Abstract
Background
Few remedies effectively treat long-term pain and disability from knee osteoarthritis. Studies suggest that Tai Chi alleviates symptoms, but no trials have directly compared Tai Chi with standard therapies for osteoarthritis.
Objective
To compare Tai Chi with standard physical therapy for patients with knee osteoarthritis.
Design
Randomized, 52-week, single-blind comparative effectiveness trial. (ClinicalTrials.gov: NCT01258985)
Setting
An urban tertiary care academic hospital.
Patients
204 participants with symptomatic knee osteoarthritis (mean age, 60 years; 70% women; 53% white).
Intervention
Tai Chi (2 times per week for 12 weeks) or standard physical therapy (2 times per week for 6 weeks, followed by 6 weeks of monitored home exercise).
Measurements
The primary outcome was Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score at 12 weeks. Secondary outcomes included physical function, depression, medication use, and quality of life.
Results
At 12 weeks, the WOMAC score was substantially reduced in both groups (Tai Chi, 167 points [95% CI, 145 to 190 points]; physical therapy, 143 points [CI, 119 to 167 points]). The between-group difference was not significant (24 points [CI, −10 to 58 points]). Both groups also showed similar clinically significant improvement in most secondary outcomes, and the benefits were maintained up to 52 weeks. Of note, the Tai Chi group had significantly greater improvements in depression and the physical component of quality of life. The benefit of Tai Chi was consistent across instructors. No serious adverse events occurred.
Limitation
Patients were aware of their treatment group assignment, and the generalizability of the findings to other settings remains undetermined.
Conclusion
Tai Chi produced beneficial effects similar to those of a standard course of physical therapy in the treatment of knee osteoarthritis.
Primary Funding Source
National Center for Complementary and Integrative Health of the National Institutes of Health.
Knee osteoarthritis is a major age-related public health problem and a leading cause of long-term pain and disability (1, 2). No effective medical treatments for the disease currently exist. Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are commonly used to treat osteoarthritis but often fail to relieve symptoms and may cause serious adverse effects (3). Physical therapy, a globally recommended element of the standard care regimen for knee osteoarthritis, produces moderate benefits for pain and physical functioning, but data on psychological well-being and durability effects are limited (4, 5). Identifying new and effective treatments for patients with knee osteoarthritis is an urgent clinical and public health priority.
Tai Chi is a multicomponent traditional Chinese mind–body practice that combines meditation with slow, gentle, graceful movements; deep diaphragmatic breathing; and relaxation (6). Previous studies have indicated that Tai Chi can reduce pain and improve physical and psychological health among patients with chronic rheumatic disorders, such as rheumatoid arthritis and fibromyalgia (7–13). In our previous randomized trial, participants with knee osteoarthritis who completed 12 weeks of Tai Chi showed greater improvements in pain, physical function, depression, and health status compared with an attention control group (12). A 2013 updated meta-analysis of 7 studies that included 348 participants with osteoarthritis showed significant reductions in pain and improvements in physical function after 8 to 24 weeks of Tai Chi training compared with a waiting list, attention control, or usual physical activity (14).
Those prior trials suggested that Tai Chi could provide a practical exercise regimen with an integrative mind–body approach to manage knee osteoarthritis. The physical component provides exercise benefits that are consistent with recommendations for knee osteoarthritis (physical function, balance, and muscle strength) (15), and the mind component promotes psychological well-being, life satisfaction, and improved perceptions of health (9, 16). To date, however, no randomized trials have directly compared Tai Chi and standard care treatments. The primary goal of this study was to compare the effectiveness of Tai Chi versus a physical therapy regimen among a large sample of patients with symptomatic and radiographic knee osteoarthritis who were seen in the clinical setting and followed for 12 months.
METHODS
Design Overview
This 52-week, single-blind, randomized, comparative effectiveness trial recruited participants with symptomatic and radiographic knee osteoarthritis. Patients were randomly assigned to either Tai Chi (2 times per week for 12 weeks) or physical therapy in a clinical setting (2 times per week for 6 weeks, followed by 6 weeks of rigorously monitored home exercise).
Details of the trial design and conduct have been published (17) and registered at ClinicalTrials.gov (NCT01258985). The study was approved by the Tufts Health Sciences Institutional Review Board and overseen by an independent data and safety monitoring board.
Setting and Participants
The trial was conducted at Tufts Medical Center in Boston, Massachusetts, between October 2010 and September 2014. Participants were recruited through multimodal strategies, including print advertisements, online media, a booth at a senior exposition, and a clinical patient database. We obtained informed consent before baseline assessments for eligibility.
We enrolled persons aged 40 years or older who met American College of Rheumatology criteria for symptomatic knee osteoarthritis and had radiographic evidence of tibiofemoral or patellofemoral osteoarthritis (defined as the presence of a definite osteophyte in the tibiofemoral compartment and/or the patellofemoral compartment, as assessed on standing anterior–posterior and lateral or sunrise views) (18). All participants were required to have a score of 40 or greater on at least 1 of the 5 questions in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (range of 0 to 100, with higher scores indicating greater pain) at baseline. A study rheumatologist (W.H.) confirmed eligibility of applicants.
We excluded persons who had participated in Tai Chi or physical therapy in the past year; those with current serious medical conditions, such as dementia, symptomatic heart or vascular disease, or recent stroke, that would limit full participation; those with intra-articular steroid or intra-articular hyaluronic acid injections in the past 3 or 6 months or reconstructive surgery before baseline screening on the most severely affected knee; and those with a score less than 24 on the Mini-Mental State Examination (19). Participants who satisfied eligibility criteria were offered enrollment.
Randomization
Participants were randomly assigned in a 1:1 ratio to Tai Chi or physical therapy after the baseline evaluation. Randomization was done in 9 consecutive cycles, comprising about 20 participants each. Within each block of patients randomly assigned in each cycle, those assigned to Tai Chi were further randomly assigned to 1 of 3 instructors so that each instructor treated 1 group every 3 blocks. This design allowed for assessment of instructor-level effects. Pseudorandom numbers were generated in advance by the statistician (C.S.) using the R statistical package (20). Assignments were concealed in sealed, opaque envelopes with date and signature labels. The study coordinator opened the consecutive envelopes individually after obtaining consent and confirming eligibility.
Research study nurses, physical function assessors, and sponsor personnel were blinded to the treatment assignments during enrollment. The blinded assessors did not have access to the data until data collection was complete.
Interventions
Tai Chi and physical therapy ran concurrently to minimize seasonal influences on disease activity. We encouraged participants to maintain routine activities but refrain from new exercises outside the study. Both groups received educational information about the importance of physical activity and home practice. We tracked reasons for missed sessions and asked participants to complete daily logs indicating duration of practice. Participants were encouraged to integrate at least 30 minutes of Tai Chi or physical therapy into their daily routine throughout follow-up. Attendance was monitored during each in-person session by using attendance forms and sign-in sheets. Study staff made monthly calls to monitor adherence throughout the 1-year follow-up.
Tai Chi
The 60-minute Tai Chi sessions occurred twice per week for 12 weeks. We recruited 3 experienced Tai Chi instructors from the greater Boston area. We developed a standardized classical Yang style Tai Chi protocol for knee osteoarthritis that was based on the literature (13). Before study initiation, the principal investigator (C.W.) and the Tai Chi master (R.R., who was also 1 of the 3 instructors) reviewed the concepts of knee osteoarthritis and trained the other instructors (17, 21). In the first session, participants received printed materials on Tai Chi principles, practice techniques, and safety precautions. The instructor explained mind–body exercise theory and procedures. Subsequent sessions started with a warm-up and a review of Tai Chi principles and movement, breathing techniques, and relaxation methods. Participants were instructed to practice Tai Chi at home for at least 20 minutes per day. The principal investigator monitored all sessions by regularly reviewing video recordings and providing feedback throughout the study. After completing the 12-week intervention (24 sessions), participants were instructed to continue Tai Chi practice for 52 weeks with the aid of provided homework materials.
Physical Therapy
The physical therapy protocol followed U.S. guidelines for knee osteoarthritis treatment (15) and consisted of two 30-minute outpatient sessions per week for 6 weeks. Before enrollment, the supervising physical therapist (M.I.) trained the 3 physical therapists. She observed evaluations and interventions during each treatment cycle to ensure consistency in documentation and provided feedback for program progression (17). Depending on the diagnostic findings in the initial musculoskeletal examination, the therapist targeted physical therapy regimens to address specific treatment goals developed collaboratively with the participant. At each session, the physical therapist examined the participant for adverse signs and symptoms before proceeding with manual therapy or exercise. Patients were encouraged to also perform exercises at home. After 6 weeks, participants were instructed to continue exercises in 30-minute sessions 4 times per week for 6 weeks. These were monitored weekly by telephone by using standardized forms to ascertain frequency, exercises completed, adverse events, and adherence.
