Summary
Phenylbutyrate is recommended in urea cycle disorders and liver injury to enhance nitrogen disposal by the urine. However, hypothetically there may be adverse responses to the use of phenylbutyrate in the treatment of liver disease because of its role as a histone deacetylase inhibitor and its stimulatory effect on branched‐chain alpha‐keto acid dehydrogenase, the rate‐limiting enzyme in the catabolism of branched‐chain amino acids (BCAA; valine, leucine and isoleucine). We report the effects of phenylbutyrate on liver regeneration and amino acid levels in plasma of partially hepatectomized (PH) rats. Phenylbutyrate or saline was administered at 12‐h intervals to PH or laparotomized rats. Phenylbutyrate delayed the onset of liver regeneration compared to the saline‐treated controls, as indicated by lower hepatic DNA specific activities 18 and 24 h post‐PH, decreased hepatic fractional protein synthesis rates 24 h post‐PH and lowered the increases in liver weights and hepatic protein and DNA contents 48 h after PH. Hepatic DNA fragmentation (a hallmark of apoptosis) was higher in the phenylbutyrate‐treated animals than in controls. Phenylbutyrate decreased the glutamine and BCAA concentrations and the ratio of the BCAA to aromatic amino acids (phenylalanine and tyrosine) in the blood plasma in both hepatectomized and laparotomized animals. In conclusion, the delayed onset of liver regeneration and the decrease in BCAA/AAA ratio in blood suggest that phenylbutyrate administration may be disastrous in subjects with acute hepatic injury and BCAA supplementation is needed when phenylbutyrate is used therapeutically.
Keywords: ammonia, branched‐chain amino acids, encephalopathy, glutamine, hepatic injury
Phenylbutyric acid is an aromatic fatty acid that provides an alternative pathway to the urea cycle for the excretion of excess nitrogen. Phenylbutyrate is oxidized to phenylacetate, which in the liver and kidneys is conjugated to glutamine to yield phenylacetylglutamine, which is quantitatively excreted by the urine. Removal of glutamine from the body prevents ammonia production from glutamine; therefore, phenylbutyrate administration may be considered as an elegant approach for the prevention and treatment of hyperammonaemia (Figure 1). Phenylbutyrate is used as sodium phenylbutyrate or glycerol phenylbutyrate in the clinical management of hyperglutaminaemia and hyperammonaemia in patients with urea cycle disorders (Brusilow 1991; Enns et al. 2007). Clinical studies suggest that phenylbutyrate may be administered safely to cirrhotic subjects, and it has the potential to lower blood ammonia and reduce hepatic encephalopathy events (McGuire et al. 2010; Rockey et al. 2014).
Figure 1.
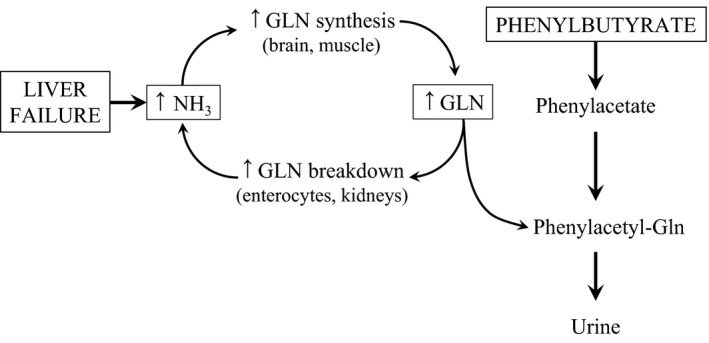
Schematic illustrating (i) a vicious cycle in which ammonia detoxification to glutamine (GLN) in skeletal muscle leads to the enhanced formation of ammonia in enterocytes and kidneys in subjects with liver injury (Holecek 2014), and (ii) proposed mechanism by which phenylbutyrate stops the cycle and enhances ammonia disposal from the body.
