Abstract
Summary
This 3-year longitudinal study among older adults showed that declining muscle mass, strength, power, and physical performance are independent contributing factors to increased fear of falling, while declines of muscle mass and physical performance contribute to deterioration of quality of life. Our findings reinforce the importance of preserving muscle health with advancing age.
Introduction
The age-associated loss of skeletal muscle quantity and function are critical determinants of independent physical functioning in later life. Longitudinal studies investigating how decrements in muscle components of sarcopenia impact fear of falling (FoF) and quality of life (QoL) in older adults are lacking.
Methods
Twenty-six healthy older subjects (age, 74.1±3.7; Short Physical Performance Battery (SPPB) score ≥10) and 22 mobility-limited older subjects (age, 77.2±4.4; SPPB score ≥9) underwent evaluations of lower extremity muscle size and composition by computed tomography, strength and power, and physical performance at baseline and after 3-year follow-up. The Falls Efficacy Scale (FES) and Short Form-36 questionnaire (SF-36) were also administered at both timepoints to assess FoF and QoL, respectively.
Results
At 3-year follow-up, muscle cross-sectional area (CSA) (p<0.013) and power decreased (p<0.001), while intermuscular fat infiltration increased (p<0.001). These decrements were accompanied with a longer time to complete 400 m by 22±46 s (p<0.002). Using linear mixed-effects regression models, declines of muscle CSA, strength and power, and SPPB score were associated with increased FES score (p<0.05 for each model). Reduced physical component summary score of SF-36 over follow-up was independently associated with decreased SPPB score (p<0.020), muscle CSA (p<0.046), and increased 400 m walk time (p<0.003).
Conclusions
In older adults with and without mobility limitations, declining muscle mass, strength, power, and physical performance contribute independently to increase FoF, while declines of muscle mass and physical performance contribute to deterioration of QoL. These findings provide further rationale for developing interventions to improve aging muscle health.
Keywords: Aging, Fear of falling, Muscle, Physical functioning, Quality of life, Sarcopenia
Introduction
By 2050, the US average life expectancy will reach 83.8 years and the population of Americans aged 65 and over is projected to more than double, from 43 to 87 million. The continued increase in longevity may be viewed as one of society’s greatest achievements, but to preserve older adults’ independence and quality of life (QoL) during those years gained remain major clinical and public health challenges.
The aging process is accompanied by a progressive loss of skeletal muscle mass and function, referred to as sarcopenia. An increase in intermuscular adipose tissue is also a typical manifestation of aging [1]. Although consensus definition and diagnosis criteria have not been reached, the bi-dimensional nature of sarcopenia is increasingly accepted, encompassing both the quantitative (muscle mass) and qualitative (muscle function) declines of skeletal muscle, and characterized by a loss of muscle mass, strength/power, and physical performance [2, 3].
Skeletal muscle dysfunction is a debilitating condition leading to—but not limited to—outright disability and mortality [4]. In particular, lower extremity muscle mass, strength, power, and physical performance are critical determinants of independent functioning in later life, a central tenet of QoL [1, 4–6]. It is thus both intuitive and widely accepted that sarcopenia may be detrimental to QoL, but evidence of a relationship between the two is scarce and drawn from cross-sectional analyses [7, 8]. To our knowledge, studies have failed so far to reveal the independent contribution of age-associated changes in muscle components of sarcopenia—including muscle mass, strength, power, and physical performance—to deterioration of QoL [2].
Another major potential threat to independent functioning in old age is fear of falling (FoF), a persistent feeling related to the risk of falling not necessarily preceded by a fall [9, 10]. Emerging evidence supports that FoF might precipitate a downward spiral of loss of confidence, restriction of activities, social withdrawal, which may ultimately lead to loss of independence [11, 12]. Cross-sectional studies have highlighted mobility impairments as contributing factors to FoF, but it remains currently largely unknown whether longitudinal age-associated changes in muscle components of sarcopenia contribute to FoF [10, 13].
In this paper, we specifically investigated the longitudinal relationships between muscle mass, strength, power, and physical performance, and FoF and QoL in older adults, using data from a recently published longitudinal study examining the physiological determinants of the loss of muscle power in healthy and mobility-limited elders [14]. Because decreased QoL was previously shown to be a consequence of FoF [12, 15–17], we also investigated the association between the two and the extent to which FoF mediates the relationship found between muscle components of sarcopenia and QoL. We hypothesized that (a) declining muscle mass, strength, power, and physical performance would contribute independently to increase FoF and deterioration of QoL over 3 years, and that (b) FoF would partially mediate the relationship between muscle components of sarcopenia and QoL. Better knowledge of the potentially modifiable factors that are independently associated with FoF and QoL in the elderly would help to develop and refine effective early-targeted interventions.
