Summary
Heparan sulphate (HS) is ubiquitously expressed and is formed of repeating glucosamine and glucuronic/iduronic acid units which are generally highly sulphated. HS is found in tissues bound to proteins forming HS proteoglycans (HSPGs) which are present on the cell membrane or in the extracellular matrix. HSPGs influence a variety of biological processes by interacting with physiologically important proteins, such as morphogens, creating storage pools, generating morphogen gradients and directly mediating signalling pathways, thereby playing vital roles during development. This review discusses the vital role HS plays in the development of tissues from the ectodermal lineage. The ectodermal layer differentiates to form the nervous system (including the spine, peripheral nerves and brain), eye, epidermis, skin appendages and tooth enamel.
Introduction
Heparan sulphate (HS), a prototype glycosaminoglycan (GAG), is a linear polysaccharide composed of repeating disaccharide units comprised of an N‐acetylglucosamine (GlcNAc) and a glucuronic acid (GlcA) (Figure 1). Following polymerization, HS chains undergo secondary modifications, which introduce immense structural diversity as a result of varying substitution with sulphate groups and glucuronic acid epimerization (Figure 2). Similar to other GAGs, HS is found in tissues bound to proteins forming HS proteoglycans (HSPGs). HS is structurally related to heparin, a highly sulphated and epimerized form of HS that is restricted to mast cells and synthesized attached to the small core protein (18 kDa) serglycin (Tantravahi et al. 1986). HSPGs are ubiquitously expressed in mammalian cells and are present on cell surfaces, in the extracellular matrix (ECM) and basement membranes.
Figure 1.
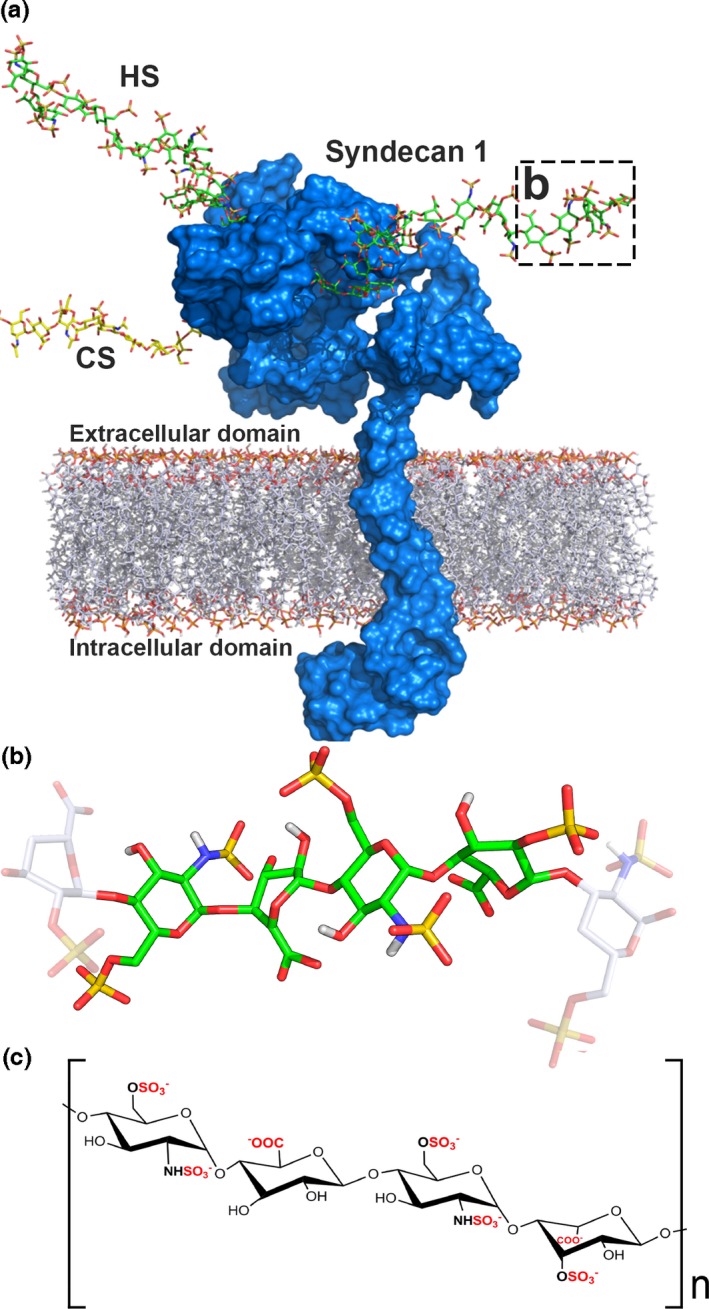
Proposed model for the HSPG syndecan‐1 and HS structure. (a) The core protein of syndecan‐1 (blue) is immersed in a lipid DPPC membrane and HS (green carbon atoms) and CS (yellow carbon atoms) chains are attached to the extracellular domain at positions Ser37 (CS), Ser45 (HS) and Ser47 (HS). The structure of syndecan‐1 was modelled using MODELLER (Marti‐Renom et al. 2000) and the DPPC membrane, tetrasaccharide linkage region, and both HS and CS chains were added using charmm version c37b2 (Brooks et al. 2009). The highlighted box (b) is represented in more detail in (b). (b) A HS tetrasaccharide is shown in detail with carbon (green sticks), oxygen (red sticks), sulphate (yellow sticks) and nitrogen atoms (blue sticks). (c) Chemical structure of HS, which is comprised of repeating disaccharide units (GlcA or IdoA and GlcN). Putative sulphation and epimerization sites are highlighted in red.
Figure 2.
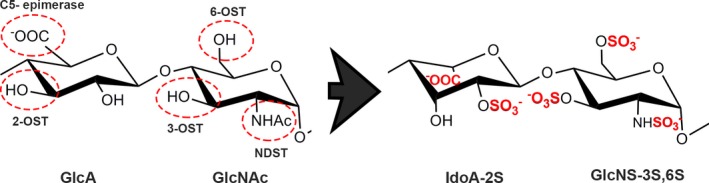
Schematic of modifications on heparan sulphate chains by biosynthetic enzymes. HS is synthesized by sequential addition of alternating GlcA and GlcNAc by EXT1 and EXT2 respectively. Thereafter, a group of enzymes further modifies the HS chain: C5 epimerase epimerizes GlcA to IdoA, and the sulfotransferases NDST, 2‐OST, 3‐OST and 6‐OST add sulphate groups.
During evolution, HS emerged in the animal kingdom with the eumetazoa, the first animal clade to display tissues organized into germ layers (Gesteira et al. 2011a; Yamada et al. 2011). HSPGs are vital in many cellular processes, ranging from development through to adult physiology, and changes in HSPGs have been correlated with a plethora of diseases. HSPGs function primarily through interactions with various protein ligands, and the fine structure of HS chains dictates binding specificity. HSPGs are required for the action of many growth factors and morphogens by localizing them to particular sites (creating a morphogen gradient and forming ‘storage pools’), by independently activating signalling pathways and by acting as coreceptors participating in the formation of ternary complexes required for the attachment of specific molecules to their receptors. The interaction of HS with growth factors in the ECM also protects these molecules from degradation and regulates their diffusion throughout the tissue (Rosengart et al. 1988; Saksela et al. 1988).
The ECM is a dynamic and complex environment that plays a critical role during development, and GAGs and PGs are integral components of this matrix. Early in development, three primary germ layers are formed: the ectoderm, mesoderm and endoderm, each with distinct characteristics giving rise to specific tissues or tissue parts. The ectodermal layer differentiates to form the nervous system (including the spine, peripheral nerves and brain), eye, epidermis, skin appendages, tooth enamel and the lining of the mouth, anus and nostrils. As previously mentioned, HSPGs are found both on the cell surface (Figure 1) and in the ECM where they influence a variety of biological processes by interacting with physiologically important proteins, such as morphogens, creating storage pools, generating morphogen gradients and directly mediating signalling pathways, thereby playing vital roles during development (Vlodavsky et al. 1991). In this review, we discuss the role of HS in the development of tissues of ectodermal origin.
Glycosaminoglycan biosynthesis
GAG biosynthesis is conserved from nematodes to man and occurs in a non‐template‐driven manner. GAG biosynthesis is a dynamic enzymatically regulated process which yields chains with a plethora of variable sulphation patterns (Prydz & Dalen 2000). HS/HEP biosynthesis requires a ‘primed’ core protein upon which the exostosin proteins EXT1/EXT2 polymerize the sugar chains. The core protein is ‘primed’ by the addition of a tetrasaccharide linkage region. The addition of this tetrasaccharide starts with the addition of a xylose by xylosyltransferase in the late endoplasmic reticulum and/or the cis‐Golgi compartment (Bourdon et al. 1987; Vertel et al. 1993; Gesteira et al. 2011b). Thereafter, two galactose monosaccharides (Galb1,4) are sequentially added to the xylose residue by galactosyltransferases I and II (Lohmander et al. 1989), and the tetrasaccharide is completed by the addition of a glucuronic acid residue by glucuronosyltransferase I (Vertel et al. 1993; Gesteira et al. 2011a). The subsequent addition of N‐acetylglucosamine by N‐acetylglucosaminyltransferase‐I (GlcNAcT‐I) determines the fate of the growing GAG to HS/HEP (Prydz and Dalen 2000). Thereafter, biosynthesis continues with the alternating addition of glucuronic acid (GlcA) and N‐acetylglucosamine (GlcNAc) residues by the HS polymerases exostosin 1 and exostosin 2 (EXT1 and EXT2) (Kitagawa & Sugahara 2000; Senay et al. 2000). The structure of this backbone composed of repeating GlcA–GlcNAc disaccharide units is then modified by a group of enzymes, which leads to the great heterogeneity shown by HS/HEP. N‐deacetylase/N‐sulfotransferase (NDST) removes the acetyl group from GlcNAc which is subsequently sulphated by the addition of a sulphate group into GlcNS (Aikawa & Esko 1999; Gesteira et al. 2011b, 2013). Evidence exists to suggest that the N‐deacetylation/N‐sulphation of GlcNAc precedes other modifications, such that NDST1 knockout mice also present a decrease in 2‐O‐sulphation to the same magnitude as N‐sulphation (Aikawa & Esko 1999; Ledin et al. 2004). C5 epimerase epimerizes GlcA to α‐L‐iduronic acid (IdoA), 2‐O‐sulfotransferase sulphates uronic acid, and both 6‐O‐sulfotransferase and 3‐O‐sulfotransferase add a sulphate group to glucosamine residues (Kitagawa et al. 1997). A strong interaction has been demonstrated between C5 epimerase and 2‐O‐sulfotransferase, and this association is essential for epimerase stability and translocation to the Golgi apparatus (Pinhal et al. 2001). Different isoforms exist for many of the modification enzymes. Four isoforms have been identified for NDST, namely NDST‐1–4. The different isoforms present different spatial–temporal distribution patterns. NDST‐1 and NDST‐2 are more widely distributed with some overlapping tissue distribution, while NDST‐3 and NDST‐4 have more restricted tissue distribution (Grobe et al. 2002). A variety of complete systemic knockout mice and also conditional knockout mice models have been developed for the different biosynthetic enzymes. In essence, HS and HEP originate from the same core structure; however, downstream modifications differentiate the two, resulting in different degrees of epimerization and levels of sulphation. As such, HEP is synthesized upon a serglycin core protein, presents most of GlcA epimerized to IdoA and is heavily sulphated (Kolset & Gallagher 1990; Kolset et al. 2004). The downstream modifications are illustrated in Figure 2. The HS chain sulphation pattern is further modified postsynthesis through the action of two extracellular endosulfatases, HS 6‐O‐endosulfatase 1 (SULF1) and 6‐O‐endosulfatase 2 (SULF2) (Lai et al. 2003; Kleinschmit et al. 2010; Higginson et al. 2012). SULFs are present in the extracellular space, and therefore, induction of their expression culminates in further alterations in the HS chain sulphation pattern. The biosynthetic pathway ensures the HS chains present a great variety of sulphation patterns which dictate their binding specificity to different morphogens (Figure 2). Often these specific sulphated motifs are interspersed with unsulphated regions, NS and NA domains respectively (Figure 3) (Rapraeger et al. 1994; Davies et al. 2003). Moreover, the degree of sulphation also confers different charges (Figure 3B) and levels of rigidity to the HS chains (Figure 3C,D), which also influences protein interactions (Gallagher et al. 1992). Torsion angles of the glycosidic linkages dictate chain flexibility, which consequently determines the optimal conformation for binding morphogens (Figure 3C) (Perkins et al. 2014). Non‐sulphated domains such as GlcA–GlcNAc (NA domains) have been shown to have higher torsion angles than highly sulphated domains such as Ido2S‐GlcNS6S (NS domains) due to the fact that the negative charges introduce greater repulsion (Figure 3C) (Perkins et al. 2014). Consequently, the NA domains flanking NS domains introduce conformational flexibility to the HS chains (Figure 3D). Thus, the level and pattern of sulphate residues along HS chains dictate their activity (Bishop et al. 2007). The binding of growth factors to HS chains present in the ECM creates storage pools, gradients and regulates growth factor diffusion, while when growth factors bind to specific HS chains on cell surface HSPGs, these proteoglycans function as cofactors for triggering signalling pathways, aid in the assembly of signalling complexes, directly trigger signalling pathways and/or may lead to the clearance of growth factors (Bishop et al. 2007; Coulson‐Thomas et al. 2014).