Unfortunately, there are some potential adverse effects of phenylbutyrate administration. Phenylbutyrate acts as a histone deacetylase inhibitor and was also investigated as an anti‐cancer agent (Bolden et al. 2006). Inhibitors of histone deacetylase modulate the chromatin structure and change transcription factor loading on DNA, which modulates the expression of various genes that control the cell cycle. Recent studies indicate that the inhibition of histone deacetylase activity may delay liver regeneration (Ke et al. 2012; Huang et al. 2013). Studies in human carcinoma cells have demonstrated that phenylbutyrate induces apoptosis (Carducci et al. 1996; Zhang et al. 2004), which may also impair the course of liver regeneration. However, other findings indicate that phenylbutyrate could protect both steatotic and non‐steatotic livers against injury and regeneration failure under ischaemia–reperfusion conditions (Ben Mosbah et al. 2010).
The effect of phenylbutyrate on the activity state of the branched‐chain α‐keto acid dehydrogenase, the rate‐limiting enzyme in the catabolism of branched‐chain amino acids (BCAA; valine, leucine and isoleucine), may also be detrimental. Brunetti‐Pierri et al. (2011) demonstrated using liver extracts from phenylbutyrate‐treated mice that phenylbutyrate enhanced the proportion of active (unphosphorylated) to inactive (phosphorylated) forms of the enzyme. The consecutive increase in BCAA oxidation may lead to a decrease in BCAA in the blood plasma and a decreased ratio of BCAA to aromatic amino acids (AAA; tyrosine and phenylalanine), which plays a deleterious role in the pathogenesis of hepatic encephalopathy (Fischer & Baldessarini 1971).
The present studies estimated the effect of phenylbutyrate on hepatocyte proliferation, which is essential for survival after acute hepatic injury, and amino acid concentrations in blood plasma, which may influence the development of hepatic encephalopathy. We used a classical model of rat liver regeneration after partial (68%) hepatectomy (PH) that manifests approximately 14 h post‐PH with a gradual increase in DNA synthesis, leading to a marked rise in liver weight and DNA and protein contents, which are useful estimates of liver regeneration rates (Bucher 1967). We measured DNA fragmentation levels to evaluate the effect of phenylbutyrate on hepatocyte apoptotic signalling. Fractional rates of protein synthesis and the activities of two major proteolytic systems within cells (the ubiquitin–proteasome and lysosomal pathways) were measured in the liver to evaluate alterations in protein metabolism.
Materials and methods
Animals
Male Wistar rats (BioTest, Konarovice, CR) weighing approximately 250 g were housed in standardized cages in quarters with controlled temperature and a 12‐h light–dark cycle. Rats were maintained on an ST‐1 (Velas, CR) standard laboratory diet containing (w/w) 24% of nitrogenous compounds, 4% fat, 70% carbohydrates and 2% minerals and vitamins, and were provided drinking water ad libitum.
Materials
L‐[3,4,5‐3H]Phenylalanine was from American Radiolabeled Chemical, Inc. (St. Louis, MO, USA). [6‐3H]Thymidine was purchased from Perkin Elmer, Inc. (Boston, MA, USA). Sodium 4‐phenylbutyrate was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Other chemicals were obtained from Sigma‐Aldrich (St. Louis, MO, USA), Lachema (Brno, CR), Waters (Milford, MA, USA), Biomol (Hamburg, Germany) and Merck (Darmstadt, Germany).
Study design
Partial hepatectomy (68% of liver tissue removed) was performed under diethyl ether narcosis according to Higgins and Anderson (1931). A midline abdominal incision was made to perform a sham operation (Sham). Phenylbutyrate (300 mg/kg b.w.) or saline was administered intraperitoneally immediately and at 12‐h intervals after surgery without anaesthesia. The dose of phenylbutyrate was based on a study by Davies et al. (2009) who observed a decrease in ammonia and an increase in urinary phenylacetylglutamine in cirrhotic rats. The animals were fasted after surgery to avoid the influence of differences in food intake, which decreases after PH more significantly when compared to sham‐treated animals (Holecek et al. 1986), and the effect of decreased appetite that has been observed in the phenylbutyrate‐treated subjects (Wilcken 2004). At the end of the experiment, rats were killed under ether anaesthesia, and blood was collected in heparinized tubes to obtain plasma.