Outcomes and Follow-up
Knee osteoarthritis outcomes, which were measured at baseline and 12, 24, and 52 weeks, were drawn from the core set recommended by the Osteoarthritis Research Society International (OARSI) and focused on pain, physical function, and patients’ overall assessment of their disease severity (22).
Primary Outcome
The primary outcome was the change in WOMAC pain subscale score between baseline and 12 weeks. The WOMAC is a validated, self-administered visual analogue scale designed specifically to evaluate osteoarthritis symptoms (23).
Secondary Outcomes
Secondary outcomes were measured at baseline and 12, 24, and 52 weeks and included WOMAC physical function and stiffness scores, Patient Global Assessment score, Beck Depression Inventory-II score (24), scores on the physical and mental components of the 36-item Short Form Health Survey (SF-36) (25), Arthritis Self-Efficacy Scale score (26), and results of the 6-minute walk test (27) and the 20-meter walk test (28). We also assessed participants’ expectations of their respective interventions by using the Outcome Expectations for Exercise Scale (29). We will report other secondary outcomes, such as muscle strength and power and Patient-Reported Outcomes Measurement Information System measures, in separate articles.
Monitoring of Adverse Events
We monitored study participants for adverse events during each encounter. Participants were also provided a study telephone number for reporting of adverse events throughout the study. We defined serious adverse events as those that were fatal, life-threatening, permanently disabling, or severely incapacitating or that required or prolonged inpatient hospitalization.
Medication Use
Participants were permitted to continue using routine medications, such as NSAIDs and acetaminophen, and maintain their usual physician visits throughout the study. Participants were not required to discontinue use of their pain medications before formal assessment visits. We kept a written record of changes in use of analgesics and NSAIDs throughout the entire intervention and evaluation period. We did not change or recommend changes in medical therapy.
Criteria for Clinical Response
We used the OMERACT-OARSI responder criteria (30) to indicate clinically meaningful improvement. The WOMAC pain and function subscores were converted to scales of 0 to 100 by dividing by 5 and 17, respectively. Patient Global Assessment scores were multiplied by 10 to convert to a scale of 0 to 100. Clinical response was defined as either 1) improvement of at least 50% in pain or function and an absolute change of at least 20 points on a scale of 0 to 100 in the WOMAC pain or function subscores, or 2) at least 2 of the following criteria: improvement of at least 20% and an absolute change greater than 10 points on a scale of 0 to 100 in the WOMAC pain score, improvement of at least 20% and an absolute change greater than 10 points on a scale of 0 to 100 in the WOMAC function score, or improvement of at least 20% in the Patient Global Assessment score and an absolute change greater than 10 points on a scale of 0 to 100.
Statistical Analysis
This study was designed to test Tai Chi for noninferiority and then for superiority if noninferiority was established with regard to the primary outcome (change in WOMAC pain score at 12 weeks). Sample sizes were chosen on the basis of effects observed in 2 prior knee osteoarthritis trials. The first of these showed an 80-point improvement in pain for physical therapy strength training compared with an attention control (31), and the second (our pilot trial [12]) found a 150-point improvement in pain for the Tai Chi group. We estimated the difference between these interventions to be 70 points (SD, 100). The noninferiority margin was set at 20 points (32) for the primary outcome of WOMAC pain score. To have 80% power for superiority with regard to this primary outcome (2-sided significance level of 0.05) and noninferiority with a margin of indifference of 20 points using a 1-sided significance level of 0.05 (32), and under the assumption of a 15% dropout rate, the study required 90 patients in each treatment group.
All analyses used all randomly assigned patients under the intention-to-treat assumption. For all outcomes, we fit longitudinal models incorporating all values recorded for each patient at baseline and 12, 24, and 52 weeks with a term for the interaction between treatment and each time point to assess differences in the effect of treatment over time. For continuous outcomes, we assumed normally distributed errors with an unstructured covariance matrix and used restricted maximum likelihood estimation. For binary outcomes, we used a generalized linear mixed model with random intercepts.
Preliminary analyses examined the association of missingness with baseline predictors and longitudinal outcomes. Participants who were lost to follow-up after completing the intervention had worse outcomes than those who remained in the study, thus invalidating the missing-completely-at-random assumption required for complete-case analysis. Several sensitivity analyses were conducted to evaluate the effect of missing data, including multiple imputation and adjustment for variables that were found to differ among participants who missed visits. These analyses are described in detail in Appendix Table 1 (available at www.annals.org).
We also explored several types of interactions. Two of these were of primary interest: the interaction of time with treatment, which indicates whether treatment has an effect, and the effect of instructor within the Tai Chi group. Potential 3-way interactions of treatment and time with 14 covariates (age, sex, body mass index [BMI], education, marital status, employment, living alone, NSAID or analgesic use, hypertension, diabetes, heart disease, weight, and class attendance) were examined to explore whether they modified the effect of treatment across time.
We used 2-sided tests at a significance level of 0.05 for all primary efficacy and interaction analyses. For the exploratory interactions, we used a significance level of 0.01 to protect against false-positive interactions. Results are presented as between-group differences with 95% CIs based on estimates from the longitudinal models. All model assumptions were checked with standard regression diagnostic evaluations (33).
All analyses were performed with SAS, version 9.4 (SAS Institute), and R.
Role of the Funding Source
This study was funded by the National Center for Complementary and Integrative Health. The authors are solely responsible for the content of the manuscript and the decision to submit it for publication.
RESULTS
Between March 2011 and June 2013, a total of 1195 patients were screened by telephone and 282 (23.6%) qualified for baseline evaluation. Of these, 204 (72.3%) consented, met inclusion criteria, and were randomly assigned to Tai Chi or physical therapy in 9 cycles (Figure 1).
Figure 1.
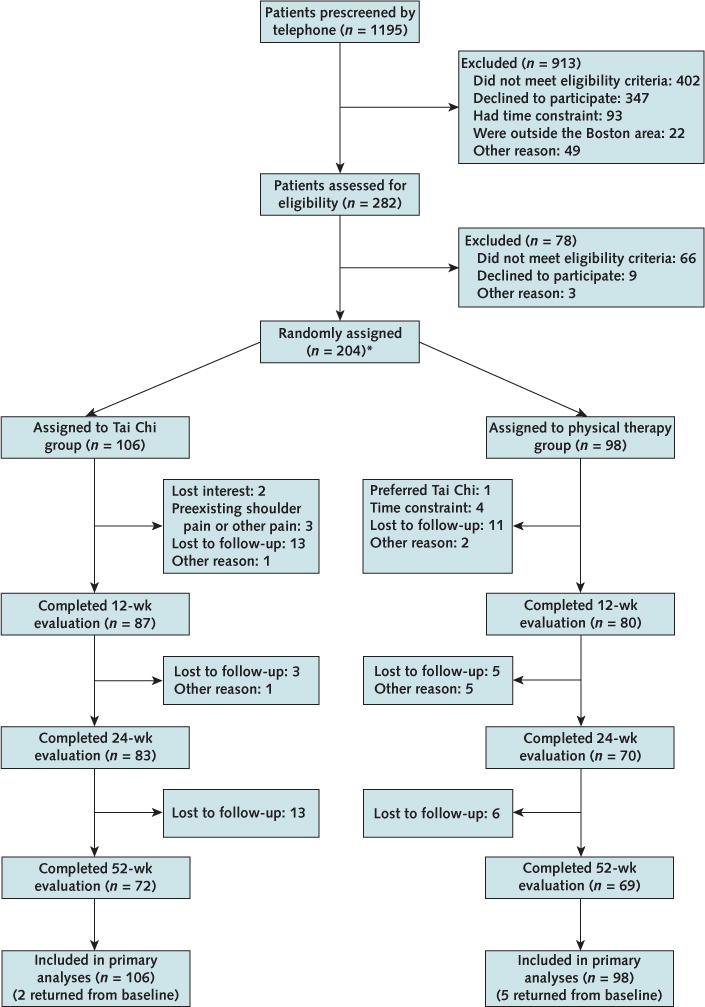
Study flow diagram.
* Two participants were inadvertently randomly assigned twice. Data from the first randomization were analyzed using the intention-to-treat principle.
Table 1 shows baseline data for the 204 participants. The mean age was 60 years, 70% were women, the racial/ethnic composition was diverse (53% white), and the mean BMI was 33 kg/m2. The average duration of knee osteoarthritis was 8 years. Participants were well-balanced between groups at baseline with respect to age, sex, race, BMI, scores of radiographic severity (Kellgren-Lawrence grade), WOMAC pain score, Beck Depression Inventory-II score, physical function, SF-36 score, and expectations of benefit from their randomly assigned regimen.
Table 1.
