Summary
Tuberculous meningitis (TBM) is an outcome of neuroinflammatory degeneration caused due to Mycobacterium tuberculosis infection and leads to death or neurological disabilities in the affected individuals. It causes the highest morbidity and mortality amongst all forms of tuberculosis. Matrix metalloproteinase‐9 levels increase and cause inflammatory destruction during progression of the disease. Although corticosteroids are usually given as an adjuvant therapy to overcome these complications, treatment outcome is contradictory. This study was designed to evaluate whether specific inhibition of MMP‐9 can be beneficial in management of the disease. MMP‐9 levels were inhibited using SB‐3CT or dexamethasone along with conventional drugs for treatment of tuberculous meningitis. Both SB‐3CT and dexamethasone decreased the elevated levels of MMP‐9 in sera and tissues of the infected mice. However, dexamethasone administration had an inhibitory effect on bacillary clearance, while SB‐3CT potentiated the bacillary clearance, suggesting that MMP‐9, if specifically inhibited, can be beneficial in the management of TBM.
Keywords: inflammation, matrix metalloproteinase‐9, Mycobacterium tuberculosis, SB‐3CT, tuberculous meningitis
Tuberculous meningitis is an extrapulmonary form of tuberculosis, associated with severe neurological complications that arise due to robust host inflammatory response against the pathogen. Although it comprises only 1% of tuberculosis cases, still it takes the highest toll amongst the various forms of TB, causing death to one‐third of infected individuals and rendering half the survivors incapacitated with lifelong neurological disabilities. The irreversible sequelae include paraplegia, blindness, motor cognitive deficits, optic atrophy, other cranial nerve involvements and secondary disabilities such as airway or urinary tract complications (Hosoglu et al. 2002; Jordán Jiménez et al. 2005; Kalita et al. 2007; Detjen & Magdorf 2009; Christensen et al. 2011). The inflammatory response involves pathogen‐driven host‐derived factors (Rich & McCordock 1933; Thwaites et al. 2003; Be et al. 2009). These include proteases secreted by activated macrophages that cause tissue destruction due to their ability to degrade extra cellular matrix (Rivera‐Marrero et al. 2002).
Matrix metalloproteinases (MMPs) are a family of proteases, which have important role in the destruction of neurological tissue and have also been implicated in a range of pathologies such as experimental autoimmune encephalomyelitis and multiple sclerosis (Gijbels et al. 1992). MMPs are capable of catabolizing all components of the extracellular matrix, and their elevated levels are often accompanied by tissue destruction. Amongst various MMPs, MMP‐9 digests type IV collagen and laminin that are the essential components of basement membrane and play a major role in the breakdown of blood–brain barrier (Paul et al. 1998). Studies have shown the increased levels of MMP‐9 in the cerebrospinal fluid (CSF) of TBM patients indicating their potential definite role in complicating the disease (Lee et al. 2004). Breakdown of type IV collagen, an important component of the blood–brain barrier, by MMP‐9, is a key initial step in the pathophysiology of central nervous system (CNS) leucocyte influx (Okada et al. 1997; van Horssen et al. 2005).
National guidelines recommend adjunctive corticosteroids in addition to anti‐tubercular drugs to manage excessive inflammatory response for the treatment of TBM. Dexamethasone or prednisolone are recommended as adjuvants for the treatment of tuberculous meningitis (Thwaites et al. 2009; World Health Organization, 2010), and dexamethasone has been found significantly to decrease the elevated levels of MMP‐9 in CSF during TBM (Green et al. 2009). However, use of dexamethasone for controlling tissue damage during TBM is limited by various factors such as host genotype specificity (Tobin et al. 2012), reactivation of infection due to immune suppression activity (Manabe et al. 2008) and other side effects of dexamethasone (Hempen et al. 2002). Besides, it is not able to control severe disabilities (Thwaites et al. 2004).
In this study, the adjunctive role of a conventional anti‐inflammatory drug, i.e. dexamethasone and SB‐3CT, a very specific inhibitor of MMP‐9, along with anti‐TB drugs in the management of experimental tuberculosis, has been evaluated. A murine model of TBM was developed by inoculating Mycobacterium tuberculosis H37Rv via intracranial route in mice (van Well et al. 2007). It has been reported previously that infection via an intravenous route did not generate TBM in mice but that infection via the intracranial route was successful (Wu et al. 2000); therefore, infection was given intracranially in mice. This model could mimic the symptoms as well as the inflammatory nature of human disease, making it a very appropriate model for the evaluation of the therapeutic efficacy of MMP‐9 inhibition in TBM.
Materials and methods
Animals
Swiss albino mice of either sex (3–4 weeks old, 20–25 g) were obtained from the Central animal house of the institute.
Chemicals
Isoniazid, rifampicin, pyrazinamide, dexamethasone, MMP‐9, SB‐3CT and gelatin were obtained from Sigma (St. Louis, MO, USA). Oleic albumin dextrose catalase (OADC) enrichment and 7H11 agar were from Difco–Becton–Dickinson (Becton, Dickinson & Company, MD, USA); Sauton's media was obtained from HiMedia Laboratories (HiMedia Laboratories Pvt. Ltd., Mumbai, India). Acrylamide, bisacrylamide, ammonium persulphate (Sigma, St. Louis, MO, USA), TEMED (HiMedia Laboratories Pvt. Ltd., India), Triton X‐100 (BIO BASIC Inc., Canada), Brij‐35 (LOBA CHEMIE Pvt. Ltd., (India)) and other reagents used in zymography were of molecular grade. Ultrapure water was used throughout the study.