Materials and methods
Study design
Data came from a 3-year, prospective, single-centre, observational longitudinal study [14, 18]. This study was approved by the Tufts University Health Sciences Institutional Review Board and each subject provided written informed consent.
Study subjects
Participants were recruited at baseline between 2006 and 2008 from the Greater Boston area and have been previously described [14, 18]. Two groups of community-dwelling older adults aged 70–85 years (mean±SD age, 75.5±4.3 years), assessed twice over a 3-year interval (35.0±6.4 months), were enrolled: (1) a healthy group of subjects which scored ≥10 on the Short Physical Performance Battery (SPPB), who took no medications (N=26; 14 males); (2) an older mobility-limited group of subjects with a SPPB score ≥9, indicative of significant mobility-deficits and an increased risk for developing disability (N=22; 10 males) [19–21]. These two distinct groups were originally constituted to test the hypothesis that leg extensor muscle power would be significantly lower in mobility-limited elders as compared to healthy counterparts and to characterize the physiological mechanisms associated with the reduction of muscle power with aging.
Lower extremity performance tests
Physical performance was evaluated using the SPPB, 400 m walk and stair climb tests. The SPPB evaluates lower extremity function by measuring three domains of physical function: usual gait speed, maintenance of standing balance, and lower extremity strength. The corresponding tasks include walking at usual speed over 4 m, three static positions with decreasing base of support to challenge balance, and the ability to rise from a chair without the use of the arms five times consecutively. Result from each test is ranked using a 0–4 scale and the composite score is summed, with the higher score reflecting a higher level of function [19–21]. Performance on the SPPB 4 m and chair stand tests were also examined separately. In the 400-m walk test, the subjects were asked to walk at a self-paced speed on a 20-m course for 10 laps, without sitting, the use of an assistive device, or the help of another person [22]. The total time was recorded with a stopwatch. Stair climb time was assessed using a standard riser of stairs as described previously [23]. The subjects were timed using a stopwatch ascending a 10 rise set of stairs as quickly as possible, using the railing as necessary. The average of two attempts was recorded.
Lower extremity muscle strength and power measurements
The detailed procedures were described previously [14, 18, 24]. In summary, lower extremity muscle strength and power measurements were performed using pneumatic bilateral leg press equipment (Leg Press A420, Keiser Corporation, Fresno, CA). Participants were seated on the leg press apparatus with knees flexed to 90° and hips flexed to approximately 110°. Knee angle was measured using an electrogoniometer (ADInstruments, Colorado Springs, CO). Leg extensor muscle strength was assessed using the one-repetition maximum (1RM) protocol and was defined as the maximum load that could be moved only once throughout the full range of motion (ROM) while maintaining proper form [24]. Following several minimally loaded repetitions to establish the subject’s ROM, the assessor progressively increased the resistance after each successful repetition until the subject could no longer move the lever arm one time through their full ROM, optimally within six to eight repetitions [18]. After the measure of 1RM, leg extensor peak muscle power was assessed. Performance of the multiple attempt peak power test has been previously described and validated [24]. Five repetitions, performed as quickly as possible and separated by 30 s of rest, were performed at 40 and 70 % of the 1RM. The highest measured power output was recorded as the leg extensor peak power. The intraclass correlation coefficients (ICCs) for repeated testing of 1RM and peak power were 0.97 and 0.89, respectively [24].
Lower extremity muscle composition measurement
As detailed previously [14, 18], computed tomography (CT) scans of the non-dominant thigh performed at the midpoint of the femur were obtained from each subject (Somatom Scanner, Siemens Medical Systems, Erlangen, Germany). The cross-sectional areas (CSAs) for normal density muscle (35–100 Houndsfield units [HU]), lipid-rich skeletal muscle, namely low-density muscle (0–34 HU), and intermuscular adipose tissue were measured using manual tracing. Total muscle CSA (0–100 HU) was calculated as the sum of low-density muscle and normal density muscle CSA. Test-retest reliability for repeated CT scan analyses has been previously demonstrated as excellent, with ICCs above 0.99 for CSA measures [25].
Total muscle CSA was used to estimate muscle-specific strength, the ratio of 1RM to total muscle CSA [18]. Subjects were characterized as having low relative muscle mass if CSA/body weight (BW) was <1 standard deviation below the mean of the CSA/BW distribution of a sex-specific young reference group aged ≥55 years [18, 26].
QoF, FoF, and other outcomes
QoL was assessed using the Short Form-36 self-administered questionnaire (SF-36) [27, 28], a generic measure of health status with well-documented psychometric properties, comprising 36 questions related to eight dimensions of QoL: limitations in physical activities because of health problems; limitations in social activities because of physical or emotional problems; limitations in usual role activities because of physical health problems; bodily pain; general mental health; limitations in usual role activities because of emotional problems; vitality; and general health perceptions. The questions are transformed into a point scale ranging from 0 (worst) to 100 (best). The scores on each of the eight domains can be combined to obtain two aggregate scores: the physical component summary (PCS) score and the mental component summary scale score. The PCS score was retained as a measure of physical QoL.
