Abstract
Purpose: In this study, we investigated the effect of a slow-releasing hydrogen sulfide (H2S) donor, GYY 4137, on intraocular pressure (IOP) in normotensive rabbits. Furthermore, we compared the IOP-lowering action of GYY 4137 with those elicited by other H2S-producing compounds, l-cysteine and ACS67 (a hybrid compound of latanoprost with an H2S-releasing moiety).
Methods: IOP was measured in New Zealand normotensive male albino rabbits using a pneumatonometer (model 30 classic; Reichert Ophthalmic Instruments, Depew, NY). At 0 h, 50 μL of test compounds were applied topically to 1 eye of each animal, while the contralateral eye received the same quantity of vehicle (saline). IOP was measured hourly until baseline IOP readings were attained and animal eyes monitored for potential side effects (i.e., tearing, hyperemia).
Results: GYY 4137 (0.1%–2%) produced a dose-dependent decrease in IOP reaching a maximum of 27.8% ± 3.14% (n = 5) after 6 h. Interestingly, a significant contralateral effect was observed in vehicle-treated controls eyes at all doses tested. l-cysteine (5%) and ACS67 (0.005%) also elicited a significant (P < 0.01) decrease in IOP that achieved a maximum of 28.84% ± 1.53% (n = 5) and 23.27% ± 0.51% (n = 5), respectively, after 3 h. All 3 H2S-producing compounds also caused a significant contralateral effect in vehicle-treated control eyes.
Conclusion: We conclude that GYY 4137 and other H2S-producing donors can reduce IOP in normotensive rabbits. However, the profile of IOP-lowering action of GYY 4137 was different from the other H2S donors affirming its ability to act as a slow-releasing gas donor.
Introduction
Hydrogen sulfide (H2S) is an odiferous water-soluble gas that is commonly released into the environment by bacterial anaerobic digestion of organic matter.1 Although known for centuries as an environmental toxicant and industrial pollutant, there is evidence that H2S can act as a gaseous neurotransmitter in mammals.1 Three enzymes have been reported to synthesize H2S from the sulfur-containing amino acid, l-cysteine, in the presence of 2 pyridoxal 5′-phosphate (vitamin B6)-dependent enzymes, cystathionine β-synthase (CBS), or cystathionine-γ-lyase (CSE) enzymes, and 3-mercaptopyruvate sulfurtransferase (3MST) along with cysteine aminotransferase.2–4
Both CBS and CSE have been localized in mammalian ocular tissues suggesting a potential physiological/pharmacological relevance for H2S in the mammalian eye.5–8 Indeed, there is a correlation between the expression of enzymes of the biosynthetic pathways for H2S production with the endogenous production of this gas in ocular tissues.6,8 Deficiency of CBS has been associated with ocular diseases such as lens dislocation, retina degeneration, retinal detachment, and acute glaucoma.9 In the bovine isolated neural retina, inhibitors of CSE and CBS have been reported to reduce endogenous production of H2S, whereas an activator of CBS, S-adenosyl-l-methionine, enhanced the biosynthesis of this gas confirming the involvement of enzymes of the transsulfuration pathway in this tissue.8
In addition to the demonstration of an in situ production of H2S, there is evidence in favor of a pharmacological role for this gas in mammalian ocular tissues. In the anterior uvea, H2S donors such as NaHS and Na2S inhibited field-stimulated [3H] norepinephrine release and reduced catecholamine concentrations from isolated porcine iris-ciliary bodies.10 The ability of H2S donors to reduce sympathetic output was blocked by inhibitors of CSE and CBS, indicating that endogenously produced H2S is involved in this response.10
At postjunctional sites, H2S donors and its substrate, l-cysteine, have also been reported relax precontracted isolated porcine irides, an effect that was partially dependent upon endogenous biosynthesis of H2S in this tissue.11,12 Furthermore, in bovine posterior ciliary arteries, H2S donors such as NaHS, GYY 4137, AP67, and AP72 have been shown to relax phenylephrine-induced tone by an action that is partially dependent upon the endogenous production of H2S, prostanoids, and KATP channels.13,14
In the retina, H2S donors can inhibit excitatory amino acid neurotransmission from both isolated bovine and porcine tissues by an effect that was partially dependent on the endogenous biosynthesis of this gas.15 There is evidence that H2S donors can increase cyclic AMP production in isolated bovine and porcine retinae and retinal pigment epithelial cells, an effect that was dependent on endogenous biosynthesis of H2S and on the functional integrity of KATP channels.16,17 Taken together, these observations affirm the fact that H2S can have both physiological and pharmacological actions on ocular tissues.