Figure 3.
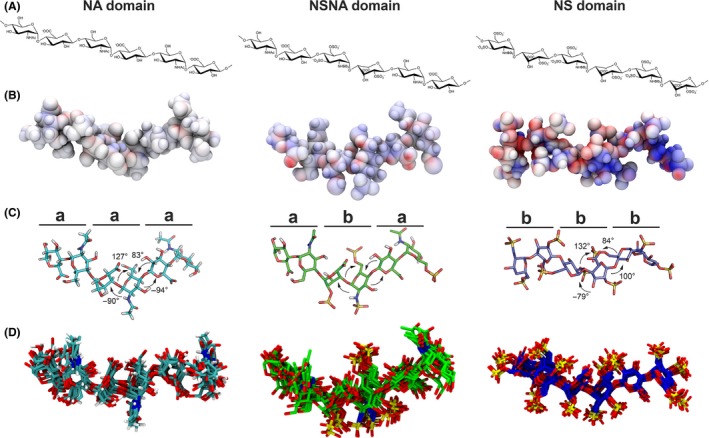
HS chemical and conformational structures. (A) The chemical structure of HS. HS is comprised of repeating disaccharide units (GlcA or IdoA and GlcN) and may display sulphated regions interspersed with unsulphated regions, creating specific domains, namely NS (N‐sulphated), NA (N‐acetylated) and NA/NS domains. (B) Electrostatic potential map of the structural domains of HS as calculated by APBS (Baker et al. 2001), demonstrating that the more negatively charged NS domain presents high electrostatic potential and the NA domain presents lower electrostatic potential (visualized as normalized volume contour at +12.0 kT/e in blue and −12.0 kT/e in red). Potential isosurfaces were visualized in VMD (Humphrey et al. 1996). (C) A representation of the crystallographic torsion angles φ–ψ of the glycosidic linkage of HS, which dictates the flexibility of the differently charged HS domains. The highly electrostatic NS domain (b) presents lower torsion angles in comparison with the less electrostatic NA domain which has higher torsion angles and is consequently more flexible than the NS domain (a) (Perkins et al. 2014). (D) Different HS domains after 20 ns molecular dynamics simulations show conformational changes of the HS domains over time revealing the reduced flexibility of NS domains in comparison with NA domains (supporting the unpublished data of Tarsis F. Gesteira). NA domains are represented as cyan sticks, NANS domains are represented as green sticks, and NS domains are represented in purple. The computer simulations and analysis were performed at the Ohio Supercomputer Center (OSC, 1987).
Heparan sulphate proteoglycans
With the exception of hyaluronan (HA), GAG synthesis requires a core protein upon which the biosynthetic process may take place, and thus HS is present in tissues bound to a core protein forming HSPGs. Two main groups of cell surface HSPGs have been described to date: the syndecans and glypicans. Syndecans are comprised of syndecan‐1, syndecan‐2, syndecan‐3 and syndecan‐4, which are a family of transmembrane proteins and play an important role in cell–matrix interactions. The syndecan family has also been shown to directly trigger intracellular signalling complexes. The cytoplasmic domain of syndecan‐4 binds specifically to phosphatidylinositol 4,5‐bisphosphate and phosphatidylinositol 4‐phosphate, consistent with findings that protein kinase C colocalizes with focal adhesions (Couchman et al. 2002). The phosphorylation of syndecan‐4 at Ser183 leads to dramatic conformational changes which serve as a molecular switch regulating the cellular functions of this molecule, such as ablation of the interaction site with syntenin (Koo et al. 2006). The other main group of cell surface HSPGs, the glypicans (GPCs), consists of six members: GPC1–6. GPCs are anchored to the membrane of eukaryotic cells by covalent linkage of their C‐terminus to glycosylphosphatidylinositol (GPI) (Brown & Waneck 1992). Similar to syndecans, GPCs also act as coreceptors in several signalling pathways and have been shown to play a vital role in biological processes such as cell division, differentiation and developmental morphogenesis. GPCs have been described as regulators for the Wnt, hedgehog (Hh), fibroblast growth factor (FGF) and bone morphogenic protein (BMP) signalling pathways.
HSPGs are also present in the ECM, such as perlecan, agrin and collagen XVIII, which are all important components of basement membranes. Perlecan is a large multidomain HSPG with a core protein of 470 kDa, comprised of five distinct protein modules. Perlecan has angiogenic bivalency, containing both pro‐ and anti‐angiogenic properties (Willis Chris, et al., 2003; Iozzo et al. 2009). Agrin is also a multimodular HSPG that may undergo alternative splicing, which confers additional complexity (Stetefeld et al. 2004). Collagen XVIII contains 10 interrupted collagenous domains flanked by non‐collagenous regions located at the N‐ and C‐termini (Iozzo et al. 2009). Upon cleavage in the ECM, perlecan, agrin and collagen XVIII generate active fragments which modulate cell behaviour (Schulze et al. 1996; Marcelo & Bix 2015).
Knockout models for heparan sulphate and heparan sulphate proteoglycans
The first studies to demonstrate the importance of HS during development were done with the Drosophila melanogaster (D. melanogaster) mutants sugarless (Binari et al. 1997; Hacker et al. 1997; Haerry et al. 1997) and sulphateless (Lin & Perrimon 1999; Lin et al. 1999). These pioneer studies demonstrated that HS is required for major signalling events. The sulphateless mutant, which lacks the only known Ndst1 in D. melanogaster, displays disrupted wingless (Wg), Hh and FGF signalling (Lin & Perrimon 1999; Lin et al. 1999; The et al. 1999). Currently, genetic knockout mouse models have been generated for most of the enzymes involved in HS biosynthesis and also for the major core proteins, thereby providing invaluable information on the role HS and HSPGs play in mammalian development. Given the importance of HS, many of the animal models are embryonic lethal and therefore many inducible conditional knockout models have also been generated to enable an evaluation of the precise role HS plays in specific cell types and/or tissues.
Ext1 and Ext2 complete systemic knockout mouse models are embryonic lethal due to gastrulation defects (Lin & Perrimon 2000; Lin et al. 2000), and conditional knockout mice have therefore been generated, Ext1 flox/flox (Inatani et al. 2003). To date, three complete systemic knockout mice have been generated for Ndst1 (Fan et al. 2000; Ringvall et al. 2000; Grobe et al. 2005), the latter being a conditional knockout mouse model. Systemic knockout of Ndst1 in the three Ndst1 knockout strains leads to perinatal death. The loss of Ndst1 results in a reduction in N‐sulphated HS by approximately one‐third, which culminates in a 50% decrease in overall HS sulphation (Humphries et al. 1999; Ringvall et al. 2000; Ledin et al. 2004). Thus, these studies provide in vivo evidence that the sulphation pattern of HS plays a vital role in the control of cell signalling events. The systemic knockout mouse model has also been developed for Ndst 2, Ndst2 −/−, and these mice are viable and fertile, however fail to synthesize sulphated heparin by mast cells which express primarily the NDST 2 isoform (Forsberg et al. 1999). Knockout models for the sulfotransferases, for example Hs2st −/− (Bullock et al. 1998), Hs2st1 flox/flox (Stanford et al. 2010), Hs6st1 flox/flox (Izvolsky et al. 2008) and Hs3st1 –/– (HajMohammadi et al. 2003), and for HS GlcUA C5 epimerase Hsepi −/− (Li et al. 2003) have also been generated. Systemic knockout mice for the endosulfatases SULF1 and SULF2, Sulf1 −/− and Sulf2 −/− mice, have also been generated and the single‐knockout mice do not display any obvious abnormalities (Forsberg et al. 1999) while the combined knockout mice, Sulf1 −/−;Sulf2 −/−, present neonatal lethality due to skeletal abnormalities and kidney hypoplasia (Ai et al. 2007; Holst et al. 2007; Ratzka et al. 2008).
Various knockout mouse models have been generated for the core protein in an attempt to elucidate the role of different PGs in development and pathogenesis. In general, the PG knockout mouse models display mild phenotypes in comparison with those targeting the HS chains, potentially indicating compensatory mechanisms, such as among the syndecan family members. The potential functional overlap or compensatory mechanism among syndecan PGs is currently being investigated in compound knockout studies. Syndecan‐1 and syndecan‐4 knockout mice, Syndecan‐1 −/− and Syndecan‐4 −/−, are viable and fertile with subtle skin and corneal wound healing defects. Syndecan‐4 −/− mice have been shown to display impaired angiogenesis (Echtermeyer et al. 2001). Glypican‐2 −/− mice are also viable and fertile with no obvious phenotype (Lander & Selleck 2000). On the other hand, glypican‐3 −/− mice have severe phenotypes with perinatal death (Cano‐Gauci et al. 1999). The functions of perlecan and agrin have also been investigated with the use of systemic knockout mouse models. Agrin −/− mice also present a subtle phenotype, and these mice are viable and fertile (Erickson & Couchman 2000). Interestingly, Perlecan −/− mice present severe defects including prenatal death, around embryonic day 10–12, due to rupture of basement membranes in the heart and brain defects, such as exencephaly (Arikawa‐Hirasawa et al. 1999; Costell et al. 1999). Animals that survive embryonic lethality ultimately develop chondrodysplasia, which leads to perinatal death (Arikawa‐Hirasawa et al. 1999). Therefore, since perlecan −/− mice are embryonic lethal or in some cases die just after birth due to skeletal dysplasia, a mouse model has been developed combining the systemic knockout with conditional site directed overexpression of perlecan, thereby overcoming the severe bone/cartilage defects and enabling the mice to survive into adulthood. Combining collagen 2α1‐driven overexpression of perlecan with systemic loss of perlecan is enough to overcome the cartilage abnormalities rescuing perinatal lethality in these compound transgenic mice (Xu et al. 2010). Some groups have opted to overexpress perlecan in order to unveil its role in organogenesis to overcome the issues with perinatal death of the Perlecan −/− mice. For example, conditional overexpression of perlecan using the keratin 5 promoter (which drives perlecan expression in epithelial cells) was used to determine the function of epithelial‐derived perlecan in odontogenesis (Ida‐Yonemochi et al. 2011).
Heparan sulphate regulates signalling pathways and is required for morphogen gradient formation during development
Both CS and HS have previously been shown to modulate the FGF, Wnt/Wg and Sonic hedgehog (SHH) signalling pathways (Ai et al. 2003; Nadanaka et al. 2008; Dani et al. 2012; Coulson‐Thomas et al. 2014). 6‐O‐sulphated HS is required for FGF receptor dimerization and tyrosine kinase activation (Guimond et al. 1993; Pye et al. 1998), and as such the selective removal of sulphate groups from the 6‐O position by the endosulfatase SULF1 has been shown to inhibit FGF2‐ and FGF4‐induced mesoderm formation (Wang et al. 2004). FGF4 and FGF8 ligands are retained in the extraembryonic ectoderm by cell surface‐tethered HS chains, and the spatiotemporal expression of cell surface‐tethered HS chains has therefore been shown to regulate local FGF signalling activity during mammalian embryogenesis (Shimokawa et al. 2011). HSPGs containing a GPC5 core protein and 2‐O‐sulpho‐iduronic acid residues at the non‐reducing ends of the glycans have been shown to be coreceptors for SHH, and promote SHH binding and signalling in the developing nervous system (Witt et al. 2013). Perlecan has also been shown to interact with SHH and is important for SHH function, particularly in the brain (Palma et al. 2011). In D. melanogaster, the coreceptors for Wg include the GPC division abnormally delayed (Dally) and Dally‐like protein (Dlp) and, interestingly, Dlp is a coreceptor with biphasic activity that can activate or repress Wg signalling, depending on the relative expression levels of Dlp and the Wg receptor, frizzled 2 (Yan et al. 2009).