FoF and depression were assessed by interview. FoF was measured by a modified ten-item version of the Fall Efficacy Scale (FES) [29, 30]. The FES is a questionnaire evaluating the level of fear or concern about falls for a range of activities of daily living containing 10 items scored from 1 (not at all concerned) to 4 (very concerned). Depression was evaluated using the Center for Epidemiologic Studies Depression Scale (CES-D) [31].
Other measures included demographic information, self-reported prescribed medications, and all self-reported diagnosed health conditions collected during interviews at each visit.
Statistical analysis
Descriptive statistics were computed as mean±standard deviation (SD) for continuous variables or as number and percentages for categorical variables. Normality was verified by using Shapiro–Francia tests. When the variables were not normally distributed, descriptive statistics was given as the median and interquartile range (IQR).
Imbalance between groups at baseline was examined using t tests or Wilcoxon rank sum tests for continuous data, and χ2 or Fisher’s exact tests for categorical data. Analysis of variance (ANOVA) was also used to adjust between-group comparisons for age. In addition, linear regression models were used to explore baseline associations between the number of medications, psychotropic medication use, and FoF and QoL.
The longitudinal association between outcome measures (first set of models, FES score; second set of models, PCS score of SF-36) over the 3-year follow-up period, in the population as a whole, was estimated by linear mixed-effects regression models, fitted using Stata’s “xtmixed” procedure. Each of the independent variables (i.e., muscle size, composition, strength, power, and physical performance variables) was included individually, along with subject as a random effect, and with group (mobility-limited or not), time, gender, and CES-D depression score entered into the models. Linear mixed-effects regression assess the slope of the dependent variable between both values (baseline and 3-year) which represents the change over time while taking into account the evolution of each individuals. Each independent variables and CES-D depression score were measured twice and both values were present in the models. Additional analyses were carried out by including medications, diagnosis, and BMI, all measured twice, in the models.
A third set of models predicted the change in physical component summary score of SF-36, with the same predictors listed above but also with FES score entered into the models.
A two-sided p value less than or equal to 0.05 was considered to indicate statistical significance. Data were analyzed using Stata version 12.1 (Stata Corp., College Station, TX) statistical software.
Results
Baseline examination
Subject characteristics are presented in Table 1 by group and gender. Mobility-limited subjects were significantly older as compared to healthy subjects. Mobility-limited participants also demonstrated lower performance on all physical function tests and significantly lower muscle strength and power values. The observed differences in physical performance, including in SPPB, remained statistically significant after controlling for age. Leg extensor 1RM strength and peak power were lower in mobility-limited older men as compared with healthy older men; in women, differences were not significant. The results of the baseline CT examination are also reported in the Table 1. Sixty-four percent of the population was classified as having low relative muscle mass according to the sarcopenic index at baseline, including 17 (81 %) mobility-limited subjects. Mobility-limited subjects had significantly higher levels of intermuscular adipose tissue CSA compared with healthy subjects, but total muscle CSA did not differ between groups. Males exhibited higher total muscle CSA values as compared with females, but the intermuscular adipose tissue CSA was not significantly different. Mobility-limited females had significantly higher levels of intermuscular adipose tissue compared with healthy females, while mobility-limited men had significantly lower levels of total muscle CSA compared with healthy counterparts.
Table 1.
