA17) | Nissl | Glia | ↔ |
| Selemon et al.113 | HTBRC, NIMH, UZ | scz 16 ctr 19 | scz 12/4 ctr 10/9 | scz 40 ctr 47 | scz 10 | PFC (BA9), OC (BA17) | Nissl | Glia | ↔ |
| Selemon et al.116 | HTBRC, UZ | scz 9 ctr 10 | scz 6/3 ctr 7/3 | scz 44 ctr 48 | scz 5 | PFC (BA9,46) | Nissl | Glia | ↔ |
| Selemon et al.114 | HBTRC, NIMH | scz 9 ctr 14 | scz 6/3 ctr 10/4 | scz 56 ctr 54 | scz 3 | FC (BA44) and DLPFC (BA9) | Nissl | Glia | ↔ |
| Selemon et al.131 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 4 | Lateral geniculate nucleus | Nissl | Glia | ↔ |
| Stark et al.105 | NA | scz 12 ctr 14 | scz 7/5 ctr 7/7 | scz 70 ctr 69 | scz 1 | ACC (BA24)*, BA32# | Giemsa stain | Glia | ↓*↔# |
| Stevens102 | SEH | scz 28 ctr 18 | scz 13/15 ctr 11/7 | scz 41 ctr 37 | NA | Multiple brain regions | Holzer's Technique | Glia | ↑ |
Abbreviations: ACC, anterior cingulate cortex; AG, amygdala; BA, Brodmann area; CA, cornu ammonis; CO, corpus callosum; CT; caudate; ctr, control; DBC; Dusseldorf Brain Collection, DLFPC; dorsolateral prefrontal cortex; EC, entorhinal cortex; escz, early onset schizophrenia; FC, frontal cortex; HBTRC; Harvard Brain Tissue Resource Centre; HPC, hippocampus; lscz; late onset schizophrenia; md, mood disturbance; MTN, mediodorsal thalamic nucleus; NA, not available; NAS, nucleus accumbens; OC, occipital cortex; OFC, orbitofrontal cortex; PC, parietal cortex; PFC, prefrontal cortex; PL, pallidum; PSB, presubiculum; PU, putamen; SB, subiculum; scz, schizophrenia; SFNC; Stanley Foundation Neuropathology Consortium; sg, subgenual prefrontal cortex; SN; substantia nigra; ST, striatum; SEH, ST. Elizabeth's Hospital; TC, temporal cortex; TH, thalamus; UZ, University of Zagreb; VIBR, Vogt Institute of Brain Research; WBC, Würzburg Brain Collection.
*, # indicate which variables results are representing.
Stevens102 published the first study which met our inclusion criteria on the effect of schizophrenia on glial cells. In a cohort of 18 schizophrenic patients, fibrous gliosis measured by Holzer's staining was more pronounced in several brain regions including the hippocampus, hypothalamus, amygdala, thalamus, and periventricular areas compared to control. Comparable effects were observed in another study, which found increased fibrous gliosis as measured by Holzer's technique in the cerebral cortex of patients with schizophrenia.103
The increase in gliosis measured by Holzer's technique appears to differ, however, with a study published shortly after the report by Stevens and colleagues, which found, using a Nissl staining technique, a decrease in glial cell density in the CA3 and CA4 of the hippocampus.104 No effect of schizophrenia, however, was observed in the CA1 and subiculum.104 Similar decreases in glia were observed by Giemsa staining in the anterior cingulate cortex105 and by cresyl violet staining in the temporal cortex106 and planum temporale.107 A layer specific decrease in glial cell density measured by cresyl violet staining was observed in three studies, where effects were only in layer V of the dorsolateral prefrontal cortex,108 layer VI of the anterior cingulate cortex (statistical significance was lost following multiple corrections)109 and layer III of the motor cortex.110 The latter study did not detect any differences in both the prefrontal and cingulate cortices.110 Gliosis measured by [3H]PK11195 binding, a ligand which binds to the TSPO receptor found on activated microglia and astrocytes, was reduced in schizophrenia in the occipital cortex, parietal cortex, and putamen but not in the prefrontal cortex, temporal cortex, thalamus, pallidum, substantia nigra and caudate.111
Twenty-five studies, however, found no effect of schizophrenia on glial cell density in postmortem brains. In a study of 13 schizophrenic postmortem brains from the Stanley Foundation Neuropathology Consortium, Nissl staining revealed no differences in glial cell density or size in the amygdala.112 Similarly, no changes in glial density were obtained in the prefrontal,113, 114, 115, 116 frontal,114 subgenual prefrontal,117 occipital113, 115 and entorhinal118 cortices in schizophrenia compared with healthy controls. By comparison, Huntington's Disease had an ~50% increase in glial cell density compared with healthy controls.113 Moreover, Huntington's disease had increased density of larger glial cells.115 When glial cell density was measured by cresyl violet staining, no changes were detected between schizophrenia patients and healthy controls in several brain regions including the fusiform cortex,119 prefrontal gyrus,120 mediodorsal thalamic nucleus,121 layer III and V of the Heschl's gyrus,122 anterior cingulate cortex,123, 124, 125 prefrontal cortex,125 insular cortex,126 orbitofrontal cortex,127 hippocampus,128 planum temporale,129 substantia nigra130 and lateral geniculate nucleus.131 It should be noted that although Bogerts et al.130 failed to detect a difference in glial cell density in schizophrenia, they did report a significant reduction in glial size in schizophrenia patients. Gallocyanin, another staining technique, also did not detect an effect of schizophrenia on glial cell density in the dorsolateral prefrontal cortex of 13 male schizophrenic patients.132 Similarly, Beckmann and Lauer133 did not find any significant differences in glial density in several brain regions including the striatum, caudate, putamen and nucleus accumbens. Crow et al.134 also did not detect a differences in gliosis in the temporal horn and in the periventricular region using Holzer's technique between schizophrenia patients and controls. This was confirmed using diazepam inhibitor binding to evaluate gliosis. In another study, Nasrallah et al.135 found no differences in glial cell density in the corpus callosum in schizophrenia compared with healthy controls using hematoxylin and eosin staining. The authors did note that gliosis rating scores were higher in late onset schizophrenia compared with early onset and control patients.135
Cytokines and chemokines
Ten studies evaluated cytokine and chemokine expression in postmortem brains of schizophrenic patients (Table 4). Two studies reported no difference in IL-1β mRNA in the prefrontal cortex,86, 136 despite measuring increases IL-1RA,136 IL-6 (ref. 86) and IL-8 mRNA.86 IFN-γ, measured by enzyme-linked immunosorbent assay, was reported to be increased in the prefrontal cortex of 35 schizophrenia patients compared to unaffected controls.137 However, Rao et al. reported 150% and 3.9 fold increases in IL-1β protein and mRNA respectively in the frontal cortex of schizophrenics. TNF-α protein and mRNA concentrations were also increased, 76% and 2.3-fold respectively, in schizophrenic patients.73 In a study of 19 schizophrenics, TNF-α receptor 1 mRNA was increased in the dorsolateral prefrontal and cingulate cortices compared to controls, whereas soluble TNF-α protein, transmembrane TNF-α protein and TNF-α receptor 2 mRNA concentrations were unchanged.138