Two separate studies were performed to evaluate the effects of phenylbutyrate on (i) liver regeneration, amino acid concentrations in blood plasma and tissues, and hepatic DNA fragmentation and (ii) protein metabolism in the liver.
DNA synthesis and extent of liver regeneration
Rats were injected intraperitoneally with [6‐3H]thymidine (0.2 mCi/kg b.w.) 1 h prior to killing by exsanguination from the abdominal aorta. The liver was quickly removed, weighed and immediately frozen in liquid nitrogen. Samples of hepatic tissue for measuring [6‐3H]thymidine radioactivity were processed according to Bucher and Swaffield (1964). The radioactivity of the samples was measured in a liquid scintillation radioactivity counter (LS 6000; Beckman Instruments, Fullerton, CA, USA). DNA content was determined using the diphenylamine reaction (Burton 1956).
The extent of liver regeneration was evaluated as a percentage of regeneration based on liver weights and the protein and DNA contents in the liver at hepatectomy and death. Residual hepatic weights and the content of protein and DNA at hepatectomy were estimated by considering the weights of the median and left lateral lobes that were resected during PH, which corresponded to 68% of the liver weight (Bucher 1967). The protein content was measured according to Lowry et al. (1951).
Alterations in apoptosis
Alterations in apoptosis were estimated by the degradation of nuclear DNA into nucleosomal units (a hallmark of apoptotic cell death) using the Cell Detection ELISA Kit (Roche Diagnostic). Briefly, samples of hepatic tissue (approximately 100 mg) were homogenized in lysis buffer and were centrifuged at 13,000 g for 15 min. The supernatant was incubated for 2 h at room temperature with an anti‐histone‐biotin/anti‐DNA‐PD reagent in a streptavidin‐coated microplate. Wells were washed three times, and 100 μl of substrate solution (ABTS) was added to each well. Absorbance was measured at 405 and 490 nm (Tecan Infinite M200). Absorbance was normalized to the protein concentration of the supernatant, and the results were expressed as arbitrary units per mg of protein.
Amino acid and ammonia concentrations
Amino acid concentrations were determined in the supernatants of deproteinized samples of blood plasma using high‐performance liquid chromatography (Alliance 2695; Waters, Milford, MA, USA) after a precolumn derivatization with 6‐aminoquinolyl‐N‐hydroxysuccinimidyl carbamate. The ammonia concentrations in blood plasma were determined enzymatically using an Ammonia Assay Kit (Sigma‐Aldrich, St. Louis, MO, USA).
Protein synthesis
Rats under ether narcosis were injected intravenously with a flooding dose of L‐[3,4,5‐3H]phenylalanine (50 μCi/100 g b.w.) combined with unlabelled L‐phenylalanine (150 μmol/100 g b.w.) 10 min before killing by exsanguination from the abdominal aorta (Garlick et al. 1980). Small samples (approximately 100 mg) of the liver were quickly removed and frozen in liquid nitrogen. The samples were treated according to the previously described procedure (Holecek & Kovarik 2011). The fractional rate of protein synthesis (FRPS) was calculated according the formula derived by McNurlan et al. (1979):
where S b and S a are the specific activities of protein‐bound phenylalanine and tissue‐free phenylalanine in the acid‐soluble fraction of tissue homogenates, respectively, and t is the time (days) between the injection of the isotope and the immersion of the tissue into liquid nitrogen. The value of 274 μmol phenylalanine/g protein was used for the calculation of protein‐bound phenylalanine specific activity (Welle 1999). The radioactivity of the samples was measured using a liquid scintillation radioactivity counter (LS 6000; Beckman Instruments, Fullerton, CA, USA).