Baseline Characteristics of the Trial Participants, by Treatment Group*
| Characteristic | Tai Chi (n = 106) | Physical Therapy (n = 98) |
|---|---|---|
| Mean age (SD), y | 60.3 (10.5) | 60.1 (10.5) |
|
| ||
| Female, n (%) | 75 (71) | 68 (69) |
| Race, n (%) | ||
|
| ||
| White | 54 (51) | 54 (55) |
|
| ||
| Black | 41 (39) | 31 (32) |
|
| ||
| Asian | 4 (4) | 2 (2) |
|
| ||
| Other | 7 (7) | 11 (11) |
|
| ||
| Mean body mass index (SD), kg/m2 | 33.0 (7.1) | 32.6 (7.3) |
|
| ||
| Mean duration of knee pain (SD), y | 8.1 (10.3) | 8.5 (9.9) |
| Kellgren-Lawrence grade, n (%)† | ||
|
| ||
| 0 | 1 (1) | 4 (4) |
|
| ||
| 1 | 7 (7) | 3 (3) |
|
| ||
| 2 | 37 (36) | 38 (39) |
|
| ||
| 3 | 41 (40) | 32 (33) |
|
| ||
| 4 | 17 (16) | 21 (21) |
|
| ||
| NSAID use before study, n (%) | 66 (62) | 57 (59) |
|
| ||
| Analgesic use before study, n (%) | 39 (37) | 31 (32) |
|
| ||
| Self-reported comorbidities, n (%) | ||
| Heart disease | 8 (8) | 8 (8) |
|
| ||
| Hypertension | 56 (53) | 46 (47) |
|
| ||
| Diabetes | 23 (22) | 15 (15) |
|
| ||
| Mean WOMAC pain score (SD) (range, 0–500), mm‡ | 254.8 (95.5) | 252.9 (101.9) |
|
| ||
| Mean WOMAC physical function score (SD) (range, 0–1700), mm‡ | 912.1 (338.5) | 884.7 (368.1) |
|
| ||
| Mean Patient Global Assessment score (SD) (range, 0–10), cm‡ | 5.3 (2.1) | 5.1 (2.3) |
|
| ||
| Mean SF-36 score (SD) (range, 0–100)§ | ||
| Physical component | 36.5 (8.3) | 36.7 (10.0) |
|
| ||
| Mental component | 52.6 (9.3) | 52.4 (9.2) |
|
| ||
| Mean Beck Depression Inventory-II score (SD) (range, 0–63)‡ | 7.8 (9.0) | 7.5 (8.3) |
|
| ||
| Mean Arthritis Self-Efficacy Scale score (SD) (range, 1–10)§ | 6.1 (2.0) | 6.3 (2.2) |
|
| ||
| Mean 6-min walk test score (SD), m | 391.2 (91.7) | 400.1 (88.7) |
|
| ||
| Mean 20-m walk test score (SD), s | 19.6 (6.3) | 18.4 (3.9) |
|
| ||
| Mean Outcome Expectations for Exercise Scale score (SD) (range, 0–5)§ | 3.9 (0.5) | 3.9 (0.6) |
NSAID = nonsteroidal anti-inflammatory drug; SF-36 = 36-Item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Percentages may not sum to 100 due to rounding.
Five participants who had a grade of 0 met eligibility criteria because they had a definite osteophyte in the patellofemoral region.
Higher scores reflect more severe symptoms.
Higher scores indicate better status.
Overall attendance rates were 74% for Tai Chi and 81% for physical therapy for participants who attended at least 1 class during the 12-week intervention. Seventy-nine percent of Tai Chi participants and 78% of physical therapy participants attended at least 50% of the sessions (Appendix Table 2, available at www.annals.org). One hundred sixty-seven (82%) participants completed their evaluation at 12 weeks, 153 (75%) completed their evaluation at 24 weeks, and 141 (69%) completed their evaluation at 52 weeks.
Table 2 shows changes from baseline at 12, 24, and 52 weeks in the 2 groups for all continuous outcomes, and Table 3 gives results for the 2 binary outcomes (NSAID and analgesic use). Results in both tables are unadjusted for covariates. For the primary outcome, both groups had improved WOMAC pain scores at 12 weeks (Tai Chi, 167 points [95% CI, 145 to 190 points]; physical therapy, 143 points [CI, 119 to 167 points]). The treatment groups did not differ significantly in pain at 12 weeks (mean difference, 24 points [CI, −10 to 58 points]). Both groups showed similar improvements in most secondary outcomes at 12 weeks and in all outcomes at 24 and 52 weeks. The upper limit of the 95% CI for the 12-week WOMAC pain score indicated that physical therapy was highly likely to be superior to Tai Chi by no more than 10 points, well within the noninferiority margin of 20 points.
Table 2.
Changes in Primary and Secondary Outcomes*
| Variable | Mean Change From Baseline
|
Between-Group Difference
|
||
|---|---|---|---|---|
| Tai Chi (n = 106) | Physical Therapy (n = 98) | Tai Chi vs. Physical Therapy | P Value† | |
| WOMAC pain score (range, 0–500), mm | 0.22 | |||
|
| ||||
| Week 12 | −167.2 (−190.4 to −144.9) | −143.0 (−167.4 to −118.6) | −24.2 (−57.9 to 9.6) | |
|
| ||||
| Week 24 | −158.6 (−182.9 to −134.3) | −124.3 (−150.0 to −98.5) | −34.3 (−69.8 to 1.1) | |
|
| ||||
| Week 52 | −138.8 (−166.7 to −110.8) | −121.0 (−150.0 to −91.9) | −17.8 (−58.1 to 22.4) | |
|
| ||||
| WOMAC physical function score (range, 0–1700), mm | 0.160 | |||
| Week 12 | −608.3 (−695.3 to −521.4) | −494.2 (−585.3 to −403.2) | −114.1 (−240.0 to 11.8) | |
|
| ||||
| Week 24 | −586.8 (−669.5 to −504.1) | −455.7 (−543.1 to −368.4) | −131.1 (−251.3 to −10.8) | |
|
| ||||
| Week 52 | −532.3 (−625.9 to −438.7) | −444.0 (−541.3 to −346.7) | −88.3 (−223.4 to 46.7) | |
| WOMAC stiffness score (range, 0–200), mm | 0.74 | |||
|
| ||||
| Week 12 | −75.3 (−86.7 to −64.0) | −70.1 (−81.9 to −58.2) | −5.3 (−21.7 to 11.2) | |
|
| ||||
| Week 24 | −72.3 (−84.0 to −60.6) | −64.9 (−77.3 to −52.4) | −7.4 (−24.5 to 9.6) | |
|
| ||||
| Week 52 | −61.6 (−74.0 to −49.1) | −60.2 (−73.1 to −47.3) | −1.4 (−19.3 to 16.6) | |
|
| ||||
| Patient Global Assessment score (range, 0–10), cm | 0.25 | |||
| Week 12 | −2.96 (−3.46 to −2.45) | −2.24 (−2.78 to −1.71) | −0.71 (−1.45 to 0.02) | |
|
| ||||
| Week 24 | −2.40 (−2.93 to −1.88) | −1.73 (−2.29 to −1.17) | −0.67 (−1.44 to 0.09) | |
|
| ||||
| Week 52 | −1.84 (−2.48 to −1.21) | −1.31 (−1.96 to −0.66) | −0.53 (−1.44 to 0.38) | |
| Beck Depression Inventory-II score (range, 0–63) | 0.049 | |||
|
| ||||
| Week 12 | −2.2 (−3.7 to −0.9) | 0.5 (−1.0 to 2.0) | −2.7 (−4.8 to −0.7) | 0.008 |
|
| ||||
| Week 24 | −1.7 (−3.1 to −0.4) | 0.2 (−1.3 to 1.7) | −1.9 (−3.9 to 0.1) | 0.059 |
|
| ||||
| Week 52 | −1.1 (−2.7 to 0.5) | −0.003 (−1.6 to 1.6) | −1.1 (−3.4 to 1.2) | 0.34 |
|
| ||||
| SF-36 scores (range, 0–100) | ||||
| Physical component | 0.034 | |||
| Week 12 | 6.3 (4.6 to 7.9) | 3.1 (1.4 to 4.8) | 3.2 (0.8 to 5.5) | 0.010 |
|
| ||||
| Week 24 | 7.1 (5.1 to 9.0) | 3.4 (1.4 to 5.5) | 3.6 (0.8 to 6.4) | 0.012 |
|
| ||||
| Week 52 | 6.3 (4.4 to 8.3) | 4.3 (2.3 to 6.4) | 2.0 (−0.8 to 4.8) | 0.162 |
| Mental component | 0.59 | |||
|
| ||||
| Week 12 | 1.6 (−0.1 to 3.2) | −0.03 (−1.7 to 1.7) | 1.6 (−0.8 to 3.9) | |
|
| ||||
| Week 24 | 0.4 (−1.5 to 2.2) | −0.7 (−2.7 to 1.4) | 1.0 (−1.8 to 3.8) | |
|
| ||||
| Week 52 | −0.1 (−1.9 to 1.8) | −1.5 (−3.4 to 0.4) | 1.4 (−1.3 to 4.1) | |
|
| ||||
| Arthritis Self-Efficacy Scale score (range, 1–10) | 0.42 | |||
| Week 12 | 1.3 (0.8 to 1.8) | 0.8 (0.3 to 1.4) | 0.4 (−0.3 to 1.2) | |
|
| ||||
| Week 24 | 0.9 (0.4 to 1.4) | 0.9 (0.4 to 1.4) | −0.2 (−0.7 to 0.7) | |
|
| ||||
| Week 52 | 1.0 (0.5 to 1.5) | 0.7 (0.2 to 1.2) | 0.3 (−0.4 to 1.0) | |
| 6-min walk test score, m | 0.97 | |||
|
| ||||
| Week 12 | 28.6 (17.9 to 39.2) | 26.1 (14.9 to 37.4) | 2.5 (−13.1 to 18.0) | |
|
| ||||
| Week 24 | 28.9 (16.6 to 41.2) | 24.5 (11.5 to 37.5) | 4.4 (−13.4 to 22.3) | |
|
| ||||
| Week 52 | 27.1 (12.2 to 42.0) | 22.8 (7.0 to 38.6) | 4.3 (−17.4 to 26.0) | |
|
| ||||
| 20-m walk test score, s | 0.194 | |||
| Week 12 | −1.6 (−2.4 to −0.8) | −1.1 (−2.0 to −0.2) | −0.5 (−1.7 to 0.7) | |
|
| ||||
| Week 24 | −2.4 (−3.4 to −1.3) | −1.2 (−2.2 to −0.1) | −1.2 (−2.7 to 0.3) | |
|
| ||||
| Week 52 | −2.4 (−3.5 to −1.4) | −1.0 (−2.1 to 0.2) | −1.5 (−3.0 to 0.1) | |
SF-36 = 36-Item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Mean values were estimated from the repeated-measures analysis. Boldface values indicate statistically significant differences between groups. Results are unadjusted for covariates.