Mycobacterial culture, maintenance and media preparation
Mycobacterium tuberculosis H37Rv culture (NCTC7416) was originally obtained from National Collection of Type Culture, London, UK. The bacteria were grown in sterile Sautons's medium and maintained in sterile Lowenstein–Jensen slants. Cultures were grown under shaking conditions at 180 rpm, 37°C.
Development of murine model of TBM
The murine model of TBM was established as described (van Well et al. 2007). Stereotaxic coordinates chosen were 0 mm dorsal (× plane), 3.5 mm rostral (y plane) and 2 mm ventral (z plane) from bregma. Mice were anaesthetized by ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotactic device. A preliminary experiment was performed using trypan blue, which was injected intracranially to mice (n = 3). Briefly, a burr hole of 1.5 mm diameter was made at the inoculation site after midline sagittal incision, trypan blue was injected using a Hamilton syringe at slow speed, and scalp incision was stitched. After 30 min the animals were sacrificed and decapitated. Brain tissues were collected and processed for further analysis. A piece of brain tissue was used to prepare cryosections and examined microscopically. Another piece of tissue was stored in 10% buffered formalin and processed for histological analysis for the successful determination of inoculation site. Mycobacterium tuberculosis infection was given at a dose of 105 CFUs in 50 μl normal saline to each animal. Mice were sacrificed at 4, 8 and 12 weeks after infection. Brain, lungs and spleen were collected, and half of each organ was fixed in 10% buffered formalin, processed for haematoxylin–eosin staining and examined microscopically. The other half was homogenized in 1 ml of sterile normal saline and analysed for CFU enumeration and MMP‐9 analysis. Bacterial load in organs was evaluated by plating the tissue homogenates (1:10, 1:100 and 1:500 serial dilutions) on Middlebrook 7H11 agar plates to determine the number of colony forming units (CFUs) after 21 days of incubation at 37°C.
Gelatin substrate zymography
Mice sera and tissue homogenates were run for zymography on 8% polyacrylamide gels containing 0.3% SDS and gelatin (1 mg/ml) as described (Matsuura et al. 2000). Protein concentration in tissue homogenates was estimated by the BCA method (Bainor et al. 2011). Diluted serum samples (1:10) and tissue homogenates containing 100 μg protein were run for zymography. Each sample was mixed with equal volumes of zymography buffer [0.50 mM Tris‐HCl, pH 6.8, 10% glycerol, 2% SDS and 0.01% bromophenol blue] and run at 20 mA for 20 min. Commercial MMP‐9 standard was also run as a control; gels were agitated in 2.5% Triton X‐100 for 1 h, washed in washing buffer (50 mM Tris‐HCl buffer, pH 7.5 containing 200 mM NaCl) and incubated overnight at 37°C in renaturation buffer (50 mM Tris‐HCl (Sigma, St. Louis, MO, USA), pH 7.5 containing 200 mM NaCl, 5 mM CaCl2, 0.02% (w/v) Brij‐35, 0.01% sodium azide). Gels were stained with Coomassie G (HiMedia Laboratories Pvt. Ltd., India) blue dye and destained in 10% acetic acid to obtain MMP‐9 bands.
Therapeutic efficacy of anti‐tubercular drugs in combination with MMP‐9 inhibitor against TBM
Chemotherapeutic treatment was given to mice after 4 weeks of infection. Mice were administered anti‐tubercular drugs (isoniazid, rifampicin and pyrazinamide) with or without dexamethasone/SB‐3CT. Mice were divided into five groups (n = 6 in each group) and given different treatment combinations: Group I: untreated controls, Group II: anti‐tubercular drugs, Group III: anti‐tubercular drugs along with dexamethasone, Group IV: anti‐tubercular drugs and dexamethasone (i.v.), Group V: anti‐tubercular drugs and SB‐3CT. All drugs were administered orally except SB‐3CT that was given i.p.; and dexamethasone in treatment group IV, which was also given i.p. to make an appropriate comparison between therapeutic efficacies of the adjuvants. Anti‐tubercular drugs and dexamethasone were administered at a dosage equivalent to the therapeutic dose given to a 70 kg adult human and SB‐3CT as described (Dunham & Miya 1957).
After 8 weeks of chemotherapy, prior to sacrifice, animals were subjected to rotarod test to determine the effect of treatment on neuromuscular coordination. Mice were first trained to balance themselves on a rotating rod at 25 rpm for a period of 3 min. After 24 h, the mice were tested to maintain their body on the rotating rod for each of the three successive trials of 3 min (Gu et al. 2005). Mice were sacrificed under sodium pentothal anaesthesia, and blood, brain, lungs and spleen were collected and processed for CFU enumeration, histopathological studies and MMP‐9 analysis.