Table 4. Cytokine and chemokine in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Dean et al.138 | VBBN | scz 19 ctr 20 | scz 15/4 ctr 16/4 | scz 48 ctr 47 | scz 8 | DLPFC (BA46), ACC (BA24) | WB, PCR | sTNF-α#, tmTNF-α#, TNF-α receptor 1*,2# | ↑*↔# |
| Durrenberger et al.98 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 5/5 | scz 66 ctr 61 | NA | TL (BA22) | PCR | IL-13RA1 | ↓. |
| Fillman et al.86 | NSWTRC | scz 37 ctr 37 | scz 24/13 ctr 30/7 | scz 51 ctr 51 | NA | DLPFC (BA46) | PCR, WB, IHC | IL-8*, IL-6*, IL-1β# | ↑*↔# |
| Fillman et al.139 | SMRIAC | scz 35 ctr 35 | scz 26/9 ctr 26/9 | scz 43 ctr 44 | scz 7 | Middle frontal gyrus | PCR | IL-6#, IL-8*, IL-1β#, IL18#, TNF-α# | ↓*↔# |
| Harris et al.137 | SMRIAC | scz 35 ctr 33 | scz 26/9 ctr 25/8 | scz 43 ctr 45 | NA | BA10 | ELISA | IFN-γ | ↑ |
| Nakatani et al.96 | VIFM | scz 7 ctr 7 | scz 3/4 ctr 3/4 | scz 61 ctr 61 | scz 1 | DLPFC (BA46), PC (BA40) | PCR | CCL3 | ↓ |
| Rao et al.73 | HBTRC | scz 10 ctr 10 | scz 6/4 ctr 7/3 | scz 59 ctr 49 | NA | FC (BA10) | PCR, WB | TNF-α, IL-1β | ↑ |
| Schmitt et al.94 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 8/2 | scz 66 ctr 61 | NA | TC (BA22) | PCR | IL-8*, IL-1α*, CCL2#, IL-1β# | ↓*↔# |
| Toyooka et al.136 | NA | scz 22 ctr 23 | scz 16/6 ctr 14/9 | scz 59 ctr 66 | NA | PFC (BA46)*, posterior hypothalamic region, PC (BA 1-3), PU | PCR, WB | IL−1β #, IL-1RA* | ↓*↔# |
| Volk et al.155 | ACOME | scz 62 ctr 62 | scz 47/15 ctr 47/15 | scz 48 ctr 49 | scz 16 | PFC (BA9) | PCR | IL-1β*, IL-6*, IL-8#, IFN-β* | ↑*↔# |
Abbreviations: ACC, anterior cingulate cortex; BA, ACOME, Allegheny County Office of the Medical Examiner; Brodmann area; BBPDGU, Brain Bank for Psychiatric Diseases at the Gottingen University, CCL, chemokine (c-c motif) ligand; CCR, chemokine (c-c motif) receptor; ctr, control; DLFPC; dorsolateral prefrontal cortex; ELISA; enzyme-linked immunoadsorbent assay; FC, frontal cortex; HBTRC; Harvard Brain Tissue Resource Centre; IHC, IFN, interferon; IL, interleukin; NA, not available; NSWTRC; New South Wales Tissue Resource Centre; PC, parietal cortex; PFC, prefrontal cortex; PU, putamen; PCR, polymerase chain reaction; scz, schizophrenia; SMRIAC, Stanley Medical Research Institute Array Collection; sTNF, soluble TNF; TC, temporal cortex; TL, temporal lobe; tmTNF, transmembrane TNF; TNF, Tumor necrosis factor; VBBN, Victorian Brain Bank Network; VIFM, Victorian Institute of Forensic Medicine; WB, western blot.
*, # indicate which variables results are representing.
A microarray analysis, followed by qPCR validation, found a decrease in IL-8 and IL-1α mRNA expression in the temporal cortex of 10 schizophrenic patients as compared with healthy control patients. However, increases detected in the microarray were not reproduced by qPCR for cytokines and chemokines such IL-1β, and CCL2.94 Another study also found a decrease in IL-8 mRNA in the middle frontal gyrus in schizophrenia, whereas IL-1β, TNF-α, IL-18 and IL-6 were not changed.139 Two more microarray studies also found decreases in expression, with CCL3 being reduced ninefold in the prefrontal cortex96 and IL-13RA reduced in the temporal lobe.98
Arachidonic acid cascade
Seven studies have evaluated the arachidonic acid cascade in postmortem schizophrenic brains (Table 5).
Table 5. Arachidonic acid cascade in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Durrenberger et al.98 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 5/5 | scz 66 ctr 61 | NA | Temporal lobe (BA22) | PCR | ALOX5AP | ↓. |
| Fillman et al.86 | NSWTRC | scz 37 ctr 37 | scz 24/13 ctr 30/7 | scz 51 ctr 51 | NA | DLPFC (BA46) | PCR | PTGS2 | ↔ |
| Fillman et al.139 | SMRIAC | scz 35 ctr 35 | scz 26/9 ctr 26/9 | scz 43 ctr 44 | scz 7 | Middle frontal gyrus | PCR | PTGS2 | ↔ |
| Maida et al.140 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 4 | PFC (BA8)*, TC (BA21 and BA22)# OC (BA18)# | WB, IHC | COX-1#, COX-2 #, cPGE2* | ↓*↔# |
| Rao et al.73 | HBTRC | scz 10 ctr 10 | scz 6/4 ctr 7/3 | scz 59 ctr 49 | NA | FC (BA10) | PCR, WB | COX-1#, COX-2*, LOX5#, LOX12#, LOX15#, cPLA2*, sPLA2*, iPLA2#, cPGES#, mPGES# | ↑*↔# |
| Tang et al.141 | VBBN | scz 38 ctr 38 | NA | scz 43 ctr 44 | NA | DLPFC (BA46) | PCR | PTGS1, PTGS2, PTGER3, CYP4Z1 | ↔ |
| Yokota et al.142 | NA | scz 17 ctr 22 | scz 12/5 ctr 13/9 | scz 69 ctr 71 | scz 0 | HPC | IHC | COX-2 | ↔ |
Abbreviations: ALOX5AP, 5-lypoxygenase activating protein; BA, Brodmann Area; BBPDGU, Brain Bank for Psychiatric Diseases at the Gottingen University; ctr, control; COX, cyclooxygenase; cPGE, cytosolic prostaglandin E; FC, frontal cortex; CYP; cytochrome P450; HBTRC, Harvard Brain Tissue Resource Centre; IHC, immunohistochemistry; LOX, lipoxygenase; NA, not available; NSWTRC, New South Wales Tissue Resource Centre; OC, occipital cortex; PCR, polymerase chain reaction; PLA, phospholipase; PFC, prefrontal cortex; PGES, prostaglandin E synthase; PTGS, prostaglandin endoperoxide synthase; PTGER, prostaglandin E receptor 3; scz, schizophrenia; SFNC, Stanley Foundation Neuropathology Consortium; SMRIAC; Stanley Medical Research Institute Array Collection, TC, temporal cortex; VBBN, Victorian Brain Bank Network; WB, western blot.
*, # indicate which variables results are representing.
Regional differences in concentration of cytosolic prostaglandin E synthase (PGES) protein were reported in schizophrenia compared with healthy controls. In schizophrenia, cytosolic PGES was elevated in the prefrontal cortex, but no changes were observed in the temporal and occipital cortices.140 COX-1 and 2, enzymes regulating the production of prostaglandin E2, were not altered in the brains of schizophrenics.140 No changes in COX-2 mRNA expression were also observed in the dorsolateral prefrontal cortex86, 141 and middle frontal gyrus,139 whereas COX-1 mRNA expression was unchanged in the dorsolateral prefrontal cortex.141 Similarly, immunohistochemical analysis of the hippocampus shows no differences in COX-2-positive cell density between schizophrenia and healthy controls.142 It should be noted that age did affect COX-1 and COX-2 mRNA expression in schizophrenia, with older schizophrenia patients having increased COX-1 and decreased COX-2 mRNA expression.141 ALOX5AP, a protein regulating 5-lypoxygenase (LOX) activity, was found to have lower mRNA expression in the temporal lobe of 66 schizophrenia patients compared with control patients.98
In contrast, Rao et al. observed no changes in cytosolic PGES mRNA and protein in the frontal cortex in schizophrenia. They also reported no changes in other arachidonic cascade enzymes, such as calcium-independent phospholipase (PLA)2, LOX5, LOX12, LOX15 and microsomal PGES. They did, however, find COX-2 to be increased in schizophrenia, along with cPLA2 and sPLA2.73
Substance P
Substance P has been measured in postmortem brains of patients with schizophrenia in 11 studies (Table 6).
