Abstract
A central problem in the treatment of drug addiction is the high risk of relapse often precipitated by drug-associated cues. The transfer of glycogen-derived lactate from astrocytes to neurons is required for long-term memory. Whereas blockade of drug memory reconsolidation represents a potential therapeutic strategy, the role of astrocyte–neuron lactate transport in long-term conditioning has received little attention. By infusing an inhibitor of glycogen phosphorylase into the basolateral amygdala of rats, we report that disruption of astrocyte-derived lactate not only transiently impaired the acquisition of a cocaine-induced conditioned place preference but also persistently disrupted an established conditioning. The drug memory was rescued by L-Lactate co-administration through a mechanism requiring the synaptic plasticity-related transcription factor Zif268 and extracellular signal-regulated kinase (ERK) signalling pathway but not the brain-derived neurotrophic factor (Bdnf). The long-term amnesia induced by glycogenolysis inhibition and the concomitant decreased expression of phospho-ERK were both restored with L-Lactate co-administration. These findings reveal a critical role for astrocyte-derived lactate in positive memory formation and highlight a novel amygdala-dependent reconsolidation process, whose disruption may offer a novel therapeutic target to reduce the long-lasting conditioned responses to cocaine.
Introduction
Drug memories that associate contextual cues with the effects of drugs of abuse are known to shape and maintain persistent drug-seeking behaviours in rodents.1 In abstinent humans, drug cues are known to evoke salient, persistent and overwhelming memories of drug-taking experiences, thereby inducing higher risks of craving and relapse.2, 3 Preclinical observations have long reported that, through predictive association with the drug's effects, drug-conditioned stimuli can precipitate the reinstatement of previously extinguished drug-seeking behaviours.4, 5, 6 However, many questions remain to be answered about the mechanisms by which long-term memories for drug-paired cues resist extinction and contribute to the enhanced drive to take drugs. A large body of evidence suggests that persistence of drug addiction depends on the remodelling of synapses and circuits that are thought to be characteristic of long-term associative memory.7
Over the past decade, converging evidence has revealed that memory and addiction share both neural circuitry and molecular mechanisms.8, 9, 10, 11, 12, 13 It has been suggested that learning the significance of a predictive cue to trigger the appropriate behavioural response requires the storage of specific patterns of information in the brain.1 As disrupting protein synthesis immediately after learning has been shown to prevent memory formation,14 a current consensus considers that the stabilization of a new memory occurs through a process known as consolidation and requires gene expression. Importantly, once consolidated, memories can again become transiently labile and sensitive to protein synthesis inhibitors if reactivated.15, 16, 17, 18 These findings may offer a critical contribution to clinical practice as they suggest that protein synthesis blockade after reactivation may selectively reduce or even eliminate long-lasting memories, including those linked to drug addiction.19 Although reconsolidation most likely contributes to updating memories,20, 21, 22 its disruption may reduce the impact of intrusive or aberrant memories on behaviour.23, 24, 25, 26, 27, 28, 29, 30, 31 As several aspects of addiction depend on mnemonic processes induced by drug experience, disrupting drug-related memories represents a promising approach to help reducing relapse propensity on subsequent exposure to drug-paired stimuli and thereby may encourage abstinence.32
Although metabolic coupling has long been considered a key mechanism through which astrocytes and neurons actively interact in response of neuronal activity,33, 34 only recent evidence revealed that interference with lactate transfer from astrocytes to neurons impairs long-term memory formation.35, 36, 37, 38 The astrocyte network, known to form highly organized anatomical domains that are interconnected through gap junctions, contact up to hundreds of thousands of synapses permitting an uninterrupted supply of energy substrates.39 Both glucose and lactate can be transported to neurons as metabolic substrates but astrocytic storage of glycogen has been considered as a supplemental energy reserve available to neurons when demand is high. In particular, the metabolic coupling between astrocytes and neurons posits that glycogenolysis-dependent lactate is released from astrocytes40, 41, 42 and imported into neurons.43, 44 Of particular interest, recent evidence demonstrated that learning resulted in lactate release in the hippocampus and that lactate transfer from astrocytes into neurons was critical for the induction of the molecular changes required for long-term memory formation.38 Disruption of the astrocytic (MCT4) and neuronal (MCT2) lactate transporters was shown to prevent the retention of an inhibitory avoidance task. However, L-Lactate administration successfully rescued the amnesia after MCT4 disruption only, suggesting that lactate import into neurons (via MCT2) is essential for long-term memory formation.38 One possible mechanism is that lactate may supply activated neurons with sufficient energy required for activation of signalling pathways underlying long-term memory formation.38 An alternative explanation would suggest that lactate may directly act as a signalling molecule for plasticity mechanisms.45, 46, 47, 48
In line with this latter assumption, recent evidence has shown that L-Lactate increases the neuronal expression of synaptic plasticity-related genes, including Arc, c-Fos, Zif268 and brain-derived neurotrophic factor (Bdnf), through a mechanism involving N-methyl-D-aspartate receptor (NMDAR) activity and its downstream signalling cascade extracellular signal–regulated kinase 1/2 (ERK1/2).45 This observation is of particular relevance given the key role played by NMDAR to initiate reconsolidation, which destabilizes memories and promotes a transient labile state following retrieval, and subsequently leads to long-term memory storage.49, 50, 51, 52 Not surprisingly, several studies have reported that NMDAR antagonists administered into the basolateral amygdala (BLA) impaired retention of appetitive53 and drug54 memories, while activation of ERK pathway31 and Zif268 (ref. 27) was shown to be necessary for the reconsolidation of addictive drug memories.