Statistical analysis
anova was used followed by student's t‐test to compare individual treatment groups with each other and untreated control. All values were mean ± S.D. Data were analysed using ms excel and spss 16 software.
Ethical approval
The plan of work was approved by the Institute Animal Ethics Committee (IAEC no.224), and all of the animal experiments were carried out according to ethical guidelines.
Results
Development of murine model of TBM
Successful inoculation and diffusion of trypan blue in the mouse brain was confirmed by microscopic examination of mice brain sections after 30 min of intracranial inoculation (Figure S1). CFU enumeration of tissues showed stable bacillary load in all tissues for up to 8 weeks postinfection. Bacillary load was significantly increased in brain and lung tissues after 12 weeks of infection (Figure 1).
Figure 1.
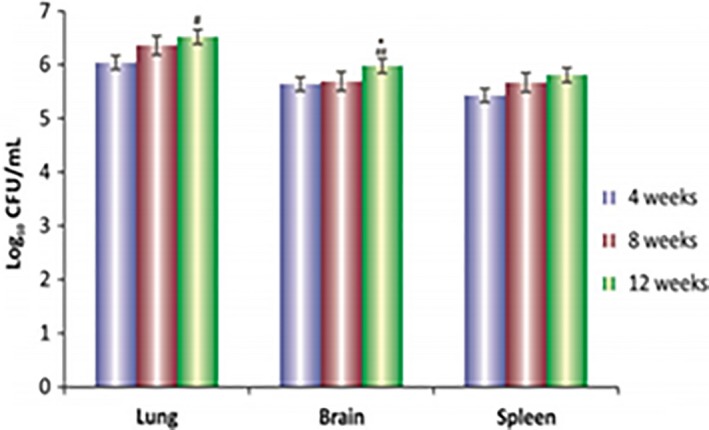
Log10 CFUs of M. tuberculosis h37r v in the brain, lungs and spleen of mice after 4, 8 and 12 weeks of infection. Values are mean ± SD of 3–5 animals at each time point * P < 0.05 with respect to 8‐week time interval of the corresponding organs, ## P < 0.001 with respect to 4‐week time interval of the corresponding organ.
Histopathological examination showed apparent meningitis on the surface of brain after 4 weeks infection which grew to denser meningitis after 8 weeks. After 12 weeks postinfection, both heavy meningitis with extensive oedema (Figure 2) and deeper brain TB pathology were observed (Figure 3). Undetectable levels of MMP‐9 were observed in tissues and sera prior to infection but were found to be elevated after four and eight weeks of infection (Figure 4). Histopathology of brain tissues showed tissue damage and oedema, which are the key features of human disease; thus, this model properly mimicked the inflammatory aspects of human disease and can be regarded as an efficient representative model system in which it is possible to evaluate the possible adjunctive role of MMP‐9 inhibition against TBM.
Figure 2.
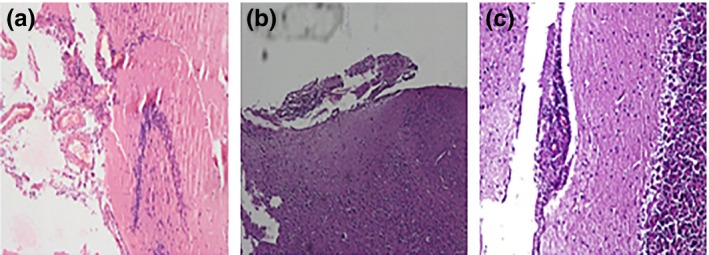
Representative histopathological micrographs of infected mice brain (a) apparent meningitis on brain surface 4 weeks after infection, (b) denser meningitis along with edema 8 weeks after infection and (c) heavy meningitis with deepening edema from submeningeal surface 12 weeks after infection.
Figure 3.
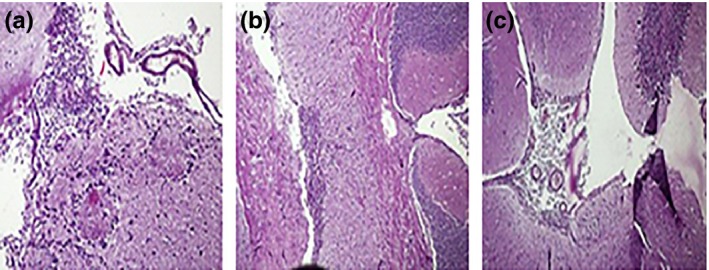
Representative histopathological micrographs of mice brain sections after 12 weeks of infection: (a) deeper brain tissue inflammatory reaction, (b) oedema of brain tissue beneath choroid plexus and (c) cerebellum part of brain with lymphocytic granuloma.
Figure 4.