Many developmental regulators bind to HS by means of HS‐/HEP‐binding domains (HBDs). SHH, for example, interacts with HSPGs via an N‐terminal Cardin‐Weintraub motif, which is characterized by a cluster of basic amino acids that allows for electrostatic interaction between the positive charges on the protein and the negatively charged sulphates on HS (Rubin et al. 2002). The ability of SHH to diffuse through HS‐containing ECM is due to N‐terminal SHH processing which targets and inactivates this HS‐binding motif (Ohlig et al. 2012). bFGF has been shown to specifically bind only to the HS chains of syndecan‐1 and syndecan‐4, and not to the CS chains of these hybrid PGs, although the CS chains are required for the formation of a ternary complex that transfers the growth factor to the corresponding cell surface receptor more efficiently in comparison with HS chains alone (Deepa et al. 2004). In the case of Wg, this regulator interacts with the Dlp core protein and the GAG chains enhance this interaction (Yan et al. 2009).
HSPGs regulate FGF gradient formation in the developing embryo, which is responsible for the positional identity of tissues, and this role of HSPGs has been reviewed by Balasubramanian and Zhang (Balasubramanian & Zhang 2015). The PGs Dally and Dlp are both essential for Wg gradient formation in D. melanogaster wing disc during development (Tsuda et al. 1999). However, Dally and Dlp have different roles, and it is the combined actions of these proteoglycans that results in the Wg concentration gradient, by means of a restricted diffusion mechanism (Han et al. 2005). Overexpression of the HS 6‐O‐endosulfatase SULF1 has been shown to inhibit the binding of Wg to Dally (Kleinschmit et al. 2013). On the other hand, the knockdown of HS 6‐O‐sulfotransferase (Hs6st) and SULF1 in D. melanogaster, which have opposite effects on HS sulphation, both increase Wg at the synaptic interface, but have opposite effects on synapse development, that is decreased and increased neurotransmission respectively (Dani et al. 2012). SULF1 has been shown to be a positive regulator of Wnt signalling; this cell surface sulfatase which removes 6‐O sulphates from HS chains decreases the affinity of HS towards the Wnt ligand, thereby promoting the binding of Wnt to the receptor frizzled (Ai et al. 2003). In a Sulf1 knockdown Xenopus laevis model, it has been shown that SULF1 is required for normal dorsoventral gradient distribution of SHH in the ventral neural tube (Ramsbottom et al. 2014). In Sulf1 and Sulf2 knockout mice, cell survival and neurite outgrowth (via FGF2, GDNF and NGF) are impaired in the postnatal cerebellum, demonstrating the importance of HSPG sulphation patterns, while SHH, which determines the laminar organization of the cerebellar cortex, is not affected (Kalus et al. 2015). The binding of SHH to PGs in mice has been shown to be required for proliferation of neural stem/precursor cells, but not for tissue patterning (Chan et al. 2009).
The role of heparan sulphate in the development of ectodermal tissues
Eye morphogenesis
The eye originates from both ectodermal and mesodermal tissues (Osipov & Vakhrusheva 1975). Eye development begins with the formation of the optic vesicle, as an outgrowth from the early brain, which eventually makes contact with the surface ectoderm and becomes the optic cup (Pei & Rhodin 1970; Buse & de Groot 1991). The retina, ciliary body, iris and optic nerves originate from the neuroepithelium tissues, while the lens and eyelid originate from the surface ectoderm. Development of the cornea also requires mesenchymal cells and neural crest cells, which invade the corneal stroma after condensation (Pei & Rhodin 1970). Therefore, corneal tissues have multiple origins: surface ectoderm (corneal epithelium), neural crest (corneal endothelium and Descemet's membrane) and mesoderm (corneal stroma and Bowman's membrane). Given that the Ext1, Ext2 and Ndst1 complete systemic mutants are embryonic lethal, the precise role of HS in eye development requires the use of inducible systems.
The important role that HS plays in eye development has previously been demonstrated using a conditional knockout system with breeding of Ext1 flox/flox with Wnt1‐cre. This system uses site‐specific recombinase technology, where Cre recombinase is expressed and removes Ext1 solely in Wnt1 expressing cells. The Wnt1‐cre;Ext1 flox/flox mice develop severe corneal defects: corneal endothelium defects, corneal stroma hypoplasia, eyelid closure failure and iridocorneal angle dysgenesis (Iwao et al. 2009b). This work demonstrates that HS mediates TGF‐β2 signalling required for neural crest cells to form the anterior chamber. Analysis of the embryos from the complete systemic Ndst1 knockout mice further revealed the importance of HS for eye development. These mice display altered HS sulphation patterns and suffer from coloboma, microphthalmia, anophthalmia, primarily on the C57BL background. Further evidence of the importance of HS, more specifically HSPGs, in eye development came from a gain of function study with agrin which led to developmental eye defects (Fuerst et al. 2007). Agrin overexpression leads to anophthalmia, failure of vitreous vessel regression, fusion of anterior chamber structures and optic stalk coloboma, which may lead to the misdifferentiation of the optic stalk as the retina (Fuerst et al. 2007). Moreover, loss of sulfotransferase function also leads to defective eye development. For example Hs2st1 −/− mice have iris coloboma (Bullock et al. 1998); and complete systemic Ndst1 −/− mice have severe eye developmental defects, which correlate with impaired SHH and FGF signalling. Interestingly perlecan (HSPG2) exon 3 knockout, which lack the three glycosylation attachment sites for potential HS side chains, have microphthalmia (Rossi et al. 2003).
Cornea
Limited studies have investigated the role of HS in the development of the anterior chamber of the eye. A number of studies have elucidated the importance of HSPGs, specifically of syndecan‐1 and perlecan, in corneal wound healing. However, the precise role HS plays in corneal development is limited by the availability of Cre‐driver transgenic mouse lines that would be able to specifically remove HS from the cornea early enough during development. For example, we used the conditional knockout model Ext1 flox/flox ;Kera‐cre in an attempt to elucidate the role HS plays in stromal development, removing Ext1 from keratocan+ cells (keratocytes). However, these mice presented no stromal phenotype (Coulson‐Thomas et al. 2015). Keratocan expression during mouse corneal development starts around embryonic day 12, and Ext1 expression would therefore only be ablated from the keratocytes of these mice approximately around embryonic day 13. Potentially slow turnover of the stromal ECM could have hampered the stromal phenotype. However, the important role HS plays in corneal epithelial development was clearly demonstrated using K14rtTA;TetOcre removing Ext1 from keratin 14+ cells (corneal epithelial cells) (Coulson‐Thomas et al. 2015). We have recently demonstrated that HS is essential for corneal epithelial stratification (Coulson‐Thomas et al. 2015). The loss of epithelial HS in the cornea leads to a loss of tight junctions, culminating in corneal dysgenesis (Coulson‐Thomas et al. 2015).
Previous studies have demonstrated the importance of syndecan‐1 and perlecan for corneal wound healing. For these studies, the authors used complete systemic knockout mice for syndecan‐1 (Syndecan‐1 −/−) and systemic perlecan knockout mice with rescued cartilage expression (Hspg2 −/− ‐Tg), which prevents perinatal death due to premature cartilage development (Arikawa‐Hirasawa et al. 1999; Costell et al. 1999). These complete systemic perlecan knockout mice, which express recombinant perlecan solely in cartilage driven by the cartilage‐specific Col2a1 promoter, survive into adulthood. Syndecan‐1 −/− mice undergo normal eye development, but present corneal epithelial defects upon wounding, including delayed re‐epithelialization and failed activation of epithelial cell proliferation after wounding (Stepp et al. 2002). The altered epithelial cell migration rates may be overcome by seeding Syndecan‐1 −/− cells on fibronectin‐ or collagen‐coated culture dishes, indicating that the epithelial‐related defects of Syndecan‐1 −/− mice could be in part due to defects in the basement membrane. Keratinocytes obtained from Syndecan‐1 −/− mice and grown in culture are more proliferative, more adherent and migrate more slowly in comparison with their wild‐type counterparts (Stepp et al. 2007). Later Stepp and collaborators have suggested a mechanism by which the loss of syndecan‐1 affects wound healing. Corneal stromal cells isolated from Syndecan‐1 −/− mice show a decrease in the size of focal adhesion complexes, decreased integrin activation and reduced assembly of fibronectin into fibrils, and consequently migrate faster (Stepp et al. 2010). Therefore, the loss of syndecan‐1 mediated integrin function leads to changes in matrix assembly, which affect cell adhesion, spread and migration of corneal stromal cells. On the other hand, perlecan knockout mice, Hspg2−/−‐Tg, do have developmental eye defects, such as microphthalmos. In the cornea, perlecan is primarily present in the basement membrane, and consequently mutant mice lacking this HSPG have a thinner corneal epithelium with a significantly reduced number of cell layers (Inomata et al. 2012).
Sulf1 −/− and Sulf2 −/− mice have been used to investigate the role of HS in corneal development (Lamanna et al. 2007; Rosen & Lemjabbar‐Alaoui 2010; Maltseva et al. 2013). The roles of SULF1 and 2 were investigated using a corneal wound healing model. This group did not describe any developmental defects that result from ablating SULF1, SULF2 or both SULF1 and SULF2 from the corneal epithelium. However, similarly to Syndecan‐1 −/− mice, Sulf1 −/− mice present delayed wound healing, while Sulf2 −/− do not have wound healing defects (Maltseva et al. 2013). Interestingly, our studies with Ndst1 flox/flox mice using the K14rtTA;TetOcre driver to remove NDST1 from the corneal epithelium resulted in a more subtle phenotype than Ext1 flox/flox ;K14rtTA;TetOcre (Coulson‐Thomas et al. 2015). The corneal phenotype of Ndst1 flox/flox ;K14rtTA;TetOcre resembled that of Sulf1 −/− mice (Coulson‐Thomas et al. 2015). The complete loss of HS in Ext1 flox/flox ;K14rtTA;TetOcre mice hinders the formation of a stratified epithelium and drastically delays corneal epithelial cell resurfacing after wounding leading to stromal damage (Coulson‐Thomas et al. 2015).
Lens
FGFs are fundamental signalling cues during early stages of lens development and HS is essential for FGF diffusion, gradient formation and morphogen–receptor interaction. Moreover, N‐sulphation is essential for the binding of FGF2 to HS (Turnbull et al. 1992). Complete systemic Ndst1 knockout mice present disrupted lens determination gene expression and the absence of lens invagination, which leads to severe lens hypoplasia or anophthalmia (Pan et al. 2006). A testament to the importance of HS for FGF signalling during lens development is that genetic disruption of either FGFR1, FGFR2 or FGFR3 leads to no developmental lens defects (Huang et al. 2003; Garcia et al. 2005; Zhao et al. 2006). The loss of the HS glycosylation site on perlecan (exon 3) leads to alterations of the lens capsule structure (Rossi et al. 2003). These lens defects become more severe when these mice are further bred with Col18α1 −/− mice, which indicates that HS chains could also have a structural function during lens development (Rossi et al. 2003).