These observations suggest that long-term memory formation requires high metabolic demands within the underlying active neuronal network and call for a better understanding of the molecular mechanisms involved in the complex reciprocal exchanges of metabolic intermediates between neurons and glia. Therefore, we investigated whether disrupting the glycogen-derived L-Lactate release from astrocytes by administering an inhibitor of glycogen phosphorylase (1,4-dideoxy-1,4-imino-D-arabinitol (DAB)) into the BLA of rats was sufficient to impair the acquisition and/or the maintenance of a cocaine-induced conditioned place preference (CPP). Our findings show that storage and retrieval of addictive drug memories require the astrocyte–neuron lactate transfer, whose disruption may offer a novel therapeutic potential to reduce the long-lasting debilitating impact of drug cues on conditioned responses to cocaine.
Materials and methods
Animals and surgery
All experiments were performed in accordance with the Swiss Federal Act on Animal Protection and the Swiss Animal Ordinance and were approved by the cantonal veterinary office (authorization 1999 to BB).
Rats were anaesthetized by inhalation of 1–3% isoflurane in oxygen and implanted bilaterally with cannula guides (home made from 22 G syringes, Terumo, Eschborn, Germany). Initial experiments were aimed at targeting the lateral ventricle (anterior–posterior A/P −0.6; mediolateral ML +/−1.9; dorsoventral D/V −3.2 mm from the skull surface). However, rats receiving intracerebroventricular administrations of the inhibitor of glycogen phosphorylase DAB still exhibited a preference for the compartment previously paired with cocaine administration (Supplementary Figure S1A). Using a similar approach, bilateral infusions of DAB into the prefrontal cortex (A/P: 3.2; ML +/−0.8; D/V −4 mm) also failed at blocking the cocaine-induced place preference (Supplementary Figure S1B). Hence, we targeted the BLA by using the following coordinates for a bilateral implantation of cannula guides (A/P −2.8 mm, ML +/−5 mm, D/V −7.5 mm; Supplementary Figure S2). Dental cement was used to anchor the cannula to the brain skull and cannulas were kept patent by insertion of a stylet to prevent obstruction. Injectors (homemade from 27 G syringes, Terumo) were placed 1 mm above the cannula guide to prevent brain tissue damage. Animals received a 0.1 mg kg−1 intraperitoneal injection of buprenorphine (Temgesic, Reckitt Benckiser, Wallisellen, Switzerland) before surgery and recovered for at least 7 days before starting the behavioural tests.
Drugs
Cocaine (Macfarlan Smith, Edinburgh, UK) was dissolved in sterile saline. Animals received 15 mg kg−1 doses, at a dose volume of 1 ml kg−1. Vehicle solution (NaCl 0.9%) was injected at a dose volume of 1 ml kg−1. All injections were given intraperitoneally.
Drugs were dissolved in sterile saline (NaCl 0.9%). DAB was administered at 150 pmol per side and L-Lactate and L-Pyruvate at 100 nmol per side (Sigma-Aldrich, Buchs, Switzerland).38 Drugs were injected using a 5 μl Hamilton syringe (Harvard Apparatus, Les Ulis, France) at a rate of 250 nl min−1 over 2 min. After infusion, the injectors were kept in place for an additional 60 s.
Conditioned place preference
The apparatus consisted of three arenas divided into two distinct chambers (45 × 45 × 30 cm3), separated by a corridor (40 × 15 × 30 cm3), whose access was closed on demand with two guillotine doors. Chambers had different floors (perforated plastic plates versus Lego base plate) and different walls (white dots versus white stripes). Time spent in each compartment was monitored with a video tracking system (Ethovision Pro 3.16 Noldus, Wageningen, The Netherlands).
Statistical analysis
Data are shown as mean±s.e.m. For most of the behavioural studies, data sets were subjected to analysis of variance, followed by Bonferroni and Tukey post hoc tests to confirm intra-session and intra-group differences, respectively. Statistical analyses were performed with Statview 5.0 (SAS Institute, Cary, NC, USA), using an α level of 0.05.
Further details about Material and methods, including statistics, are described in Supplementary Information.