In preliminary studies, we found that the H2S donor, NaHS, can reduce intraocular pressure (IOP) on normotensive conscious albino rabbits.18 Similarly, ACS67, an H2S-releasing derivative of latanoprost, induced a much higher decrease in IOP than the parent compound in 2 glaucomatous rabbit models, indicating a role for H2S in the regulation of aqueous humor dynamics.19 The aim of this study is to compare the IOP-lowering effect of 3 different categories of H2S-releasing compounds (Fig. 1) in normotensive rabbits: GYY 4137, a slow H2S-releasing compound; ACS67, a fast-releasing hybrid of latanoprost; and an H2S-producing moiety and l-cysteine, a substrate for the endogenous production of H2S. Parts of the data presented in this article have been communicated in an abstract form.20
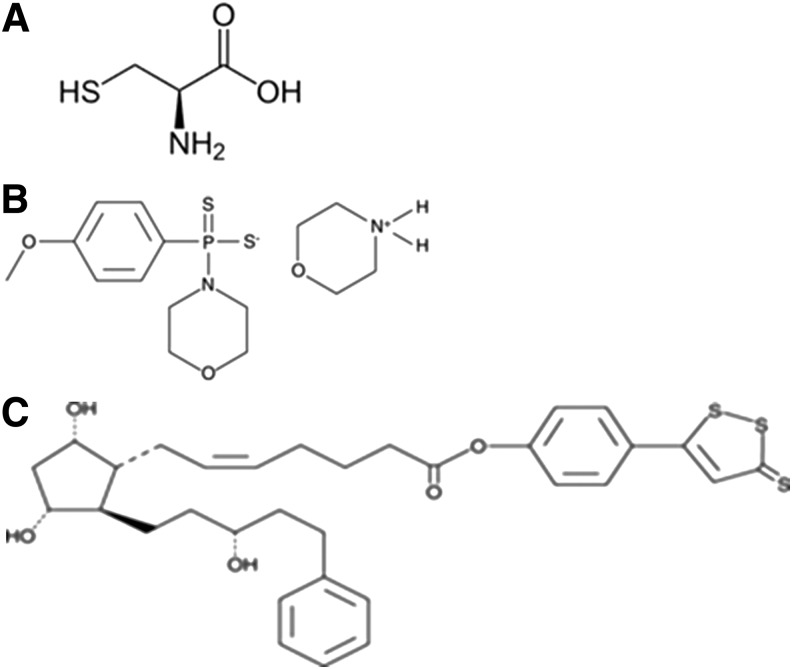
FIG. 1.
Chemical structures for hydrogen sulfide-producing compounds. (A) l-cysteine; (B) GYY 4137; and (C) ACS67.
Methods
Chemicals
ACS67 [7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl ester, 5Z-heptenoic acid] and GYY 4137 [(p-methoxyphenyl)morpholino-phosphinodithioic acid] were purchased from Cayman Chemicals (Ann Arbor, MI). l-cysteine was purchased from Sigma-Aldrich (St. Louis, MO; 63103).
Measurement of IOP
Animal protocols were approved by the Institutional Animal Care and Use Committee. Animal studies were conducted in adherence to the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
New Zealand normotensive male albino rabbits (weighing about 2 kgs) were purchased from Charles River Laboratories and conditioned to 12-h light–12-h dark cycles and divided into groups of 5 animals each. Each concentration of test drug was tested in 5 different animals. When utilized multiple times, animals were allowed a wash-out period of at least 7 days before they were used to evaluate the IOP effects of other test drugs. Test drugs were applied at 8:00 am in the morning and were unmasked to the investigators.