Values aligned with row headings are for the interaction of treatment group and time.
Table 3.
Changes in Use of NSAIDs and Analgesics*
| Variable | Odds Ratio (95% CI) Compared With Baseline
|
Odds Ratio (95% CI) for Between-Group Difference
|
||
|---|---|---|---|---|
| Tai Chi (n = 106) | Physical Therapy (n = 98) | Tai Chi vs. Physical Therapy | P Value† | |
| NSAIDs | 0.179 | |||
|
| ||||
| Week 12 | 0.39 (0.18–0.87) | 0.54 (0.24–1.21) | 0.73 (0.23–2.26) | |
|
| ||||
| Week 24 | 0.17 (0.07–0.40) | 0.61 (0.26–1.42) | 0.27 (0.08–0.91) | |
|
| ||||
| Week 52 | 0.39 (0.17–0.92) | 0.75 (0.32–1.77) | 0.52 (0.16–1.74) | |
|
| ||||
| Analgesics | 0.059 | |||
| Week 12 | 0.51 (0.21–1.25) | 0.45 (0.17–1.21) | 1.14 (0.30–4.31) | |
|
| ||||
| Week 24 | 0.42 (0.17–1.05) | 0.65 (0.24–1.76) | 0.64 (0.16–2.52) | |
|
| ||||
| Week 52 | 0.22 (0.08–0.63) | 1.24 (0.47–3.26) | 0.18 (0.04–0.75) | |
NSAID = nonsteroidal anti-inflammatory drug.
Odds ratios and 95% CIs were estimated from the repeated-measures analysis. Boldface values indicate statistically significant differences between groups. Results are unadjusted for covariates.
For the interaction of treatment group and time.
The Tai Chi group showed greater improvement than the physical therapy group for most outcomes, but these differences were not statistically significant except for the SF-36 physical component summary and the Beck Depression Inventory-II scores. Figure 2 shows the between-group mean differences for all outcomes at all time points. Results were similar after adjustment for BMI, which was higher among those who dropped out.
Figure 2.
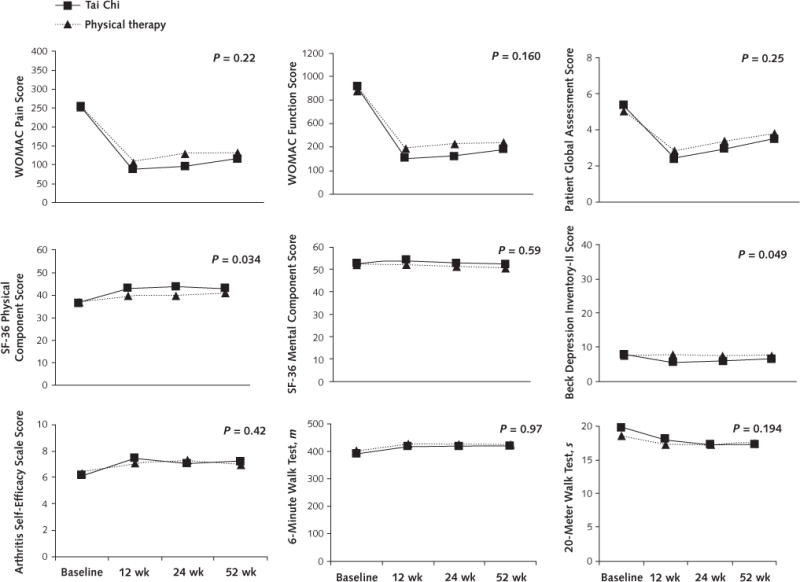
Mean change in outcomes, by treatment group.
Measurements were obtained at baseline and at 12, 24, and 52 wk, but data points are offset slightly for clarity. SF-36 = 36-item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Regression diagnostic evaluations indicated that some observations had large residuals. Transformation of some outcomes to the logarithmic scale reduced the number of outliers but did not qualitatively change results, and the Beck Depression Inventory-II score remained significant in favor of Tai Chi. Results of analyses on the logarithmic scale are shown in Appendix Table 3 (available at www.annals.org).
Both treatment groups had high proportions of participants meeting OARSI response criteria. For the Tai Chi group, the proportions at 12, 24, and 52 weeks were 72%, 67%, and 49%, respectively. For the physical therapy group, the corresponding percentages were 63%, 48%, and 51%.
Only 1 of the 154 interactions among the 14 covariates with 11 separate outcomes (attendance rate and WOMAC pain score) was significant at the 0.01 level. Effects were consistent across Tai Chi instructors. Although use of NSAIDs and analgesics in the 7 days before the evaluation visit was generally lower than at baseline at all follow-up times in each treatment group, these reductions did not statistically significantly differ between groups over time (Table 3). No serious adverse events were observed.
Comparisons of baseline characteristics between participants with missing versus complete data at each evaluation visit revealed differences by race, BMI, analgesic use, 6-minute walk test result, and SF-36 physical component summary score (Appendix Table 4, available at www.annals.org). Several sensitivity analyses were done to examine the effect of missing observations (Appendix Table 1). Adjustment for these variables in the longitudinal analyses did not change any of our results. Additional sensitivity analyses using multiple imputation and assigning no effect to participants missing the 12-week visit also yielded results similar to those described in Table 2.
DISCUSSION
This comparative effectiveness trial of 2 active therapies showed that despite a substantial difference in intensity of contact for participants between groups, Tai Chi and physical therapy each confer clinically significant improvements in pain and related health outcomes by 12 weeks, with the benefits maintained up to 52 weeks. Both treatment groups showed similar improvement in most secondary outcomes, but the Tai Chi group had significantly greater improvements in depression and the physical component of quality of life. Of note, the benefit of Tai Chi was consistent across experienced instructors treating patients with knee osteoarthritis.
A major strength of this trial is the enrollment of a representative sample of participants with knee osteoarthritis, including older and obese persons with risk factors and comorbidities commonly associated with the disease (34). Such persons typically face limited options due to ineffectiveness of and contraindications to osteoarthritis treatments. Indeed, the overall positive findings among adults who were representative of those seen in routine practice strengthen the evidence that the effectiveness and durability of both Tai Chi and physical therapy extend to obese older adults with knee osteoarthritis. Of note, instead of using inactive comparators, our study directly compared the effectiveness of 2 therapies that were each known to have health benefits for knee osteoarthritis, and the study had high adherence to assigned treatments and high follow-up rates. In addition, we found no difference in effectiveness among the Tai Chi instructors, implying that the intervention can be delivered standardly and effectively by appropriately trained instructors.