Baseline characteristics of the study population by group and gender
| Healthy older
|
Mobility-limited older
|
|||
|---|---|---|---|---|
| N=26 | N=22 | |||
| Male | Female | Male | Female | |
| N=14 | N=12 | N=10 | N=12a | |
| Age (years) | 73.9±3.6 | 74.2±3.9 | 78.5±3.8* | 76.1±4.7‡ |
| BMI (kg/m2) | 26.3±3.0 | 24.1±4.4 | 27.2±2.6 | 26.7±4.0 |
| MMSE (score) | 29.3±0.8 | 29.2±0.8 | 28.7±1.5 | 28.9±0.9 |
| Number of medications | – | – | 3.6±2.5 | 2.1±2.2 |
| Number of diagnoses | – | – | 2.7±1.8 | 1.8±1.9 |
| Gait speed (m/s) | 1.2±0.3 | 1.2±0.1 | 0.8±0.2* | 0.9±0.1*‡ |
| Chair stand (s) | 10.9±2.1 | 12.0±2.4 | 15.7±3.5* | 16.1±4.5*‡ |
| SPPB (score) | 11.3±1.2 | 10.8±0.9 | 7.8±1.2* | 7.9±1.4*‡ |
| 400 m (s) | 292.0±38.1 | 309.4±38.7 | 395.7±57.2* | 364.4±47.5*‡ |
| Stair climb (s) | 5.1±0.7 | 5.8±0.7† | 7.6±1.8* | 7.7±2.8‡ |
| 1RM strength (N) | 1629±200 | 869±211† | 1275±394* | 921±194†‡+ |
| Peak power (W) 40 % 1RM | 612±114 | 264±73† | 329±109* | 200(82) †‡+ |
| Peak power (W) 70 % 1RM | 656±140 | 256±71† | 358±110* | 230±88†‡+ |
| Total muscle CSA (cm2) | 131±13 | 82±10† | 110±14* | 82±21†+ |
| Total intermuscular adipose tissue CSA (cm2) | 2.8(1.8) | 2.2±1.6 | 4.8±2.6 | 4.0±1.7*‡ |
| Specific strength (N/cm2) | 12.5±1.6 | 10.5±2.2 | 11.5±2.5 | 11.2±1.2+ |
Results are reported as mean±standard deviation or median (interquartile range)
BMI body mass index, MMSE mini-mental state examination, SPPB Short Physical Performance Battery, 40 % 1RM 40 % of a one-repetition maximum, 70 % 1RM 70 % of a one-repetition maximum, 1RM one-repetition maximum, CSA cross-sectional area
p<0.05 (mobility-limited older as compared to healthy older by gender);
p<0.05 (male vs female within group);
p<0.05 (mobility-limited older as compared to healthy older group);
p<0.05 (male vs female)
Only 11 subjects with CT scan available
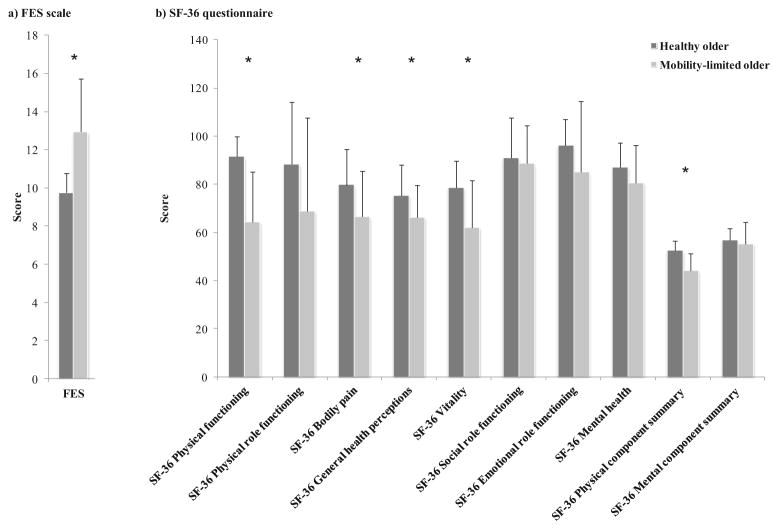
The level of FoF evaluated by the FES score was higher in mobility-limited subjects as compared with healthy elders (13 ±3 vs 10±1, p<0.0001). Several scores for domains of the SF-36 related to physical status and the PCS score were significantly lower in mobility-limited subjects compared to healthy subjects (Fig. 1). Linear regression models showed that individuals with higher number of medication had reduced quality of life and increased fear of falling (p<0.001 and p<0.03, respectively), while individuals taking psychotropic medication had reduced quality of life, only (p<0.001).
Fig. 1.
Baseline characteristics: FES and SF-36 scores by group. Mobility-limited subjects displayed higher FES score than healthy subjects, and lower scores for several domains of the SF-36 related to physical status. a FES scale; b SF-36 questionnaire\FES = Fall Efficacy Scale; SF-36 = Short Form 36 questionnaire. *p<0.05, mobility-limited older (N=22) as compared to healthy older (N=26)
Prospective follow-up examination
Longitudinal changes by group had been described previously in detail [14]. Considering the population as a whole, we observed at follow-up a significant increase in the time to complete 400 m by 22±46 s (p<0.002), corresponding to a decrease in gait speed by 5.8 % (p<0.005). The decline by 2 % in 1RM strength did not reach statistical significance. Conversely, muscle power at 40 and 70 % of the 1RM decreased significantly by 16 and 9 %, respectively (p<0.05). Total intermuscular adipose tissue depots increased (p<0.001) while total muscle CSA decreased by 3 % (p<0.013). No significant changes in specific strength or BMI were observed (p>0.05).
Finally, FES score increased significantly from 11±3 to 14 ±5 (p<0.0001). The increase tended to predominate in the mobility-limited group (p<0.09). The decrease of PCS score of SF-36 from 49±7 to 47±9 failed to reach statistical significance.
