Abstract
Purpose:
Over the last decade, the use of oral chemotherapy (OC) for the treatment of cancer has dramatically increased. Despite their route of administration, OCs pose many of the same risks as intravenous agents. In this quality improvement project, we sought to examine our current process for the prescription of OC at the Abramson Cancer Center of the University of Pennsylvania and to improve on its safety.
Methods:
A multidisciplinary team that included oncologists, advanced-practice providers, and pharmacists was formed to analyze the current state of our OC practice. Using Lean Six Sigma quality improvement tools, we identified a lack of pharmacist review of the OC prescription as an area for improvement. To address these deficiencies, we used our electronic medical system to route OC orders placed by treating providers to an oncology-specific outpatient pharmacist at the Abramson Cancer Center for review.
Results:
Over 7 months, 63 orders for OC were placed for 45 individual patients. Of the 63 orders, all were reviewed by pharmacists, and, as a result, 22 interventions were made (35%). Types of interventions included dosage adjustment (one of 22), identification of an interacting drug (nine of 22), and recommendations for additional drug monitoring (12 of 22).
Conclusion:
OC poses many of the same risks as intravenous chemotherapy and should be prescribed and reviewed with the same oversight. At our institution, involvement of an oncology-trained pharmacist in the review of OC led to meaningful interventions in one third of the orders.
INTRODUCTION
Over the last decade, the number of oral chemotherapeutics available for the treatment of cancer has significantly increased, and more than 25% of the 400 neoplastic drugs currently in development are oral agents.1-3 Despite the route of administration, oral chemotherapy (OC) poses many of the same risks as intravenous (IV) agents, and it may even have greater risks of drug-drug interactions because of inconsistency in absorption and metabolism.3 Although most institutions have strict guidelines for the administration of IV chemotherapy, similar protocols generally do not exist for OC. In 2007, an evaluation of 42 comprehensive cancer centers in the United States revealed significant variability in the OC prescription process, with only nine centers requiring a double check of the prescription by a second provider.4 More recent data from a cross-sectional survey in 2012 among French oncology physicians demonstrated that only 8% of prescribers had the OC prescription verified by another clinician.3
As a result of this shifting paradigm in oncology care and the lack of standardization in OC, ASCO collaborated with the Oncology Nursing Society to update their chemotherapy safety standards in 2013 to specifically include OC. These standards redefined the term chemotherapy to include all nonhormonal oral agents, such as targeted therapies, and created consensus recommendations to improve on the safety of the prescription and administration of OC.5 At the Abramson Cancer Center (ACC) of the University of Pennsylvania, the prescription process for OC was individualized by each provider and lacked standardization. As a response to the new OC safety standards, a quality improvement (QI) project was begun to decrease the risk of drug-drug interactions or dosing errors by means of a pharmacist's review of the OC prescription.
METHODS
A multidisciplinary team composed of three physicians, two ACC pharmacists, and one nurse practitioner was assembled to lead this QI initiative. The team used Lean Six Sigma performance improvement methods for this QI project. Lean Six Sigma is a QI method that focuses on systematically reducing waste and using a data-based approach to improve the design and execution of a process in any industry.6,7 Key steps in this approach are defining the problem from the perspective of the customer or patient, which, in this case, was to reduce the risk of prescription errors and drug-drug interactions with OC to improve patient safety. After the problem is identified, the next steps include acquisition of data to clearly describe the current condition, recognition and analysis of the root causes of the problem, and, last, development of targeted measures to improve the process. This project was reviewed by the institutional review board of the University of Pennsylvania and was determined to be a QI initiative.
Baseline Condition
To understand the current process, several practitioners were interviewed and then observed while prescribing OC. During these observations, we learned that oncology-specific ACC pharmacists reviewed all outpatient IV chemotherapy before its administration, but they were not involved in the review of outpatient OC prescriptions. In general, the only pharmacist who reviewed the OC prescription was a retail or specialty pharmacist who may not have access to vital patient information, such as recent laboratory test results or a current medication list for the patient. With regard to retail pharmacies specifically, these pharmacists may lack oncology-related pharmacology training, and they may not be familiar with infrequently prescribed OC.