Chymotrypsin‐like activity (CHTLA) of proteasome and cathepsin B and L activities
The CHTLA of the proteasome and cathepsin B and L activities were determined using the fluorogenic substrates Suc‐LLVY‐MCA (Gomes‐Marcondes & Tisdale 2002) and Z‐FA‐MCA (Koohmaraie & Kretchmar 1990), respectively, as previously described in detail (Holecek & Kovarik 2011). The fluorescence of the samples was measured at the excitation wavelength of 340 nm and the emission wavelength of 440 nm (Tecan Infinite M200). A standard curve was established for 7‐amino‐4‐methylcoumarin (AMC), which allowed expression of the enzyme activities in nmol of AMC/g protein/hour.
Statistical analysis
The results are expressed as means ± SE. F‐test and one‐way analysis of variance followed by Mann–Whitney test or Bonferroni multiple comparisons procedure were used to detect significant differences. The statistical software NCSS 2001 (Kaysville, UT, USA) was used for the analyses. Differences were considered significant at P ˂ 0.05.
Ethical approval statement
The Animal Care and Use Committee of Charles University in Prague, Faculty of Medicine in Hradec Kralove specifically approved this study (Licence No. 144879/2011‐MZE‐17214). All applicable international, national, local and/or institutional guidelines governing the use of experimental animals were followed. Animals were treated carefully by animal experts educated and trained on how to manipulate animals to maintain a healthy environment and to reduce distress and minimize potential pain and suffering.
Results
Alterations in liver regeneration
Hepatic DNA specific activities were significantly lower 18 and 24 h after PH (i.e. in the period of progressive increase in DNA synthesis) and higher 48 h post‐PH in the phenylbutyrate‐treated animals than in the saline‐treated controls (Figure 2).
Figure 2.
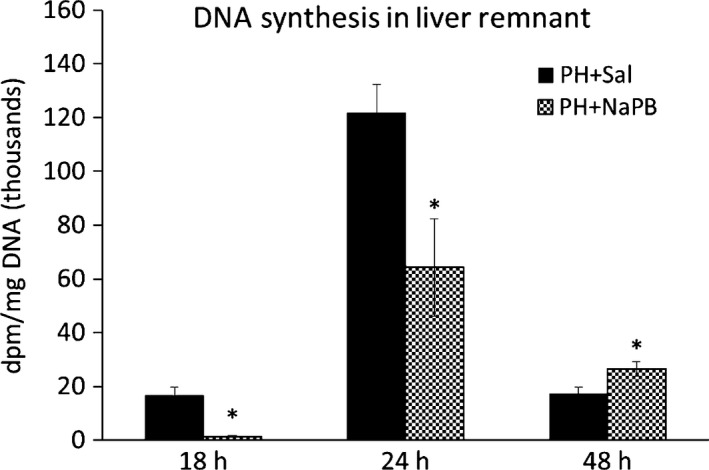
Effect of phenylbutyrate on DNA specific activity in the liver regenerating after PH. Means ± SE, Mann–Whitney test, *P < 0.05. NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
Partial hepatectomy enhanced CHTLA in the liver, but the effect on protein synthesis and cathepsin B and L activities was not significant at 24 h after PH. Phenylbutyrate treatment decreased FRPS in the liver in sham‐operated and PH rats and enhanced hepatic cathepsin B and L activities of PH rats (Table 1).
Table 1.
Effects of phenylbutyrate on protein metabolism in liver at 24 h after PH
| Sham + Sal | Sham + NaPB | PH + Sal | PH + NaPB | |
|---|---|---|---|---|
| FRPS (% per day) | 26.73 ± 1.35 | 17.94 ± 1.08* | 32.70 ± 2.60 | 23.41 ± 1.34* |
| CHTLA (nmol AMC/mg/h) | 6.23 ± 0.19 | 6.39 ± 0.13 | 8.61 ± 0.30# | 9.24 ± 0.52# |
| Cathepsins B and L (nmol AMC/mg/h) | 697 ± 35 | 715 ± 24 | 577 ± 40 | 778 ± 54* |
Means ± SE, anova and Bonferroni multiple comparisons. *P ˂ 0.05, effect of NaPB (compared to the corresponding saline‐treated group); #P ˂0.05, effect of PH (compared to the corresponding sham‐operated group). CHTLA, chymotrypsin‐like activity; FRPS, fractional rate of protein synthesis; NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
DNA fragmentation (a hallmark of apoptosis) in the regenerating remnant of the liver tissue was higher in the phenylbutyrate‐treated animals compared to the saline‐treated group (Figure 3).