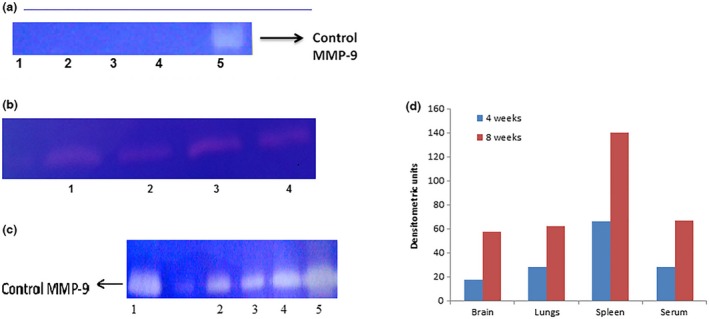
(a) Gelatin zymograms showing MMP‐9 levels in normal mice (1) brain, (2) lungs, (3) spleen, (4) serum and (5) control MMP‐9. (b) Gelatin zymograms showing MMP‐9 levels in mice after 4 weeks of infection (1) brain, (2) lungs, (3) spleen and (4) serum. (c) Gelatin zymograms showing MMP‐9 levels in organ homogenates and serum of untreated mice 8 week after TBM infection (1) control MMP‐9, (2) lungs, (3) brain, (4) serum and (5) spleen. (d) Densitometric representation of MMP‐9 in mice organs and sera after 4 and 8 weeks of infection.
Therapeutic efficacy of anti‐tubercular drugs in combination with MMP‐9 inhibitor (SB‐3CT) or dexamethasone against mice model of TBM
Bacillary load was decreased in all treatment groups; however, addition of dexamethasone to anti‐tubercular drugs was not effective in clearing the bacillary load. Rather it increased the bacterial burden in some of the organs. Interestingly, addition of then MMP‐9 inhibitor (SB‐3CT) to anti‐tubercular drugs leads to significant decrease in bacillary load inside the brain (Figure 5). Addition of adjuvants to drugs improved the tissue pathology to normal (Figure 6). A significant decrease in levels of MMP‐9 was also seen when SB‐3CT/dexamethasone was used along with anti‐tubercular drugs. MMP‐9 levels were high in tissues of untreated infected animals (Figure 4) and undetectable in mice treated with MMP‐9 inhibitors (Figure 7). Muscle coordination was severely affected after infection as infected mice could not maintain themselves on the rotating rod and fell down more frequently. Treatment with MMP‐9 inhibitors decreased the number of falls in mice after 4–8 weeks of chemotherapy. Mice treated with SB‐3CT showed recovery in muscle coordination as the number of falls was significantly decreased after 4‐week treatment (Figure 8). Results of histopathology and zymography confirm the effective role of adjuvants in decreasing inflammatory tissue degradation in experimental mice. CFU enumeration led to the interesting observation that dexamethasone slowed down clearance of bacilli, hence limiting its usage as a good adjuvant but that SB‐3CT enhanced bacillary clearance significantly, thus giving it an extra advantage.
Figure 5.
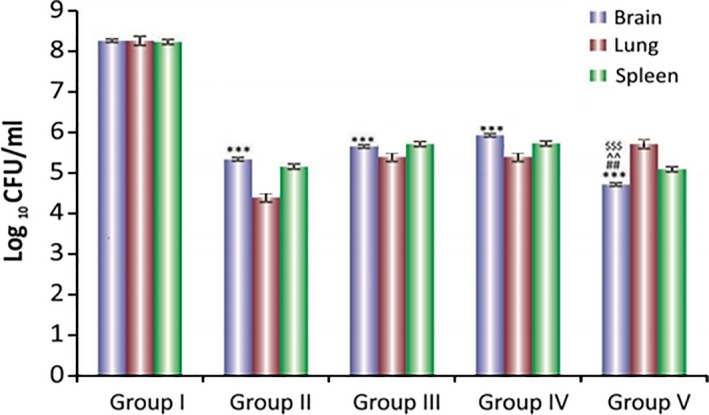
Log10 CFUs of M. tuberculosis H37Rv in brain, lungs and spleen of mice after 8 weeks of chemotherapy Group I: untreated, Group II: antitubercular drugs, Group III: antitubercular drugs + dexamethasone (orally), Group IV: antitubercular drugs + dexamethasone (i.p), Group V: free drug + SB‐3CT. Results are mean ± SD of 3–5 animals. ***P < 0.001 with respect to group I, ### P < 0.001 with respect to group II, ^^^P < 0.01, ^P < 0.5 with respect to group III. $$$ P < 0.001, $$ P < 0.01 with respect to group IV.
Figure 6.
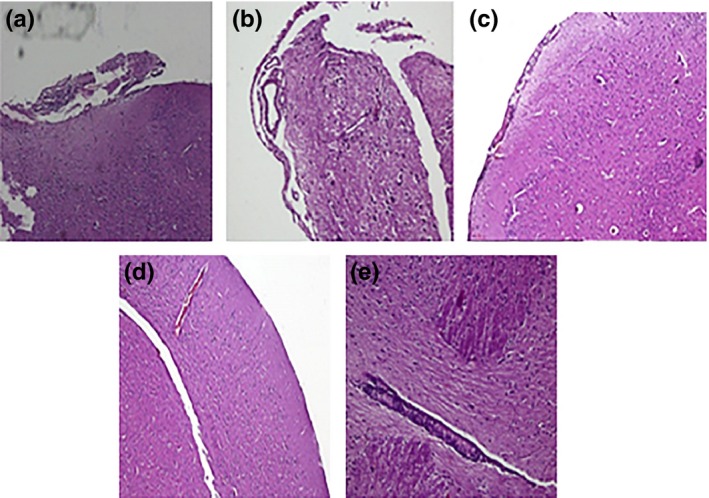
Representative micrographs of mice brains after 8 weeks of chemotherapy. (a) Untreated controls showing highly inflamed meninges, (b) antitubercular drugs showing meningitis, (c) antitubercular drugs + dexamethasone (orally) showing improved meninges, (d) antitubercular drugs + dexamethasone (i.v) showing improved meninges and (e) antitubercular drugs + SB‐3CT (i.v) showing normal meninges after treatment.