Table 6. Substance P in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Burnet and Harrison151 | SFNC | scz 13 ctr 14 | scz 8/5 ctr 9/5 | scz 44 ctr 47 | scz 3 | ACC | AR | [125I]BH–substance P binding (NK1 receptor) | ↔ |
| Carletti et al.143 | SFNC | scz 14 ctr 15 | scz 9/5 ctr 9/6 | scz 44 ctr 48 | scz 4 | AG*, TC# | In situ hybridization binding assay | Preprotachykinin A*, NK1 receptor# | ↓*↔# |
| Harrington et al.144 | NA | scz 4 ctr 5 | scz 0/4 ctr 4/1 | scz 71 ctr 69 | NA | CT, PU | In situ hybridization | Preprotachykinin A | ↔ |
| Iadarola145 | DCCO | scz 12 ctr 9 | scz 10/2 ctr 8/1 | scz 33 ctr 45 | scz 9 | SN | RIA | Substance P | ↔ |
| Kleinman et al.146 | DCCO | scz 40 ctr 18 | NA | scz 48 ctr 50 | NA | FC, CT, NAS, PU, GB, HPL | RIA | Substance P | ↔ (increased in other non schizophrenia psychotic disorders) |
| Rioux et al.150 | NA | scz 5 ctr 5 | NA | scz 70 ctr 70 | NA | NAS#, PU*, CT# | AR | [125I]BH–substance P binding (NK1 receptor) | ↔*↑# |
| Roberts et al.149 | NA | scz 14 ctr 12 | scz 8/6 ctr 7/5 | scz 62 ctr 82 | scz 2 | TC (BA21/22)*, FC (BA4)*, PC (BA7)*, CI (BA24)*, HPC#, AG*, TH*, BG* | RIA | Substance P | ↔*↑# |
| Tooney et al.152 | NSWTRC | scz 6 ctr 6 | scz 6/0 ctr 5/1 | scz 44 ctr 43 | scz 2 ctr 3 | PFC (BA9) | IHC | NK1 receptor | ↑ (except layer VI) |
| Toru et al.148 | NA | scz 14 ctr 10 | scz 9/5 ctr 7/3 | scz 58 ctr 67 | NA | BG*, SN*, TH*, HPC*, TC*, PFC*, PC*, OFC# | RIA | Substance P | ↔*↑# |
| Weidenhofer et al.153 | NSWTRC | scz 12 ctr 15 | scz 10/2 ctr 13/2 | scz 48 ctr 48 | scz 3 | AG | IHC, PCR | NK1 receptor | ↔ |
| Zech and Bogerts147 | NA | scz 6 ctr 5 | scz 1/5 ctr 3/2 | scz 31 ctr 45 | NA | BG | IHC | Substance P | ↔ |
Abbreviations: ACC, anterior cingulate cortex; AG, amygdala; AR, autoradiography; BA, Brodmann Area; BG, basal ganglia; CI; cingulate cortex; CT, caudate; ctr, control; DCCO, District of Colombia Coroner's Office; FC, frontal cortex; HPC, hippocampus; HPL, hypothalamus; IHC, immunohistochemistry; NA, not available; NAS, nucleus accumbens; NK1, neurokinin 1; NSWTRC, New south wales tissue resource centre; OFC, orbitofrontal cortex; PC, parietal cortex; PCR, polymerase chain reaction; PFC, prefrontal cortex; PU, putamen; RIA, radioimmunoassay; scz, schizophrenia; SN, substantia nigra: SFNC, Stanley Foundation Neuropathology Consortium; TC, temporal cortex; TH, thalamus.
*, # indicate which variables results are representing.
One study evaluated preprotachykinin A, a precursor to substance P, and reported that mRNA measured by in situ hybridization is decreased in the basal and lateral nuclei of the amygdala, whereas no changes were measured in the temporal cortex.143 Similarly, the density of cells containing preprotachykinin A mRNA measured by in situ hybridization is also not changed in the caudate and putamen in schizophrenia.144 Substance P density in multiple brain regions, including substantia nigra,145 caudate nucleus,146 frontal cortex,146 basal ganglia146 and hypothalamus,146 detected by radioimmunoassay, is not different in schizophrenia compared with healthy controls. Psychosis without schizophrenia, such as affective disorder and unspecified functional psychosis, did exhibit higher substance P protein concentrations.146 An immunohistochemical study also did not detect any changes in substance P in the basal ganglia of six schizophrenia patients compared with unaffected controls.147
Two studies, however, have reported differences in substance P concentration in schizophrenia. Toru et al.148 found a significant increase in substance P detected by radioimmunoassay in the orbitofrontal cortex and hippocampus, and in antipsychotic medication users in the thalamus, substantia nigra and temporal cortex.148 Similarly, Roberts et al. found increased hippocampal substance P, but no changes were seen in multiple brain regions including the amygdala, thalamus, basal ganglia, and temporal, frontal, parietal and cingulate cortices.149
Five studies evaluated substance P binding to substance P neurokinin 1 receptor. Autoradiography found no changes in neurokinin 1 receptor density in the putamen,150 anterior cingulate cortex151 and temporal cortex.143 There was, however, an increase in receptor density in the caudate150 and nucleus accumbens.150 Immunohistochemical analysis found similar increases in substance P receptor in the prefrontal cortex in schizophrenia,152 but not in the amygdala.153 This lack of change in the amygdala cell density expressing substance P receptor was consistent with mRNA expression.153
Other markers
Multiple other markers associated with inflammation that do not fit the categories mentioned above have also been measured in postmortem brains of scizophrenic patients to evaluate a potential link between neuroinflammation and schizophrenia. We identified 16 studies evaluating miscellaneous markers in postmortem brains in schizophrenia (Table 7).
Table 7. Other markers in postmortem schizophrenia brain.
| Author | Brain bank | n | Sex (m/f) | Age | Death from suicide | Brain region | Technique | Inflammatory markers | Results |
|---|---|---|---|---|---|---|---|---|---|
| Arion et al.157 | UPCNMDBB | scz 14 ctr 14 | scz 12/2 ctr 12/2 | scz 43 ctr 42 | scz 3 | PFC (BA9) | PCR | SERPINA3, IFITM1, IFITM3, CHI3L1, HSPB1, MT2A | ↑ |
| Catts and Weickert161 | SMRIAC, NSWTRC | scz 72 ctr 71 | scz 50/22 ctr 55/16 | scz 47 ctr 48 | scz 15 | DLPFC*, OFC# | PCR | TNFSF13 | ↑*, ↔# |
| Durrenberger et al.98 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 5/5 | scz 66 ctr 61 | NA | TL (BA22) | PCR | TIMP1, TNFRSF1A, TYROBP | ↓ |
| Fillman et al.86 | NSWTRC | scz 37 ctr 37 | scz 24/13 ctr 26/9 | scz 51 ctr 48 | NA | DLPFC (BA46) | PCR | NFκB#, SERPINA3*, IL6ST# | ↑*↔# |
| Fillman et al.139 | SMRIAC | scz 35 ctr 35 | scz 26/9 ctr 26/9 | scz 43 ctr 44 | scz 7 | Middle frontal gyrus | PCR | SERPINA3*, IL-1 RL1# | ↑*↔# |
| Harris et al.137 | SMRIAC | scz 35 ctr 33 | scz 26/9 ctr 25/8 | scz 43 ctr 45 | NA | BA10 | ELISA | TIMP1 | ↔ |
| Hwang et al.77 | SNFC, SMRIAC | scz 33 ctr 34 | scz 23/10 ctr 23/11 | scz 44 ctr 46 | NA | HPC | PCR | CD163, S100a8, S100a9, IFITM1, IFITM2, IFITM3 | ↑ |
| Iwamoto et al.158 | SFNC | scz 13 ctr 15 | scz 8/5 ctr 9/6 | scz 44 ctr 48 | scz 4 | PFC (BA10) | PCR | IFITM3 | ↑ |
| Rao et al.73 | HBTRC | scz 10 ctr 10 | scz 6/4 ctr 7/3 | scz 59 ctr 49 | NA | FC (BA10) | PCR, WB | IL-1R#, NFκBp50*, NFκBp65*, iNOS# | ↑*↔# |
| Saetre et al.93 | SFNC, HBTRC, MBB | scz 55 ctr 55 | NA | scz 59 ctr 55 | NA | FC (BA8 and 9), superior frontal gyrus | PCR | IFITM2, IFITM3, SERPINA3, GPB1 | ↑ |
| Schmitt et al.94 | BBPDGU | scz 10 ctr 10 | scz 5/5 ctr 8/2 | scz 66 ctr 61 | NA | TC (BA22) | PCR | LPL*, CFD*, PTGER4*, EDG3* ITGA1#, LCP1#, LTC4S#, MTHFD2#, SOD2#, CCR1#, IL1RAP#, IFI16#, IFNAR2#, CD84#, GPX# | ↓* ↔# |
| Shao and Vawter160 | SMRIAC | scz 32 ctr 27 | scz 23/9 ctr 23/6 | scz 43 ctr 44 | NA | DLPFC (BA46) | PCR | TNFSF8, TNFSF10 | ↔ |
| Siegel et al.159 | ACOME | scz 57 ctr 57 | scz 42/15 ctr 42/15 | scz 47 ctr 48 | scz 16 | PFC (BA9) | PCR, in situ hybridization | IFITM1, IFITM2/3 | ↑ |
| Sun et al.156 | SFNC | NA | NA | NA | NA | FC | PCR | NFκB2 | ↔ |
| Thomas et al.154 | SFNC | scz 15 ctr 15 | scz 9/6 ctr 9/6 | scz 45 ctr 48 | scz 4 | DLFPC (BA9,46), ACC (BA24) | IHC | ICAM-1 | ↔ |