Lacrimal gland
Extensive studies have demonstrated the important role HS plays in lacrimal gland morphogenesis. More importantly, the HS/FGF signalling axis dictates vital cues for lacrimal gland formation. Ndst1 expression at the tip of the lacrimal gland bud has been shown to be required for lacrimal gland development, and the loss of NDST1 leads to limited lacrimal gland outgrowth and, in some mice, no lacrimal gland budding. Complete systemic Ndst1 knockout (Ndst1 −/−) and conditional knockout of Ndst1 flox/flox with the LE‐Cre transgenic driver (Ndst1 flox/flox ;LE‐Cre) were used to demonstrate that NDST1 expression is required in lacrimal gland epithelial cells and not in mesenchymal cells. Moreover, NDST1 modifications of HS chains are required for FGF10–FGFR2b complex formation, but not for FGF1–FGFR2b (Pan et al. 2008). Another study has demonstrated that both FGF7 and FGF10 are dependent on HS for the gradient formation required for lacrimal and salivary gland morphogenesis. FGF7 has been correlated during gland development with the budding of prebranched epithelial buds, while FGF10 is primarily correlated with epithelial bud elongation, with both events requiring HS. Interestingly, a single amino acid mutation in the FGF10 binding pocket, which reduces its binding affinity to HS, turns FGF10 into a functional mimic of FGF7, and thereby this mutant form of FGF10 leads to lacrimal gland budding rather than elongation (Makarenkova et al. 2000). The interaction between FGF10 and FGFR2b and HS was further investigated using knockout mice for HS 2‐O‐sulfotransferase, Hs2st flox/flox ;LECre, and 6‐O‐sulfotransferase, Hs6st1 flox/flox ;LECre;Hs6st2 −/−, to modify the fine structure of HS (Qu et al. 2011). Both Hs2st flox/flox ;LECre and Hs6st1 flox/flox ;LECre;Hs6st2 −/− mice presented lacrimal gland hypoplasia; however, mice lacking 6‐O‐sulfotransferases presented a more severe phenotype with strong FGF10 genetic interaction, and finally, the triple knockout mice, Hs2st flox/flox ;Hs6st1 flox/flox ;LECre;Hs6st2 −/−, presented the most severe phonotype with abolished lacrimal gland development and disruption of FGF10–FGFR2b–HS complex formation on the cell surface (Qu et al. 2011). This study demonstrates that O‐sulphation of HS is also required for FGF signalling. Lacrimal gland development starts with the formation of the lacrimal gland bud, which invades the FGF10 expressing mesenchyme (Makarenkova et al. 2000; Entesarian et al. 2005). The lacrimal gland bud invades the mesenchyme until E15.5 when secondary branching initiates to create a complex tubuloalveolar structure. As mentioned above, NDST1 expression by epithelial cells at the tip of the invading bud is required for lacrimal gland morphogenesis (Pan et al. 2008). However, a study also elucidated the role of HS, more specifically HS sulphation, in the FGF‐producing mesenchyme during lacrimal gland morphogenesis. Mesenchymal ablation of GAGs leads to excessive diffusion of FGF10, which culminates in a loss of FGF signalling response and, consequently, stunts lacrimal gland budding in the presumptive epithelium (Qu et al. 2012). This work further demonstrates that lacrimal gland budding requires mesenchymal expression of NDST1 and NDST2, and not Hs2st, Hs6st1 and Hs6st2, to create a FGF10 gradient and limit its diffusion (Qu et al. 2012).
Retina
HS is also required for retinal development, and early on, HSPGs were found to be expressed in neurites of retinal neuronal cells and are potentially involved in synaptogenesis (Chai & Morris 1994). Changes in the fine structure of HS lead to retinal axon targeting defects in Xenopus laevis (Irie et al. 2002). HS is expressed throughout retinal development and is required for retinal ganglion cell axon projection towards the optic nerve head (Ogata‐Iwao et al. 2011). Regional expression of 2‐ and 6‐O‐sulfotransferase culminates in specific HS species required for axon guidance towards the tectum (Irie et al. 2002). Moreover, Netrin‐1 and Slit‐mediated intraretinal RGC axon guidance require HS (Ogata‐Iwao et al. 2011). The role of HS, more specifically of HS fine structure, in retinal ganglion cell axon projection to the optic disc was investigated using Ndst1 flox/flox ;Ndst2 −/−;Six3‐cre and Hs2st flox/flox ;Hs6st flox/flox;Six3‐cre compound mice. The compound mutants exhibit normal retinal neurogenesis and optic fissure closure; however, these mice present defective optic disc and stalk development leading to optic nerve hypoplasia and aplasia (Cai et al. 2014). Ndst1 flox/flox ;Ndst2 −/−;Six3‐cre and Hs2st flox/flox ;Hs6st flox/flox;Six3‐cre mice express undersulphated HS chains in Six3‐expressing cells, including in the retina, hypothalamus and layer 4 of sensory cortical areas (Furuta et al. 2000). These mice present retinal ganglion cell axon projection misrouting in part due to the loss of FGF signalling (Cai et al. 2014). Ext1 flox/flox ;Wnt1‐cre mice present disrupted neural crest cell migration due to disturbed TGF‐β2 signalling (Iwao et al. 2009a). Moreover, Ext1 flox/flox ;Dkk3‐cre mice, which lack HS synthesis in retinal progenitor cells (Dickkopf‐3+ cells), display severe intraretinal guidance errors, ectopic axon penetration through the neural retina, optic nerve hypoplasia and disturbed centrifugal RGC axon projection towards the optic nerve head (Ogata‐Iwao et al. 2011).
CNS morphogenesis
The ectodermal tissue differentiates to form the tooth enamel, epidermis and skin appendages and finally the nervous system, including the spinal cord, peripheral nerves and brain. Early studies had already suggested that PGs play an important role in neurite outgrowth, neuronal cell adhesion and differentiation (Oohira et al. 1994; Margolis et al. 1996). However, the vital role HS plays in the development of the central nervous system (CNS) was first demonstrated with genetic studies using D. melanogaster. Wg, the Wnt homologue gene in D. melanogaster, is vital for determining segment polarity during embryonic development (Sharma et al. 1973; Klaus & Birchmeier 2008). The sugarless and sulphateless D. melanogaster mutants, which have impaired HS biosynthesis, have revealed that HS is required for Wg signalling (Binari et al. 1997; Hacker et al. 1997; Haerry et al. 1997; Lin et al. 1999). Moreover, syndecan and glypican have been demonstrated to be required for morphogen gradient formation and for mediating signalling cues for CNS histogenesis and axon guidance. Both Wg and Hh distributions require glypican during CNS development (Han et al. 2004; Johnson et al. 2004; Franch‐Marro et al. 2005; Hacker et al. 2005) and Slit–glypican‐1 interactions govern histogenesis during certain stages of CNS development (Liang et al. 1999). The Slit–Robo signalling axis has been shown to require syndecans for midline axon guidance (Hu 2001; Johnson et al. 2004; Steigemann et al. 2004). FGF‐HS signalling pathways are also required for CNS development. D. melanogaster mutants for the perlecan homologue present reduced FGF signalling and larval brain neuroblast cell cycle arrest (Voigt et al. 2002; Park et al. 2003). Thereafter, genetic studies using Caenorhabditis elegans (C. elegans) HS and HSPG mutants have further demonstrated the important role of HS in CNS development. In C. elegans, mutations in the HS biosynthetic machinery and core proteins result in drastic effects on ectoneuronal development and axonal guidance demonstrating that the fine structure of HS has information necessary for CNS development (Kinnunen et al. 1998; Bulow & Hobert 2004; Rhiner & Hengartner 2006; Bulow et al. 2008; Edwards & Hammarlund 2014).
The role HS plays in the development of the mammalian CNS remains to be fully elucidated. All syndecans‐1–4 have been shown to be expressed in the mammalian CNS (Bandtlow & Zimmermann 2000); however, syndecan‐3 is the most highly expressed syndecan in nervous tissues during early postnatal development (Carey 1996). HS synthesis and secondary modifications are tightly regulated during mammalian CNS development. Expression of the major HS biosynthetic polymerases, EXT1 and EXT2, has been detected during CNS development in mice from E9.5 and peaks in the cerebral cortex during the early postnatal period (Inatani & Yamaguchi 2003). As with D. melanogaster and C. elegans, HS is required for FGF, BMP, Wnt and Hh signalling during mammalian CNS development. Embryonic stem cells fail to differentiate into Pax6‐positive neural precursor cells in Ext1 −/− mice due to disrupted FGF and BMP signalling (Kraushaar et al. 2012). The 6‐O‐sulphation pattern, number of sulphates and chain length of HS have been shown to change in the developing neuroepithelium, which functionally correlates with an altered ability to activate FGF2 and FGF8 signalling (Brickman et al. 1998). This same group later demonstrated that there is differential expression of HS sulfotransferases in the developing brain, leading to the distribution of variant HS species which orchestrate FGF and FGFR signalling complexes (Ford‐Perriss et al. 2002). Ext1 flox/flox ;Nestin‐Cre leads to severe nerve patterning defects encompassing those of HS‐binding morphogens. HS is required for midline axon guidance and Ext1 flox/flox ;Nestin‐Cre mice present caudal midbrain–cerebellum malformations, small cerebral cortex, loss of major commissural tracts, commissural axon pathfinding defects and lack of the olfactory bulb (Inatani & Yamaguchi 2003; Matsumoto et al. 2007). Ext1 flox/flox ;Wnt1‐Cre mice also present commissural axon pathfinding defects (Matsumoto et al. 2007). HS, more specifically 2‐O‐sulphated HS, is required for the formation of signalling complexes between HS, FGF2 and FGFR1, and consequent Erk1/2 activation at the developing telencephalic midline (Chan et al. 2015). This same group demonstrated that 2‐O‐ and 6‐O‐sulfotransferases generate a HS‐containing environment which regulates Erk signalling, which is conducive with corpus callosum development (Clegg et al. 2014). The important role that GAGs, including HS, play in CNS function and pathogenesis has recently been reviewed (Smith et al. 2015).
Skin morphogenesis
The skin serves many important roles, such as serving as the first barrier against environmental insults (requiring survival and regeneration) and also providing the necessary cues for skin appendage formation. The development of skin and skin appendages, including teeth, hair and nails/claws, is regulated by the formation of a placode which requires reciprocal interactions between epidermal and mesenchymal tissues. The skin is composed of the epidermis followed by the basement membrane and the ECM‐rich dermis (of mesenchymal origin). The skin basement membrane is composed of a PG‐rich specialized ECM. It has been suggested that the skin basement membrane, specifically at the dermal–epidermal junction and hair follicle epithelium, is primarily secreted by epithelial cells (Yamane et al. 1996). Skin integrity requires the formation of anchoring complexes by the basement membrane, such as hemidesmosomes, which are required for linking the epidermis to the dermis. HS side chains have been demonstrated as integral players in the assembly of anchoring complexes (Iriyama et al. 2011b). Moreover, HSPGs at the epidermal–dermal junction regulate epidermal differentiation and proliferation and are essential for maintaining epidermal homeostasis (Iriyama et al. 2011a; Behrens et al. 2012).
As mentioned above, Syndecan‐1 −/− mice are healthy and fertile. Syndecan‐1 −/− mice present increased skin epithelial cell proliferation rates, but do not display any morphological changes to their skin or increases in epithelial thickness (Stepp et al. 2002). However, upon injury Syndecan‐1 −/− mice present impaired wound healing. Syndecan‐1 −/− mice display proliferation delays after full thickness wounds leading to prolonged hypoplasia, but differently to the corneal epithelium, no migration defects are observed (Stepp et al. 2002). Syndecan‐4 −/− mice are also healthy and fertile with normal skin development; however, fibroblasts isolated from Syndecan‐4 −/− mice display reduced wound healing rates in vitro and Syndecan‐4 −/− mice present impaired wound healing. In the skin of naïve mice, syndecan‐4 is expressed exclusively by epidermal cells; however, upon injury, there is an increase in syndecan‐4 expression throughout the dermis. Syndecan‐1 and syndecan‐4 seem to play a synergistic role in wound healing, where syndecan‐1 is primarily involved in keratocyte function, re‐epithelialization and stratification, while syndecan‐4 is associated with fibroblast migration, wound contraction and angiogenesis. Recently, (Gopal et al. 2015) demonstrated that syndecans control TRP channels, thereby controlling fibroblast calcium influx, which in turn impacts the phosphorylation status of focal adhesion kinase. This study indicates that syndecan regulation of TRPC‐type channels may control myofibroblast phenotype influencing wound repair and fibrosis. Perlecan has also been demonstrated to be essential for epidermal formation. Precocious epidermal cell apoptosis causes incomplete stratification in human in vitro engineered skin with perlecan‐deficient keratinocytes, which leads to a thin and poorly organized epidermis (Sher et al. 2006). Further evidence for the role of HSPGs in epidermis morphogenesis has come from studies administering heparanase to skin explants (Vlodavsky et al. 1999; Zcharia et al. 2005). Moreover, our recent work has demonstrated that knocking out HS in the epidermis leads to epidermal hyperplasia (Coulson‐Thomas et al. 2014).