Figure 7.
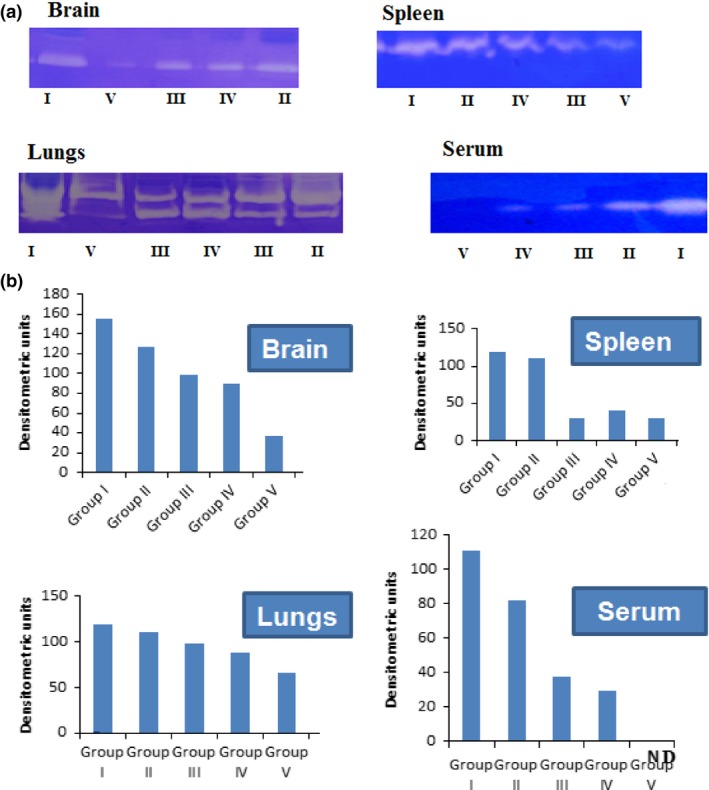
(a) Gelatin zymograms showing MMP‐9 levels in mice tissues after 8 weeks of therapy. In each gel, i: untreated controls, ii: antitubercular drug treated, III: treated with antitubercular drugs and dexamethasone, iv: treated with antitubercular drugs and dexamethasone (i.p), V: treated with antitubercular drugs and SB‐3CT. (b) Densitometric representation of MMP‐9 in mice organs and sera after 8 weeks of chemotherapy. Samples (tissue/sera) of six animals from each group were pooled and run for zymography.
Figure 8.
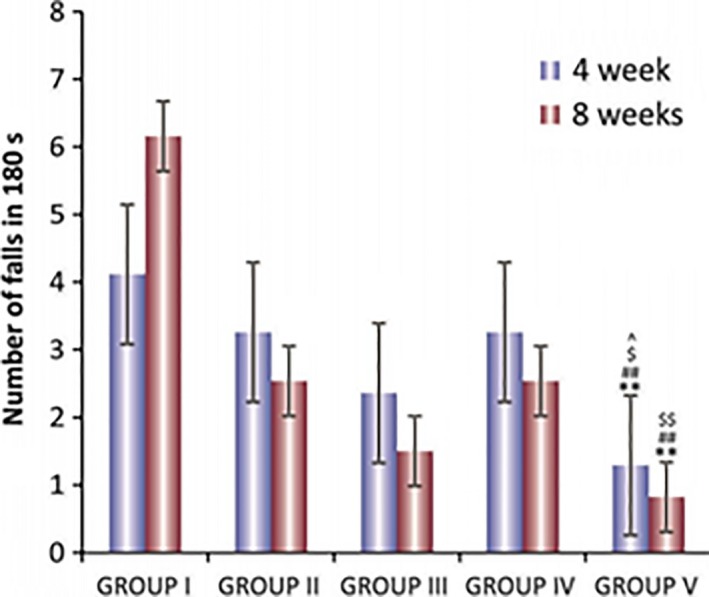
Graphical representation showing number of falls in Rota‐rod test after 4 and 8 week chemotherapy of TBM model of mice. (a) Group I: untreated control, (b) Group II: treated with antitubercular drugs, (c) Group III: treated with antitubercular drugs and dexamethasone, (d) Group IV: treated with antitubercular drugs and dexamethasone (intraperitoneally) and (e) Group V: treated with antitubercular drugs and SB‐3CT. The values are mean ± SD of six animals in each group. **P < 0.01. ***P < 0.001 with respect to group I in corresponding time interval, # P < 0.05, ## P < 0.01 with respect to group II in corresponding time interval, ^P < 0.05 with respect to group IV in corresponding time interval, $ P < 0.05, $$ P < 0.01 with respect to group V in corresponding time interval.