| Volk et al.155 | ACOME | scz 62 ctr 62 | scz 47/15 ctr 47/15 | scz 48 ctr 49 | scz 16 | PFC (BA9) | PCR | NFκB1#, NFκB2#, Shn-2* | ↓*↑# |
Abbreviations: ACC, anterior cingulate cortex; ACOME, Allegheny County Office of the Medical Examiner; APOL, apoliprotein L; BA, Brodmann area; BBPDGU, Brain Bank for Psychiatric Diseases at the Gottingen University; CCR, chemokine (c-c motif) receptor: CD, cluster of differentiation; CFD, complement factor D; CHI3L1, chitinase-3 like protein 1; ctr, control; DLFPC; dorsolateral prefrontal cortex; EDG3, endothelial differentiation, sphingolipid g-coupled-receptor; FC, frontal cortex; GPX, glutathione peroxidase; GPB, guanylate binding protein; HBTRC, Harvard Brain Tissue Resource Centre; HPC, hippocampus; HSPB, heat shock protein beta; ICAM, intercellular adhesion molecule; IFI, interferon gamma-inducible; IFITM; interferon-induced transmembrane; IFNAR, interferon (alpha, beta and omega) receptor: IHC, immunohistochemistry; IL1RAP; interleukin 1 receptor accessory protein; IL1RL, interleukin 1 receptor like; IL6ST, glycoprotein 130; ITGA, integrin alpha; iNOS, inducible nitric oxide; LCP, lymphocyte cytosolic protein; LPL, lipoprotein lipase; LTC4S, leukotriene C4 synthase; MBB, Maudsley Brain Bank; MT2A, metallothionein 2A; MTHFD, methylenetetrahydrofolate dehydrogenase; NA, not available; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NSWTRC; New South Wales Tissue Resource Centre; OFC, orbitofrontal cortex; PCR, polymerase chain reaction; scz, schizophrenia; PFC, prefrontal cortex; PTGER, prostaglandin E receptor 4; SERPIN, serine protease inhibitor; SFNC; Stanley Foundation Neuropathology Consortium; Shn-2, Schnurri-2; SMRIAC, Stanley Medical Research Institute Array Collection; SOD, superoxide dismutase; TC, temporal cortex; TIMP; tissue inhibitor of metalloproteinases; TL, Temporal lobe; TNFSF, tumor necrosis factor superfamily; TYROBP, TYRO protein tyrosine kinase binding protein; UPCNMDBB, University of Pittsburgh's center for the neuroscience of mental disorder brain bank.
*, # indicate which variables results are representing.
ICAM-1 is a marker of neuroinflammation, associated with blood–brain barrier disruption. Thomas et al.154 found no differences in ICAM-1 labeled cells in both the dorsolateral prefrontal cortex and anterior cingulate cortex of 15 schizophrenia patients of the Stanley Foundation Neuropathology Consortium compared to healthy controls.
Four studies investigated the NF-κB pathway in postmortem schizophrenic brains. Rao et al.73 measured increases in both NF-κB p50 and p65 subunits mRNA expression in the BA10 of schizophrenia patients. A second study evaluating the prefrontal cortex of schizophrenics reported increased NF-κB1 and 2 mRNA expression.155 However, 2 separate studies could not detect any differences in NF-κB2 expression in the frontal cortex156 and NF-κB in the dorsolateral prefrontal cortex86 between schizophrenics and healthy controls. Schnurri-2, a NF-κB site binding protein inhibiting downstream transcription, has been reported to be decreased in the prefrontal cortex of schizophrenia patients.155
Microarray analyses followed by qPCR have proposed markers associated with the immune system or inflammatory response being associated with schizophrenia. One such marker, which was reported in four microarray analyses, is SERPINA3, a protease inhibitor that is involved in inflammatory processes and connective tissue turnover. In the dorsolateral prefrontal cortex, SERPINA3 mRNA expression was significantly higher in the brains of schizophrenics compared with healthy controls.86 The same group confirmed this finding in a second cohort, finding increased SERPINA3 mRNA expression in the medial frontal gyrus in schizophrenia, whereas changes in IL-1RL1 expression were not detected.139 Similar increases of SERPINA3 mRNA expression were reported in two other microarray studies in the frontal cortices of 55 (ref. 93) and 14 (ref. 157) schizophrenia patients and were confirmed by qPCR.
These two microarray studies also found elevated interferon-induced transmembrane protein (IFITM)1, 2 and 3, proteins involved in regulation of the immune response, mRNA expression in the prefrontal cortex in schizophrenia.93, 157 A third study confirmed the increased IFITM3 mRNA expression in the prefrontal cortex.158 Similar overexpression of IFITM1, 2 and 3 was observed by microarray and confirmed by qPCR in the hippocampus of schizophrenic patients.77 A fifth study targeted IFITM1 and 2/3 expression in a separate cohort of prefrontal cortices of schizophrenia patients and reported an increase in both markers independent of antipsychotic use.159
Other markers that either increased or decreased in microarrays include CD163 and S100a8 and 9 in the hippocampus,77 CHI3L1 (ref. 157) and GBP1 (ref. 93) in the prefrontal cortex, TNFSF8, 10 and 13 (although 8 and 13 were not significant in PCR validation) in the dorsolateral prefrontal cortex,160, 161 and TIMP1, TYROB and TNFSRF1A in the temporal lobe.98 However, unlike the decrease in TIMP1 mRNA expression measured in the temporal lobe, TIMP1 protein concentration, measured by enzyme-linked immunosorbent assay, was not changed in the prefrontal cortex in another study.137 Schmitt et al. reported 6 out of 23 immune-related genes are downregulated in the superior temporal cortex in schizophrenia. The 23 immune-related genes include cytokines and microglial markers, discussed above, and other markers including LPL, CFD, PTGER4 and EDG3 being downregulated and ITGA1, LCP1, LTC4S, MTHFD2, CD84, GPX, IFI16 and SOD2 being unchanged.94
Discussion
Schizophrenia has been linked to neuroinflammation.8, 9, 10 Schizophrenic patients have been shown to have elevated cytokines in blood17, 18 and elevated microglia activation in the brain as measured by PET analysis in some22, 23 but not all25, 26 reports. This paper systematically reviewed the literature covering neuroinflammatory analyses in postmortem brains from schizophrenic patients.
Multiple studies evaluating neuroinflammation in postmortem brain samples found evidence of neuroinflammation in schizophrenia. However, a definitive statement cannot be made on whether neuroinflammation is present in schizophrenic postmortem brain samples due to the large number of null studies. For example, out of 33 studies evaluating GFAP, 21 studies did not find any effect of schizophrenia on GFAP expression, whereas 6 studies found a decrease in GFAP and 6 studies had elevated GFAP expression. Similarly, out of 34 studies that evaluated glial cell density, 25 studies found no effect of schizophrenia, whereas 7 studies found a decrease in glial cells and 2 studies found an increase. Variability is also observed for four microglial markers (HLA, CD11b, CD68 and calprotectin), where 11 studies had elevated expression of microglial markers, 8 studies found no differences and 3 found a decrease. SERPINA3, a protease inhibitor that is involved in inflammatory processes and connective tissue turnover, however, was elevated in the 4 studies, which have reported on its mRNA expression. IFITM, a viral restriction factor, was also reported elevated in four microarrays, and confirmed in one targeted study.