Sweat glands
Sweat glands, or eccrine sweat glands, are composed of a spiralled intraepidermal duct, a dermal duct comprised of a straight and coiled portion, and a secretory tubule, which is a coiled duct within the dermis. In human embryos, the development of sweat glands begins at ~12–13 weeks primarily on the palms and soles followed by the rest of the body at week 20 (Sato et al. 1989), and morphogenesis is only complete after birth. Sweat glands play a vital role in thermoregulation, primarily for cooling and hydrating the skin. Thermoregulation is a vital evolutionary requirement for maintaining homeostasis in endothermal animals, such as horses and camels, where sweating is vital for survival (Schmidt‐Nielsen et al. 1957; Jenkinson 1973). In contrast to humans that have sweat glands throughout the skin, mice have sweat glands exclusively on the pads of their paws making them more sensitive to extreme temperatures (Lu & Fuchs 2014). In mice, the development of sweat glands commences at embryonic day 17.5–18.5. Initial stages of sweat gland morphogenesis involve epidermal invagination of K14 expressing cells (Sun et al. 1979; Moll & Moll 1992), and gradually the inner layer of the developing sweat gland differentiates into luminal cells and expresses K8/K18 in lieu of K5/K14 (Lu et al. 2012). Research on the involvement of HSPGs in sweat gland morphogenesis is scarce. However, we have recently shown that Ext1 knock out in K14 expressing cells, thereby causing invaginating epidermal cells to be void of HS side chains, induces sweat gland morphogenesis in adult mice (Coulson‐Thomas et al. 2014).
Hair follicles
The hair follicle is a composite organ composed of compartments of both epithelial and dermal origin. Similarly to the development of teeth and nails/claws, the development of hair follicles requires intricate epithelial–mesenchymal interactions between the underlying dermis and epidermis culminating in a dermal signal that induces formation of the follicle placode (Biggs & Mikkola 2014). It has been well established that the ECM composition plays a major role in epithelial–mesenchymal interactions (Watt & Huck 2013). Moreover, the presence of a basement membrane physically separating the epidermis and dermis (mesenchyme) is an indicative that basement membrane HSPGs could play an important role in dictating the initial cues for hair follicle morphogenesis. The spatial and temporal distribution of perlecan changes during hair follicle morphogenesis. All human fetal skin basement membranes contain perlecan, but it is concentrated in the dermal papilla during later stages of hair follicle morphogenesis, coinciding with NCAM (calcium‐independent neural cell adhesion molecule), which interacts with HSPGs and is involved in cell–cell adhesion (Kaplan & Holbrook 1994). In mice, hair follicle morphogenesis starts around embryonic day 13 and is completed by postnatal day 20 after which morphogenesis of new hair follicles ceases and the existing hair follicles cycle to maintain hair health. Interestingly, we have recently shown that knocking out HS from the epidermis is enough to remove the inhibitory signals that restrict further follicle placode formation in adult mice, and unrestrained hair follicle morphogenesis takes place in mature mice (Coulson‐Thomas et al. 2014). Studies have also demonstrated the presence of HSPGs other than perlecan in the hair follicle; hair follicles have been shown to express high levels of syndecan‐1 (Bayer‐Garner et al. 2002; Richardson et al. 2009; Coulson‐Thomas et al. 2014) and also, although to a lesser extent, syndecan‐3 (Coulson‐Thomas et al. 2014). Many morphogens that regulate hair follicle morphogenesis are known to require HS for both triggering signalling cascades and correct tissue distribution. An example of this is SHH, which is known to mediate various developmental processes including hair follicle morphogenesis and cycling (Zhang et al. 2007; Chang et al. 2011; Ohlig et al. 2012). Mice lacking epidermal HS present disrupted SHH distribution with drastically increased deposition in the underlying dermis (Figure 4) (Coulson‐Thomas et al. 2014).
Figure 4.
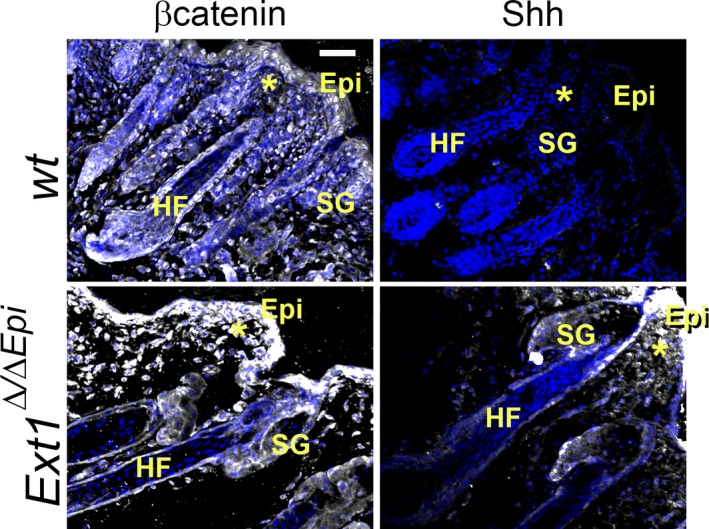
Loss of epidermal HS disrupts the distribution of morphogens. The knockout of Ext1 in keratin 14 expressing cells (Ext1 Δ/ΔEpi) leads to a loss of epidermal HS disrupting β‐catenin and SHH distribution at the epidermal–mesenchymal junction in comparison with wild‐type mice (wt). The epidermis (Epi), a sebaceous gland (SB), a hair follicle (HF) and the underlying dermis (*) are shown in the figure.
In mature skin, given the impossibility to develop new hair follicles, healthy hair is made possible by the hair follicle undergoing repeated cycles of growth (anagen), regression (catagen) and quiescence (telogen). Both anagen and catagen require significant remodelling of the surrounding ECM to enable growth and regression of the hair follicle. Of significant importance is the matrix remodelling that enables the downward growth of anagen hair follicles, which requires enzymatic degradation of the surrounding ECM. Heparanase expression has been reported during both anagen and catagen, primarily in the inner root sheath (Malgouries et al. 2008). Moreover, heparanase inhibition induces cycling into the catagen phase (Malgouries et al. 2008). Interestingly, our work knocking out Ext1 and consequently HS from the epithelial compartment of the hair follicle inhibited cycling into catagen, therefore sequestering all follicles in anagen (Coulson‐Thomas et al. 2014). Also, heparanase overexpression increased hair growth rate during the anagen phase further supporting the notion that removal of HS chains is required for downward growth during anagen (Vlodavsky et al. 1999; Zcharia et al. 2005).
Sebaceous glands
The sebaceous glands produce sebum, which plays a vital role in hair and skin homeostasis. The lack of sebaceous glands and sweat glands, such as in scar tissue, leaves the skin severely dry, fragile and significantly more prone to infection. Sebaceous glands are attached to hair follicles and are formed as part of the same developmental process. Limited research has investigated the significance of HSPGs in sebaceous gland morphogenesis and cycling. However, we have found that the loss of HS expression in sebaceous glands leads to hyperplasia and increased sebum production (Coulson‐Thomas et al. 2014).
Nail morphogenesis
Nail development begins with the formation of primordial nails, which appear on the dorsal surface of developing distal digits. Surrounding cells form the nail folds, which are succeeded by the nail matrix containing proliferating keratinocytes. Keratinocytes dorsal to the matrix undergo apoptosis, resulting in keratinization of the proximal nail folds forming the nail plates. Many developmental regulators modulated by HSPG play a role in nail/claw development, including Wnt, Shh and bone morphogenetic protein 4 (BMP4) (Hamrick 2001; Cui et al. 2013); however, the direct role of HS remains to be investigated.
Tooth morphogenesis
Tooth morphogenesis involves various stages, namely the bud, cap, bell and late bell stages, which are regulated by sequential and reciprocal interactions between the epithelial and mesenchymal tissues (Thesleff 2003). It is during the bell stage that the hard tissue‐forming cells of the tooth (odontoblasts and ameloblasts) differentiate at the interface of the epithelium and mesenchyme and deposit the dentin and enamel matrices respectively (Thesleff 2003). The distribution of basement membrane PGs has been shown to change during cell differentiation and matrix secretion in the developing tooth (Thesleff et al. 1981; Bronckers et al. 1989; Kogaya et al. 1990).
HSPG sulphation and desulphation play a role in dentinogenesis; odontogenic cells are highly sulphated on the cell surface and become desulphated during their differentiation to odontoblasts (Hayano et al. 2012). Sulf1 −/− ;Sulf2 −/− double null mutant mice exhibit defective dentin phenotypes, whereas single SULF mutants do not show such defective phenotypes (Hayano et al. 2012). This is due to the fact that SULF1 and SULF2 are essential for temporally and spatially regulating the 6‐O‐desulphation of odontogenic cells during their differentiation. Desulphation of HS decreases the binding affinity of Wnt10a towards HSPGs, which facilitates the binding of Wnt10a to its receptor resulting in activation of the Wnt signalling pathway and upregulation of dentin sialophosphoprotein expression, which is a dentin‐specific matrix protein that plays a role in the formation of mineralized dentin (Hayano et al. 2012). During cementogenesis, the period in which dental follicular cells penetrate the ruptured Hertwig's epithelial root sheath (HERS) and differentiate into cementoblasts, there is an upregulation of the endoglucuronidase heparanase by HERS cells and degradation of the HSPG perlecan in the dental basement membrane, which could result in the release of growth factors such as bFGF bound to perlecan (Hirata & Nakamura 2006). The overexpression of perlecan in epithelial cells in transgenic mice results in an irregular alignment of HERS cells, dull‐ended crowns, outward‐curved tooth roots and poorly crystallized enamel (Ida‐Yonemochi et al. 2011). During mouse incisor amelogenesis, syndecans‐1–4 are spatially and temporally expressed; syndecan‐1 is expressed in undifferentiated epithelial and mesenchymal cells, and syndecan‐2, syndecan‐3 and syndecan‐4 in more differentiated cells (Muto et al. 2007). There is a loss of syndecan‐4 expression when inner enamel epithelial cells gave rise to ameloblasts (Yan et al. 2014). The knockdown of syndecan‐4 has been shown to result in reduced cell proliferation and increased expression of amelogenin, ameloblastin, kallikrein 4 and matrix metalloproteinase 20, which are molecules that participate in the formation of enamel (Yan et al. 2014).
Conclusion
A plethora of information has amounted on the vital role HS plays in the development of ectodermal tissues. A vital role has been established for HS in the development of the CNS, retina, cornea and lacrimal gland, which opens new research avenues in regenerative medicine for targeting HS and HSPG core protein expression. However, further research is necessary to unveil additional roles of HS in CNS, retina and cornea pathogenesis. Furthermore, current research has clearly revealed that HS plays a vital role in hair follicle morphogenesis; however, the precise mechanism remains elusive and, exciting future research targeting epidermal HS could provide a cure for alopecia. Plenty of research is still required to unveil the precise role of HS and HSPGs in the formation of skin appendages, such as sebaceous glands and sweat glands, teeth and nails. This research could have important pharmaceutical implications for targeting HS expression.
Acknowledgements
I would like to thank both Dr. Yvette May Coulson‐Thomas and Dr. Tarsis F. Gesteira for their invaluable contributions to this review. The compiled data on conformational changes of the HS domains have been included with permission from Dr. Tarsis F. Gesteira. This review was made possible through the Young Investigator Award from the BSMB to VJCT. This review was funded in part through the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisa (CNPq). The Ohio Supercomputer Center provided High Performance Computing resources.