Root-Cause Analysis
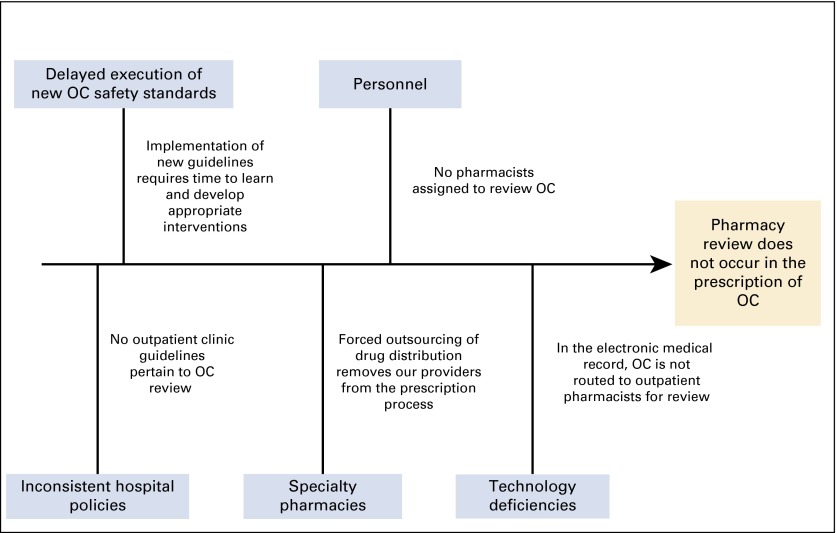
The QI team used a fishbone diagram (Fig 1) as a root-cause analysis tool to identify the causes of the lack of pharmacist oversight during the OC process. We determined that both policy and practice varied between OC prescriptions for inpatients in our hospital and outpatients in the ACC. Although inpatient orders for OC in the hospital required verification in the pharmacy before its administration, this was not required in the outpatient setting. The lack of pharmacy oversight in the outpatient setting was caused by the absence of pharmacy personnel dedicated to the review of OC and the lack of routing of OC prescriptions through EPIC (EPIC Systems, Verona, WI), our electronic medical record (EMR), to an ACC pharmacist for review.
FIG 1.
Fishbone diagram depicts the root causes of the lack of review of oral chemotherapy (OC) prescriptions.
Interventions
Given the aforementioned findings, targeted measures were implemented in the melanoma disease group of the ACC. We worked with our information technology team to develop a system in our EMR where all specified OC drug orders were identified and subsequently routed to an outpatient ACC oncology-trained pharmacist for review immediately after it was prescribed by the oncology provider. The ACC pharmacist then thoroughly evaluated the drug using an OC review form created by the QI team. The 2013 chemotherapy safety standards were used to develop this form, which included pertinent information such as body surface area calculations, current medication list, and potential drug-drug interactions. Renal and hepatic dosing adjustments and additional monitoring required were reported by using Food and Drug Administration labeling recommendations (Appendix, online only). Pharmacists completed this form, placed it into our EMR, and subsequently routed the note to the prescribing provider. Pharmacists contacted physicians directly at their own discretion regarding dosing and/or monitoring recommendations or about potential drug-drug interactions. After 2 months of application in the melanoma practice, these interventions were expanded to include additional disease practices, specifically the breast and thoracic oncology groups.