In the first set of linear mixed-effects regression models, changes in muscle strength, power, and CSA were inversely associated with change in FES score (p<0.05 for each model, with group, gender, CES-D depression score, and time entered into the model) (Table 2). A significant association between change in SPPB score and change in FES score was also found (p<0.035). In the second set of models, reduced PCS score of SF-36 over follow-up was significantly determined by decreasing SPPB score (p<0.020), muscle CSA (p<0.046) and increasing 400 m walk time (p<0.003) (Table 3). All associations remained significant after additional adjustment for other potential confounders including medications, diagnosis, and BMI, except for the association between SPPB and total muscle CSA, and PCS score which just failed to reach statistical significance when adjusting for medications (p= 0.064 and p=0.057, respectively) (data not shown).
Table 2.
Association between muscle size, composition, strength, power and physical performances, and fear of falling in the whole population (N=48)
| Fall Efficacy Scale score | Adjusted coefficient | Standard error | [95 % confidence interval | p>z unadjusted | p>z adjusted |
|---|---|---|---|---|---|
| 1RM strength (N) | −0.003 | 0.001 | [−0.005; −0.0008] | 0.021 | 0.008 |
| Peak power (W) 40 % 1RM | −0.006 | 0.003 | [−0.011; −0.001] | 0.270 | 0.022 |
| Peak power (W) 70 % 1RM | −0.004 | 0.002 | [−0.009; 0.0006] | 0.326 | 0.086 |
| Specific strength (N/cm2) | −0.194 | 0.149 | [−0.485; 0.097] | 0.158 | 0.192 |
| SPPB (score) | −0.428 | 0.203 | [−0.825; −0.030] | 0.0001 | 0.035 |
| Gait speed (m/s) | −1.109 | 1.585 | [−4.215; 1.999] | 0.002 | 0.484 |
| Chair stand (s) | 0.016 | 0.102 | [−0.183; 0.216] | 0.261 | 0.872 |
| 400 m (s) | 0.007 | 0.005 | [−0.004; 0.018] | 0.0001 | 0.190 |
| Total muscle CSA (cm2) | −0.050 | 0.022 | [−0.094; −0.007] | 0.029 | 0.024 |
| Total intermuscular adipose tissue CSA (cm2) | −0.104 | 0.152 | [−0.403; 0.194] | 0.367 | 0.493 |
For each variable, change of Fall Efficacy Scale score over the follow-up was assessed using linear mixed-effects regression models, with subject included as random effect, and with group (mobility-limited or not), time, gender, and CES-D depression score entered into the model
1RM one-repetition maximum, 40 % 1RM 40 % of a one-repetition maximum, 70 % 1RM 70 % of a one-repetition maximum, SPPB short physical performance battery, CSA cross-sectional area
Table 3.
Association between muscle size, composition, strength, power and physical performances, and quality of life in the whole population (N=48)
| Physical component summary score of SF-36 | Adjusted coefficient | Standard error | [95 % confidence interval] | p>z unadjusted | p>z adjusted |
|---|---|---|---|---|---|
| 1RM strength | 0.004 | 0.002 | [−0.0004; 0.009] | 0.080 | 0.076 |
| Peak power (W) 40 % 1RM | 0.006 | 0.006 | [−0.006; 0.017] | 0.223 | 0.346 |
| Peak power (W) 70 % 1RM | 0.006 | 0.005 | [−0.004; 0.017] | 0.244 | 0.235 |
| Specific strength (N/cm2) | 0.411 | 0.315 | [−0.207; 1.029] | 0.213 | 0.192 |
| SPPB (score) | 1.031 | 0.445 | [0.159; 1.903] | 0.0001 | 0.020 |
| Gait speed (m/s) | 4.496 | 3.488 | [−2.340; 11.331] | 0.0001 | 0.197 |
| Chair stand (s) | −0.010 | 0.218 | [−0.438; 0.418] | 0.189 | 0.965 |
| 400 m (s) | −0.037 | 0.012 | [−0.061; −0.012] | 0.0001 | 0.003 |
| Total muscle CSA (cm2) | 0.102 | 0.051 | [0.002; 0.201] | 0.031 | 0.046 |
| Total intermuscular adipose tissue CSA (cm2) | 0.206 | 0.348 | [−0.477; 0.889] | 0.451 | 0.555 |
For each variable, change of physical component summary score over the follow-up was assessed using linear mixed-effects regression models, with subject included as random effect, and with group (mobility-limited or not), time, gender, and CES-D depression score entered into the model
SF-36 Short Form-36 questionnaire, 1RM one-repetition maximum, 40 % 1RM 40 % of a one-repetition maximum, 70 % 1RM 70 % of a one-repetition maximum, SPPB Short Physical Performance Battery, CSA cross-sectional area
A significant association between change in FES score and change in PCS score was observed (p<0.0001, data not shown). Addition of FES score to the models (third set of models) attenuated, but did not eliminate, all associations seen with PCS scores of SF-36 (400 m walk; β=−0.031; p<0.010) (data not shown).