These discrepancies may be explained, at least partly, by the heterogeneity in study designs across studies. One of the heterogeneous variable across studies is brain region analyzed. For example, studies evaluating GFAP expression have analyzed 34 brain regions, including the hippocampus, prefrontal cortex, enthorhinal cortex, orbitofrontal cortex and cingulate cortex among others. Whereas all five studies analyzing GFAP expression in the entorhinal cortex found no differences in schizophrenia, 4 of the 13 studies evaluating GFAP expression in the frontal cortex, prefrontal cortex or dorsolateral prefrontal cortex (BA9, 10 or 46) identified differences between schizophrenia and healthy controls. However, classification of the frontal cortices varied between studies and may explain differing results. Moreover, four out of six studies examining the cingulate cortex, subgenual cingulate cortex or anterior cingulate cortex found significant changes in GFAP in schizophrenia. It is possible that certain brain regions, such as the cingulate cortex, are more susceptible to change in schizophrenia compared to other regions such as the entorhinal cortex. Nevertheless, despite more studies pointing to a decrease in GFAP expression in the cingulate cortex in schizophrenia, not all studies show decreases despite evaluating the same brain region and marker.53, 58
Consideration of the cortical layer in which the markers are measured may be needed in order to tease out the differences across studies. Many studies found layer-specific effects in various brain regions and markers. For example, in two studies, GFAP expression was increased solely in layer V of the dorsolateral prefrontal cortex70 and layer I in subgenual cingulate cortex.67 This could explain differences across studies measuring GFAP in the whole prefrontal cortex mentioned above. Similarly, layer-specific effects of schizophrenia on glial cell density measured by cresyl violet were observed in several studies evaluating the motor cortex (layer III), planum temporale (layer IV), cingulate cortex (layer IV) and dorsolateral prefrontal cortex (layer V).
Differences in methodological approaches also warrant consideration when evaluating the results of the studies mentioned above. Stereological analysis, an unbiased cell counting method, was applied to approximately half of the studies measuring glial cells. Only one study utilizing stereology measured differences in glial cell density, whereas seven studies using other methods reported differences. However, the use of stereology is not always clear in the methods section and therefore the results above should be considered with caution. Similarly, double labeling could be utilized to detect different subtypes of cells. However, few studies in this review utilized double labeling, which should be considered when no differences in cell densities are detected. Thus, the lack of changes in cell densities may not reflect changes in subtypes of cells.
Another variable that may contribute to the heterogeneous results is the stage of the disorder. By separating paranoid schizophrenia from residual schizophrenia, differences in S100b-positive cells were observed.79 Microglia are also elevated in paranoid schizophrenia, where HLA-DR-positive cell density is higher in paranoid schizophrenia compared with residual schizophrenia.88 Moreover, differences in gliosis score are seen between early onset and late onset schizophrenia.135 Similarly, the three patients with microgliosis in the study by Bayer et al.85 were all defined to have late onset schizophrenia.
Suicide is common in schizophrenia. This is important to consider as postmortem brains from suicide victims may present elevated pro-inflammatory cytokines.162, 163 This is in agreement with Steiner and colleagues where the two schizophrenia patients that committed suicide had the highest HLA-DR-positive cell density.91 When accounting for suicide victims, the same group found no differences between diagnosis groups. They did, however, find a relation between suicide and HLA-DR-positive cells.92 Similarly, GFAP cell density is elevated in the dorsolateral prefrontal cortex of suicide victims compared with non-suicide schizophrenic patients.55 This effect on GFAP, in the dorsolateral prefrontal cortex of suicide victims, however, was not seen in another study measuring GFAP by western blot.65 No effect of suicide was also observed for ICAM-1 expression.154 This is also an important consideration for control group selection. Tooney et al.152 found an effect of schizophrenia on neurokinin 1 receptor compared with a control group that contained suicide victims, which may potentially confound the results. Although a few studies considered the effect of suicide on their measurements, many studies do not report this data or include it in their statistical analysis, making it a limitation and should be considered in future studies.
Several other confounding factors have been associated with potential effects on neuroinflammatory markers in schizophrenia in postmortem brains. Antipsychotics have been associated with modulation of inflammation.164 Typical antipsychotics generally reduce pro-inflammatory markers while atypical antipsychotics generally increase them.164, 165 In our systematic review, antipsychotics were reported to raise GFAP,53, 71, 74 substance P148 and HLA.53 No effect of medication, however, was seen on IL-1β.136 This is important to note, as not all studies measured antipsychotic levels at time of death or corrected for this potential confounder. Moreover, even when measured, separation of typical and atypical antipsychotics was not considered in the statistical analysis. Also, control subjects would not have been exposed to antipsychotic medication, potentially creating a confounder between controls and the experimental group. Similarly, age is positively correlated to the expression of GFAP,66 S10058 and substance P receptor binding.151 Lifestyle choices, such as smoking and alcohol abuse, may also contribute to neuroinflammation. In one study, decreases in MHC I observed in the dorsolateral prefrontal cortex of non-smoking schizophrenia patients were no longer apparent in the smoking population.100 Interestingly, lifestyle choices and antipsychotic use are also risk factors for the development of type II diabetes,166 which is more prevalent in schizophrenia167 and has been associated with neuroinflammation.168, 169 Although not reported in the studies in this review, it would be of interest for future studies to investigate a potential link between diabetes in schizophrenia and neuroinflammation.
The source of the brains also needs consideration. Several brain banks produced multiple studies utilizing several different brain regions from the same brains. Brain banks may have different diagnosis methods, inclusion and exclusion criteria, storage, and demographics among many other variables. Thus, it is possible that the results may be biased by where the brains samples used were provided from. For example, the 33 studies on GFAP reported in this paper were generated from brains from 15 separate brain banks. Of those 33 studies, 6 studies reported a decrease in GFAP. Of those 6 studies, 2 studies utilized the Stanley Foundation Neuropathology Consortium whereas 3 other studies used the Corsellis Brain Collection.
Despite the heterogeneity across studies, the expression of both SERPINA3 and IFITM was repeatedly found to be increased in microarray studies. SERPINA3, a member of the serine protein inhibitor family, is an acute-phase protein which increases during inflammatory episodes170 and is expressed in reactive astrocytes.171 SERPINA3 has previously been linked with decreased age of onset of Alzheimer's symptoms.172 Moreover, SERPINA3 expression is correlated with GFAP positive cells in Alzheimer's disease.173 Patients with multiple sclerosis have elevated SERPINA3 CFS concentration.174 In depression, no association was reported between blood levels of SERPINA3 and symptoms.175 IFITM, on the other hand, is an immune-related protein involved in viral replication. In animal models of inflammation, IFITM1 is increased in the cortex of mice lacking the NF-κB site binding protein Schnurri-2.176 Similarly, IFITM1 and 3 expression is upregulated in the hippocampus following centrally administered lipopolysaccharide injection,177 suggesting its involvement in neuroinflammatory processes.
In conclusion, although the majority of studies note a lack of change in neuroinflammatory markers in postmortem brain samples of patients with schizophrenia, there are still multiple studies indicating either increases or decreases in neuroinflammatory markers. Although ~70% of studies evaluating astrocytes or glial cells in schizophrenia found no change, there were still ~30% of studies showing either an increase or decrease in astrocytic markers and glial cell density. The changes in microglial markers in schizophrenia is more variable across studies, with ~45% of studies showing an increase and 40% of studies showing no change. Similarly, pro-inflammatory cytokine concentration in the postmortem schizophrenia brain is also variable across studies, with studies showing both elevated and decreased cytokine levels in schizophrenia. The cause of this heterogeneity in results is not clear at the moment, but may be due to several factors including brain region measured, stage of disorder, source of the brain and medication. Despite this heterogeneity, microarray analyses have consistently indicated markers such as SERPINA3 and IFITM to be elevated in schizophrenia. Future studies should consider these potential sources of heterogeneity when measuring neuroinflammatory markers in postmortem brain samples of schizophrenia patients.