RESULTS
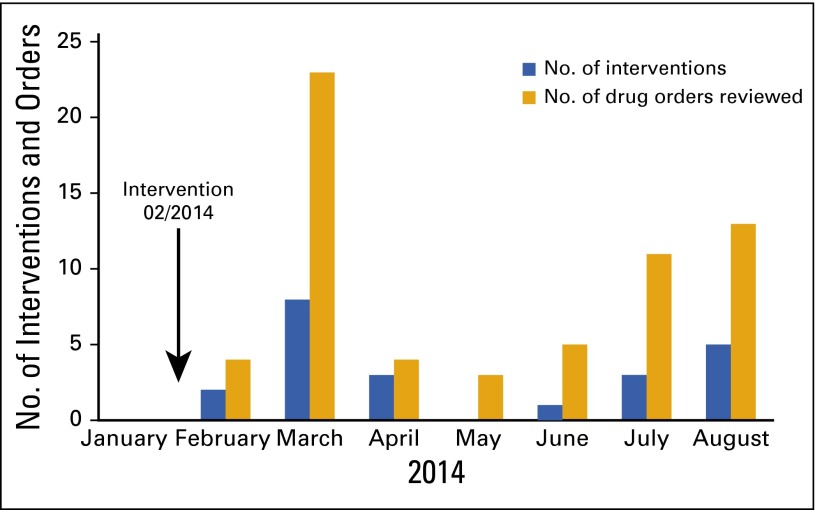
From February 2014 to August 2014, all orders for OC drugs that were placed electronically for the treatment of melanoma, breast cancer, and lung cancer were routed to an outpatient ACC pharmacist for review. Handwritten prescriptions were unable to be reviewed because they were not captured electronically. In total, 63 orders for OC were reviewed for 45 individual patients. For the 63 orders, ACC pharmacists made 22 interventions (Fig 2). A median of five (range, three to 23) OC orders were reviewed each month. We believe that the peak of 23 orders reviewed was a result of the recent Food and Drug Administration approval of two oral melanoma drugs that occurred during this period. No significant dosing errors were discovered.
FIG 2.
Number of oral chemotherapy agents reviewed and intervened upon by pharmacists at the Abramson Cancer Center of the University of Pennsylvania from January to August 2014.
Types of pharmacy interventions included recommendations regarding dosage modification (one of 22), discontinuation of an interacting drug (nine of 22), and guidance regarding necessary monitoring tests (12 of 22). Of these 22 interventions, the pharmacist directly contacted the ordering provider for 16 of them. The remaining six were communicated by means of messaging in our EMR, with electronic acknowledgment of receipt of the routed note. In the 16 circumstances in which the provider was directly contacted, the physicians agreed with the recommendations in all cases but one. Specific examples of pharmacists’ recommendations included echocardiographic monitoring with the prescription of trametinib, a dosage reduction of vemurafenib for a patient with concurrent liver dysfunction, increased ECG monitoring for a patient receiving concurrent dronaderone and vemurafenib, and modification of proton-pump inhibitor therapy in the setting of erlotinib and dabrafenib. In the performance of these reviews, ACC pharmacists spent a mean of 22 minutes per patient.
DISCUSSION
With the increasing use of OC in the treatment of cancer, standardized protocols will be important to improve the safety and reliability of the prescription process. The lack of a consistent process for OC has led national organizations such as ASCO and the Oncology Nursing Society to create new safety standards for OC.5 At our institution, a QI project was implemented to minimize the risk of OC prescription errors and drug-drug interactions by having an oncology-trained pharmacist review the prescription.
At the ACC, the inclusion of outpatient, oncology-trained pharmacists in the review process led to meaningful interventions in one third of OC orders. Although most of these interventions were not dosage related, ACC pharmacists were able to identify drugs that had the potential to interact with OC and recommended appropriate monitoring that may improve the safety of OC. Our QI initiative did not include a control group. Therefore, we cannot state with certainty which interacting drugs may have been discontinued or which monitoring tests may have been ordered without the involvement of a pharmacist or if adverse events or hospitalizations were avoided. However, the number of recognized issues highlights the complexity of OC and the need for review by a pharmacist. Although results of our project suggest benefits achieved with the review of orders for OC, potential costs must be considered. Specifically, these include the increased utilization of limited resources. To comprehensively review each OC prescription, ACC pharmacists spent approximately 22 minutes per patient. Given this new workload in addition to their preexisting responsibilities, our QI team determined that additional pharmacy staff would be needed before this protocol can be widely disseminated throughout our cancer center. However, as a result of the data obtained from this QI project, the ACC has created additional pharmacy staff positions, with one position specifically dedicated to the review of OC. Pending the hiring and training of this new staff member, we plan to make pharmacist review of OC prescriptions a new safety standard at our institution.