Results
Inhibition of glycogen metabolism in the BLA impairs the acquisition of cocaine-induced CPP, while L-Lactate co-administration restores the appetitive memory through a mechanism requiring Zif268
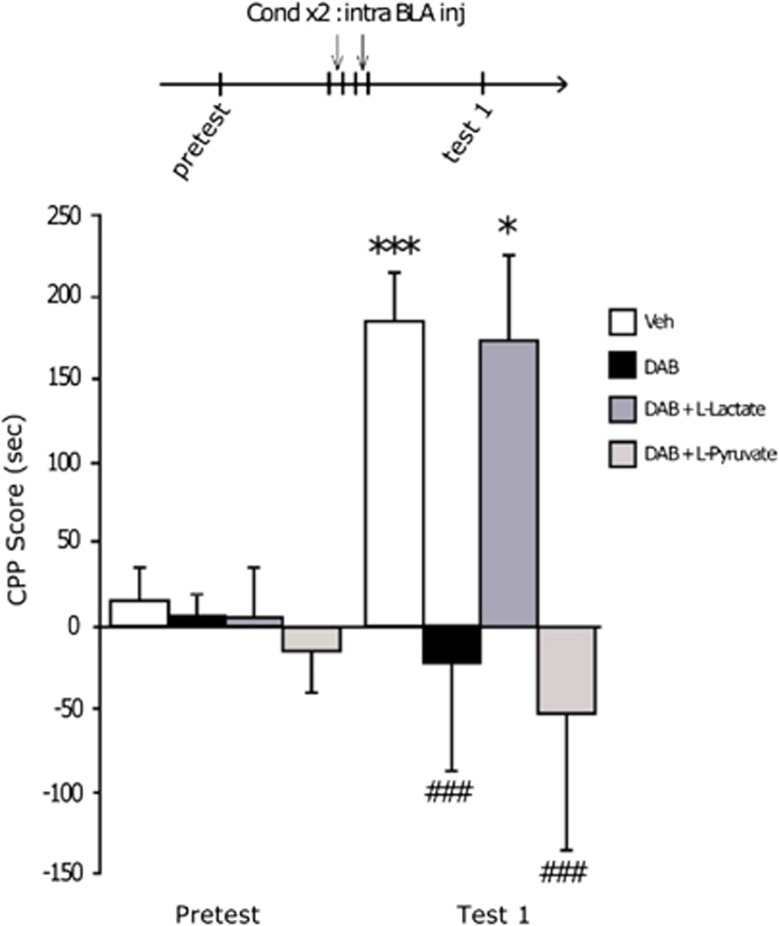
After having explored the CPP apparatus during the pretest session, rats implanted bilaterally with chronic indwelling cannulas targeting the BLA were infused either with vehicle or with the inhibitor of glycogen phosphorylase DAB, 15 min prior to being injected with cocaine (15 mg kg−1, intraperitoneal), and confined in one chamber for 20 min. Animals received two cocaine and two saline injections on alternative days (see Material and methods in Supplementary Information). On the test day, DAB-treated rats, unlike vehicle-treated animals, did not show any preference for the compartment previously paired with cocaine administration (Figure 1). Co-administration of DAB and L-Lactate rescued the preference for the cocaine-paired compartment. In contrast, co-administration of DAB and L-Pyruvate (an energetic equivalent to L-Lactate) failed to rescue this preference. These data provide evidence for a critical role of glycogen-derived lactate in cocaine-induced CPP. The reduced preference for the previously cocaine paired compartment was not the consequence of undesirable or aversive side effects as bilateral administrations of DAB into the BLA did not reduce the locomotor activity in an open field apparatus; it also did not induce any place aversion (Supplementary Figure S3). The reduced preference for the cocaine side was not the consequence of damaged brain tissue owing to local injections as rats, transiently unresponsive to cocaine cues after DAB treatments, exhibited a marked preference for the cocaine compartment after another conditioning 1 week later (Supplementary Figure S4).
Figure 1.
Astrocyte-derived lactate is required for the acquisition of a cocaine-induced conditioned place preference (CPP). Experimental timeline is shown above the graphic. Data represent CPP mean score (±s.e.m.) expressed in seconds as time spent in cocaine compartment minus time spent in saline compartment. A two-way repeated-measures analysis of variance revealed a significant session × treatment interaction (F3,44=4.467, P<0.05), and post hoc analyses demonstrated a significant preference for the compartment previously paired with cocaine injections in vehicle and 1,4-dideoxy-1,4-imino-D-arabinitol (DAB)+L-Lactate-treated animals (*P<0.05, ***P<0.001 compared with pretest score, n=15 and 11, respectively), while DAB- and DAB+L-Pyruvate-treated rats did not exhibit any preference (###P<0.001, n=13 and 11, respectively) compared with respective pretest conditions. BLA, basolateral amygdala.