Discussion
This study highlights the role of MMP‐9 inhibition in efficient management of tuberculous meningitis. An experimental mice model of TBM was generated to study adjuvant effect of MMP‐9 inhibition. The intracranial route was used for inducing infection in mouse brain as based upon previous studies which had reported that intravenous inoculation of M. tuberculosis did not lead to onset of meningitis in mice (Rich & McCordock 1933; Be et al. 2008). The most recent study for generation of a mouse model of TBM emphasized the use of the intracranial route for the successful development of meningitis in mice (van Well et al. 2007). The inoculation point chosen was safe for animals as rodents can tolerate frontal lobectomy and survive with behavioural and cognitive defective functions only (Karganov et al. 1998). 1 × 105 CFUs of Mycobacterium tuberculosis H37Rv were used for infection as per the earlier study (van Well et al. 2007) and generated a stable bacillary load in the brain for 12 weeks after infection.
Results of the CFU counts were in agreement with the earlier study (van Well et al. 2007); however, bacillary load in tissues was not as high as reported (van Well et al. 2007). Histopathologically, onset of meningitis was seen after 4 weeks of infection as reported earlier for the TBM model generated using the BCG strain of Mycobacterium tuberculosis (Zucchi et al. 2012). The apparent meningeal inflammation and oedema after 4 weeks of infection confirmed the successful onset of the disease. Tuberculous pathology in organs was more prominent after 8 and 12 weeks of infection, which supports breach of the blood–brain barrier and spread of infection to other tissues as previously reported in the rabbit model of TB meningitis (Lin et al. 2008). The spread of inflammation to deeper brain sections, oedema of the cerebellum and tissue destruction at the choroid plexus reflects development of TBM in mice (Figure 3). Most of the studies have shown only lymphocytic filtration as a marker of TBM and no study (to the best of our knowledge) has shown the histopathology of other brain or lung sections depicting the established disease (van Well et al. 2007; Be et al. 2008). However, CSF analysis could not be performed in this study because of the insufficient sample volume collected. Some studies have confirmed the presence of bacilli in CSF (Elkington et al. 2011) and inflammatory markers in CSF and sera of the infected mice (van Well et al. 2007). MMP‐9 levels were elevated in the infected mice. Previous studies have also reported elevated levels of MMPs and suggested a role in progression of tuberculosis (Elkington et al. 2011; Salgame 2011). It has been shown that MMP‐9 helps in granuloma formation by recruiting uninfected macrophages around the infected ones and contributing to growth of the pathogen inside cells (Volkman et al. 2010). The fact that M. tuberculosis infection can generate MMP‐9 has been demonstrated both in vitro and in vivo studies (Price et al. 2001). Moreover, MMP‐9 level is also elevated in patients with TBM as well as pulmonary tuberculosis (Lee et al. 2004; Sheen et al. 2009). TNF‐α, a major inflammatory marker, is known to have direct role in stimulating and generating MMP‐9 (Lee et al. 2007, 2008), thus reflecting the role of MMP‐9 as a mediator of inflammatory response and phenotypic degradation.
Chemotherapeutic treatment alone decreased bacillary load in mice tissues, but addition of SB‐3CT to the regimen enhanced the bacillary clearance significantly. In contrast, dexamethasone addition to chemotherapeutic regimen inhibited the bacillary clearance (Figure 5). These results are in agreement with other studies that have shown increased cultivable bacillary load or reactivation of bacilli in animals after dexamethasone treatment (Mazzolla et al. 2002; Singhal et al. 2011). Although, the mechanism behind the role of dexamethasone in inhibiting bacillary clearance has not been deciphered as yet, but the immuno suppressive role of dexamethasone is well known (Mazzolla et al. 2002; Singhal et al. 2011), and this could be the possible reason for higher bacillary load in dexamethasone‐treated mice. Interestingly, addition of SB‐3CT instead of dexamethasone significantly decreased bacillary load in mice tissues. The histology of infected tissue was improved with use of either SB‐3CT or dexamethasone indicating that MMP‐9 inhibition can prevent inflammatory degradation in TBM. This observation is supported by an earlier study in the rabbit model of TBM in which use of adjuvant and thalidomide, along with anti‐tubercular drugs improved survival rate of infected animals by decreasing inflammatory markers (Cheigh et al. 2010). The inhibition of MMP‐9 using either dexamethasone or SB‐3CT is well supported by previous studies (Tsenova et al. 1998; Verhoef et al. 1999; Lin et al. 2008). However, SB‐3CT seems to be a better adjuvant, which overcomes inflammation as well as augments the effect of anti‐tubercular drugs in clearing bacillary load.
The infected mice in this study also showed signs of physical weakness after 8 weeks of infection, the movement of hindlimbs was affected, and some mice showed imbalanced posture and impaired muscle coordination. The use of SB‐3CT or dexamethasone along with anti‐tubercular drugs improved the muscle coordination in mice. These results are in agreement with previous studies showing improved long‐term neurobehavioural outcomes including sensorimotor function and hippocampus‐associated spatial learning and memory after MMP‐9 inhibition (Yang et al. 2011; Hadass et al. 2013). Thus, this study reveals the importance of controlling MMP‐9 levels with use of proper adjuvants in the treatment of neurological complications in tuberculous meningitis.