On the day of the experiment, baseline IOP was measured 30 min before and 0 h after topical application of proparacaine 0.5% (local anesthetic). Measurements of IOP were taken in gently restrained conscious animals using a pneumatonometer (model 30 classic; Reichert Ophthalmic Instruments, Depew, NY). At 0 h, 50 μL of compounds (GYY 4137, ACS67, and l-cysteine) were applied topically to 1 eye of each animal, while the contralateral eye received the same quantity of vehicle (saline). IOP was measured hourly until baseline IOP readings were attained to observe the complete profile of action of the compounds administered. Eyes of animals treated with the various compounds were also monitored for potential side effects (i.e., tearing, hyperemia).
Data analysis
Results were expressed as change in IOP (mmHg) and/or percentage inhibition of IOP. Except where indicated otherwise, values given are arithmetic mean ± standard error of the mean. Significance of differences between control and agent-treated preparations was evaluated using analysis of variance followed by Tukey's post-test. Differences with P-values <0.05 were accepted as statistically significant.
Results
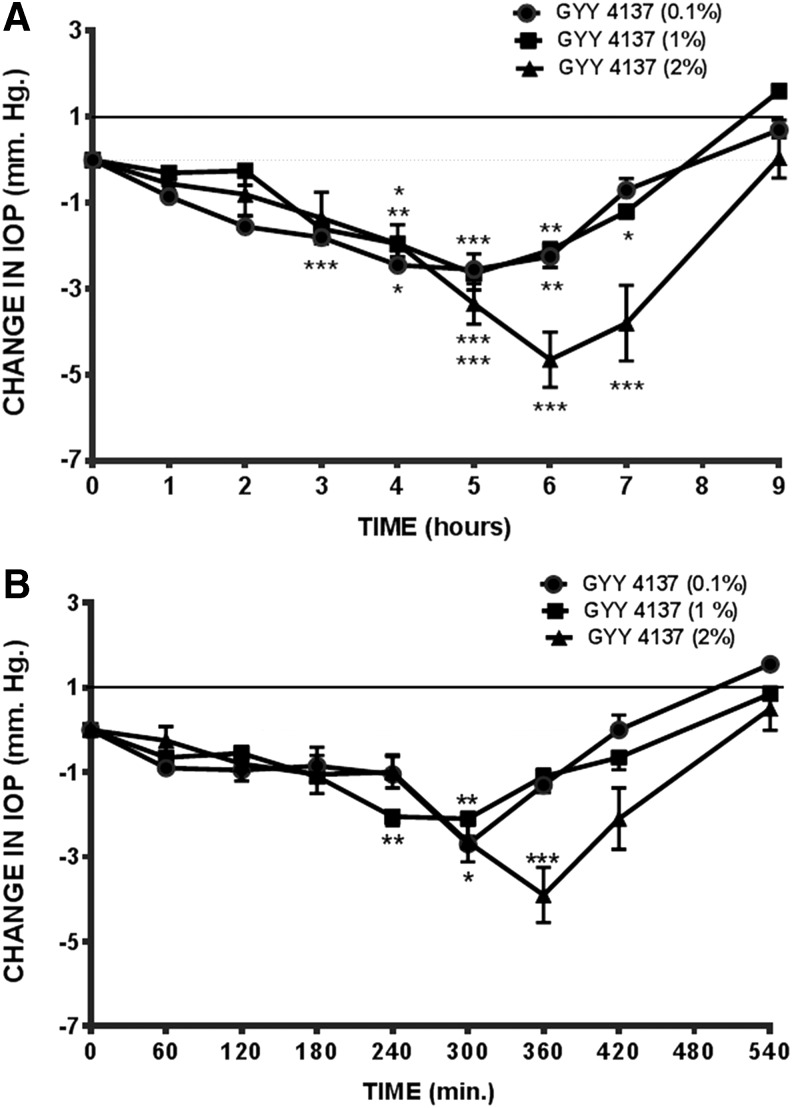
As illustrated in Fig. 2A, topical application of GYY 4137 (0.1%–2%) elicited a dose-dependent decrease in IOP. Interestingly, a fall in IOP was also observed in the contralateral eyes treated with the vehicle (Fig. 2B). At a dose of 2%, GYY 4137 caused a 27.48% ± 3.14% (n = 5) maximal decrease in IOP with a duration of action that lasted up to 9 h. The decrease in IOP observed in the contralateral (vehicle control) eyes of animals treated with GYY 4137 also exhibited a similar pattern of response and recovery (Fig. 2B). We observed that eyes treated with GYY 4137 and its vehicle did not exhibit any ocular side effects (hyperemia, tearing, etc.).