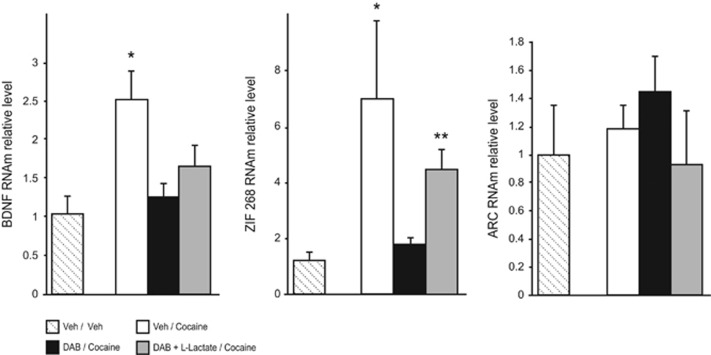
We then examined whether disrupting astrocytic glycogen mobilization influenced the expression levels of molecular markers of memory consolidation. We replicated the conditioning with four groups of rats: three groups were conditioned with cocaine (vehicle/cocaine; DAB/cocaine; DAB+L-Lactate/cocaine) and the last rats received vehicle treatments during the CPP procedure (vehicle/vehicle). Arc and Zif268 expression was assessed 1 h after the last cocaine conditioning,55 and Bdnf expression was measured 3 h after.56
Although recent evidence implicated Arc in the consolidation of explicit and implicit forms of memory, and in maladaptive plasticity associated with drug addiction,57, 58 Arc expression remained unchanged in the BLA following cocaine CPP (Figure 2), whereas cocaine conditioning effectively increased Arc expression in the nucleus accumbens (Supplementary Figure S5). Meanwhile, data revealed that rats conditioned with cocaine administrations and injected with vehicle showed a significant increase in Zif268 and Bdnf expression in the BLA compared with unconditioned rats (Figure 2). Not only the increased expression of Zif268 and Bdnf was abolished by DAB treatment but also L-Lactate treatment rescued the inhibitory effect of DAB on Zif268 expression without, however, any effect on Bdnf mRNA levels (Figure 2).
Figure 2.
Astrocyte-derived lactate modulates gene expression involved in conditioned responses to cocaine. mRNA levels are expressed relative to control animals. Cocaine-induced place preference correlated with increased Bdnf and Zif268 mRNA expression (*P<0.05, compared with Veh/Veh). This increase was abolished after 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) treatment (P>0.05 compared with Veh/Veh animals). Co-administration of DAB and L-Lactate rescued the expression of Zif268 mRNA (**P<0.01, compared with Veh/Veh) but not that of Bdnf (P>0.05, compared with Veh/Veh). Cocaine conditioning or intra-basolateral amygdala treatments did not alter Arc mRNA expression (P>0.05, compared with Veh/Veh). Bdnf measures, Veh/Veh n=5, Veh/cocaine n=5, DAB/cocaine n=8, DAB+L-Lactate/cocaine n=10; Zif268 measures, n=4, 4, 5 and 8, respectively; Arc measures, n=4, 7, 6 and 5, respectively.
Blocking glycogen metabolism in the BLA before and after contextual re-exposure persistently disrupts cocaine CPP
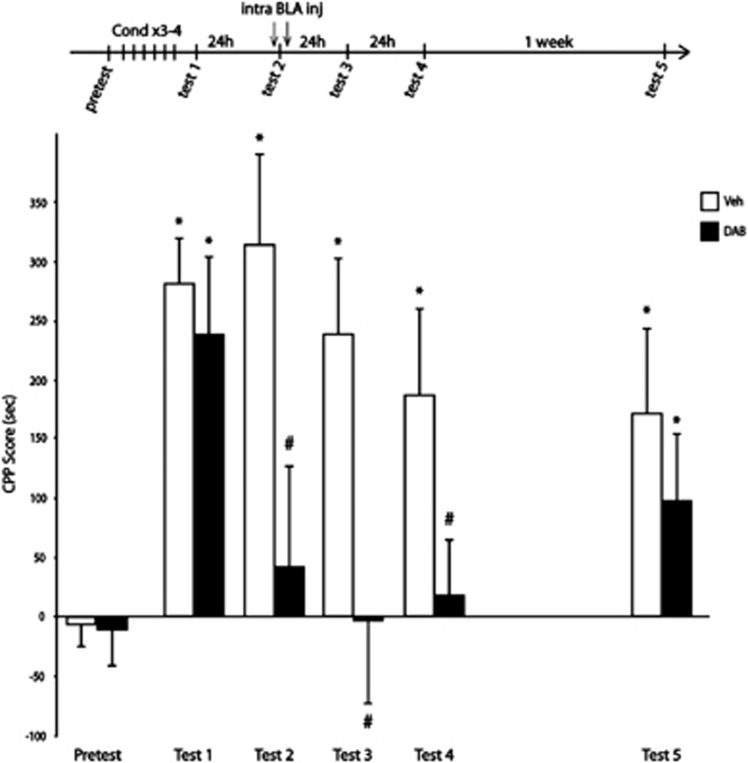
We then investigated whether acquired cocaine CPP was sensitive to glycogen blocking in the BLA. As shown in Figure 3, two groups of rats were conditioned with cocaine injections for 3 days. Twenty-four hours after the end of conditioning, rats were tested for CPP (test 1) to assess their preference for the compartment previously paired with cocaine administration. Twenty-four hours after test 1, one group received vehicle infusions and the other received DAB infusions into the BLA prior being tested once again in the CPP arena (test 2). Animals were tested again twice, at 24-h intervals (tests 3 and 4), and finally, their preference for the cocaine side was assessed 1 week later (test 5).
Figure 3.