Conflict of interest
Authors do not have any conflict of interest in this paper.
Funding source
Financial support from Indian Council of Medical research, New Delhi, India is acknowledged.
Supporting information
Figure S1. Representative gross section of mouse brain showing diffusion of trypan blue from site of injection.
References
- Bainor A., Chang L., McQuade T.J., Webb B. & Gestwicki J.E. (2011) Bicinchoninic acid (BCA) assay in low volume. Anal. Biochem. 410, 310–312. [DOI] [PubMed] [Google Scholar]
- Be N.A., Lamichhane G., Grosset J. et al (2008) Murine model to study the invasion and survival of Mycobacterium tuberculosis in the central nervous system. J. Infect. Dis. 198, 1520–1528. [DOI] [PubMed] [Google Scholar]
- Be N.A., Kim K.S., Bishai W.R. & Jain S.K. (2009) Pathogenesis of central nervous system tuberculosis. Curr. Mol. Med. 9, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheigh C.I., Senaratne R., Uchida Y., Casali N., Kendall L.V. & Riley L.W. (2010) Post treatment reactivation of tuberculosis in mice caused by Mycobacterium tuberculosis disrupted in mce1R. J. Infect. Dis. 202, 752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A.S., Roed C., Omland L.H., Andersen P.H., Obel N. & Andersen Å.B. (2011) Long‐term mortality in patients with tuberculous meningitis: a Danish nationwide cohort study. PLoS ONE 6, e27900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detjen A.K. & Magdorf K. (2009) Characteristics of childhood tuberculosis. Pneumologie 63, 207–218. [DOI] [PubMed] [Google Scholar]
- Dunham N.W. & Miya T.S. (1957) A note on a simple apparatus for detecting neurological deficit in rats and mice. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 46, 208–209. [DOI] [PubMed] [Google Scholar]
- Elkington P.T., Ugarte‐Gil C.A. & Friedland J.S. (2011) Matrix metalloproteinases in tuberculosis. Eur. Respir. J. 38, 456–464. [DOI] [PubMed] [Google Scholar]
- Gijbels K., Masure S., Carton H. & Opdenakker G. (1992) Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J. Neuroimmunol. 41, 29–34. [DOI] [PubMed] [Google Scholar]
- Green J.A., Tran C.T., Farrar J.J. et al (2009) Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS ONE 4, e7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Cui J., Brown S. et al (2005) A highly specific inhibitor of matrix metalloproteinase‐9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J. Neurosci. 25, 6401–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadass O., Tomlinson B.N., Gooyit M. et al (2013) Selective inhibition of matrix metalloproteinase‐9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 8, e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempen C., Weiss E. & Hess C.F. (2002) Dexamethasone treatment in patients with brain metastases and primary brain tumors: do the benefits outweigh the side‐effects? Support. Care Cancer 10, 322–328. [DOI] [PubMed] [Google Scholar]
- van Horssen J., Bö L., Vos C.M., Virtanen I. & de Vries H.E. (2005) Basement membrane proteins in multiple sclerosis‐associated inflammatory cuffs: potential role in influx and transport of leukocytes. J. Neuropathol. Exp. Neurol. 64, 722–729. [DOI] [PubMed] [Google Scholar]
- Hosoglu S., Geyik M.F., Balik I. et al (2002) Predictors of outcome in patients with tuberculous meningitis. Int. J. Tuberc. Lung Dis. 6, 64–70. [PubMed] [Google Scholar]
- Jordán Jiménez A., Tagarro García A., Baquero Artigao F. et al (2005) Tuberculous meningitis: a review of 27 years. An. Pediatr. (Barc) 62, 215–220. [DOI] [PubMed] [Google Scholar]
- Kalita J., Misra U.K. & Ranjan P. (2007) Predictors of long‐term neurological sequelae of tuberculous meningitis: a multivariate analysis. Eur. J. Neurol. 14, 33–37. [DOI] [PubMed] [Google Scholar]
- Karganov M., Romanova G., Braslawsky W., Tarshitz D. & Telegdy G. (1998) Neuromodulator role of VIP in recovery of rat behavior and brain neurotransmitters level after frontal lobectomy. Ann. N. Y. Acad. Sci. 865, 519–522. [DOI] [PubMed] [Google Scholar]
- Lee K.Y., Kim E.H., Yang W.S. et al (2004) Persistent increase of matrix metalloproteinases in cerebrospinal fluid of tuberculous meningitis. J. Neurol. Sci. 220, 73–78. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Lin C.C., Lin W.N. et al (2007) TNF‐alpha induces MMP‐9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF‐kappaB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L799–L812. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Park S.S., Lee U.S., Kim W.J. & Moon S.K. (2008) Signaling pathway for TNF‐alpha‐induced MMP‐9 expression: mediation through p38 MAP kinase, and inhibition by anti‐cancer molecule magnolol in human urinary bladder cancer 5637 cells. Int. Immunopharmacol. 8, 1821–1826. [DOI] [PubMed] [Google Scholar]
- Lin C.W., Shen S.C., Hou W.C., Yang L.Y. & Chen Y.C. (2008) Heme oxygenase‐1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase‐9. Mol. Cancer Ther. 7, 1195–1206. [DOI] [PubMed] [Google Scholar]