Acknowledgments
MOT holds a studentship from the Natural Sciences and Engineering Research Council of Canada (PGSD-442373-2013). RPB acknowledges funding from the Canadian Institutes of Health Research (#303157) and holds a Canada Research Chair in Brain Lipid Metabolism. The authors would also acknowledge Dr. JL Sievenpiper for the helpful discussion on systematic reviews.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, ‘just the facts' what we know in 2008. 2. Epidemiology and etiology. Schizophr Res 2008; 102: 1–18. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet 2009; 374: 635–645. [DOI] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature 2010; 468: 187–193. [DOI] [PubMed] [Google Scholar]
- Roussos P, Haroutunian V. Schizophrenia: susceptibility genes and oligodendroglial and myelin related abnormalities. Front Cell Neurosci 2014; 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoutzou G, Emrich HM, Dietrich DE. The myelin-pathogenesis puzzle in schizophrenia: a literature review. Mol Psychiatry 2008; 13: 245–260. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 2009; 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry 2014; 75: e31. [DOI] [PubMed] [Google Scholar]
- Leza JC, Garcia-Bueno B, Bioque M, Arango C, Parellada M, Do K et al. Inflammation in schizophrenia: a question of balance. Neurosci Biobehav Rev 2015; 55: 612–626. [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation 2013; 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015; 300: 141–154. [DOI] [PubMed] [Google Scholar]
- Muller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci 2015; 9: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev 2006; 213: 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 2014; 11: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci 2014; 5: 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Neerven S, Nemes A, Imholz P, Regen T, Denecke B, Johann S et al. Inflammatory cytokine release of astrocytes in vitro is reduced by all-trans retinoic acid. J Neuroimmunol 2010; 229: 169–179. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015; 14: 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 2008; 63: 801–808. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamne M, Wood J, Chowdari K, Watson AM, Celik C, Mansour H et al. Evaluation of HLA polymorphisms in relation to schizophrenia risk and infectious exposure. Schizophr Bull 2012; 38: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue WH, Wang HF, Sun LD, Tang FL, Liu ZH, Zhang HX et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet 2011; 43: 1228–1231. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Kubicki M, Shenton ME. In vivo imaging of neuroinflammation in schizophrenia. Schizophr Res 2015; 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008; 64: 820–822. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 2016; 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in vivo PET study with [18 F]-FEPPA. Schizophr Bull 2015; 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 2010; 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry 2002; 159: 1029–1034. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res 2007; 90: 179–185. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2010; 71: 520–527. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry 2005; 57: 1594–1596. [DOI] [PubMed] [Google Scholar]
- Nitta M, Kishimoto T, Muller N, Weiser M, Davidson M, Kane JM et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull 2013; 39: 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier MO, Hopperton KE, Orr SK, Bazinet RP. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: an update. Eur J Pharmacol 2016; e-pub ahead of print. [DOI] [PubMed]
- Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013; 75: 645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Chiliza B, Asmal L, du Plessis S, Phahladira L, van Niekerk E et al. A randomized, controlled trial of omega-3 fatty acids plus an antioxidant for relapse prevention after antipsychotic discontinuation in first-episode schizophrenia. Schizophr Res 2014; 158: 230–235. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry 2010; 67: 146–154. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res 2004; 76: 834–845. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature 2006; 440: 1054–1059. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol 2012; 33: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302: 1760–1765. [DOI] [PubMed] [Google Scholar]
- Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia 1999; 28: 114–127. [PubMed] [Google Scholar]
- Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 2015; 161: 102–112. [DOI] [PubMed] [Google Scholar]
- Tooyama I, Kimura H, Akiyama H, McGeer PL. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer's disease. Brain Res 1990; 523: 273–280. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Colter N, Lofthouse R, Bogerts B, Zech M, Crow TJ. Gliosis in schizophrenia: a survey. Biol Psychiatry 1986; 21: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Colter N, Lofthouse R, Johnstone EC, Crow TJ. Is there gliosis in schizophrenia? Investigation of the temporal lobe. Biol Psychiatry 1987; 22: 1459–1468. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Trojanowski JQ, Moberg PJ, Gur RE. Glial fibrillary acidic protein-immunoreactive astrocytosis in elderly patients with schizophrenia and dementia. Acta Neuropathol 1996; 91: 269–277. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry 1998; 55: 225–232. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo TUW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 2010; 67: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Abulseoud OA, Foland-Ross L, Bartzokis G, Chang S, Mintz J et al. Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord 2010; 12: 541–549. [DOI] [PubMed] [Google Scholar]
- Falkai P, Honer WG, David S, Bogerts B, Majtenyi C, Bayer TA. No evidence for astrogliosis in brains of schizophrenic patients. A post-mortem study. Neuropathol Appl Neurobiol 1999; 25: 48–53. [DOI] [PubMed] [Google Scholar]
- Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res 2000; 93: 103–110. [DOI] [PubMed] [Google Scholar]
- Stevens CD, Altshuler LL, Bogerts B, Falkai P. Quantitative study of gliosis in schizophrenia and Huntington's chorea. Biol Psychiatry 1988; 24: 697–700. [DOI] [PubMed] [Google Scholar]
- Williams MR, Marsh R, Macdonald CD, Jain J, Pearce RK, Hirsch SR et al. Neuropathological changes in the nucleus basalis in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013; 263: 485–495. [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol 2000; 59: 137–150. [DOI] [PubMed] [Google Scholar]
- Damadzic R, Bigelow LB, Krimer LS, Goldenson DA, Saunders RC, Kleinman JE et al. A quantitative immunohistochemical study of astrocytes in the entorhinal cortex in schizophrenia, bipolar disorder and major depression: absence of significant astrocytosis. [References]. Brain Res Bull 2001; 55: 611–618. [DOI] [PubMed] [Google Scholar]
- Hercher C, Chopra V, Beasley CL. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J Psychiatry Neurosci 2014; 39: 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun 2001; 15: 388–400. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet 2003; 362: 798–805. [DOI] [PubMed] [Google Scholar]
- Katsel P, Byne W, Roussos P, Tan W, Siever L, Haroutunian V. Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology 2011; 36: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Dwork AJ, Rosoklija G, Mann JJ, Mancevski B, Jakovski Z et al. Metabolic abnormalities in fronto-striatal-thalamic white matter tracts in schizophrenia. Schizophr Res 2009; 109: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Laurence JA, Araghi-Niknam M, Stary JM, Schulz SC, Lee S et al. Glial fibrillary acidic protein is reduced in cerebellum of subjects with major depression, but not schizophrenia. Schizophr Res 2004; 69: 317–323. [DOI] [PubMed] [Google Scholar]
- Karson CN, Casanova MF, Kleinman JE, Griffin WST. Choline acetyltransferase in schizophrenia. Am J Psychiatry 1993; 150: 454–459. [DOI] [PubMed] [Google Scholar]
- Dean B, Gray L, Scarr E. Regionally specific changes in levels of cortical S100beta in bipolar 1 disorder but not schizophrenia. Aust N Z J Psychiatry 2006; 40: 217–224. [DOI] [PubMed] [Google Scholar]
- Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality'. Mol Psychiatry 1999; 4: 39–45. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci USA 1996; 93: 14182–14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feresten AH, Barakauskas V, Ypsilanti A, Barr AM, Beasley CL. Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr Res 2013; 150: 252–257. [DOI] [PubMed] [Google Scholar]
- Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res 2008; 103: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Hampton T, Pearce RK, Hirsch SR, Ansorge O, Thom M et al. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013; 263: 41–52. [DOI] [PubMed] [Google Scholar]
- Williams M, Pearce RK, Hirsch SR, Ansorge O, Thom M, Maier M. Fibrillary astrocytes are decreased in the subgenual cingulate in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2014; 264: 357–362. [DOI] [PubMed] [Google Scholar]