A single injection of 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) into the basolateral amygdala (BLA) transiently disrupts already established cocaine-induced conditioned place preference (CPP). Experimental timeline is shown above the graphic. Data represent CPP mean score (±s.e.m.) expressed in seconds as time spent in cocaine compartment minus time spent in saline compartment. A two-way repeated-measures analysis of variance revealed a significant session × treatment interaction and post hoc analysis revealed a preference for the compartment previously paired with cocaine administration in all rats (F5,19=3.301, *P<0.05 compared with pretest score, at test 1). DAB injections into the BLA 15 min prior test 2 prevented the expression of the place preference. This effect was prolonged for up to 2 days (on tests 3 and 4, #P<0.05 compared with vehicle-treated animals, *P<0.05 compared with pretest conditions). One week after treatment, both groups expressed a clear-cut preference for the compartment previously paired with cocaine administration (*P<0.05 compared with pretest conditions, Veh, n=10; DAB, n=11).
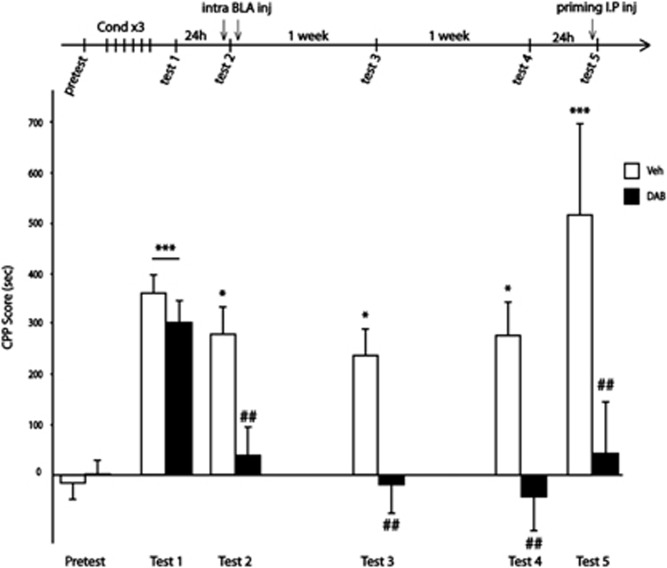
In contrast to a single injection performed 5 h after test 2 that had no effect (Supplementary Figure S6), DAB administered into the BLA 15 min prior test 2 immediately blocked place preference expression. This effect was prolonged for up to 2 days (tests 3 and 4), but 1 week after treatment, both groups expressed a marked preference for the compartment previously paired with cocaine administration (test 5). Hence, inhibition of glycogen phosphorylase immediately prior to re-exposure to the context transiently impaired the cocaine-induced CPP. One possible explanation is that each cocaine conditioning session activated glycogen metabolism, therefore blocking glycogen phosporylase prior the re-exposure trial only delayed but did not persistently disrupt the reconsolidation process. Interestingly, previous reports have established that a consolidated memory can become transiently labile and sensitive to protein synthesis inhibitors if reactivated.15, 16, 17, 18 To test the hypothesis that glycogen breakdown is necessary for reconsolidation of contextual appetitive memories, DAB was administered into the BLA before and after the re-exposure. As shown in Figure 4, two groups of rats were conditioned for 3 days. Twenty-four hours after the end of conditioning, rats were tested for CPP (test 1) and tested again 24 h later (test 2), which served as a re-exposure trial to reactivate the memory. Rats received intra-BLA administrations of vehicle or DAB just prior and 5 h after test 2. Animals were tested again twice, at 7-day intervals (tests 3 and 4), and finally, their preference for the cocaine side was challenged with a cocaine priming injection 24 h later (test 5). In line with the above-mentioned observations, DAB injections into the BLA 15 min prior to test 2 blocked place preference expression. Of critical importance, the second DAB administration into BLA extended the amnesia for up to 2 weeks (tests 3 and 4), and the indifference to cocaine compartment persisted even after a cocaine priming injection (test 5), suggesting a permanent disruption of the contextual appetitive memories. However, the same procedure replicated in rats conditioned with highly palatable chocolate flavoured food pellets failed to block the CPP (Supplementary Figure S7), suggesting that glycogen breakdown may be necessary for reconsolidation of contextual appetitive memories specific to cocaine.
Figure 4.
A double injection of 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) into the basolateral amygdala (BLA) permanently disrupt an already established cocaine-induced conditioned place preference (CPP). Experimental timeline is shown above the graphic. Data represent CPP mean score (±s.e.m.) expressed in seconds as time spent in cocaine compartment minus time spent in saline compartment. A two-way repeated-measures analysis of variance revealed a significant session × treatment interaction and post hoc analysis revealed a preference for the compartment previously paired with cocaine in all rats (F5,10=3.126, ***P<0.001 compared with pretest score, at test 1). DAB injections into the BLA 15 min prior to test 2 immediately blocked the preference for the cocaine-paired compartment compared with vehicle-treated animals. A second bilateral administration of DAB into the BLA 5 h after test 2 prolonged the effect for up to 2 weeks (on tests 3 and 4, ##P<0.01 compared with vehicle animals, *P<0.05 compared with pretest conditions). Twenty-four hours after test 4, all rats were challenged with a cocaine priming injection (15 mg kg−1 intraperitoneal (IP)). Whereas rats formerly treated with vehicle still exhibited a clear-cut preference for the compartment previously paired with cocaine administration, DAB-treated animals still did not exhibit any preference for the cocaine-paired compartment (test 5, ##P<0.01 compared with vehicle animals, ***P<0.001 compared with pretest conditions, Veh, n=11; DAB, n=11).