References
- Ai X., Do A.T., Lozynska O., Kusche‐Gullberg M., Lindahl U. & Emerson C.P. Jr (2003) QSulf1 remodels the 6‐O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. J. Cell Biol. 162, 341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai X., Kitazawa T., Do A.T., Kusche‐Gullberg M., Labosky P.A. & Emerson C.P. Jr (2007) SULF1 and SULF2 regulate heparan sulfate‐mediated GDNF signaling for esophageal innervation. Development 134, 3327–3338. [DOI] [PubMed] [Google Scholar]
- Aikawa J. & Esko J.D. (1999) Molecular cloning and expression of a third member of the heparan sulfate/heparin GlcNAc N‐deacetylase/N‐sulfotransferase family. J. Biol. Chem. 274, 2690–2695. [DOI] [PubMed] [Google Scholar]
- Arikawa‐Hirasawa E., Watanabe H., Takami H., Hassell J.R. & Yamada Y. (1999) Perlecan is essential for cartilage and cephalic development. Nat. Genet. 23, 354–358. [DOI] [PubMed] [Google Scholar]
- Baker N.A., Sept D., Joseph S., Holst M.J. & McCammon J.A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. USA 98, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian R. & Zhang X. (2015) Mechanisms of FGF gradient formation during embryogenesis. Semin. Cell Dev. Biol. 18, 14119–14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C.E. & Zimmermann D.R. (2000) Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 80, 1267–1290. [DOI] [PubMed] [Google Scholar]
- Bayer‐Garner I.B., Sanderson R.D. & Smoller B.R. (2002) Syndecan‐1 is strongly expressed in the anagen hair follicle outer root sheath and in the dermal papilla but expression diminishes with involution of the hair follicle. Am. J. Dermatopathol. 24, 484–489. [DOI] [PubMed] [Google Scholar]
- Behrens D.T., Villone D., Koch M. et al (2012) The epidermal basement membrane is a composite of separate laminin‐ or collagen IV‐containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 287, 18700–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs L.C. & Mikkola M.L. (2014) Early inductive events in ectodermal appendage morphogenesis. Semin. Cell Dev. Biol. 25–26, 11–21. [DOI] [PubMed] [Google Scholar]
- Binari R.C., Staveley B.E., Johnson W.A., Godavarti R., Sasisekharan R. & Manoukian A.S. (1997) Genetic evidence that heparin‐like glycosaminoglycans are involved in wingless signaling. Development 124, 2623–2632. [DOI] [PubMed] [Google Scholar]
- Bishop J.R., Schuksz M. & Esko J.D. (2007) Heparan sulphate proteoglycans fine‐tune mammalian physiology. Nature 446, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Bourdon M.A., Krusius T., Campbell S., Schwartz N.B. & Ruoslahti E. (1987) Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc. Natl Acad. Sci. USA 84, 3194–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman Y.G., Ford M.D., Gallagher J.T., Nurcombe V., Bartlett P.F. & Turnbull J.E. (1998) Structural modification of fibroblast growth factor‐binding heparan sulfate at a determinative stage of neural development. J. Biol. Chem. 273, 4350–4359. [DOI] [PubMed] [Google Scholar]
- Bronckers A.L., Lyaruu D.M. & Woltgens J.H. (1989) Immunohistochemistry of extracellular matrix proteins during various stages of dentinogenesis. Connect. Tissue Res. 22, 65–70. [PubMed] [Google Scholar]
- Brooks B.R., Brooks C.L. 3rd, Mackerell A.D. Jr et al (2009) CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. & Waneck G.L. (1992) Glycosyl‐phosphatidylinositol‐anchored membrane proteins. J. Am. Soc. Nephrol. 3, 895–906. [DOI] [PubMed] [Google Scholar]
- Bullock S.L., Fletcher J.M., Beddington R.S. & Wilson V.A. (1998) Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2‐sulfotransferase. Genes Dev. 12, 1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow H.E. & Hobert O. (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 41, 723–736. [DOI] [PubMed] [Google Scholar]
- Bulow H.E., Tjoe N., Townley R.A., Didiano D., van Kuppevelt T.H. & Hobert O. (2008) Extracellular sugar modifications provide instructive and cell‐specific information for axon‐guidance choices. Curr. Biol. 18, 1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse E. & de Groot H. (1991) Generation of developmental patterns in the neuroepithelium of the developing mammalian eye: the pigment epithelium of the eye. Neurosci. Lett. 126, 63–66. [DOI] [PubMed] [Google Scholar]
- Cai Z., Grobe K. & Zhang X. (2014) Role of heparan sulfate proteoglycans in optic disc and stalk morphogenesis. Dev. Dyn. 243, 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano‐Gauci D.F., Song H.H., Yang H. et al (1999) Glypican‐3‐deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson‐Golabi‐Behmel syndrome. J. Cell Biol. 146, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey D.J. (1996) N‐syndecan: structure and function of a transmembrane heparan sulfate proteoglycan. Perspect. Dev. Neurobiol. 3, 331–346. [PubMed] [Google Scholar]
- Chai L. & Morris J.E. (1994) Distribution of heparan sulfate proteoglycans in embryonic chicken neural retina and isolated inner limiting membrane. Curr. Eye Res. 13, 669–677. [DOI] [PubMed] [Google Scholar]
- Chan J.A., Balasubramanian S., Witt R.M. et al (2009) Proteoglycan interactions with Sonic Hedgehog specify mitogenic responses. Nat. Neurosci. 12, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W.K., Howe K., Clegg J.M. et al (2015) 2‐O heparan sulfate sulfation by Hs2st is required for Erk/Mapk signalling activation at the mid‐gestational mouse telencephalic midline. PLoS ONE 10, e0130147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.C., Mulloy B., Magee A.I. & Couchman J.R. (2011) Two distinct sites in sonic Hedgehog combine for heparan sulfate interactions and cell signaling functions. J. Biol. Chem. 286, 44391–44402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J.M., Conway C.D., Howe K.M. et al (2014) Heparan sulfotransferases Hs6st1 and Hs2st keep Erk in check for mouse corpus callosum development. J. Neurosci. 34, 2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M., Gustafsson E., Aszodi A. et al (1999) Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 147, 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman J.R., Vogt S., Lim S.T. et al (2002) Regulation of inositol phospholipid binding and signaling through syndecan‐4. J. Biol. Chem. 277, 49296–49303. [DOI] [PubMed] [Google Scholar]
- Coulson‐Thomas V.J., Gesteira T.F., Esko J. & Kao W. (2014) Heparan sulfate regulates hair follicle and sebaceous gland morphogenesis and homeostasis. J. Biol. Chem. 289, 25211–25226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson‐Thomas V.J., Chang S.H., Yeh L.K. et al (2015) Loss of corneal epithelial heparan sulfate leads to corneal degeneration and impaired wound healing. Invest. Ophthalmol. Vis. Sci. 56, 3004–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C.Y., Klar J., Georgii‐Heming P. et al (2013) Frizzled6 deficiency disrupts the differentiation process of nail development. J Invest Dermatol 133, 1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N., Nahm M., Lee S. & Broadie K. (2012) A targeted glycan‐related gene screen reveals heparan sulfate proteoglycan sulfation regulates WNT and BMP trans‐synaptic signaling. PLoS Genet. 8, e1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.A., Yates E.A. & Turnbull J.E. (2003) Structural determinants of heparan sulphate modulation of GDNF signalling. Growth Factors 21, 109–119. [DOI] [PubMed] [Google Scholar]
- Deepa S.S., Yamada S., Zako M., Goldberger O. & Sugahara K. (2004) Chondroitin sulfate chains on syndecan‐1 and syndecan‐4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. J. Biol. Chem. 279, 37368–37376. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F., Streit M., Wilcox‐Adelman S. et al (2001) Delayed wound repair and impaired angiogenesis in mice lacking syndecan‐4. J Clin Invest 107, R9–R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T.J. & Hammarlund M. (2014) Syndecan promotes axon regeneration by stabilizing growth cone migration. Cell Rep. 8, 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entesarian M., Matsson H., Klar J. et al (2005) Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat. Genet. 37, 125–127. [DOI] [PubMed] [Google Scholar]
- Erickson A.C. & Couchman J.R. (2000) Still more complexity in mammalian basement membranes. J. Histochem. Cytochem. 48, 1291–1306. [DOI] [PubMed] [Google Scholar]
- Fan G., Xiao L., Cheng L., Wang X., Sun B. & Hu G. (2000) Targeted disruption of NDST‐1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 467, 7–11. [DOI] [PubMed] [Google Scholar]
- Ford‐Perriss M., Guimond S.E., Greferath U. et al (2002) Variant heparan sulfates synthesized in developing mouse brain differentially regulate FGF signaling. Glycobiology 12, 721–727. [DOI] [PubMed] [Google Scholar]
- Forsberg E., Pejler G., Ringvall M. et al (1999) Abnormal mast cells in mice deficient in a heparin‐synthesizing enzyme. Nature 400, 773–776. [DOI] [PubMed] [Google Scholar]
- Franch‐Marro X., Marchand O., Piddini E., Ricardo S., Alexandre C. & Vincent J.P. (2005) Glypicans shunt the Wingless signal between local signalling and further transport. Development 132, 659–666. [DOI] [PubMed] [Google Scholar]
- Fuerst P.G., Rauch S.M. & Burgess R.W. (2007) Defects in eye development in transgenic mice overexpressing the heparan sulfate proteoglycan agrin. Dev. Biol. 303, 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Lagutin O., Hogan B.L. & Oliver G.C. (2000) Retina‐ and ventral forebrain‐specific Cre recombinase activity in transgenic mice. Genesis 26, 130–132. [PubMed] [Google Scholar]
- Gallagher J.T., Turnbull J.E. & Lyon M. (1992) Heparan sulphate proteoglycans: molecular organisation of membrane–associated species and an approach to polysaccharide sequence analysis. Adv. Exp. Med. Biol. 313, 49–57. [DOI] [PubMed] [Google Scholar]
- Garcia C.M., Yu K., Zhao H. et al (2005) Signaling through FGF receptor‐2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev. Dyn. 233, 516–527. [DOI] [PubMed] [Google Scholar]
- Gesteira T.F., Coulson‐Thomas V.J., Ogata F.T. et al (2011a) A novel approach for the characterisation of proteoglycans and biosynthetic enzymes in a snail model. Biochim. Biophys. Acta 1814, 1862–1869. [DOI] [PubMed] [Google Scholar]
- Gesteira T.F., Coulson‐Thomas V.J., Taunay‐Rodrigues A. et al (2011b) Inhibitory peptides of the sulfotransferase domain of the heparan sulfate enzyme, N‐deacetylase‐N‐sulfotransferase‐1. J. Biol. Chem. 286, 5338–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteira T.F., Pol‐Fachin L., Coulson‐Thomas V.J., Lima M.A., Verli H. & Nader H.B. (2013) Insights into the N‐sulfation mechanism: molecular dynamics simulations of the N‐sulfotransferase domain of NDST1 and mutants. PLoS ONE 8, e70880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal S., Sogaard P., Multhaupt H.A.B. et al (2015) Transmembrane proteoglycans control stretch‐activated channels to set cytosolic calcium levels. J. Cell Biol. 210, 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe K., Ledin J., Ringvall M. et al (2002) Heparan sulfate and development: differential roles of the N‐acetylglucosamine N‐deacetylase/N‐sulfotransferase isozymes. Biochim. Biophys. Acta 1573, 209–215. [DOI] [PubMed] [Google Scholar]
- Grobe K., Inatani M., Pallerla S.R., Castagnola J., Yamaguchi Y. & Esko J.D. (2005) Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132, 3777–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B.B., Lindahl U. & Rapraeger A.C. (1993) Activating and inhibitory heparin sequences for FGF‐2 (basic FGF). Distinct requirements for FGF‐1, FGF‐2, and FGF‐4. J. Biol. Chem. 268, 23906–23914. [PubMed] [Google Scholar]
- Hacker U., Lin X. & Perrimon N. (1997) The Drosophila sugarless gene modulates Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development 124, 3565–3573. [DOI] [PubMed] [Google Scholar]
- Hacker U., Nybakken K. & Perrimon N. (2005) Heparan sulphate proteoglycans: the sweet side of development. Nat. Rev. Mol. Cell Biol. 6, 530–541. [DOI] [PubMed] [Google Scholar]
- Haerry T.E., Heslip T.R., Marsh J.L. & O'Connor M.B. (1997) Defects in glucuronate biosynthesis disrupt Wingless signaling in Drosophila. Development 124, 3055–3064. [DOI] [PubMed] [Google Scholar]