Discussion
In the present study, we examined the association between muscle mass, strength and power, physical performance, FoF, and QoL over a 3-year follow-up period in older adults. We are unaware of any study that has comprehensively investigated these prospective relationships. Our results revealed that decreasing muscle mass and physical performance were independently associated with declining physical QoL. We also observed independent associations between decreased muscle mass, strength, power, and physical performance, and increased FoF. These findings underline the importance of preserving skeletal muscle health with advancing age and may provide a framework for future interventions in this population. By targeting the distinct muscle components of sarcopenia, therapeutic and preventive interventions have the potential to reduce FoF and improve QoL in older adults. There is increasing evidence that muscle components of sarcopenia are potentially modifiable factors, including through progressive or high-velocity resistance training and with potential additional benefits of nutritional interventions.
Association between muscle components of sarcopenia and FoF
Our finding of an independent association between decreased muscle strength and increased FoF is consistent with the sparse data available in the literature showing that lower extremity strength of older adults fearful of falling is lower than that of non-fearful older individuals [17]. Delbaere et al. [32] also revealed that quadriceps strength independently contributed to explain perceived fall risk in community-dwelling older people.
The association found between decreasing performance on the SPPB and increasing FoF extends those of previous investigations highlighting the relationship of mobility impairments to FoF [9, 13], including a recent study that found that lower performance on the SPPB (and the individual SPPB components) were related to FoF [33]. Nevertheless, of the five physical performance tests examined in our study, only change in SPPB score was associated with FoF; decreased gait speed and 400 m walk performances were associated with increased FoF in unadjusted analysis but did not retain a statistically significance after adjustment. Despite the finding of Delbaere et al.[34] that FoF may not be associated with gait speed in normal or non-challenging conditions in community-dwelling older adults, our results contradict those of cross-sectional or prospective studies which found that poorer performance on chair stand test or lower gait speed was associated with increased FoF [10, 17, 35–37]. Difference in populations, physical assessment methods, or constructs used to measure FoF (e.g., dichotomous response to a single question) may partly contribute to this discrepancy. Also, although gait speed is widely used as a proxy for muscle performance, it may integrate disturbances in numerous organ systems (e.g., cognition and neural control); this was not taken into account in the present analyses.
Regarding the lack of data looking at the relationship between muscle mass, power, and FoF, study results are important and relevant from a clinical perspective. The underlying mechanisms for the associations between muscle quantity and function and FoF remain unclear and of potential interest for further studies. In particular, FoF may arise at various levels of the disablement process [5] and/or exacerbate this pathway, but it is likely that functional limitations and disability represent mediating conditions between muscle pathology (e.g., reduced muscle CSA) or impairments (e.g., reduced muscle power) and FoF.
Of note, the associations observed with FoF may have been driven by unmeasured potential confounders, especially falls or reduced physical activity level. Also, because mobility impairments (e.g., including gait impairments) have been identified both as predictors and consequences of FoF, a bidirectional causal relationship between muscle impairments and FoF cannot be excluded. Unfortunately, the current data set precluded confident analyses to fully delineate the dynamic and temporal nature of the relationships between components of muscle decline and FoF.
Association between components of sarcopenia and QoL
While few studies evaluated QoL in populations of older community-dwelling individuals with a diagnosis of sarcopenia, all failed to investigate the independent contribution of declines in muscle quantity and function parameters to decreased QoL [2]. In the current study, changes in muscle mass and physical performance (i.e., SPPB and 400 m tests) represented independent determinants of change of PCS score of SF-36 over follow-up.
Our finding of a relationship between physical performance and QoL is consistent with previous reports, including Groessl et al. study [38] showing that 400 m walk time is a strong correlate of QoL in older adults at risk for disability, independent of a comorbidity index. An abnormal SPPB score was also recently associated with lower QoL in older adults [37]. Whether higher physical performances bring improvements in QoL requires further studies in large trials.
We failed to find a significant association between change in muscle strength and change in physical QoL. This is in agreement with a previous study highlighting that isometric hip and abductor strengths were significantly correlated with PCS score of SF-36 in older adults but not knee extensor strength [39]. In contrast, a large cross-sectional study conducted by Sayer et al. [40] among community-dwelling older adults showed a relationship between muscle strength and physical domains of SF-36, but using grip strength as an indicator of muscle strength. Our study, by its longitudinal design, is better suited to understand the independent association between muscle strength and impaired QoL.
No study to date has examined the association between muscle power and QoL. Although previous research indicates that high-velocity power training may influence multiple levels of quality of life [41], we failed to find any independent association. Also, no association between intermuscular adipose tissue and QoL, nor FoF, reached statistical significance. In a recent cross-sectional investigation in ageing men, SF-36 scores on several physical domains were inversely associated with subcutaneous adipose tissue (measured by magnetic resonance imaging) [42]. Caution should be taken when interpreting the findings because the limited sample size and follow-up duration, and the mixed gender cohort of the present study precludes definitive evaluation.