Importantly, disruption of cocaine memory was found to be critically dependent upon re-exposure of the conditioning context as conditioned rats injected with DAB and returned to their home cages (not in the CPP apparatus) still exhibited a strong preference for the compartment previously paired with cocaine administrations when returned in the CPP apparatus 24 h later (Supplementary Figure S8). These data strongly support the key role of glycogen breakdown in the formation of the predictive association between the context and the drug effects.
The preference for the cocaine compartment is impaired following disruption of glycogen metabolism in the BLA but is rescued by L-Lactate
To demonstrate the key role of glycogen-derived lactate in the reconsolidation of memories for drug-associated cues during cocaine CPP, we examined the effect of DAB+L-Lactate co-administration.
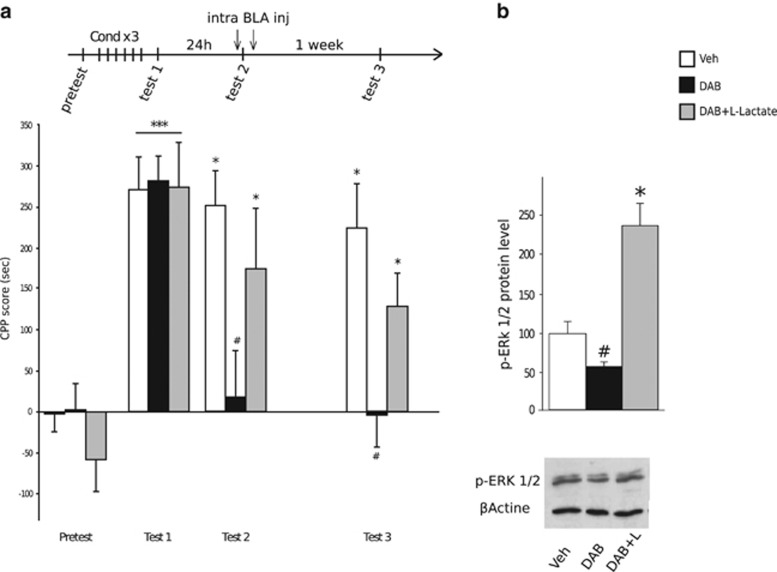
As shown in Figure 5a, three groups of rats were conditioned with cocaine for 3 days. Twenty-four hours after the end of conditioning, preference for the cocaine compartment was assessed (test 1). Twenty-four hours later, rats were administered twice into the BLA, 15 min prior test 2 and again 5 h later, with either vehicle, DAB or DAB+L-Lactate. Confirming our former observations, all rats exhibited a preference for the cocaine compartment after 1 week, with the exception of those previously treated with DAB (Figure 5a, test 3).
Figure 5.
L-Lactate co-administration abolishes the 1,4-dideoxy-1,4-imino-D-arabinitol (DAB)-induced disruption of conditioned responses to cocaine and restores the expression of p-ERK (phosphorylated extracellular signal–regulated kinase) in the basolateral amygdala (BLA). (a) Experimental timeline is shown above the graphic. Data represent conditioned place preference (CPP) mean score (±s.e.m.) expressed in seconds as time spent in cocaine compartment minus time spent in saline compartment. A two-way repeated-measures analysis of variance revealed a significant session × treatment interaction and post hoc analysis revealed a preference for the compartment previously paired with cocaine in all rats (F2,36=3.820, ***P<0.001 compared with pretest score at test 1). DAB injections into the BLA 15 min prior to test 2 immediately blocked the preference for the cocaine-paired compartment compared with vehicle-treated animals, whereas co-administration of DAB and L-Lactate permitted the expression of the cocaine-induced place preference (*P<0.05 compared with pretest conditions). A second bilateral administration of DAB into the BLA 5 h after test 2 prolonged the indifference for the cocaine compartment for up to 1 week, whereas rats that received a second (DAB+L-Lactate) administration exhibited a significant cocaine-seeking behaviour (test 3, #P<0.01 compared with vehicle animals, *P<0.05 compared with pretest conditions). (b) ERK protein phosphorylation (measured 15 min after test 3) was significantly decreased after the double administration of DAB into the BLA (#P<0.05, compared with the Veh group), while the double co-administration of (DAB+L-Lactate) restored the expression of p-ERK (*P<0.05, compared with the Veh group. Veh, n=7; DAB, n=8; DAB+L-Lactate, n=9).
As ERK activity has a key role in the long-term alterations in synaptic plasticity that result from repeated cocaine exposure,59 we examined ERK phosphorylation in the BLA after test 3 (Figure 5b). Although DAB administration did not influence the phosphorylation of ERK in naive rats (Supplementary Figure S9), our data show reduced p-ERK protein levels in cocaine-conditioned rats receiving DAB treatments compared with animals receiving vehicle or DAB+L-Lactate. This observation strongly supports that glycogen breakdown triggers ERK activation that correlates with long-lasting cocaine-induced CPP.