Our results are commensurate with our prior attention-controlled study (12) and with other studies that have demonstrated the efficacy of Tai Chi compared with various interventions for pain and physical and mental health over a range of chronic conditions (7–13). By integrating physical, psychosocial, emotional, spiritual, and behavioral elements, Tai Chi may systematically promote health by its effect on both the body and the mind. For example, pain has been found to correlate with the degree of quadriceps muscle weakness in knee osteoarthritis (35, 36). The physical exercise associated with Tai Chi enhances muscular strength, cardiovascular fitness, endurance, and coordination, thus improving functional capacity and joint stability (12, 13). In addition, central nervous system factors have recently been recognized as playing a prominent role in influencing osteoarthritis pain perception (37). Tai Chi may elicit behavioral responses by activating neuroendocrine and autonomic functioning and navigating neurochemical and analgesic pathways, which in turn may modulate the inflammatory response of the immune system and modify susceptibility to chronic pain (38, 39). Furthermore, by improving self-efficacy, social function, and depression, Tai Chi can help patients bolster their self-confidence and overcome their fear of pain (the latter of which often leads to physical malfunction and debility [7]).
Limitations of this research include patients’ awareness of their treatment group assignment and preconceived notions of treatment benefit potentially influencing their health and functional outcomes. For a study involving complex, multicomponent mind–body therapy, searching for and finding a feasible, useful, and valid sham comparison group remains challenging, with no well-accepted solution (40). Our study was necessarily single-blinded, a design with well-known limitations. To try to mitigate the influence of preexisting beliefs and expectations about the relative benefits of the interventions, we explicitly informed potential participants that the study was designed to test the effects of 2 different types of exercise programs. By emphasizing equipoise, we hoped to decrease expectations and minimize bias by not mentioning Tai Chi specifically. Using the Outcome Expectations for Exercise Scale to assess the possibility of bias, we found that participants’ expectations of benefit from their randomly assigned treatment regimen were similar (Tai Chi, 3.9 [SD, 0.5]; physical therapy, 3.9 [SD, 0.6]). Furthermore, total session attendance rates were similar between groups (74% for Tai Chi and 81% for physical therapy). Because the trial was conducted in a single academic center, we did not directly evaluate the generalizability of this intervention to other settings.
In conclusion, the results of this comparative effectiveness trial support the supposition that Tai Chi, a multicomponent mind–body exercise, improves pain and well-being in patients with knee osteoarthritis. Despite the substantial differences in delivering a mind–body intervention to a group and physical therapy to individual persons, both interventions produced similar effects in the treatment of knee osteoarthritis. Therefore, standardized Tai Chi should be considered as an effective therapeutic option for knee osteoarthritis. Future examination of the disease-modifying mechanisms of successful mind–body medicine will better inform clinical decision making for patients with this therapeutically challenging disorder.
Supplementary Material
Acknowledgments
The authors thank the data and safety monitoring board members (Drs. Wenjun Li, Yvonne Lee, and Kristin Baker) for their insightful suggestions and comments on the study protocol; Marcie Griffith, Fatima Shahzad, Tressa Gamache, Dr. John Griffith, Dr. Ronenn Roubenoff, and the Clinical and Translational Research Center nurses for their help with various aspects of the study; Dorri Li and Brian Muccio for their expertise in teaching the Tai Chi groups; Megan Whitmore, Marie Boneparth, and Jane Lucas for their expert physical therapy instruction; and the study participants, whose cooperation, encouragement, and enthusiasm are an inspiration to the authors.
Financial Support: By the National Center for Complementary and Integrative Health of the National Institutes of Health (R01 AT005521 and K24 AT007323), the National Center for Research Resources of the National Institutes of Health (UL1 RR025752), and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000073 and UL1TR001064). Dr. Fielding was partially supported by the U.S. Department of Agriculture under agreement 58-1950-4-003, the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679), and the Boston Rehabilitation Outcomes Center (1R24HD 065688-01A1).
Appendix Table 1.
Sensitivity Analyses for Primary Outcome (WOMAC Pain Score*)
| Variable | Mean Change From Baseline (95% CI), mm
|
Between−Group Difference
|
||
|---|---|---|---|---|
| Tai Chi (n = 106) | Physical Therapy (n = 98) | Tai Chi vs. Physical Therapy (95% CI), mm | PValue† | |
| Main results from Table 2 for comparison | 0.22 | |||
|
| ||||
| Week 12 | −167.2 (−190.4 to −144.9) | −143.0 (−167.4 to −118.6) | −24.2 (−57.9 to 9.6) | |
|
| ||||
| Week 24 | −158.6 (−182.9 to −134.3) | −124.3 (−150.0 to −98.5) | −34.3 (−69.8 to 1.1) | |
|
| ||||
| Week 52 | −138.8 (−166.7 to −110.8) | −121.0 (−150.0 to −91.9) | −17.8 (−58.1 to 22.4) | |
|
| ||||
| Score assigned baseline value if no 12-wk data (last observation carried forward) | ||||
| Week 12 | −137.6 (−160.6 to −114.7) | −116.0 (−140.0 to −92.1) | −21.6 (−54.6 to 11.4) | 0.20 (t test) |
| Via multiple imputation | 0.24–0.80 | |||
|
| ||||
| Week 12 | −158.6 (−184.4 to −132.9) | −141.0 (−168.9 to −113.1) | −17.6 (−54.7 to 19.5) | |
|
| ||||
| Week 24 | −152.0 (−179.2 to −124.8) | −127.0 (−157.3 to −96.7) | −25.0 (−65.1 to 15.0) | |
|
| ||||
| Week 52 | −132.2 (−164.4 to −100.1) | −116.9 (−147.5 to −86.3) | −15.4 (−60.9 to 30.2) | |
|
| ||||
| Adjusted for baseline body mass index | 0.23 | |||
| Week 12 | −166.8 (−190.2 to −143.4) | −143.1 (−167.5 to −118.8) | −23.7 (−57.5 to 10.1) | |
|
| ||||
| Week 24 | −157.1 (−181.6 to −132.6) | −123.9 (−149.7 to −98.1) | −33.2 (−68.8 to 2.4) | |
|
| ||||
| Week 52 | −137.4 (−165.6 to −109.3) | −120.8 (−149.9 to −91.7) | −16.6 (−57.1 to 23.9) | |
| Adjusted for race | 0.18 | |||
|
| ||||
| Week 12 | −166.6 (−189.9 to −143.3) | −141.1 (−165.5 to −116.7) | −25.5 (−59.3 to 8.3) | |
|
| ||||
| Week 24 | −157.9 (−182.3 to −133.5) | −121.6 (−147.4 to −95.7) | −36.4 (−71.9 to −0.81) | |
|
| ||||
| Week 52 | −137.8 (−165.7 to −109.9) | −118.2 (−147.2 to −89.2) | −19.6 (−59.8 to 20.7) | |
|
| ||||
| Adjusted for analgesic use at the corresponding visit | 0.21 | |||
| Week 12 | −165.5 (−188.4 to −142.5) | −140.9 (−164.9 to −116.8) | −24.6 (−57.8 to 8.6) | |
|
| ||||
| Week 24 | −157.2 (−181.4 to −132.9) | −125.8 (−151.4 to −100.1) | −31.4 (−66.7 to 3.9) | |
|
| ||||
| Week 52 | −134.8 (−162.7 to −106.8) | −121.9 (−150.7 to −93.1) | −12.9 (−53.0 to 27.2) | |
| Adjusted for 6-min walk (in meters) at the corresponding visit | 0.52 | |||
|
| ||||
| Week 12 | −156.7 (−179.1 to −134.3) | −142.2 (−165.7 to −118.7) | −14.5 (−46.7 to 17.7) | |
|
| ||||
| Week 24 | −146.0 (−171.1 to −121.0) | −119.5 (−145.8 to −93.2) | −26.5 (−62.6 to 9.5) | |
|
| ||||
| Week 52 | −133.9 (−161.0 to −106.8) | −116.0 (−144.5 to −87.5) | −17.9 (−57.0 to 21.2) | |
|
| ||||
| Adjusted for SF-36 physical component summary at the corresponding visit | 0.84 | |||
| Week 12 | −142.3 (−163.4 to −121.2) | −134.1 (−155.8 to −112.4) | −8.2 (−38.0 to 21.7) | |
|
| ||||
| Week 24 | −130.0 (−152.8 to −107.2) | −115.9 (−139.6 to −92.2) | −14.1 (−46.5 to 18.3) | |
|
| ||||
| Week 52 | −116.5 (−141.5 to −91.5) | −103.2 (−129.0 to −77.4) | −13.3 (−48.7 to 22.1) | |
SF-36 = 36-Item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Range, 0–500 mm. Sensitivity analyses were performed using the multiple imputation method (multivariate chained equation) to examine the effect of missing observations. All variables shown in Appendix Table 3 were included in the multiple imputation. There were 5 imputed data sets in total. Higher scores for all variables indicate more severe disease status.
Interaction P value unless otherwise indicated.