Finally, the association found between FoF and physical QoL agrees with and extends data from previous studies showing the detrimental impact of FoF on QoL [12, 15–17]. Adjustment for FoF attenuated but did not eliminate all associations seen with QoL, suggesting that FoF may only partially mediate the relationship found between muscle mass and physical performances, and QoL.
The most prominent strengths of the present study were its longitudinal design and the use of well validated and robust assessment tools to capture several markers of muscle quantity and function in older adults with distinct aging phenotypes. However, several limitations need to be acknowledged. At first, as noted above, the relatively small sample size may have hampered our ability to detect subtle associations between outcomes and prevented us to further assess whether there were sex-specific associations. Second, we were unable to examine the influence of a number of potential moderators or confounding factors, such as fall history, physical activity level, or nutritional intake, because these data were not prospectively collected. In addition, medications and diagnoses data were self-reported and not verified by medical records, and types of medication were not available at 3-year visit for all subjects. Third, self-reported measures of FoF and QoL are prone to reporting bias, and FES and SF-36 instruments are not without weakness (e.g., ceiling effect), although they still remain “broad-use” generic instruments. SF-36 has gained widespread acceptance and has been recently suggested to serve as a generic core for sarcopenia studies [2]. Fourth, the follow-up period was only 3 years. The clinical relevance of the observed associations might increase over the long term. Finally, this study involved community-dwelling volunteer subjects and excluded non-completers from the analyses. Therefore, it is possible that our overall findings were influenced by the nature of the sample; generalization of the results should be done with caution.
In summary, this is the first comprehensive longitudinal investigation of the relationship between age-associated changes in skeletal muscle quantity and function parameters, and FoF and QoL in older adults. The results suggest that declining muscle mass, strength, power, and physical performance are independent contributing factors to increase FoF, while declines of muscle mass and physical performance contribute to deterioration of QoL. These findings suggest that therapeutic and preventive approaches targeting these distinct factors may have the potential to counteract the onset or alleviate FoF and its ensuing downward spiral, at least to some extent, and overall enhance QoL in older population.
Acknowledgments
This work was supported by the National Institute on Aging grant AG18844 and based upon work supported by the US Department of Agriculture (58-1950-0-014), the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679), and the Boston Rehabilitation Outcomes Center, funded by National Institutes of Health infrastructure grant (1R24HD065688-01A1). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflicts of interest Andrea Trombetti, Kieran F. Reid, Mélany Hars, François R. Herrmann, Evan Pasha, Edward M. Phillips and Roger A. Fielding declare that they have no conflict of interest.
References
- 1.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dam TT, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J Gerontol A Biol Sci Med Sci. 2014;69:584–590. doi: 10.1093/gerona/glu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, Waite LM, Seibel MJ, Sambrook PN. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 7.Go SW, Cha YH, Lee JA, Park HS. Association between sarcopenia, bone density, and health-related quality of life in Korean men. Korean J Fam Med. 2013;34:281–288. doi: 10.4082/kjfm.2013.34.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kull M, Kallikorm R, Lember M. Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in Northern Europe. J Clin Densitom. 2012;15:32–38. doi: 10.1016/j.jocd.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing. 2008;37:19–24. doi: 10.1093/ageing/afm169. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande N, Metter EJ, Bandinelli S, Lauretani F, Windham BG, Ferrucci L. Psychological, physical, and sensory correlates of fear of falling and consequent activity restriction in the elderly: the InCHIANTI study. Am J Phys Med Rehabil. 2008;87:354–362. doi: 10.1097/PHM.0b013e31815e6e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vellas B, Cayla F, Bocquet H, de Pemille F, Albarede JL. Prospective study of restriction of activity in old people after falls. Age Ageing. 1987;16:189–193. doi: 10.1093/ageing/16.3.189. [DOI] [PubMed] [Google Scholar]
- 12.Cumming RG, Salkeld G, Thomas M, Szonyi G. Prospective study of the impact of fear of falling on activities of daily living, SF-36 scores, and nursing home admission. J Gerontol A Biol Sci Med Sci. 2000;55:M299–M305. doi: 10.1093/gerona/55.5.m299. [DOI] [PubMed] [Google Scholar]