Discussion
Although astrocytes have long been considered to have mainly a supportive function for neurons, a growing body of evidence suggests that they fulfil other active roles, including information processing, signal transmission and regulation of neural and synaptic plasticity.46, 47, 48 In particular, a recent study revealed that lactate transfer from astrocytes into neurons was essential for the induction of the molecular changes required for long-term aversive memory formation.38 In the present study, we extend these findings by revealing that disrupting glycogen breakdown in the BLA impairs the acquisition, retrieval and long-term maintenance of positive affective memories associated with cocaine-paired cues. Importantly, we show that L-Lactate, but not L-Pyruvate, administered into the BLA rescued the preference for the cocaine compartment during conditioning. In addition, our results confirm that lactate also mediates intracellular responses for cell signalling and regulation of gene expression required for long-term positive memory formation.38, 39, 45, 60 Of particular relevance, we demonstrate that DAB-induced disruption of glycogenolysis in the BLA impairs the expression of Bdnf and Zif268, known to modulate the synaptic morphology and plasticity underlying the learning processes that strengthen conditioned responses to cocaine.61, 62 Interestingly, L-Lactate rescued the expression of Zif268 but not that of Bdnf, suggesting that lactate-mediated rescuing of cocaine-associated memories during cocaine conditioning may not depend on Bdnf in the BLA. A similar observation reported a double dissociation in the hippocampus, where Bdnf was specifically required for consolidation, whereas Zif268 was required for strengthening contextual fear conditioning.63, 64 Assuming that L-Lactate co-administration compensated the effect of DAB and permitted the stabilization of a novel memory following the first cocaine conditioning (consolidation phase), one can speculate that L-Lactate co-administration prior to the second cocaine conditioning permitted the reconsolidation phase that strengthens the association between the drug effects and the context.26, 27, 28 Hence, the preference for the cocaine compartment coincided with an increased expression of Zif268 but not of Bdnf. Analysis of Bdnf and Zif268 expression in rats treated with DAB and L-Lactate following one cocaine injection would be necessary to confirm this double dissociation assumption.64 However, one unique cocaine administration remains a weak conditioning, and the acquisition of a CPP would be uncertain. Nevertheless, our striking observation suggests that if L-Lactate, unlike L-Pyruvate, mediates intracellular responses for cell signalling and regulation of gene expression required for long-term positive memory formation, this is probably the result of increasing intracellular levels of NADH, thereby influencing the redox state of neurons and possibly redox-sensitive NMDA receptor subunits.45
In contrast to these results collected with a CPP paradigm, the conditioned responses to cocaine in rats trained for self-administering intravenous cocaine remained unaffected after bilateral administration of DAB into the BLA (Supplementary Figure S10), an observation that has already been reported using NMDA antagonism.65
The second demonstration of this study is that disruption of glycogen metabolism not only transiently impairs acquisition of cocaine-induced CPP but also persistently disrupts an established conditioning. Indeed, rats with a strong cocaine preference showed transient amnesia after inhibition of glycogen phosphorylase prior to re-exposure to the drug context. Unexpectedly, rats exhibited a spontaneous recovery a week after treatment. One possible explanation is that blocking glycogen phosphorylase prior to the re-exposure trial most likely impaired the retrieval process and thus contributed to delay but did not persistently disrupt the reconsolidation process.
To test the hypothesis that glycogenolysis is necessary for reconsolidating contextual appetitive memories, we blocked glycogen breakdown twice, prior and after the memory retrieval window. Hence, we showed a long-lasting amnesia for cocaine-associated memory after prolonged disruption of glycogen metabolism in the BLA. Furthermore, the unresponsiveness to cocaine cues persisted even after cocaine priming injection, suggesting a permanent disruption of the contextual appetitive memories.
The ERK pathway is stimulated by drugs of abuse in striatal neurons through coincident activation of dopamine D1 and glutamate NMDA receptors and is considered to be critical for drug-induced long-lasting behavioural effects.8 Further, converging evidence has shown that ERK phosphorylation and immediate-early gene expression are stimulated by drug-associated cues in the absence of drugs and have a key role in long-lasting drug-seeking behaviours.59, 66, 67 In line with these observations, we showed that not only the long-lasting indifference for the cocaine compartment following inhibition of glycogenolysis in the BLA was associated with decreased phosphorylated ERK1/2 protein levels but we also demonstrated that lactate-induced recovery of contextual appetitive memories correlated with restored levels of phosphorylated ERK1/2 in the BLA. In line with recent in vitro observations from ours45 and the demonstration that application of the NMDAR-agonist, D-cycloserine, potentiates the reconsolidation of appetitive memories,68 our data strongly support the idea that L-Lactate stimulates the neuronal expression of synaptic plasticity-related gene through a mechanism involving NMDAR activity and its downstream signalling cascade ERK1/2.45
However, upstream of the protein synthesis required for reconsolidation, there may be an initial destabilization process, named deconsolidation,69 which most likely requires NR2B-containing NMDA receptors49, 50, 51 and protein degradation.70 Hence, further studies are required to assess whether lactate may supply activated neurons with NADH, stimulating redox-sensitive NMDAR subunits possibly triggering destabilization of appetitive memories. Lactate may also supply activated neurons with sufficient energy required for synaptic protein degradation. In a second step, lactate may also contribute to the subsequent phase of the plasticity process by providing sufficient energy required for activation of signalling pathways and protein synthesis underlying long-term memory formation.