Appendix Table 2.
Intervention Attendance Rates
| Intervention Cycle | Tai Chi | Physical Therapy | Total |
|---|---|---|---|
| Cycle 1 | |||
|
| |||
| Participants, n | 11 | 11 | 22 |
|
| |||
| Attendance rate, % | 76 | 90 | 83 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 8 | 9 | 17 |
|
| |||
| 50%–69% | 0 | 1 | 1 |
|
| |||
| 1%–49% | 2 | 0 | 2 |
|
| |||
| 0% | 1 | 1 | 2 |
| Cycle 2 | |||
|
| |||
| Participants, n | 9 | 11 | 20 |
|
| |||
| Attendance rate, % | 66 | 77 | 72 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 5 | 8 | 13 |
|
| |||
| 50%–69% | 0 | 0 | 0 |
|
| |||
| 1%–49% | 2 | 2 | 4 |
|
| |||
| 0% | 2 | 1 | 3 |
| Cycle 3 | |||
|
| |||
| Participants, n | 13 | 10 | 23 |
|
| |||
| Attendance rate, % | 83 | 71 | 78 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 10 | 6 | 16 |
|
| |||
| 50%–69% | 1 | 1 | 2 |
|
| |||
| 1%–49% | 2 | 2 | 4 |
|
| |||
| 0% | 0 | 1 | 1 |
| Cycle 4 | |||
|
| |||
| Participants, n | 12 | 11 | 23 |
|
| |||
| Attendance rate, % | 76 | 89 | 81 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 10 | 9 | 19 |
|
| |||
| 50%–69% | 0 | 0 | 0 |
|
| |||
| 1%–49% | 2 | 0 | 2 |
|
| |||
| 0% | 0 | 2 | 2 |
| Cycle 5 | |||
|
| |||
| Participants, n | 13 | 10 | 23 |
|
| |||
| Attendance rate, % | 83 | 89 | 86 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 9 | 9 | 18 |
|
| |||
| 50%–69% | 1 | 1 | 2 |
|
| |||
| 1%–49% | 1 | 0 | 1 |
|
| |||
| 0% | 2 | 0 | 2 |
| Cycle 6 | |||
|
| |||
| Participants, n | 12 | 9 | 21 |
|
| |||
| Attendance rate, % | 80 | 88 | 83 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 9 | 6 | 15 |
|
| |||
| 50%–69% | 2 | 2 | 4 |
|
| |||
| 1%–49% | 1 | 0 | 1 |
|
| |||
| 0% | 0 | 1 | 1 |
| Cycle 7 | |||
|
| |||
| Participants, n | 12 | 10 | 22 |
|
| |||
| Attendance rate, % | 70 | 85 | 78 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 7 | 9 | 16 |
|
| |||
| 50%–69% | 1 | 0 | 1 |
|
| |||
| 1%–49% | 2 | 1 | 3 |
|
| |||
| 0% | 2 | 0 | 2 |
| Cycle 8 | |||
|
| |||
| Participants, n | 12 | 14 | 26 |
|
| |||
| Attendance rate, % | 64 | 78 | 70 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 7 | 9 | 16 |
|
| |||
| 50%–69% | 1 | 0 | 1 |
|
| |||
| 1%–49% | 4 | 2 | 6 |
|
| |||
| 0% | 0 | 3 | 3 |
| Cycle 9 | |||
|
| |||
| Participants, n | 12 | 12 | 24 |
|
| |||
| Attendance rate, % | 68 | 66 | 67 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 7 | 7 | 14 |
|
| |||
| 50%–69% | 1 | 1 | 2 |
|
| |||
| 1%–49% | 3 | 4 | 7 |
|
| |||
| 0% | 1 | 0 | 1 |
| Total | |||
|
| |||
| Participants, n | 106 | 98 | 204 |
|
| |||
| Attendance rate, %* | 74 | 81 | 7 |
| Participation by attendance rate, n | |||
|
| |||
| 70%–100% | 72 | 72 | 144 |
|
| |||
| 50%–69% | 7 | 6 | 13 |
|
| |||
| 1%–49% | 19 | 11 | 30 |
|
| |||
| 0% | 8 | 9 | 17 |
Treated participants only.
Appendix Table 3.
Relative Change in Primary and Secondary Outcomes*
| Variable | Proportional Change From Baseline†
|
Between-Group Change‡
|
||
|---|---|---|---|---|
| Tai Chi (n = 106) | Physical Therapy (n = 98) | Tai Chi vs. Physical Therapy | P Value§ | |
| WOMAC pain score (range, 0–500 mm) | 0.36 | |||
|
| ||||
| Week 12 | 0.20 (0.15–0.25) | 0.24 (0.18–0.31) | 0.84 (0.58–1.21) | |
|
| ||||
| Week 24 | 0.24 (0.19–0.31) | 0.33 (0.26–0.42) | 0.74 (0.52–1.04) | |
|
| ||||
| Week 52 | 0.26 (0.20–0.35) | 0.33 (0.25–0.44) | 0.79 (0.53–1.18) | |
|
| ||||
| WOMAC physical function score (range, 0–1700 mm) | 0.44 | |||
| Week 12 | 0.17 (0.13–0.23) | 0.23 (0.17–0.31) | 0.74 (0.50–1.12) | |
|
| ||||
| Week 24 | 0.22 (0.17–0.29) | 0.28 (0.21–0.37) | 0.79 (0.53–1.16) | |
|
| ||||
| Week 52 | 0.23 (0.17–0.31) | 0.32 (0.23–0.43) | 0.72 (0.47–1.11) | |
| WOMAC stiffness score (range, 0–200 mm) | 0.66 | |||
|
| ||||
| Week 12 | 0.19 (0.15–0.25) | 0.24 (0.19–0.32) | 0.79 (0.55–1.14) | |
|
| ||||
| Week 24 | 0.22 (0.17–0.29) | 0.26 (0.20–0.35) | 0.86 (0.58–1.26) | |
|
| ||||
| Week 52 | 0.28 (0.21–0.37) | 0.33 (0.25–0.45) | 0.83 (0.55–1.25) | |
|
| ||||
| Patient Global Assessment score (range, 0–10 cm) | 0.20 | |||
| Week 12 | 0.81 (0.78–0.84) | 0.85 (0.82–0.88) | 0.95 (0.90–1.00) | |
|
| ||||
| Week 24 | 0.84 (0.81–0.87) | 0.88 (0.85–0.92) | 0.95 (0.90–1.00) | |
|
| ||||
| Week 52 | 0.87 (0.83–0.91) | 0.91 (0.87–0.95) | 0.96 (0.90–1.02) | |
| Beck Depression Inventory-II score (range, 0–63) | 0.012 | |||
|
| ||||
| Week 12 | 0.68 (0.57–0.80) | 1.01 (0.85–1.21) | 0.67 (0.52–0.86) | 0.002 |
|
| ||||
| Week 24 | 0.81 (0.68–0.97) | 0.93 (0.77–1.13) | 0.87 (0.67–1.12) | 0.28 |
|
| ||||
| Week 52 | 0.78 (0.64–0.96) | 1.00 (0.81–1.23) | 0.78 (0.58–1.04) | 0.093 |
|
| ||||
| SF-36 scores (range, 0–100) | ||||
| Physical component | 0.079 | |||
| Week 12 | 1.17 (1.12–1.23) | 1.09 (1.04–1.14) | 1.08 (1.01–1.15) | |
|
| ||||
| Week 24 | 1.19 (1.13–1.26) | 1.09 (1.03–1.15) | 1.10 (1.01–1.18) | |
|
| ||||
| Week 52 | 1.17 (1.11–1.24) | 1.12 (1.06–1.18) | 1.05 (0.97–1.13) | |
| Mental component | 0.47 | |||
|
| ||||
| Week 12 | 1.03 (0.99–1.07) | 0.99 (0.95–1.03) | 1.04 (0.98–1.10) | |
|
| ||||
| Week 24 | 1.01 (0.97–1.06) | 0.96 (0.92–1.01) | 1.05 (0.98–1.12) | |
|
| ||||
| Week 52 | 1.00 (0.96–1.05) | 0.96 (0.92–1.01) | 1.04 (0.98–1.11) | |
|
| ||||
| Arthritis Self-Efficacy Scale score (range, 1–10) | 0.73 | |||
| Week 12 | 1.20 (1.09–1.32) | 1.14 (1.03–1.26) | 1.05 (0.92–1.21) | |
|
| ||||
| Week 24 | 1.12 (1.02–1.24) | 1.16 (1.04–1.29) | 0.97 (0.84–1.12) | |
|
| ||||
| Week 52 | 1.14 (1.04–1.25) | 1.13 (1.03–1.24) | 1.01 (0.89–1.15) | |
| 6-min walk score (in meters) | 0.86 | |||
| Week 12 | 1.08 (1.05–1.11) | 1.07 (1.04–1.10) | 1.01 (0.97–1.05) | |
|
| ||||
| Week 24 | 1.08 (1.05–1.12) | 1.06 (1.03–1.10) | 1.02 (0.97–1.07) | |
|
| ||||
| Week 52 | 1.06 (1.02–1.11) | 1.06 (1.02–1.11) | 1.00 (0.94–1.06) | |
|
| ||||
| 20-m walk score (in seconds) | 0.058 | |||
| Week 12 | 0.93 (0.90–0.96) | 0.95 (0.91–0.98) | 0.98 (0.94–1.03) | |
|
| ||||
| Week 24 | 0.89 (0.85–0.92) | 0.95 (0.91–0.99) | 0.94 (0.88–0.99) | |
|
| ||||
| Week 52 | 0.88 (0.84–0.92) | 0.95 (0.90–0.99) | 0.93 (0.87–0.99) | |
SF-36 = 36-Item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Values are estimated means (95% CIs) from longitudinal regressions, including time, treatment, and their interaction only with outcome variables on a logarithmic scale. Boldface values indicate statistically significant differences between groups.