Figure 3.
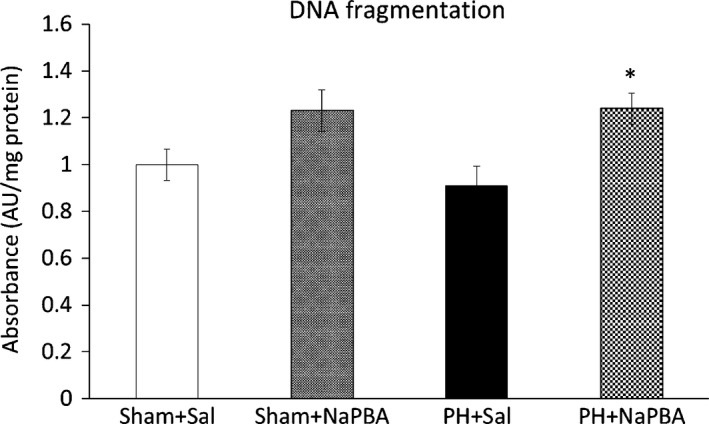
Effect of phenylbutyrate on DNA fragmentation at 24 h after PH. Means ± SE, anova and Bonferroni multiple comparisons. *P ˂ 0.05, effect of NaPB (compared to the corresponding saline‐treated group). NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
Alterations in DNA synthesis, protein metabolism and apoptosis observed in the phenylbutyrate‐treated animals resulted in the decrease of all estimates of the liver regeneration rates calculated on the basis of liver weights and hepatic protein and DNA contents at 48 h in the phenylbutyrate‐treated rats compared to the saline‐treated rats (Figure 4).
Figure 4.
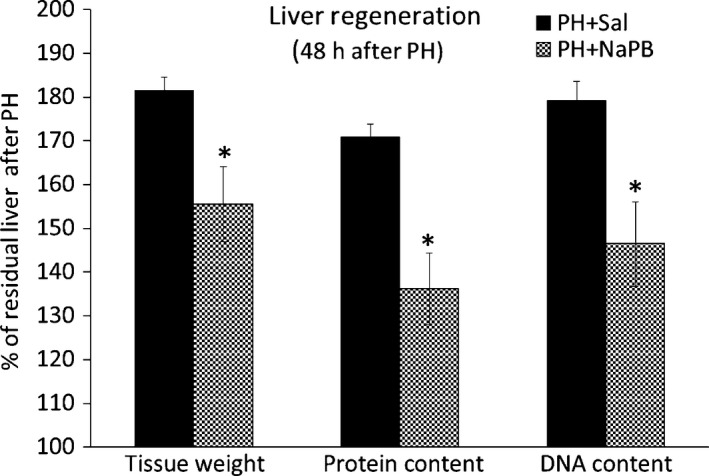
Effect of phenylbutyrate on the liver regeneration rate. The liver regeneration rates were calculated on the basis of the liver weights and protein and DNA contents in the liver at the times of hepatectomy and killing. Means ± SE, Mann–Whitney test, *P < 0.05. NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
Alterations in amino acid concentrations in blood plasma
No remarkable alterations in the ammonia or amino acid concentrations in blood plasma were observed in PH animals. However, a decrease in urea at 18 h (3.81 ± 0.28 vs. 6.56 ± 0.35) and 24 h (3.40 ± 0.31 vs. 6.70 ± 0.39) post‐PH (Table 2) and a decrease in the BCAA/AAA ratios at all intervals after PH (Figure 5) were found.