Overall, we show that glycogenolysis-dependent lactate release is essential for mediating intracellular responses underlying long-term regulation of gene expression required for cocaine-induced long-lasting behavioural effects. Our results confirm the importance of astrocyte–neuron metabolic interactions in cognitive functions and, for the first time, demonstrate the key role of the astrocyte–neuron metabolic coupling in positive affective memory storage and retrieval. These findings open novel therapeutic avenues to reduce the long-lasting impact of drug cues on conditioned responses to cocaine.
Acknowledgments
The financial support of the NCCR Synapsy and the Préfargier Foundation is gratefully acknowledged.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry 2005; 162: 1414–1422. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann NY Acad Sci 1992; 654: 400–415. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr 1988; 84: 25–43. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 1981; 75: 134–143. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 1996; 7: 754–763. [PubMed] [Google Scholar]
- Weiss F. Advances in Animal Models of Relapse for Addiction Research. In: Kuhn CM, Koob GF (eds). Advances in the Neuroscience of Addiction (2nd edn), 2010. [PubMed]
- Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 2014; 35: 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2001; 2: 695–703. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 2004; 44: 161–179. [DOI] [PubMed] [Google Scholar]
- Landauer TK. Reinforcement as consolidation. Psychol Rev 1969; 76: 82–96. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem 2002; 78: 637–647. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 2002; 78: 625–636. [DOI] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Ann Rev Neurosci 1996; 19: 405–436. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull 1984; 96: 518–559. [PubMed] [Google Scholar]
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 2005; 28: 51–56. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron 2004; 44: 93–100. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000; 406: 722–726. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 2000; 7: 73–84. [DOI] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev 2012; 36: 1400–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol 2006; 16: 174–178. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Nadel L. Episodic memory reconsolidation: updating or source confusion? Memory 2009; 17: 502–510. [DOI] [PubMed] [Google Scholar]
- Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci 2009; 32: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 2008; 42: 503–506. [DOI] [PubMed] [Google Scholar]
- Fan HY, Cherng CG, Yang FY, Cheng LY, Tsai CJ, Lin LC et al. Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res 2010; 208: 522–527. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 2009; 12: 256–258. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 2006; 26: 5881–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 2005; 47: 795–801. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM. Persistent disruption of an established morphine conditioned place preference. J Neurosci 2006; 26: 3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Effects of anisomycin on consolidation and reconsolidation of a morphine-conditioned place preference. Behav Brain Res 2007; 178: 146–153. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biol Psychiatry 2009; 65: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 2006; 103: 2932–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 2012; 336: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol 2006; 209: 2304–2311. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci 1996; 16: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, O'Dowd BS, Hertz E, Hertz L. Astrocytic energy metabolism consolidates memory in young chicks. Neuroscience 2006; 141: 9–13. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 2006; 54: 214–222. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One 2011; 6: e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011; 144: 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 2011; 14: 724–738. [DOI] [PubMed] [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J Neurochem 2004; 89: 537–552. [DOI] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int 2004; 45: 529–536. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 1993; 623: 208–214. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science 1999; 283: 496–497. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 1994; 91: 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci USA 2014; 111: 12228–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 2010; 72: 335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature 2010; 463: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009; 32: 421–431. [DOI] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 2006; 9: 1237–1239. [DOI] [PubMed] [Google Scholar]
- Finnie PS, Nader K. The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation. Neurosci Biobehav Rev 2012; 36: 1667–1707. [DOI] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci 2009; 12: 905–912. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 2009; 10: 224–234. [DOI] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ. Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem 2008; 90: 147–154. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 2008; 28: 8230–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci 2001; 21: 5089–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998; 20: 709–726. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci 2008; 28: 11760–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaghband Y, O'Dell SJ, Azarnia S, Khalaj AJ, Guzowski JF, Marshall JF. Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol Learn Mem 2014; 116: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci 2006; 29: 695–703. [DOI] [PubMed] [Google Scholar]
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 2011; 34: 76–87. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 2007; 8: 262–275. [DOI] [PubMed] [Google Scholar]
- Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P et al. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci 2006; 26: 4956–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 2004; 304: 839–843. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci 2008; 11: 1264–1266. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem 2008; 15: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 2005; 47: 873–884. [DOI] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM et al. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology 2013; 38: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Gardner RJ, Butler VJ, Everitt BJ. D-cycloserine potentiates the reconsolidation of cocaine-associated memories. Learn Mem 2009; 16: 82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ledoux JE. Memory reconsolidation. Curr Biol 2013; 23: R746–R750. [DOI] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 2008; 319: 1253–1256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.