FIG. 2.
Effect of GYY 4137 on IOP in normotensive conscious albino rabbits in vivo. (A) GYY 4137 (0.1%–2%)-treated and (B) contralateral, vehicle-treated eyes. Vertical bars represent means ± SEM of data obtained from 5 rabbits. *P < 0.05, **P < 0.001; ***P < 0.001, significantly different from baseline IOP. IOP, intraocular pressure; SEM, standard error of the mean.
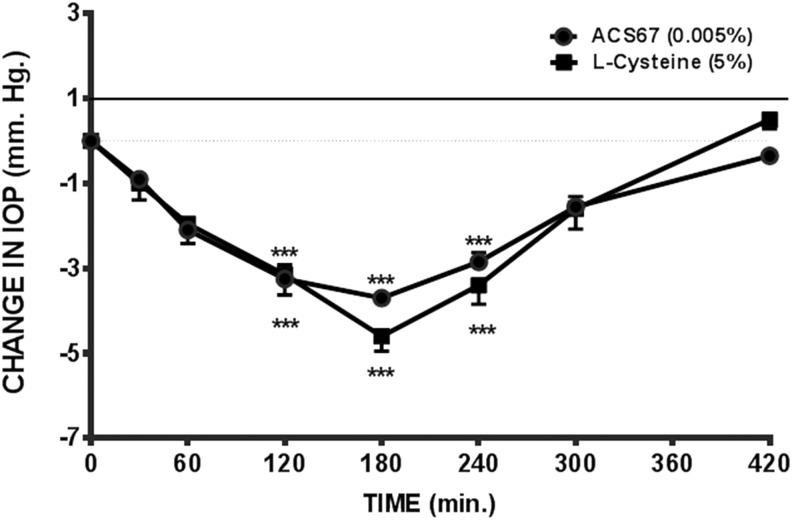
We compared the pharmacological actions of GYY 4137 with those of l-cysteine, a substrate for the production of H2S. Topical application of l-cysteine (5%) caused a 28.84% ± 1.53% (n = 5) decrease in IOP, which reached a maximum in 3 h and lasted up to 7 h (Fig. 3). A contralateral decrease in IOP was observed in the vehicle-treated (control) eyes. The fall in IOP in the control eyes displayed a similar pattern of response seen in compound-treated ones. In addition, no ocular side effects were observed in eyes treated with l-cysteine or in the vehicle (control) ones.
FIG. 3.
Effect of hydrogen sulfide-producing compounds, l-cysteine (5%) and ACS67 (0.005%), on IOP in normotensive conscious albino rabbits in vivo. Vertical bars represent means ± SEM of data obtained from 5 rabbits. ***P < 0.001, significantly different from baseline IOP.
We next examined the effect of a topical application of 2 doses of ACS67 on IOP. ACS67 [0.005% (Fig. 3) and 0.01%] elicited a 23.3% ± 0.51% (n = 5) and a 22.84% ± 3.41% (n = 5) reduction in baseline IOP that reached a maximum in ∼3 h and lasted up to 7 h. A corresponding decrease in IOP was observed in the contralateral vehicle-treated (control) eyes of animals exposed to both doses of ACS67. We did not observe ocular side effects in animals treated with both doses of ACS67. Table 1 summarizes the effects of doses of GYY 4137, l-cysteine, and ACS67 that produced an equivalent maximal reduction of IOP in normotensive animals.
Table 1.