Values are relative change, defined as follow-up score as a proportion of baseline score.
Values are change in Tai Chi group relative to change in physical therapy group. For example, a value of 0.95 means that the Tai Chi score decreased by 5% more than the physical therapy score.
Values aligned with row headings are for the interaction of treatment group and time.
Appendix Table 4.
Baseline Characteristics Associated With Missingness at Follow-up Evaluation Visits*
| Baseline Characteristic | Week 12
|
Week 24
|
Week 52
|
|||
|---|---|---|---|---|---|---|
| Missing Data (n = 38) | Data Present (n = 166) | Missing Data (n = 51) | Data Present (n = 153) | Missing Data (n = 63) | Data Present (n = 141) | |
| Mean age (SD), y | 58.1 (9.9) | 60.7 (10.5) | 58.5 (9.7) | 60.8 (10.7) | 59.3 (11.6) | 60.6 (9.9) |
|
| ||||||
| Female, % | 63.2 | 71.7 | 66.7 | 71.2 | 66.7 | 71.6 |
| Race, % | ||||||
|
| ||||||
| White | 39.5 | 56.0 | 35.3 | 58.8 | 38.1 | 59.6 |
|
| ||||||
| Black | 44.7 | 33.1 | 45.1 | 32.0 | 47.6 | 29.8 |
|
| ||||||
| Other | 15.8 | 10.8 | 19.6 | 9.2 | 14.3 | 10.6 |
|
| ||||||
| Education, % | ||||||
| High school or less | 15.8 | 16.9 | 19.6 | 15.7 | 19.1 | 15.6 |
|
| ||||||
| Some college/trade | 50.0 | 34.4 | 45.1 | 34.6 | 47.6 | 32.6 |
|
| ||||||
| College graduate | 10.5 | 23.5 | 15.7 | 22.9 | 17.5 | 22.7 |
|
| ||||||
| Graduate school | 23.7 | 25.3 | 19.6 | 26.8 | 15.9 | 29.1 |
|
| ||||||
| Married/living with partner, % | 39.5 | 28.9 | 33.3 | 30.1 | 36.5 | 28.4 |
|
| ||||||
| Living alone, % | 50.0 | 53.0 | 52.9 | 52.3. | 47.6 | 54.6 |
|
| ||||||
| Employment, % | ||||||
| Full-time | 10.5 | 19.9 | 17.7 | 18.3 | 19.1 | 17.7 |
|
| ||||||
| Part-time | 13.2 | 11.5 | 13.7 | 11.1 | 14.3 | 10.6 |
|
| ||||||
| Retired | 31.6 | 28.9 | 27.5 | 30.1 | 23.8 | 31.9 |
|
| ||||||
| Other | 44.7 | 39.8 | 41.2 | 40.5 | 42.9 | 39.7 |
|
| ||||||
| History of knee injury, % | 47.4 | 42.8 | 43.1 | 43.8 | 42.9 | 44.0 |
|
| ||||||
| Arthroscopy, % | 23.7 | 18.1 | 21.6 | 18.3 | 25.4 | 16.3 |
|
| ||||||
| Effusion, % | 44.7 | 46.3 | 46.0 | 46.0 | 45.9 | 46.0 |
|
| ||||||
| Randomly assigned to Tai Chi, % | 50.0 | 52.4 | 45.1 | 54.3 | 54.0 | 51.1 |
|
| ||||||
| Mean body mass index (SD), kg/m2 | 34.4 (8.2) | 32.4 (6.9) | 35.4 (8.1) | 31.9 (6.6) | 33.8 (7.9) | 32.4 (6.8) |
|
| ||||||
| Mean pain duration (SD), y | 6.9 (5.6) | 8.6 (10.8) | 8.8 (11.0) | 8.2 (9.8) | 8.6 (11.1) | 8.2 (9.6) |
|
| ||||||
| Kellgren-Lawrence grade, %† | ||||||
| 0–2 | 35.1 | 47.0 | 40.0 | 46.4 | 34.4 | 49.3 |
|
| ||||||
| 3 | 43.2 | 34.8 | 40.0 | 35.1 | 42.6 | 33.6 |
|
| ||||||
| 4 | 21.6 | 18.3 | 20.0 | 18.5 | 23.0 | 17.1 |
|
| ||||||
| Joint symptoms, % | 68.4 | 68.1 | 72.6 | 66.7 | 76.2 | 64.5 |
|
| ||||||
| Mean Arthritis Self-Efficacy Scale score (SD) (range, 1–10)‡ | 6.0 (2.2) | 6.3 (2.1) | 6.3 (2.1) | 6.2 (2.1) | 6.1 (2.0) | 6.3 (2.2) |
|
| ||||||
| Mean Outcome Expectations Scale for Randomized Intervention score (SD) (range, 1–5) | 3.9 (0.7) | 3.9 (0.6) | 3.9 (0.7) | 3.9 (0.6) | 3.9 (0.7) | 3.9 (0.6) |
|
| ||||||
| Self-reported comorbidities, % | ||||||
| Heart disease | 5.3 | 8.4 | 7.8 | 7.8 | 11.1 | 6.4 |
|
| ||||||
| Hypertension | 44.7 | 51.2 | 49.0 | 50.3 | 50.8 | 49.7 |
|
| ||||||
| Diabetes | 18.4 | 18.7 | 19.6 | 18.3 | 17.5 | 19.2 |
|
| ||||||
| Mean WOMAC pain score (SD) (range, 0–500), mm§ | 256.5 (89.0) | 253.3 (100.7) | 261.1 (92.7) | 251.5 (100.4) | 261.7 (89.3) | 250.4 (102.3) |
|
| ||||||
| Mean WOMAC physical function score (SD) (range, 0–1700), mm§ | 958.2 (274.2) | 885.4 (367.3) | 971.0 (294.9) | 875.0 (367.3) | 951.6 (310.4) | 875.5 (368.2) |
|
| ||||||
| Mean WOMAC stiffness score (SD) (range, 0–200), mm§ | 119.0 (38.3) | 116.6 (47.6) | 119.5 (39.2) | 116.2 (48.1) | 115.9 (42.5) | 117.5 (47.6) |
|
| ||||||
| Mean Patient Global Visual Analogue Scale score (SD) (range, 0–10), cm§ | 5.4 (2.3) | 5.2 (2.2) | 5.5 (2.1) | 5.1 (2.2) | 5.6 (2.1) | 5.0 (2.2) |
| Mean SF-36 score (SD) (range, 0–100)‡ | ||||||
|
| ||||||
| Physical component | 34.4 (9.5) | 37.1 (9.0) | 34.1 (9.3) | 37.4 (8.9) | 34.9 (8.8) | 37.3 (9.2) |
|
| ||||||
| Mental component | 52.5 (8.0) | 52.5 (9.5) | 52.1 (7.8) | 52.7 (9.7) | 52.6 (8.6) | 52.5 (9.5) |
|
| ||||||
| Mean Beck Depression Inventory-II score (SD) (range, 0–63)§ | 7.4 (8.9) | 7.7 (8.6) | 6.8 (8.0) | 7.9 (8.8) | 7.1 (8.6) | 7.9 (8.7) |
|
| ||||||
| Mean perceived stress score (SD) (range, 0–40)§ | 13.0 (6.6) | 13.3 (7.1) | 12.6 (6.9) | 13.5 (7.0) | 13.3 (6.9) | 13.3 (7.0) |
|
| ||||||
| Mean 6-min walk score (SD), m | 368.8 (99.3) | 401.6 (87.1) | 369.0 (93.3) | 404.4 (87.5) | 371.7 (102.1) | 406.0 (82.5) |
|
| ||||||
| Mean 20-m walk score (SD), s | 19.8 (5.0) | 18.8 (5.4) | 19.9 (4.8) | 18.7 (5.5) | 19.8 (5.0) | 18.6 (5.4) |
|
| ||||||
| Analgesic use, % | 50.0 | 31.1 | 41.2 | 32.5 | 42.9 | 30.9 |
|
| ||||||
| NSAID use, % | 59.5 | 61.2 | 56.0 | 62.5 | 58.1 | 62.1 |
NSAID = nonsteroidal anti-inflammatory drug; SF-36 = 36-Item Short Form Health Survey; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Boldface values indicate statistically significant differences between groups. The chi-square test was used for categorical variables, and the t test was used for continuous variables.