- Williams MR, Galvin K, O'Sullivan B, MacDonald CD, Ching EW, Turkheimer F et al. Neuropathological changes in the substantia nigra in schizophrenia but not depression. Eur Arch Psychiatry Clin Neurosci 2014; 264: 285–296. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 2002; 57: 127–138. [DOI] [PubMed] [Google Scholar]
- Toro CT, Hallak JE, Dunham JS, Deakin JF. Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci Lett 2006; 404: 276–281. [DOI] [PubMed] [Google Scholar]
- Markova E, Markov I, Revishchin A, Okhotin V, Sulimov G. 3-D Golgi and image analysis of the olfactory tubercle in schizophrenia. Anal Quant Cytol Histol 2000; 22: 178–182. [PubMed] [Google Scholar]
- Rao JS, Kim HW, Harry GJ, Rapoport SI, Reese EA. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in the postmortem frontal cortex from schizophrenia patients. Schizophr Res 2013; 147: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 2009; 112: 54–64. [DOI] [PubMed] [Google Scholar]
- Catts VS, Wong J, Fillman SG, Fung SJ, Shannon Weickert C. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z J Psychiatry 2014; 48: 722–734. [DOI] [PubMed] [Google Scholar]
- Webster MJ, O'Grady J, Kleinman JE, Weickert CS. Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 2005; 133: 453–461. [DOI] [PubMed] [Google Scholar]
- Hwang Y, Kim J, Shin JY, Kim JII, Seo JS, Webster MJ et al. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry 2013; 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Schmitt A, Schroeter ML, Bogerts B, Falkai P, Turck CW et al. S100B is downregulated in the nuclear proteome of schizophrenia corpus callosum. Eur Arch Psychiatry Clin Neurosci 2014; 264: 311–316. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Bielau H, Farkas N, Winter J, Dobrowolny H et al. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. J Psychiatr Res 2008; 42: 868–876. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Stevens JR, Kleinman JE. Astrocytosis in the molecular layer of the dentate gyrus: a study in Alzheimer's disease and schizophrenia. Psychiatry Res 1990; 35: 149–166. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 2009; 117: 395–407. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Uranova N. Ultrastructural abnormalities of astrocytes in the hippocampus in schizophrenia and duration of illness: a postortem morphometric study. World J Biol Psychiatry 2010; 11: 282–292. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry 2010; 11: 567–578. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett 1999; 271: 126–128. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 2013; 18: 206–214. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 2005; 43: 81–89. [PubMed] [Google Scholar]
- Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 2012; 26: 1273–1279. [DOI] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Kosno-Kruszewska E, Lechowicz W, Pasennik E, Schmidt-Sidor B. Degeneration of microglial cells in frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 2004; 42: 157–165. [PubMed] [Google Scholar]
- Foster R, Kandanearatchi A, Beasley C, Williams B, Khan N, Fagerhol MK et al. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: evidence for an inflammatory process? Eur J Neurosci 2006; 24: 3561–3566. [DOI] [PubMed] [Google Scholar]
- Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein HG et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol 2006; 112: 305–316. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 2008; 42: 151–157. [DOI] [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry 2007; 7: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Leonardi-Essmann F, Durrenberger PF, Parlapani E, Schneider-Axmann T, Spanagel R et al. Regulation of immune-modulatory genes in left superior temporal cortex of schizophrenia patients: a genome-wide microarray study. World J Biol Psychiatry 2011; 12: 201–215. [DOI] [PubMed] [Google Scholar]
- Comte I, Kotagiri P, Szele FG. Regional differences in human ependymal and subventricular zone cytoarchitecture are unchanged in neuropsychiatric disease. Dev Neurosci 2012; 34: 299–309. [DOI] [PubMed] [Google Scholar]
- Nakatani N, Hattori E, Ohnishi T, Dean B, Iwayama Y, Matsumoto I et al. Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum Mol Genet 2006; 15: 1949–1962. [DOI] [PubMed] [Google Scholar]
- Connor CM, Guo Y, Akbarian S. Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry 2009; 66: 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger PF, Fernando FS, Kashefi SN, Bonnert TP, Seilhean D, Nait-Oumesmar B et al. Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm (Vienna) 2015; 122: 1055–1068. [DOI] [PubMed] [Google Scholar]
- Gos T, Myint AM, Schiltz K, Meyer-Lotz G, Dobrowolny H, Busse S et al. Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav Immun 2014; 41: 59–64. [DOI] [PubMed] [Google Scholar]
- Kano S, Nwulia E, Niwa M, Chen Y, Sawa A, Cascella N. Altered MHC class I expression in dorsolateral prefrontal cortex of nonsmoker patients with schizophrenia. Neurosci Res 2011; 71: 289–293. [DOI] [PubMed] [Google Scholar]
- Sinkus ML, Adams CE, Logel J, Freedman R, Leonard S. Expression of immune genes on chromosome 6p21.3-22.1 in schizophrenia. Brain Behav Immunity 2013; 32: 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR. Neuropathology of schizophrenia. Arch Gen Psychiatry 1982; 39: 1131–1139. [DOI] [PubMed] [Google Scholar]
- Bruton CJ, Crow TJ, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia and the brain: a prospective clinico-neuropathological study. Psychol Med 1990; 20: 285–304. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B. Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236: 154–161. [DOI] [PubMed] [Google Scholar]
- Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry 2004; 161: 882–888. [DOI] [PubMed] [Google Scholar]
- Brauch RA, Adnan El-Masri M, Parker JC Jr., El-Mallakh RS. Glial cell number and neuron/glial cell ratios in postmortem brains of bipolar individuals. J Affect Disord 2006; 91: 87–90. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honavar M, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the superior temporal white matter in schizophrenia, major depressive disorder and bipolar disorder. Schizophr Res 2009; 115: 156–162. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 2002; 12: 386–394. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 2001; 58: 545–553. [DOI] [PubMed] [Google Scholar]
- Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 1986; 43: 31–35. [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Wakai T, Toru M. Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm (Vienna) 1997; 104: 1361–1370. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk YB, Sun X, Wang JF, MacQueen GM, McEwen BS, Young LT. Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. J Psychiatry Neurosci 2007; 32: 203–210. [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry 1995; 52: 805–818; discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Mrzljak J, Kleinman JE, Herman MM, Goldman-Rakic PS. Regional specificity in the neuropathologic substrates of schizophrenia: a morphometric analysis of Broca's area 44 and area 9. Arch Gen Psychiatry 2003; 60: 69–77. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry 1998; 55: 215–224. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol 1998; 392: 402–412. [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 1998; 95: 13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region—a morphometric study. Biol Psychiatry 1988; 24: 515–521. [DOI] [PubMed] [Google Scholar]
- Di Rosa E, Crow TJ, Walker MA, Black G, Chance SA. Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res 2009; 166: 102–115. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry 2006; 188: 26–31. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Everall I, Cotter D. Glial cell number and nuclear size in the mediodorsal thalamic nucleus (MDNT) in schizophrenia. Schizophr Res 2008; 102: 344–345. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Frangou S, Hudson L, Landau S. Cell density and cortical thickness in Heschl's gyrus in schizophrenia, major depression and bipolar disorder. Br J Psychiatry 2004; 185: 258–259. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry 2001; 50: 395–406. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry 2003; 53: 1086–1098. [DOI] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulate cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry 1991; 48: 996–1001. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Hudson L, Cotter DR. Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophr Res 2008; 106: 164–171. [DOI] [PubMed] [Google Scholar]
- Cotter D, Hudson L, Landau S. Evidence for orbitofrontal pathology in bipolar disorder and major depression, but not in schizophrenia. Bipolar Disord 2005; 7: 358–369. [DOI] [PubMed] [Google Scholar]
- Jonsson SA, Luts A, Guldberg-Kjaer N, Brun A. Hippocampal pyramidal cell disarray correlates negatively to cell number: implications for the pathogenesis of schizophrenia. Eur Arch Psychiatry Clin Neurosci 1997; 247: 120–127. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Chana G, Honavar M, Landau S, Everall IP, Cotter D. Evidence for altered neuronal organisation within the planum temporale in major psychiatric disorders. Schizophr Res 2005; 73: 69–78. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Hantsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry 1983; 18: 951–969. [PubMed] [Google Scholar]