Peak Activity, Duration of Action, and Maximum IOP Reduction (%) of Hydrogen Sulfide Donors
| Drug | Concentration (%) | Peak activity (h) | Duration of action (h) | Max ± SEM (%) IOP reduction |
|---|---|---|---|---|
| GYY 4137 | 0.1 | 5 | 9 | 16.34 ± 2.37 |
| GYY 4137 | 1 | 5 | 9 | 17.04 ± 1.79 |
| GYY 4137 | 2 | 6 | 9 | 27.84 ± 3.14 |
| ACS67 | 0.005 | 3 | 7 | 23.27 ± 0.51 |
| ACS67 | 0.01 | 3–4 | 7 | 22.84 ± 3.41 |
| l-Cysteine | 5 | 3 | 7 | 28.84 ± 1.53 |
IOP, intraocular pressure; SEM, standard error of the mean.
Discussion
It is well established that exposure of the mammalian eye to high concentrations of H2S can lead to deleterious side effects.21 For instance, concentrations of H2S above 50 ppm at the mucus membrane can lead to keratoconjunctivitis.22 Recently, the possible physiological and pharmacological actions of this gas have been demonstrated in ocular tissues from several mammalian species. The presence and distribution of enzymes responsible for the biosynthesis of H2S in ocular tissues (such as CBS and CSE) have been reported by several investigators.5–8 Interestingly, insufficiency of CBS due to a deficiency in the gene encoding this enzyme has been linked to some ocular disorders such as retinal detachment and acute glaucoma.9 In addition to enzymes of the transsulfuration pathway involved in the production of H2S, substrates such as cysteine and homocysteine are present in ocular tissues. Indeed, elevated concentrations of homocysteine have been reported in the aqueous humor, tear fluid, and plasma of patients with primary open-angle glaucoma.23,24 Furthermore, Roedl et al. found an increased level of homocysteine in the tear fluid and plasma of patients with pseudoexfoliation glaucoma.25 In 2014, Ran et al. reported elevated concentrations of H2S in the vitreous body and plasma of patients with proliferative diabetic retinopathy.26
In addition to its potential physiological and pathophysiological roles in the eye, H2S has been shown to exert pharmacological actions in mammalian ocular anterior and posterior segments. For instance, H2S can alter neurotransmitter release from tissues of the anterior uvea and retina and can exert a neuroprotective action in the retina.10,15–17,19,27,28 There is evidence that H2S can relax both isolated mammalian irides and posterior ciliary arteries.11–14 ACS67, a novel H2S-releasing derivative of latanoprost, was found to exert a greater fall in IOP than the parent compound in 2 glaucomatous rabbit models, indicating a role for H2S in aqueous humor dynamics as well.19
In this study, we compared the pharmacological actions of a slow-releasing H2S donor, GYY 4137, with those of other donors, l-cysteine and ACS67, on IOP in normotensive rabbits. GYY 4137 has been reported to act as a water soluble, slow-releasing H2S donor.29,30 We observed that topical administration of GYY 4137 caused a dose-dependent decrease in IOP that reached a maximum in 5–6 h and lasted up to 9 h before its return to baseline pressure. Interestingly, a corresponding fall in IOP was observed in the contralateral vehicle (control) eyes at all doses tested. The observed consensual ophthalmotonic reaction observed in the control eyes could be due to a systemic action of the absorbed topical dose of GYY 4137 or by a central action on IOP control mechanisms31.
NaHS is a widely used H2S donor that has been shown to release large amounts of the gas in a short duration of time.29,30 In preliminary studies, a dose of 1%, topically applied NaHS caused a drop in IOP of 28.13% (n = 6), which peaked in 3 h and lasted up to 7 h.18 A comparable effect was observed for l-cysteine, a substrate for the production of H2S, which also caused a maximal fall in IOP in 3 h that lasted up to 7 h before its return to baseline pressure. Similar to NaHS, the contralateral vehicle-treated (control) eyes of animals exposed to l-cysteine also displayed a concomitant parallel decrease in IOP that lasted up to 7 h.18 The observed contralateral effect in the vehicle-treated eyes may be due to a systemic action of these compounds or their central effect on IOP control mechanisms. It is pertinent to note that the maximal reduction in IOP elicited by l-cysteine occurred earlier and the duration of action was shorter than that observed with GYY 4137.