Table 2.
Effect of phenylbutyrate on amino acids, ammonia and urea in blood plasma following PH
| Hours | Sham + Sal | Sham + NaPB | PH + Sal | PH + NaPB | |
|---|---|---|---|---|---|
| Glutamine | 18 h | 634 ± 6 | 530 ± 16 | 663 ± 28 | 1526 ± 145* , # |
| 24 h | 616 ± 9 | 476 ± 14 | 600 ± 17 | 735 ± 95# | |
| 48 h | 620 ± 13 | 518 ± 28* | 687 ± 33 | 671 ± 24# | |
| Glutamate | 18 h | 100 ± 4 | 76 ± 10 | 68 ± 5 | 130 ± 15* , # |
| 24 h | 86 ± 3 | 76 ± 8 | 65 ± 3 | 68 ± 9 | |
| 48 h | 106 ± 4 | 176 ± 20 * | 72 ± 4 | 140 ± 5 * | |
| Alanine | 18 h | 368 ± 13 | 305 ± 16 | 319 ± 18 | 997 ± 161* , # |
| 24 h | 247 ± 15 | 285 ± 21 | 315 ± 19 | 504 ± 63* , # | |
| 48 h | 375 ± 13 | 494 ± 52 * | 419 ± 20 | 408 ± 22 | |
| Phenylalanine | 18 h | 63 ± 1 | 57 ± 2 | 88 ± 4 | 151 ± 29* , # |
| 24 h | 67 ± 3 | 56 ± 2 | 76 ± 3 | 100 ± 20# | |
| 48 h | 62 ± 2 | 67 ± 4 | 66 ± 4 | 81 ± 3* , # | |
| Tyrosine | 18 h | 93 ± 3 | 77 ± 5 | 108 ± 7 | 236 ± 27* , # |
| 24 h | 79 ± 3 | 81 ± 4 | 104 ± 6 | 210 ± 67# | |
| 48 h | 84 ± 4 | 67 ± 6 | 106 ± 7# | 103 ± 4# | |
| Valine | 18 h | 171 ± 4 | 104 ± 7 * | 167 ± 10 | 220 ± 23* , # |
| 24 h | 179 ± 12 | 113 ± 6 * | 153 ± 6 | 109 ± 16 * | |
| 48 h | 167 ± 8 | 141 ± 9 | 139 ± 7 | 103 ± 10* , # | |
| Isoleucine | 18 h | 93 ± 3 | 44 ± 3 * | 89 ± 5 | 71 ± 8# |
| 24 h | 105 ± 6 | 47 ± 3 * | 79 ± 3# | 36 ± 6 * | |
| 48 h | 87 ± 4 | 65 ± 5 * | 67 ± 4# | 35 ± 2* , # | |
| Leucine | 18 h | 150 ± 4 | 84 ± 6 * | 147 ± 9 | 148 ± 16# |
| 24 h | 159 ± 10 | 90 ± 5 * | 129 ± 6# | 74 ± 11 * | |
| 48 h | 142 ± 6 | 113 ± 8* | 112 ± 6# | 62 ± 3* , # | |
| BCAA | 18 h | 414 ± 10 | 232 ± 16 * | 402 ± 23 | 438 ± 47# |
| 24 h | 443 ± 28 | 250 ± 14 * | 361 ± 14 | 191 ± 22 * | |
| 48 h | 397 ± 17 | 319 ± 21 | 318 ± 16# | 201 ± 14* , # | |
| ∑ of all amino acids | 18 h | 3629 ± 64 | 3216 ± 123 | 3539 ± 135 | 7603 ± 815* , # |
| 24 h | 3387 ± 92 | 2919 ± 93 | 3310 ± 99 | 4385 ± 762# | |
| 48 h | 3476 ± 65 | 3419 ± 217 | 3811 ± 175 | 3715 ± 91 | |
| Ammonia | 18 h | 27.5 ± 6.3 | 51.7 ± 11.9 | 33.5 ± 6.3 | 99.4 ± 15.7* , # |
| 24 h | 33.2 ± 9.5 | 39.2 ± 7.9 | 22.8 ± 4.0 | 52.4 ± 9.6 | |
| 48 h | 39.7 ± 8.0 | 39.1 ± 5.6 | 52.1 ± 10.1 | 48.9 ± 6.6 | |
| Urea | 18 h | 6.56 ± 0.35 | 7.13 ± 0.43 | 3.81 ± 0.28# | 8.85 ± 0.59* , # |
| 24 h | 6.70 ± 0.39 | 6.03 ± 0.35 | 3.40 ± 0.31# | 5.92 ± 0.43* | |