- Selemon LD, Begovic A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res 2007; 151: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoistad M, Heinsen H, Wicinski B, Schmitz C, Hof PR. Stereological assessment of the dorsal anterior cingulate cortex in schizophrenia: absence of changes in neuronal and glial densities. Neuropathol Appl Neurobiol 2013; 39: 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann H, Lauer M. The human striatum in schizophrenia. II. Increased number of striatal neurons in schizophrenics. Psychiatry Res 1997; 68: 99–109. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N et al. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry 1989; 46: 1145–1150. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, McCalley-Whitters M, Bigelow LB, Rauscher FP. A histological study of the corpus callosum in chronic schizophrenia. Psychiatry Res 1983; 8: 251–260. [DOI] [PubMed] [Google Scholar]
- Toyooka K, Watanabe Y, Iritani S, Shimizu E, lyo M, Nakamura R et al. A decrease in interleukin-1 receptor antagonist expression in the prefrontal cortex of schizophrenic patients. Neurosci Res 2003; 46: 299–307. [DOI] [PubMed] [Google Scholar]
- Harris LW, Pietsch S, Cheng TM, Schwarz E, Guest PC, Bahn S. Comparison of peripheral and central schizophrenia biomarker profiles. PLoS ONE 2012; 7: e46368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Gibbons AS, Tawadros N, Brooks L, Everall IP, Scarr E. Different changes in cortical tumor necrosis factor-alpha-related pathways in schizophrenia and mood disorders. Mol Psychiatry 2013; 18: 767–773. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry 2014; 4: e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida ME, Hurley SD, Daeschner JA, Moore AH, O'Banion MK. Cytosolic prostaglandin E2 synthase (cPGES) expression is decreased in discrete cortical regions in psychiatric disease. Brain Res 2006; 1103: 164–172. [DOI] [PubMed] [Google Scholar]
- Tang B, Capitao C, Dean B, Thomas EA. Differential age- and disease-related effects on the expression of genes related to the arachidonic acid signaling pathway in schizophrenia. Psychiatry Res 2012; 196: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota O, Terada S, Ishihara T, Nakashima H, Kugo A, Ujike H et al. Neuronal expression of cyclooxygenase-2, a pro-inflammatory protein, in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 715–721. [DOI] [PubMed] [Google Scholar]
- Carletti R, Corsi M, Melotto S, Caberlotto L. Down-regulation of amygdala preprotachykinin A mRNA but not 3H-SP receptor binding sites in subjects affected by mood disorders and schizophrenia. Eur J Neurosci 2005; 21: 1712–1718. [DOI] [PubMed] [Google Scholar]
- Harrington KA, Augood SJ, Faull RL, McKenna PJ, Emson PC. Dopamine D1 receptor, D2 receptor, proenkephalin A and substance P gene expression in the caudate nucleus of control and schizophrenic tissue: a quantitative cellular in situ hybridisation study. Brain Res Mol Brain Res 1995; 33: 333–342. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Ofri D, Kleinman JE. Enkephalin, dynorphin and substance P in postmortem substantia nigra from normals and schizophrenic patients. Life Sci 1991; 48: 1919–1930. [DOI] [PubMed] [Google Scholar]
- Kleinman JE, Hong J, Iadarola M, Govoni S, Gillin CJ. Neuropeptides in human brain—postmortem studies. Prog Neuropsychopharmacol Biol Psychiatry 1985; 9: 91–95. [DOI] [PubMed] [Google Scholar]
- Zech M, Bogerts B. Methionine-enkephalin and substance P in the basal ganglia of normals, Parkinson patients, Huntington patients, and schizophrenics. A qualitative immunohistochemical study. Acta Neuropathol 1985; 68: 32–38. [DOI] [PubMed] [Google Scholar]
- Toru M, Watanabe S, Shibuya H, Nishikawa T, Noda K, Mitsushio H et al. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand 1988; 78: 121–137. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Ferrier IN, Lee Y, Crow TJ, Johnstone EC, Owens DG et al. Peptides, the limbic lobe and schizophrenia. Brain Res 1983; 288: 199–211. [DOI] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Joyce JN. Increased number of [125I] BH-substance p receptors in schizophrenia. Prog Neuro-psychopharmacol Biol Psychiatry 1998; 22: 1295–1299. [Google Scholar]
- Burnet PW, Harrison PJ. Substance P (NK1) receptors in the cingulate cortex in unipolar and bipolar mood disorder and schizophrenia. Biol Psychiatry 2000; 47: 80–83. [DOI] [PubMed] [Google Scholar]
- Tooney PA, Crawter VC, Chahl LA. Increased tachykinin NK(1) receptor immunoreactivity in the prefrontal cortex in schizophrenia. Biol Psychiatry 2001; 49: 523–527. [DOI] [PubMed] [Google Scholar]
- Weidenhofer J, Yip J, Zavitsanou K, Huang XF, Chahl LA, Tooney PA. Immunohistochemical localisation of the NK1 receptor in the human amygdala: preliminary investigation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Davis S, Ferrier IN, Kalaria RN, O'Brien JT. Elevation of cell adhesion molecule immunoreactivity in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry 2004; 55: 652–655. [DOI] [PubMed] [Google Scholar]
- Volk DW, Chitrapu A, Edelson JR, Roman KM, Moroco AE, Lewis DA. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am J Psychiatry 2015; 172: 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhang L, Johnston NL, Torrey EF, Yolken RH. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br J Psychiatry Suppl 2001; 41: s137–s141. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Kakiuchi C, Bundo M, Ikeda K, Kato T. Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders. Mol Psychiatry 2004; 9: 406–416. [DOI] [PubMed] [Google Scholar]
- Siegel BI, Sengupta EJ, Edelson JR, Lewis DA, Volk DW. Elevated viral restriction factor levels in cortical blood vessels in schizophrenia. Biol Psychiatry 2014; 76: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Vawter MP. Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry 2008; 64: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catts VS, Weickert CS. Gene expression analysis implicates a death receptor pathway in schizophrenia pathology. PLoS ONE 2012; 7: e35511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res 2012; 46: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand 2008; 117: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2014; 48: 277–286. [DOI] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS. Cytokines in schizophrenia and the effects of antipsychotic drugs. Brain Behav Immun 2006; 20: 532–545. [DOI] [PubMed] [Google Scholar]
- Ventriglio A, Gentile A, Stella E, Bellomo A. Metabolic issues in patients affected by schizophrenia: clinical characteristics and medical management. Front Neurosci 2015; 9: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvisaari J, Perala J, Saarni SI, Harkanen T, Pirkola S, Joukamaa M et al. Type 2 diabetes among persons with schizophrenia and other psychotic disorders in a general population survey. Eur Arch Psychiatry Clin Neurosci 2008; 258: 129–136. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab 2013; 2: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk G, van Heijningen S, Reijne AC, Nyakas C, van der Zee EA, Eisel UL. Integrative neurobiology of metabolic diseases, neuroinflammation, and neurodegeneration. Front Neurosci 2015; 9: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsheker NA. Alpha 1-antichymotrypsin. Int J Biochem Cell Biol 1996; 28: 961–964. [DOI] [PubMed] [Google Scholar]
- Gopalan SM, Wilczynska KM, Konik BS, Bryan L, Kordula T. Astrocyte-specific expression of the alpha1-antichymotrypsin and glial fibrillary acidic protein genes requires activator protein-1. J Biol Chem 2006; 281: 1956–1963. [DOI] [PubMed] [Google Scholar]
- Kamboh MI, Minster RL, Kenney M, Ozturk A, Desai PP, Kammerer CM et al. Alpha-1-antichymotrypsin (ACT or SERPINA3) polymorphism may affect age-at-onset and disease duration of Alzheimer's disease. Neurobiol Aging 2006; 27: 1435–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Mallory M, Hansen LA, Masliah E. Increased levels of alpha-1-antichymotrypsin in brains of patients with Alzheimer's disease correlate with activated astrocytes and are affected by APOE 4 genotype. J Neuroimmunol 1998; 88: 105–110. [DOI] [PubMed] [Google Scholar]
- Ottervald J, Franzen B, Nilsson K, Andersson LI, Khademi M, Eriksson B et al. Multiple sclerosis: identification and clinical evaluation of novel CSF biomarkers. J Proteomics 2010; 73: 1117–1132. [DOI] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJ, Tiemeier H, Carvalho LA. Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology 2015. [DOI] [PMC free article] [PubMed]
- Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 2013; 38: 1409–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonow RH, Aid S, Zhang Y, Becker KG, Bosetti F. The brain expression of genes involved in inflammatory response, the ribosome, and learning and memory is altered by centrally injected lipopolysaccharide in mice. Pharmacogenomics J 2009; 9: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.