- HajMohammadi S., Enjyoji K., Princivalle M. et al (2003) Normal levels of anticoagulant heparan sulfate are not essential for normal hemostasis. J Clin Invest 111, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick M.W. (2001) Development and evolution of the mammalian limb: adaptive diversification of nails, hooves, and claws. Evol Dev 3, 355–363. [DOI] [PubMed] [Google Scholar]
- Han C., Belenkaya T.Y., Wang B. & Lin X. (2004) Drosophila glypicans control the cell‐to‐cell movement of Hedgehog by a dynamin‐independent process. Development 131, 601–611. [DOI] [PubMed] [Google Scholar]
- Han C., Yan D., Belenkaya T.Y. & Lin X. (2005) Drosophila glypicans Dally and Dally‐like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132, 667–679. [DOI] [PubMed] [Google Scholar]
- Hayano S., Kurosaka H., Yanagita T. et al (2012) Roles of heparan sulfate sulfation in dentinogenesis. J. Biol. Chem. 287, 12217–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginson J.R., Thompson S.M., Santos‐Silva A., Guimond S.E., Turnbull J.E. & Barnett S.C. (2012) Differential sulfation remodelling of heparan sulfate by extracellular 6‐O‐sulfatases regulates fibroblast growth factor‐induced boundary formation by glial cells: implications for glial cell transplantation. J. Neurosci. 32, 15902–15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A. & Nakamura H. (2006) Localization of perlecan and heparanase in Hertwig's epithelial root sheath during root formation in mouse molars. J. Histochem. Cytochem. 54, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst C.R., Bou‐Reslan H., Gore B.B. et al (2007) Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS ONE 2, e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. (2001) Cell‐surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat. Neurosci. 4, 695–701. [DOI] [PubMed] [Google Scholar]
- Huang J.X., Feldmeier M., Shui Y.B. & Beebe D.C. (2003) Evaluation of fibroblast growth factor signaling during lens fiber cell differentiation. Invest. Ophthalmol. Vis. Sci. 44, 680–690. [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A. & Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14(33–38), 27–38. [DOI] [PubMed] [Google Scholar]
- Humphries D.E., Wong G.W., Friend D.S., Gurish M.F. & Stevens R.L. (1999) 14 heparin‐null transgenic mice are unable to store certain granule proteases in their mast cells. J. Histochem. Cytochem. 47, 1645D–1646. [PubMed] [Google Scholar]
- Ida‐Yonemochi H., Satokata I., Ohshima H. et al (2011) Morphogenetic roles of perlecan in the tooth enamel organ: an analysis of overexpression using transgenic mice. Matrix Biol. 30, 379–388. [DOI] [PubMed] [Google Scholar]
- Inatani M. & Yamaguchi Y. (2003) Gene expression of EXT1 and EXT2 during mouse brain development. Brain Res. Dev. Brain Res. 141, 129–136. [DOI] [PubMed] [Google Scholar]
- Inatani M., Irie F., Plump A.S., Tessier‐Lavigne M. & Yamaguchi Y. (2003) Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science 302, 1044–1046. [DOI] [PubMed] [Google Scholar]
- Inomata T., Ebihara N., Funaki T. et al (2012) Perlecan‐deficient mutation impairs corneal epithelial structure. Invest. Ophthalmol. Vis. Sci. 53, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Iozzo R.V., Zoeller J.J. & Nystrom A. (2009) Basement membrane proteoglycans: modulators Par Excellence of cancer growth and angiogenesis. Mol. Cells 27, 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A., Yates E.A., Turnbull J.E. & Holt C.E. (2002) Specific heparan sulfate structures involved in retinal axon targeting. Development 129, 61–70. [DOI] [PubMed] [Google Scholar]
- Iriyama S., Hiruma T., Tsunenaga M. & Amano S. (2011a) Influence of heparan sulfate chains in proteoglycan at the dermal‐epidermal junction on epidermal homeostasis. Exp. Dermatol. 20, 810–814. [DOI] [PubMed] [Google Scholar]
- Iriyama S., Tsunenaga M., Amano S. & Adachi E. (2011b) Key role of heparan sulfate chains in assembly of anchoring complex at the dermal‐epidermal junction. Exp. Dermatol. 20, 953–955. [DOI] [PubMed] [Google Scholar]
- Iwao K., Inatani M., Matsumoto Y. et al (2009a) Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF‐beta2 signaling. J Clin Invest 119, 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao K., Inatani M., Ogata‐Iwao M., Takihara Y., Tanihara H. (2009b) Restricted post‐trabeculectomy bleb formation by conjunctival scarring. Graefes Arch. Clin. Exp. Ophthalmol. 247, 1095–1101. [DOI] [PubMed] [Google Scholar]
- Izvolsky K.I., Lu J., Martin G., Albrecht K.H. & Cardoso W.V. (2008) Systemic inactivation of Hs6st1 in mice is associated with late postnatal mortality without major defects in organogenesis. Genesis 46, 8–18. [DOI] [PubMed] [Google Scholar]
- Jenkinson D.M. (1973) Comparative phisiology of sweating. Br. J. Dermatol. 88, 397–406. [DOI] [PubMed] [Google Scholar]
- Johnson K.G., Ghose A., Epstein E., Lincecum J., O'Connor M.B. & Van Vactor D. (2004) Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr. Biol. 14, 499–504. [DOI] [PubMed] [Google Scholar]
- Kalus I., Rohn S., Puvirajesinghe T.M. et al (2015) Sulf1 and Sulf2 differentially modulate heparan sulfate proteoglycan sulfation during postnatal cerebellum development: evidence for neuroprotective and neurite outgrowth promoting functions. PLoS ONE 10, e0139853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E.D. & Holbrook K.A. (1994) Dynamic expression patterns of tenascin, proteoglycans, and cell adhesion molecules during human hair follicle morphogenesis. Dev. Dyn. 199, 141–155. [DOI] [PubMed] [Google Scholar]
- Kinnunen A., Kinnunen T., Kaksonen M., Nolo R., Panula P. & Rauvala H. (1998) N‐syndecan and HB‐GAM (heparin‐binding growth‐associated molecule) associate with early axonal tracts in the rat brain. Eur J Neuorsci 10, 635–648. [DOI] [PubMed] [Google Scholar]
- Kitagawa H. & Sugahara K. (2000) [Biosynthesis of heparan sulfate and the tumor suppressor EXT gene family]. Tanpakushitsu Kakusan Koso 45, 579–586. [PubMed] [Google Scholar]
- Kitagawa H., Tsutsumi K., Tone Y. & Sugahara K. (1997) Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J. Biol. Chem. 272, 31377–31381. [DOI] [PubMed] [Google Scholar]
- Klaus A. & Birchmeier W. (2008) Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398. [DOI] [PubMed] [Google Scholar]
- Kleinschmit A., Koyama T., Dejima K., Hayashi Y., Kamimura K. & Nakato H. (2010) Drosophila heparan sulfate 6‐O endosulfatase regulates Wingless morphogen gradient formation. Dev. Biol. 345, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmit A., Takemura M., Dejima K., Choi P.Y. & Nakato H. (2013) Drosophila heparan sulfate 6‐O‐endosulfatase Sulf1 facilitates wingless (Wg) protein degradation. J. Biol. Chem. 288, 5081–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogaya Y., Kim S. & Akisaka T. (1990) Changes in ultrastructural distribution of dental basement membrane heparan sulfate during early mouse tooth development. J Biol Buccale 18, 109–115. [PubMed] [Google Scholar]
- Kolset S.O. & Gallagher J.T. (1990) Proteoglycans in haemopoietic cells. Biochim. Biophys. Acta 1032, 191–211. [DOI] [PubMed] [Google Scholar]
- Kolset S.O., Prydz K. & Pejler G. (2004) Intracellular proteoglycans. Biochem J 379, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B.K., Jung Y.S., Shin J. et al (2006) Structural basis of syndecan‐4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 355, 651–663. [DOI] [PubMed] [Google Scholar]
- Kraushaar D.C., Rai S., Condac E. et al (2012) Heparan sulfate facilitates FGF and BMP signaling to drive mesoderm differentiation of mouse embryonic stem cells. J. Biol. Chem. 287, 22691–22700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Chien J., Staub J. et al (2003) Loss of HSulf‐1 up‐regulates heparin‐binding growth factor signaling in cancer. J. Biol. Chem. 278, 23107–23117. [DOI] [PubMed] [Google Scholar]
- Lamanna W.C., Kalus I., Padva M., Baldwin R.J., Merry C.L. & Dierks T. (2007) The heparanome – the enigma of encoding and decoding heparan sulfate sulfation. J. Biotechnol. 129, 290–307. [DOI] [PubMed] [Google Scholar]
- Ledin J., Staatz W., Li J.P. et al (2004) Heparan sulfate structure in mice with genetically modified heparan sulfate production. J. Biol. Chem. 279, 42732–42741. [DOI] [PubMed] [Google Scholar]
- Li J.P., Gong F., Hagner‐McWhirter A. et al (2003) Targeted disruption of a murine glucuronyl C5‐epimerase gene results in heparan sulfate lacking L‐iduronic acid and in neonatal lethality. J. Biol. Chem. 278, 28363–28366. [DOI] [PubMed] [Google Scholar]
- Liang Y., Annan R.S., Carr S.A. et al (1999) Mammalian homologues of the Drosophila slit protein are ligands of the heparan sulfate proteoglycan glypican‐1 in brain. J. Biol. Chem. 274, 17885–17892. [DOI] [PubMed] [Google Scholar]
- Lin X. & Perrimon N. (1999) Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400, 281–284. [DOI] [PubMed] [Google Scholar]
- Lin X. & Perrimon N. (2000) Role of heparan sulfate proteoglycans in cell‐cell signaling in Drosophila . Matrix Biol. 19, 303–307. [DOI] [PubMed] [Google Scholar]
- Lin X., Buff E.M., Perrimon N. & Michelson A.M. (1999) Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development 126, 3715–3723. [DOI] [PubMed] [Google Scholar]
- Lin X., Wei G., Shi Z. et al (2000) Disruption of gastrulation and heparan sulfate biosynthesis in EXT1‐deficient mice. Dev. Biol. 224, 299–311. [DOI] [PubMed] [Google Scholar]
- Lohmander L.S., Shinomura T., Hascall V.C. & Kimura J.H. (1989) Xylosyl transfer to the core protein precursor of the rat chondrosarcoma proteoglycan. J. Biol. Chem. 264, 18775–18780. [PubMed] [Google Scholar]
- Lu C. & Fuchs E. (2014) Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb. Perspect Med. 4, a015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Lu Q., Zheng Y. & Li Q. (2012) Notch signaling promotes the corneal epithelium wound healing. Mol Vis 18, 403–411. [PMC free article] [PubMed] [Google Scholar]
- Makarenkova H.P., Ito M., Govindarajan V. et al (2000) FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 127, 2563–2572. [DOI] [PubMed] [Google Scholar]
- Malgouries S., Donovan M., Thibaut S. & Bernard B.A. (2008) Heparanase 1: a key participant of inner root sheath differentiation program and hair follicle homeostasis. Exp. Dermatol. 17, 1017–1023. [DOI] [PubMed] [Google Scholar]
- Maltseva I., Chan M., Kalus I., Dierks T. & Rosen S.D. (2013) The SULFs, extracellular sulfatases for heparan sulfate, promote the migration of corneal epithelial cells during wound repair. PLoS ONE 8, e69642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo A. & Bix G. (2015) The potential role of perlecan domain V as novel therapy in vascular dementia. Metab. Brain Dis. 30, 1–5. [DOI] [PubMed] [Google Scholar]
- Margolis R.K., Rauch U., Maurel P. & Margolis R.U. (1996) Neurocan and phosphacan: two major nervous tissue‐specific chondroitin sulfate proteoglycans. Perspect. Dev. Neurobiol. 3, 273–290. [PubMed] [Google Scholar]
- Marti‐Renom M.A., Stuart A.C., Fiser A., Sanchez R., Melo F. & Sali A. (2000) Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29, 291–325. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Irie F., Inatani M., Tessier‐Lavigne M. & Yamaguchi Y. (2007) Netrin‐1/DCC signaling in commissural axon guidance requires cell‐autonomous expression of heparan sulfate. J Neurosc 27, 4342–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I. & Moll R. (1992) Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. J Invest Dermatol 98, 777–785. [DOI] [PubMed] [Google Scholar]
- Muto T., Miyoshi K., Munesue S. et al (2007) Differential expression of syndecan isoforms during mouse incisor amelogenesis. J Med Invest 54, 331–339. [DOI] [PubMed] [Google Scholar]
- Nadanaka S., Ishida M., Ikegami M. & Kitagawa H. (2008) Chondroitin 4‐O‐sulfotransferase‐1 modulates Wnt‐3a signaling through control of E disaccharide expression of chondroitin sulfate. J. Biol. Chem. 283, 27333–27343. [DOI] [PubMed] [Google Scholar]