| 48 h | 5.19 ± 0.55 | 4.78 ± 0.49 | 4.66 ± 0.31 | 4.44 ± 0.35 |
Means ± SE, anova and Bonferroni multiple comparisons. *P ˂ 0.05, effect of NaPB (compared to the corresponding saline‐treated group); # P ˂ 0.05, effect of PH (compared to the corresponding sham‐operated group). Amino acid and ammonia concentrations are expressed in μmol/L; concentrations of urea in mmol/L. BCAA, branched‐chain amino acids; NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
Figure 5.
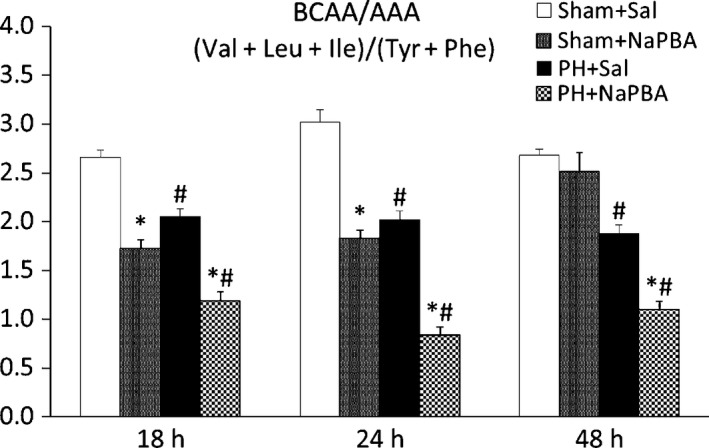
Effect of phenylbutyrate on the BCAA/AAA ratios in the blood plasma. Means ± SE, anova and Bonferroni multiple comparisons. *P ˂ 0.05, effect of NaPB (compared to the corresponding saline‐treated group); # P ˂ 0.05, effect of PH (compared to the corresponding sham‐operated group). NaPB, sodium phenylbutyrate; PH, partial hepatectomy; Sal, saline.
Phenylbutyrate treatment decreased the plasma glutamine (518 ± 28 vs. 620 ± 13 at 48 h) and BCAA concentrations (232 ± 16 vs. 414 ± 10 at 18 h and 250 ± 14 vs. 443 ± 28 at 24 h) in the sham‐operated animals and increased the concentrations of urea, ammonia and most amino acids at 18 and 24 h after PH. The ratio of BCAA to AAA was markedly lower in PH animals treated with phenylbutyrate than in animals treated with saline (Figure 5).
Discussion
The data reported here indicate that phenylbutyrate administration to subjects with impaired hepatic function may exert adverse effects on liver repair and amino acid concentrations in the blood.
Alterations in liver regeneration
Markedly lower liver DNA specific activities at 18 and 24 h and lower fractional rates of protein synthesis and higher DNA fragmentation (a hallmark of apoptosis) 24 h after PH indicate a delayed onset of regeneration in the phenylbutyrate‐treated animals compared to controls. The consequence of this delay was lower values of all estimates of liver regeneration based on liver weights and DNA and protein contents 48 h after PH. A delayed onset of liver regeneration may be practically important, particularly during acute hepatocellular damage when the capability of the remaining hepatocytes to regenerate is essential for survival. Alternative research approaches that use more advanced methods may provide important details about the response of liver regenerating after PH to phenylbutyrate treatment.