In conclusion, a QI intervention related to improvement of the safety and rigor of the OC prescription process was successfully implemented at a large, tertiary academic oncology center. As more novel OC drugs are introduced, the importance of prescription verification by a second provider will be vital to patient safety. Our process could serve as a model for other similar organizations that wish to include a review process for the prescription of OC.
Acknowledgment
Supported by the Cancer Center Research Training Program Grant No. NCI 5-T32 CA09615-25 and the Center for Health Care Improvement and Patient Safety at the University of Pennsylvania. Presented at the ASCO Quality Improvement Symposium, Boston, MA, October 17-18, 2014.
Appendix
Sample pharmacist's note for review of oral chemotherapy.
Pharmacist Medication Manager's Note for Oral Chemotherapy
Attending physician:
Patient's height: ________ Weight:__________ Body surface area:_________
WBC___ Absolute neutrophil count_____ Platelets _____ Serum creatinine_____ Total bilirubin ______
Current medication list:
Oral chemotherapy proposed (drug and dosage):
Primary oncologic diagnosis:
Brief history of present illness:
Renal/hepatic adjustment? Yes/No, comments:
Laboratory monitoring needed? Yes/No, comments:
Drug, food, and/or supplement interactions? Yes/No, comments:
Specific administration? (ie, with or without food, on empty stomach, with full glass of water, hydration requirement) Yes/No, comments:
Are premedications required? Yes/No, comments:
Additional comments:
Time spent preparing this note:
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Nirav N. Shah, Erica Casella
Data analysis and interpretation: Nirav N. Shah, Erica Casella, Jennifer S. Myers
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Improving the Safety of Oral Chemotherapy at an Academic Medical Center
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Nirav N. Shah
Stock or Other Ownership: Exelixis, ONCS, GERN
Erica Casella
Employment: Prime Healthcare Services
Leadership: Prime Healthcare Services
Donna Capozzi
No relationship to disclose
Suzanne McGettigan
Speakers' Bureau: Merck, BMS
Tara C. Gangadhar
Honoraria: Merck Sharp & Dohme
Lynn Schuchter
Research Funding: GlaxoSmithKline, Merck, Bristol-Myers Squibb, Genentech, Roche
Jennifer S. Myers
No relationship to disclose
References
- 1.Aisner J. Overview of the changing paradigm in cancer treatment: Oral chemotherapy. Am J Health Syst Pharm. 2007;64:S4–S7. doi: 10.2146/ajhp070035. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 2.Weingart SN, Brown E, Bach PB, et al. NCCN Task Force report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6:S1–S14. (suppl 3) [PubMed] [Google Scholar]
- 3.Bourmaud A, Pacaut C, Melis A, et al. Is oral chemotherapy prescription safe for patients? A cross-sectional survey. Ann Oncol. 2014;25:500–504. doi: 10.1093/annonc/mdt553. [DOI] [PubMed] [Google Scholar]
- 4.Weingart SN, Flug J, Brouillard D, et al. Oral chemotherapy safety practices at US cancer centres: Questionnaire survey. BMJ. 2007;334:407. doi: 10.1136/bmj.39069.489757.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuss MN, Polovich M, McNiff K, et al. 2013 updated American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards including standards for the safe administration and management of oral chemotherapy. J Oncol Pract. 2013;9:5S–13S. doi: 10.1200/JOP.2013.000874. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimmerson C. A3 Problem Solving for Healthcare: A Practical Method for Eliminating Waste. New York, NY: Productivity Press; 2007. [Google Scholar]
- 7.Scoville R, Little K. Cambridge, MA: Institute for Healthcare Improvement; 2014. Comparing Lean and Quality Improvement: IHI White Paper. [Google Scholar]