- Ogata‐Iwao M., Inatani M., Iwao K. et al (2011) Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 52, 6671–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlig S., Pickhinke U., Sirko S. et al (2012) An emerging role of Sonic hedgehog shedding as a modulator of heparan sulfate interactions. J. Biol. Chem. 287, 43708–43719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oohira A., Matsui F., Watanabe E., Kushima Y. & Maeda N. (1994) Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody IG2 in the rat cerebrum. Neuroscience 60, 145–157. [DOI] [PubMed] [Google Scholar]
- Osipov V.V. & Vakhrusheva M.P. (1975) Interaction of the ectomesenchyme and optic vesicle in mice. Sov J Dev Biol 5, 353–355. [PubMed] [Google Scholar]
- Palma V., Carrasco H., Reinchisi G., Olivares G., Faunes F. & Larrain J. (2011) SHh activity and localization is regulated by perlecan. Biol. Res. 44, 63–67. [DOI] [PubMed] [Google Scholar]
- Pan Y., Woodbury A., Esko J.D., Grobe K. & Zhang X. (2006) Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development 133, 4933–4944. [DOI] [PubMed] [Google Scholar]
- Pan Y., Carbe C., Powers A. et al (2008) Bud specific N‐sulfation of heparan sulfate regulates Shp2‐dependent FGF signaling during lacrimal gland induction. Development 135, 301–310. [DOI] [PubMed] [Google Scholar]
- Park Y., Rangel C., Reynolds M.M. et al (2003) Drosophila perlecan modulates FGF and hedgehog signals to activate neural stem cell division. Dev. Biol. 253, 247–257. [DOI] [PubMed] [Google Scholar]
- Pei Y.F. & Rhodin J.A. (1970) The prenatal development of the mouse eye. Anat. Rec. 168, 105–125. [DOI] [PubMed] [Google Scholar]
- Perkins S.J., Fung K.W. & Khan S. (2014) Molecular interactions between complement factor H and its heparin and heparan sulfate ligands. Front Immunol. 5, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhal M.A., Smith B., Olson S., Aikawa J., Kimata K. & Esko J.D. (2001) Enzyme interactions in heparan sulfate biosynthesis: uronosyl 5‐epimerase and 2‐O‐sulfotransferase interact in vivo. Proc. Natl Acad. Sci. USA 98, 12984–12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz K. & Dalen K.T. (2000) Synthesis and sorting of proteoglycans. J. Cell Sci. 113(Pt 2), 193–205. [DOI] [PubMed] [Google Scholar]
- Pye D.A., Vives R.R., Turnbull J.E., Hyde P. & Gallagher J.T. (1998) Heparan sulfate oligosaccharides require 6‐O‐sulfation for promotion of basic fibroblast growth factor mitogenic activity. J. Biol. Chem. 273, 22936–22942. [DOI] [PubMed] [Google Scholar]
- Qu X., Carbe C., Tao C. et al (2011) Lacrimal gland development and Fgf10‐Fgfr2b signaling are controlled by 2‐O‐ and 6‐O‐sulfated heparan sulfate. J. Biol. Chem. 286, 14435–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X., Pan Y., Carbe C., Powers A., Grobe K. & Zhang X. (2012) Glycosaminoglycan‐dependent restriction of FGF diffusion is necessary for lacrimal gland development. Development 139, 2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsbottom S.A., Maguire R.J., Fellgett S.W. & Pownall M.E. (2014) Sulf1 influences the Shh morphogen gradient during the dorsal ventral patterning of the neural tube in Xenopus tropicalis . Dev. Biol. 391, 207–218. [DOI] [PubMed] [Google Scholar]
- Rapraeger A.C., Guimond S., Krufka A. & Olwin B.B. (1994) Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 245, 219–240. [DOI] [PubMed] [Google Scholar]
- Ratzka A., Kalus I., Moser M., Dierks T., Mundlos S. & Vortkamp A. (2008) Redundant function of the heparan sulfate 6‐O‐endosulfatases Sulf1 and Sulf2 during skeletal development. Dev. Dyn. 237, 339–353. [DOI] [PubMed] [Google Scholar]
- Rhiner C. & Hengartner M.O. (2006) Sugar antennae for guidance signals: syndecans and glypicans integrate directional cues for navigating neurons. The Scientific World Journal 6, 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G.D., Fantauzzo K.A., Bazzi H., Maatta A. & Jahoda C.A. (2009) Dynamic expression of Syndecan‐1 during hair follicle morphogenesis. Gene Expr. Patterns 9, 454–460. [DOI] [PubMed] [Google Scholar]
- Ringvall M., Ledin J., Holmborn K. et al (2000) Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N‐deacetylase/N‐sulfotransferase‐1. J. Biol. Chem. 275, 25926–25930. [DOI] [PubMed] [Google Scholar]
- Rosen S.D. & Lemjabbar‐Alaoui H. (2010) Sulf‐2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets 14, 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengart T.K., Johnson W.V., Friesel R., Clark R. & Maciag T. (1988) Heparin protects heparin‐binding growth factor‐I from proteolytic inactivation in vitro. Biochem. Biophys. Res. Commun. 152, 432–440. [DOI] [PubMed] [Google Scholar]
- Rossi M., Morita H., Sormunen R. et al (2003) Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 22, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J.B., Choi Y. & Segal R.A. (2002) Cerebellar proteoglycans regulate sonic hedgehog responses during development. Development 129, 2223–2232. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A. & Rifkin D.B. (1988) Endothelial cell‐derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 107, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Kang W.H., Saga K. & Sato K.T. (1989) Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 20, 537–563. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Nielsen B., Schmidt‐Nielsen K., Houpt T.R. & Jarnum S.A. (1957) Urea excretion in the camel. Am. J. Physiol. 188, 477–484. [DOI] [PubMed] [Google Scholar]
- Schulze B., Sasaki T., Costell M., Mann K. & Timpl R. (1996) Structural and cell‐adhesive properties of three recombinant fragments derived from perlecan domain III. Matrix Biol. 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Senay C., Lind T., Muguruma K. et al (2000) The EXT1/EXT2 tumor suppressors: catalytic activities and role in heparan sulfate biosynthesis. EMBO Rep. 1, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B., Sharma R.P. & Prakash R. (1973) Enhancement of ethylemethane sulfunate‐induced frequency in Drosophila by dimethyl sulphoxide. Experientia 29, 1029–1030. [DOI] [PubMed] [Google Scholar]
- Sher I., Zisman‐Rozen S., Eliahu L. et al (2006) Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 281, 5178–5187. [DOI] [PubMed] [Google Scholar]
- Shimokawa K., Kimura‐Yoshida C., Nagai N. et al (2011) Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev. Cell 21, 257–272. [DOI] [PubMed] [Google Scholar]
- Smith P.D., Coulson‐Thomas V.J., Foscarin S., Kwok J.C. & Fawcett J.W. (2015) “GAG‐ing with the neuron”: the role of glycosaminoglycan patterning in the central nervous system. Exp. Neurol. 3, 100–114. [DOI] [PubMed] [Google Scholar]
- Stanford K.I., Wang L., Castagnola J. et al (2010) Heparan sulfate 2‐O‐sulfotransferase is required for triglyceride‐rich lipoprotein clearance. J. Biol. Chem. 285, 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigemann P., Molitor A., Fellert S., Jackle H. & Vorbruggen G. (2004) Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr. Biol. 14, 225–230. [DOI] [PubMed] [Google Scholar]
- Stepp M.A., Gibson H.E., Gala P.H. et al (2002) Defects in keratinocyte activation during wound healing in the syndecan‐1‐deficient mouse. J. Cell Sci. 115, 4517–4531. [DOI] [PubMed] [Google Scholar]
- Stepp M.A., Liu Y., Pal‐Ghosh S. et al (2007) Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J. Cell Sci. 120, 2851–2863. [DOI] [PubMed] [Google Scholar]
- Stepp M.A., Daley W.P., Bernstein A.M. et al (2010) Syndecan‐1 regulates cell migration and fibronectin fibril assembly. Exp. Cell Res. 316, 2322–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetefeld J., Alexandrescu A.T., Maciejewski M.W. et al (2004) Modulation of agrin function by alternative splicing and Ca2+ binding. Structure 12, 503–515. [DOI] [PubMed] [Google Scholar]
- Sun T.T., Shih C. & Green H. (1979) Keratin cytoskeletons in epithelial cells of internal organs. Proc. Natl Acad. Sci. USA 76, 2813–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantravahi R.V., Stevens R.L., Austen K.F. & Weis J.H. (1986) A single gene in mast cells encodes the core peptides of heparin and chondroitin sulfate proteoglycans. Proc. Natl Acad. Sci. USA 83, 9207–9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The I., Bellaiche Y. & Perrimon N. (1999) Hedgehog movement is regulated through tout velu‐dependent synthesis of a heparan sulfate proteoglycan. Mol. Cell 4, 633–639. [DOI] [PubMed] [Google Scholar]
- Thesleff I. (2003) Epithelial‐mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Barrach H.J., Foidart J.M., Vaheri A., Pratt R.M. & Martin G.R. (1981) Changes in the distribution of type IV collagen, laminin, proteoglycan, and fibronectin during mouse tooth development. Dev. Biol. 81, 182–192. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Kamimura K., Nakato H. et al (1999) The cell‐surface proteoglycan Dally regulates Wingless signalling in Drosophila . Nature 400, 276–280. [DOI] [PubMed] [Google Scholar]
- Turnbull J.E., Fernig D.G., Ke Y., Wilkinson M.C. & Gallagher J.T. (1992) Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J. Biol. Chem. 267, 10337–10341. [PubMed] [Google Scholar]
- Vertel B.M., Walters L.M., Flay N., Kearns A.E. & Schwartz N.B. (1993) Xylosylation is an endoplasmic reticulum to Golgi event. J. Biol. Chem. 268, 11105–11112. [PubMed] [Google Scholar]
- Vlodavsky I., Bashkin P., Ishai‐Michaeli R. et al (1991) Sequestration and release of basic fibroblast growth factor. Ann. N. Y. Acad. Sci. 638, 207–220. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Friedmann Y., Elkin M. et al (1999) Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 5, 793–802. [DOI] [PubMed] [Google Scholar]
- Voigt A., Pflanz R., Schafer U. & Jackle H. (2002) Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev. Dyn. 224, 403–412. [DOI] [PubMed] [Google Scholar]
- Wang S., Ai X., Freeman S.D. et al (2004) QSulf1, a heparan sulfate 6‐O‐endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc. Natl Acad. Sci. USA 101, 4833–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F.M. & Huck W.T. (2013) Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 14, 467–473. [DOI] [PubMed] [Google Scholar]
- Willis Chris D., Schaefer L., Iozzo Renato V. (2003) The biology of perlecan and its bioactive modules In Pathobiology and Signaling, pp 472–493 (ed N. Karamanos ), Germany: Walter De Gruyter, Walter de Gruyter GmbH. [Google Scholar]
- Witt R.M., Hecht M.L., Pazyra‐Murphy M.F. et al (2013) Heparan sulfate proteoglycans containing a glypican 5 core and 2‐O‐sulfo‐iduronic acid function as Sonic Hedgehog co‐receptors to promote proliferation. J. Biol. Chem. 288, 26275–26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Ichikawa N., Kosaki K. et al (2010) Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol. 29, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Sugahara K. & Ozbek S. (2011) Evolution of glycosaminoglycans: comparative biochemical study. Commun Integr Biol 4, 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane Y., Yaoita H. & Couchman J.R. (1996) Basement membrane proteoglycans are of epithelial origin in rodent skin. J Invest Dermatol 106, 531–537. [DOI] [PubMed] [Google Scholar]
- Yan D., Wu Y., Feng Y., Lin S.C. & Lin X. (2009) The core protein of glypican Dally‐like determines its biphasic activity in wingless morphogen signaling. Dev. Cell 17, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Chen G., Yang Y. et al (2014) Expression and roles of syndecan‐4 in dental epithelial cell differentiation. Int. J. Mol. Med. 34, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Zcharia E., Philp D., Edovitsky E. et al (2005) Heparanase regulates murine hair growth. Am. J. Pathol. 166, 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., McLellan J.S., Ayala A.M., Leahy D.J. & Linhardt R.J. (2007) Kinetic and structural studies on interactions between heparin or heparan sulfate and proteins of the hedgehog signaling pathway. Biochemistry 46, 3933–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Yang Y., Partanen J., Ciruna B.G., Rossant J. & Robinson M.L. (2006) Fibroblast growth factor receptor 1 (Fgfr1) is not essential for lens fiber differentiation in mice. Mol Vis 12, 15–25. [PMC free article] [PubMed] [Google Scholar]