- Manabe Y.C., Kesavan A.K., Lopez‐Molina J. et al (2008) The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis 88, 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura E., Umehara F., Hashiguchi T., Fujimoto N., Okada Y. & Osame M. (2000) Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J. Neurol. Sci. 173, 45–52. [DOI] [PubMed] [Google Scholar]
- Mazzolla R., Puliti M., Barluzzi R. et al (2002) Differential microbial clearance and immunoresponse of Balb/c (Nramp1 susceptible) and DBA2 (Nramp1 resistant) mice intracerebrally infected with Mycobacterium bovis BCG (BCG). FEMS Immunol. Med. Microbiol. 32, 149–158. [DOI] [PubMed] [Google Scholar]
- Okada S., Kita H., George T.J., Gleich G.J. & Leiferman K.M. (1997) Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase‐9. Am. J. Respir. Cell Mol. Biol. 17, 519–528. [DOI] [PubMed] [Google Scholar]
- Paul R., Lorenzl S., Koedel U. et al (1998) Matrix metalloproteinases contribute to the blood‐brain barrier disruption during bacterial meningitis. Ann. Neurol. 44, 592–600. [DOI] [PubMed] [Google Scholar]
- Price N.M., Farrar J., Tran T.T., Nguyen T.H., Tran T.H. & Friedland J.S. (2001) Identification of a matrix‐degrading phenotype in human tuberculosis in vitro and in vivo. J Immunol 166, 4223–4230. [DOI] [PubMed] [Google Scholar]
- Rich A.R. & McCordock H.A. (1933) The pathogenesis of tuberculous meningitis. Bull. Johns Hopkins Hosp. 52, 5–37. [Google Scholar]
- Rivera‐Marrero C.A., Schuyler W., Roser S., Ritzenthaler J.D., Newburn S.A. & Roman J. (2002) M. tuberculosis induction of matrix metalloproteinase‐9: the role of mannose and receptor‐mediated mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L546–L555. [DOI] [PubMed] [Google Scholar]
- Salgame P. (2011) MMPs in tuberculosis: granuloma creators and tissue destroyers. J Clin Invest 121, 1686–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen P., O'Kane C.M., Chaudhary K. et al (2009) High MMP‐9 activity characterises pleural tuberculosis correlating with granuloma formation. Eur. Respir. J. 33, 134–141. [DOI] [PubMed] [Google Scholar]
- Singhal A., el Aliouat M., Hervé M. et al (2011) Experimental tuberculosis in the Wistar rat: a model for protective immunity and control of infection. PLoS One 6, e18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites G.E., Simmons C.P., Than Ha Quyen N. et al Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J. Infect. Dis. 2003; 188:1105–1115. [DOI] [PubMed] [Google Scholar]
- Thwaites G.E., Nguyen D.B., Nguyen H.D. et al (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351, 1741–1751. [DOI] [PubMed] [Google Scholar]
- Thwaites G., Fisher M., Hemingway C., Scott G., Solomon T. & Innes J. (2009) British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J. Infect. 59, 167–187. [DOI] [PubMed] [Google Scholar]
- Tobin D.M., Roca F.J., Oh S.F. et al (2012) Host genotype‐specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148, 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsenova L., Sokol K., Freedman V.H. & Kaplan G. (1998) A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis‐associated death. J. Infect. Dis. 177, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Verhoef C.M., van Roon J.A., Vianen M.E., Lafeber F.P. & Bijlsma J.W. (1999) The immune suppressive effect of dexamethasone in rheumatoid arthritis is accompanied by upregulation of interleukin 10 and by differential changes in interferon gamma and interleukin 4 production. Ann. Rheum. Dis. 58, 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman H.E., Pozos T.C., Zheng J., Davis J.M., Rawls J.F. & Ramakrishnan L. (2010) Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327, 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Well G.T., Wieland C.W., Florquin S., Roord J.J., van der Poll T. & van Furth A.M. (2007) A new murine model to study the pathogenesis of tuberculous meningitis. J. Infect. Dis. 195, 694–697. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2010) Guidelines for Treatment of Tuberculosis, 4th edn. Geneva: WHO. [Google Scholar]
- Wu H.S., Kolonoski P., Chang Y.Y. & Bermudez L.E. (2000) Invasion of the brain and chronic central nervous system infection after systemic Mycobacterium avium complex infection in mice. Infect. Immun. 68, 2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.T., Lee T.H., Lee I.N., Chung C.Y., Kuo C.H. & Weng H.H. (2011) Dexamethasone inhibits ICAM‐1 and MMP‐9 expression and reduces brain edema in intracerebral hemorrhagic rats. Acta Neurochir. (Wien) 153, 2197–2203. [DOI] [PubMed] [Google Scholar]
- Zucchi F.C., Pelegrini‐da‐Silva A., Neder L., Silva C.L., Tsanaclis A.M. & Takayanagui O.M. (2012) The contribution of a murine CNS‐TB model for the understanding of the host‐pathogen interactions in the formation of granulomas. J. Neurosci. Methods 206, 88–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative gross section of mouse brain showing diffusion of trypan blue from site of injection.