Five participants who had a score of 0 met eligibility criteria because they had a definite osteophyte in the patellofemoral region.
Higher scores indicate better status.
Higher scores indicate more severe symptoms.
Footnotes
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Integrative Health at the National Institutes of Health. Any opinions, findings, conclusions, or recommendations expressed in this article are those of the authors and do not necessarily reflect the views of the U.S. Department of Agriculture.
Disclosures: Dr. Schmid reports grants from Brown University during the conduct of the study and personal fees from Pfizer outside the submitted work. Dr. Harvey reports grants from Samumed, AbbVie, and Fidia Pharma outside the submitted work. Dr. Fielding reports grants from the National Institutes of Health during the conduct of the study; grants from Nestlé, Regeneron, Astellas Pharma, and Pronutria Biosciences outside the submitted work; personal fees from Nestlé, Regeneron, Astellas Pharma, Pronutria Biosciences, GlaxoSmith-Kline, ICON, and Biophytis outside the submitted work; and nonfinancial support from Pronutria and Myosyntax outside the submitted work. Dr. Wong reports grants from the Patient-Centered Outcomes Research Institute and the Rheumatology Research Foundation of the American College of Rheumatology and nonfinancial support from the European League Against Rheumatism outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M15-2143.
Reproducible Research Statement: Study protocol: See the Supplement (available at www.annals.org). Statistical code and data set: Available from Dr. Wang (e-mail, cwang2 @tuftsmedicalcenter.org).
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: C. Wang, C.H. Schmid, M.D. Iversen, J.B. Wong, R. Rones, T. McAlindon. Analysis and interpretation of the data: C. Wang, C.H. Schmid, M.D. Iversen, W.F. Harvey, R.A. Fielding, J.B. Driban, L.L. Price, J.B. Wong, K.F. Reid, R. Rones, T. McAlindon.
Drafting of the article: C. Wang, C.H. Schmid, M.D. Iversen, W.F. Harvey, R.A. Fielding, J.B. Driban, L.L. Price, K.F. Reid, T. McAlindon.
Critical revision of the article for important intellectual content: C. Wang, C.H. Schmid, M.D. Iversen, W.F. Harvey, R.A. Fielding, J.B. Driban, L.L. Price, J.B. Wong, K.F. Reid, T. McAlindon.
Final approval of the article: C. Wang, C.H. Schmid, M.D. Iversen, W.F. Harvey, R.A. Fielding, J.B. Driban, L.L. Price, J.B. Wong, K.F. Reid, R. Rones, T. McAlindon.
Provision of study materials or patients: W.F. Harvey.
Statistical expertise: C.H. Schmid, L.L. Price, J.B. Wong.
Obtaining of funding: C. Wang, J.B. Wong.
Administrative, technical, or logistic support: C. Wang, J.B. Driban.
Collection and assembly of data: C.H. Schmid, W.F. Harvey, R.A. Fielding, J.B. Driban, L.L. Price, K.F. Reid.
References
- 1.Murphy L, Schwartz TA, Helmick CG, Renner JB, Tudor G, Koch G, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Smalley WE, Griffin MR. The risks and costs of upper gastrointestinal disease attributable to NSAIDs. Gastroenterol Clin North Am. 1996;25:373–96. doi: 10.1016/s0889-8553(05)70253-3. [DOI] [PubMed] [Google Scholar]
- 4.Iversen MD. Rehabilitation interventions for pain and disability in osteoarthritis. Am J Nurs. 2012;112:S32–7. doi: 10.1097/01.NAJ.0000412649.02926.35. [DOI] [PubMed] [Google Scholar]
- 5.Jamtvedt G, Dahm KT, Christie A, Moe RH, Haavardsholm E, Holm I, et al. Physical therapy interventions for patients with osteoarthritis of the knee: an overview of systematic reviews. Phys Ther. 2008;88:123–36. doi: 10.2522/ptj.20070043. [DOI] [PubMed] [Google Scholar]
- 6.Wayne PM, Kaptchuk TJ. Challenges inherent to t’ai chi research: part I—t’ai chi as a complex multicomponent intervention. J Altern Complement Med. 2008;14:95–102. doi: 10.1089/acm.2007.7170A. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, et al. A randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363:743–54. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C. Role of Tai Chi in the treatment of rheumatologic diseases. Curr Rheumatol Rep. 2012;14:598–603. doi: 10.1007/s11926-012-0294-y. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Bannuru R, Ramel J, Kupelnick B, Scott T, Schmid CH. Tai Chi on psychological well-being: systematic review and meta-analysis. BMC Complement Altern Med. 2010;10:23. doi: 10.1186/1472-6882-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C. Tai chi and rheumatic diseases. Rheum Dis Clin North Am. 2011;37:19–32. doi: 10.1016/j.rdc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Roubenoff R, Lau J, Kalish R, Schmid CH, Tighiouart H, et al. Effect of Tai Chi in adults with rheumatoid arthritis [Letter] Rheumatology (Oxford) 2005;44:685–7. doi: 10.1093/rheumatology/keh572. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Schmid CH, Hibberd PL, Kalish R, Roubenoff R, Rones R, et al. Tai Chi is effective in treating knee osteoarthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1545–53. doi: 10.1002/art.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Collet JP, Lau J. The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med. 2004;164:493–501. doi: 10.1001/archinte.164.5.493. [DOI] [PubMed] [Google Scholar]
- 14.Yan JH, Gu WJ, Sun J, Zhang WX, Li BW, Pan L. Efficacy of Tai Chi on pain, stiffness and function in patients with osteoarthritis: a meta-analysis. PLoS One. 2013;8:e61672. doi: 10.1371/journal.pone.0061672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–88. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Axford J, Heron C, Ross F, Victor CR. Management of knee osteoarthritis in primary care: pain and depression are the major obstacles. J Psychosom Res. 2008;64:461–7. doi: 10.1016/j.jpsychores.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Iversen MD, McAlindon T, Harvey WF, Wong JB, Fielding RA, et al. Assessing the comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis: design and rationale for a randomized trial. BMC Complement Altern Med. 2014;14:333. doi: 10.1186/1472-6882-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 21.China Sports. Simplified “Taijiquan”. 2nd. Beijing, China: China Publications Center; 1983. [Google Scholar]
- 22.Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol. 1997;24:799–802. [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 25.Ware J, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: Health Assessment Lab; 1994. [Google Scholar]
- 26.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 27.King S, Wessel J, Bhambhani Y, Maikala R, Sholter D, Maksy-mowych W. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J Rheumatol. 1999;26:2233–7. [PubMed] [Google Scholar]
- 28.Motyl JM, Driban JB, McAdams E, Price LL, McAlindon TE. Test-retest reliability and sensitivity of the 20-meter walk test among patients with knee osteoarthritis. BMC Musculoskelet Disord. 2013;14:166. doi: 10.1186/1471-2474-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick B, Zimmerman SI, Orwig D, Furstenberg AL, Magaziner J. Outcome expectations for exercise scale: utility and psychometrics. J Gerontol B Psychol Sci Soc Sci. 2000;55:S352–6. doi: 10.1093/geronb/55.6.s352. [DOI] [PubMed] [Google Scholar]
- 30.Pham T, Van Der Heijde D, Lassere M, Altman RD, Anderson JJ, Bellamy N, et al. OMERACT-OARSI Outcome variables for osteoarthritis clinical trials: the OMERACT-OARSI set of responder criteria. J Rheumatol. 2003;30:1648–54. [PubMed] [Google Scholar]
- 31.Baker KR, Nelson ME, Felson DT, Layne JE, Sarno R, Roubenoff R. The efficacy of home based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655–65. [PubMed] [Google Scholar]
- 32.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Ann Rheum Dis. 2005;64:34–7. doi: 10.1136/ard.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzmaurice G, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd. New York: J Wiley; 2011. [Google Scholar]
- 34.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Hall MC, Mockett SP, Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis. 2006;65:865–70. doi: 10.1136/ard.2005.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slemenda C, Brandt KD, Heilman DK, Mazzuca S, Braunstein EM, Katz BP, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states—maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–54. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One. 2014;9:e100903. doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Goldenberg D, McAlindon T. Correspondence: a randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363:2266–7. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