Since ACS67 has been reported to decrease IOP in 2 models of induced glaucoma in rabbits, this study investigated the pharmacological actions of this compound on IOP in normotensive animals.19 We found that ACS67 elicited a fall in IOP that reached a maximum in ∼3 h and lasted up to 7 h. Our data support the observation made by Perrino et al. that ACS67 can lower IOP in both normotensive and glaucomatous animals.19 A corresponding decrease in IOP was observed in the contralateral vehicle (control)-treated eyes at both doses tested. The consensual ophthalmotonic reaction may be due to a systemic action of ACS or its central effect on IOP control mechanisms. Since New Zealand albino rabbits are reportedly resistant to the IOP-lowering effects of latanoprost,19 it is conceivable that the H2S accounts for the observed ocular hypotensive effect elicited by ACS67. In comparison with GYY 4137, ACS67 displayed a profile of reduction in IOP that reached its maximum earlier and had a shorter duration of action.
It is of interest to note that all 3 H2S-releasing compounds tested lowered IOP and caused a corresponding contralateral effect in vehicle-treated eyes. The ability of ocular hypotensive compounds to elicit a parallel consensual ophthalmotonic reaction has been reported for β-adrenoceptor antagonists such as timolol and for muscarinic receptor agonists such as pilocarpine.31 The contralateral effects observed with H2S donors may be due to the inherent ability of the gas to easily cross biological membranes. The exact mechanism of action of H2S in regulating aqueous humor dynamics is unknown. It is tempting to speculate that due to its ability to modify sympathetic neurotransmission, an effect of H2S on nerve activity and/or neurotransmitter pools in the anterior uvea may account, at least in part, for its action on IOP.10
We conclude that H2S-releasing compounds can lower IOP in normotensive animals. The slow-releasing H2S compound, GYY 4137, displayed a profile of action that took a longer time to reach maximal reduction in IOP and a longer time to recover from its action. On the other hand, the substrate for H2S biosynthesis, l-cysteine and ACS67, elicited a fall in IOP that reached its maximum in a shorter time and recovered much faster than GYY 4137. The profile of IOP-lowering action of GYY 4137 was different from the other H2S donors confirming its ability to act as a slow-releasing H2S donor.
Acknowledgments
The project described was supported by Grant Number G20RR024001 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. This project was also supported by Grant Number 1R15EY022215-01 from the National Institute of Health, National Eye Institute.
Author Disclosure Statement
The authors, S.E.O. and C.A.O., hold U.S. Patent 8092838, 2012 for “Use of hydrogen sulfide in the treatment of eye diseases.”
References
- 1.Wagner F., Asfar P., Calzia E., Radermacher P., and Szabo C. Bench-to-bedside review: hydrogen sulfide-the third gaseous transmitter: applications for critical care. Crit. Care. 13:213, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper A.J.L. Biochemistry of sulfur-containing amino acids. Annu. Rev. Biochem. 52:187–222, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Shibuya N., Mikami Y., Kimura Y., and Nagahara N.K.H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biol. Chem. 146:623–626, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Shibuya N., Tanaka M., Yoshida M., Ogasaware Y., Togawa T., and Ishii K.K.H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 11:703–714, 2009 [DOI] [PubMed] [Google Scholar]
- 5.De L.G., Ruggeri P., and Macaione S. Cystathionase activity in rat tissues during development. Ital. J. Biochem. 23:371–379, 1974 [PubMed] [Google Scholar]
- 6.Persa C., Osmotherly K., Chao-Wei C.K., Moon S., and Lou M.F. The distribution of cystathionine beta-synthase (CBS) in the eye: implication of the presence of a trans-sulfuration pathway for oxidative stress defense. Exp. Eye Res. 83:817–823, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Pong W.W., Stouracova R., Frank N., Kraus J.P., and Eldred W.D. Comparative localization of cystathionine beta-synthase and cystathionine gamma-lyase in retina: differences between amphibians and mammals. J. Comp. Neurol. 505:158–165, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni K., Njie-Mbye Y.F., Okpobiri I., Zhao M., Opere C.A., and Ohia S.E. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem. Res. 36:1540–1545, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Kraus J.P., and Kozich V. Cystathionine B synthase and its deficiency. In: Carmel R., and Jacobsen D.W., eds. Homocysteine in Health and Disease. Cambridge: Cambridge University Press; 2001; p. 223–243 [Google Scholar]