- 13.Denkinger MD, Lukas A, Nikolaus T, Hauer K. Factors associated with fear of falling and associated activity restriction in community-dwelling older adults: a systematic review. Am J Geriatr Psychiatr. 2014;23:72–86. doi: 10.1016/j.jagp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114:29–39. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillemin F, Martinez L, Calvert M, et al. Fear of falling, fracture history, and comorbidities are associated with health-related quality of life among European and US women with osteoporosis in a large international study. Osteoporos Int. 2013;24:3001–3010. doi: 10.1007/s00198-013-2408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang NT, Chi LY, Yang NP, Chou P. The impact of falls and fear of falling on health-related quality of life in Taiwanese elderly. J Community Health Nurs. 2010;27:84–95. doi: 10.1080/07370011003704958. [DOI] [PubMed] [Google Scholar]
- 17.Brouwer B, Musselman K, Culham E. Physical function and health status among seniors with and without a fear of falling. Gerontology. 2004;50:135–141. doi: 10.1159/000076771. [DOI] [PubMed] [Google Scholar]
- 18.Reid KF, Doros G, Clark DJ, Patten C, Carabello RJ, Cloutier GJ, Phillips EM, Krivickas LS, Frontera WR, Fielding RA. Muscle power failure in mobility-limited older adults: preserved single fiber function despite lower whole muscle size, quality and rate of neuromuscular activation. Eur J Appl Physiol. 2012;112:2289–2301. doi: 10.1007/s00421-011-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Am Geriatr Soc. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 22.Sayers SP, Guralnik JM, Thombs LA, Fielding RA. Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc. 2005;53:467–471. doi: 10.1111/j.1532-5415.2005.53166.x. [DOI] [PubMed] [Google Scholar]
- 23.Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 24.Callahan D, Phillips E, Carabello R, Frontera WR, Fielding RA. Assessment of lower extremity muscle power in functionally-limited elders. Aging Clin Exp Res. 2007;19:194–199. doi: 10.1007/BF03324689. [DOI] [PubMed] [Google Scholar]
- 25.Lustgarten MS, Price LL, Phillips EM, Fielding RA. Serum glycine is associated with regional body fat and insulin resistance in functionally-limited older adults. PLoS One. 2013;8:e84034. doi: 10.1371/journal.pone.0084034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochi M, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, Miki T, Kohara K. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle-aged to elderly population. Geriatr Gerontol Int. 2010;10:233–243. doi: 10.1111/j.1447-0594.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36)I Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 28.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol. 1990;45:P239–P243. doi: 10.1093/geronj/45.6.p239. [DOI] [PubMed] [Google Scholar]
- 30.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, Koch ML, Trainor K, Horwitz RI. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Delbaere K, Close JC, Brodaty H, Sachdev P, Lord SR. Determinants of disparities between perceived and physiological risk of falling among elderly people: cohort study. BMJ. 2010;341:c4165. doi: 10.1136/bmj.c4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, Cho H, Shin JH, Kim T, Park SB, Choi BY, Kim MJ. Relationship among fear of falling, physical performance, and physical characteristics of the rural elderly. Am J Phys Med Rehabil. 2014;93(5):379–386. doi: 10.1097/PHM.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 34.Delbaere K, Sturnieks DL, Crombez G, Lord SR. Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. J Gerontol A Biol Sci Med Sci. 2009;64:237–242. doi: 10.1093/gerona/gln014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rochat S, Bula CJ, Martin E, Seematter-Bagnoud L, Karmaniola A, Aminian K, Piot-Ziegler C, Santos-Eggimann B. What is the relationship between fear of falling and gait in well-functioning older persons aged 65 to 70 years? Arch Phys Med Rehabil. 2010;91:879–884. doi: 10.1016/j.apmr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Binda SM, Culham EG, Brouwer B. Balance, muscle strength, and fear of falling in older adults. Exp Aging Res. 2003;29:205–219. doi: 10.1080/03610730303711. [DOI] [PubMed] [Google Scholar]
- 37.Oh B, Cho B, Choi HC, Son KY, Park SM, Chun S, Cho SI. The influence of lower-extremity function in elderly individuals’ quality of life (QOL): an analysis of the correlation between SPPB and EQ-5D. Arch Gerontol Geriatr. 2014;58:278–282. doi: 10.1016/j.archger.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Glynn NW, Hsu FC, Walkup M, Pahor M. Health-related quality of life in older adults at risk for disability. Am J Prev Med. 2007;33:214–218. doi: 10.1016/j.amepre.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuel D, Rowe P, Hood V, Nicol A. The relationships between muscle strength, biomechanical functional moments and health-related quality of life in non-elite older adults. Age Ageing. 2012;41:224–230. doi: 10.1093/ageing/afr156. [DOI] [PubMed] [Google Scholar]
- 40.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire cohort study. Age Ageing. 2006;35:409–415. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 41.Katula JA, Rejeski WJ, Marsh AP. Enhancing quality of life in older adults: a comparison of muscular strength and power training. Health Qual Life Outcomes. 2008;6:45. doi: 10.1186/1477-7525-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glintborg D, Nielsen TL, Wraae K, Hougaard D, Gudex C, Brixen K, Andersen M. The relationship between health-related quality of life, obesity and testosterone levels in older men. Age Ageing. 2014;43:280–284. doi: 10.1093/ageing/aft203. [DOI] [PubMed] [Google Scholar]