- 10.Kulkarni M., Kulkarni K.H., Monjok E.M., et al. Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem. Res. 34:400–406, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Monjok E.M., Kulkarni K.H., Kouamou G., et al. Inhibitory action of hydrogen sulfide on muscarinic receptor-induced contraction of isolated porcine irides. Exp. Eye Res. 87:612–616, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Ohia S.E., Opere C.A., Monjok E.M., Kouamou G., LeDay A.M., and Nije-Mbye Y.F. Role of hydrogen sulfide production in inhibitory action of L-cysteine on isolated porcine irides. Curr. Eye Res. 35:402–407, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Chitnis M.K., Nije-Mbye Y.F., Opere C.A., Wood M.E., Whiteman M., and Ohia S.E. Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp. Eye Res. 116C:350–354, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni-Chitnis M., Njie-Mbye Y.F., Mitchell L., et al. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp. Eye Res. 134:73–79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opere C.A., Monjok E.M., Kulkarni K.H., and Nije Y.F. Regulation of [3H]D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem. Res. 34:1962–1968, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Nije-Mbye Y.F., Bongmba O.N., Onyema C.C., et al. Effect of hydrogen sulfide on cyclic AMP production in isolated bovine and porcine neural retinae. Neurochem. Res. 35:487–494, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Nije-Mbye Y.F., Kulkarni M., Opere C.A., and Ohia S.E. Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp. Eye Res. 98:16–22, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Ohia S.E., Opere C.A., Zhan G.L., Monjok E.M., and Kulkarni K. Use of hydrogen sulfide in the treatment of eye diseases. U.S. Patent 8092838, 2012
- 19.Perrino E., Uliva C., Lanzi C., Del Soldato P., Masini E., and Sparatore A. New prostaglandin derivative for glaucoma treatment. Bioorg. Med. Chem. Lett. 19:1639–1642, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Salvi A, Bankhele P, Jamal J, Njie Mbye YF, Kulkarni M, Ohia SE, Opere CA. “Regulation of mammalian sympathetic neurotransmitter release and intraocular pressure by hydrogen sulfide donor, GYY 4137.” Association for Research in Vision and Ophthalmology Annual Meeting, Invest Ophthalmol Vis Sci, Vol 54, Issue 15, Abstract #1976, 2013 [Google Scholar]
- 21.Lambert T.W., Goodwin V.M., Stefani D., and Strosher L. Hydrogen sulfide (H2S) and sour gas effects on the eye. A Historical perspective. Sci. Total Environ. 367:1–22, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Guidotti T.L. Hydrogen sulphide. Occup. Med. (Lond.). 46:367–371, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Roedl J.B., Bleich S., Reulbach U., et al. Homocystein levels in aqueous humor and plasma of patients with primary open-angle glaucoma. J. Neural Trans. 114:445–450, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Roedl J.B., Bleich S., Schlotzer-Schrehardt U., et al. Increased homocysteine levels in tear fluid of patients with primary open-angle glaucoma. Ophthalmic Res. 40:249–256, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Roedl J.B., Bleich S., Reulbach U., et al. Homocysteine in tear fluid of patients which pseudoexfoliation glaucoma. J. Glaucoma. 16:234–239, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ran R., Du L., Zhang X., et al. Elevated hydrogen sulfide levels in vitreous body and plasma in patients with proliferative diabetic retinopathy. Retina. 34:2003–2009, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Osborne N.N., Ji D., Abdul Majid A.S., Fawcett R.J., Sparatore A., and Del Soldato P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest. Ophthalmol. Vis. Sci. 51:284–294, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Mikami Y., Shibuya N., Kimura Y., Nagahara N.K.H., Yamada M., and Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 286:39379–39386, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Whiteman M., Guan Y.Y., et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137); new insights into the biology of hydrogen sulfide. Circulation. 117:2351–2360, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Martelli A., Testai L., Citi V., et al. Arylthioamides as H2S donors: 1-cysteine-activated releasing properties and vascular effects in vitro and in vivo.. ACS Med. Chem. Lett. 4:904–908, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibbens M.V. The consensual ophthalmotonic reaction. Br. J. Oththalmol. 72:746–749, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]