The delayed onset of regeneration and impaired protein synthesis in the liver are the main causes of the enhanced concentrations of several amino acids in the blood plasma of the phenylbutyrate‐treated animals. Hyperaminoacidaemia is probably the cause of enhanced levels of ammonia and urea in blood plasma of phenylbutyrate‐treated animals at 18 and 24 h after PH. Markedly elevated plasma concentrations of alanine may be linked to stimulatory effect of phenylbutyrate on oxidation of the BCAA, which act as the main source of nitrogen for synthesis of alanine from pyruvate in skeletal muscle.
We assume that phenylbutyrate plays its role as a histone deacetylase inhibitor in the treatment of liver disease, which may be implicated both in the delayed onset of DNA synthesis and in the enhanced apoptosis. The mouse PH model showed that hepatic histone deacetylase activity was significantly increased in the nuclear and cytoplasmic fractions following PH, and the treatment using suberoylanilide hydroxamic acid (a specific inhibitor of zinc‐dependent histone deacetylase) suppressed hepatocellular bromodeoxyuridine incorporation (Huang et al. 2013). Impairment of liver regeneration and induction of liver cell cycle arrest was observed recently in partially hepatectomized mice treated with valproic acid, another inhibitor of histone deacetylase (Ke et al. 2012). There are several studies using carcinoma cells showing that phenylbutyrate treatment induces apoptosis (Carducci et al. 1996; Zhang et al. 2004).
Reduced levels of glutamine and BCAA observed in the phenylbutyrate‐treated animals may also contribute to impaired liver regeneration. Glutamine is an important precursor for the synthesis of nucleic acids and is a regulator of cell hydration and cell volume, which affect cell function, including cell proliferation and protein metabolism. BCAA are essential substrates and regulators of protein synthesis, particularly leucine, which stimulates protein synthesis through the mammalian target of rapamycin (mTOR) signalling pathway (Nair & Short 2005). Favourable effects of glutamine or BCAA on liver regeneration were demonstrated previously (Holecek et al. 1985, 1991 and Yoshida et al. 1995).
Alterations in BCAA levels
The marked decreases in the plasma BCAA levels after phenylbutyrate treatment are consistent with other experimental studies and observations of subnormal plasma BCAA levels in phenylbutyrate‐treated patients with urea cycle disorders (Scaglia et al. 2004; Burrage et al. 2014). This effect may be explained by enhanced activity of the branched‐chain α‐keto acid dehydrogenase, which is the rate‐limiting enzyme in BCAA metabolism (Brunetti‐Pierri et al. 2011).
Decreased BCAA concentrations and the decreased ratio of the BCAA to aromatic amino acids may have deleterious effects in patients with hepatic disease. The decrease in plasma BCAA may precipitate hepatic encephalopathy due to increased influx of aromatic amino acids to the brain, which causes an imbalance in the synthesis of dopamine, norepinephrine and serotonin or formation of false neurotransmitters, such as tyramine, phenylethanolamine and octopamine (Fischer & Baldessarini 1971). Subnormal levels of BCAA may contribute to muscle wasting, hypoalbuminaemia, impaired immunity and impaired repair capability of liver tissue.
Conclusions
The impaired rate of liver regeneration and the pronounced decrease in BCAA/AAA ratios in the blood plasma of the phenylbutyrate‐treated animals are causes for concern that phenylbutyrate administration may be disastrous in subjects with acute hepatic injury and indicate that BCAA supplementation should be recommended when phenylbutyrate is used therapeutically. Considering the favourable effects of phenylbutyrate on the course of liver injury reported in other conditions (Ben Mosbah et al. 2010), further studies are warranted to determine the pros and cons of phenylbutyrate use in the treatment of hepatic disease.
Author contributions
The authors meet ICMJE authorship criteria, and nobody who qualifies for authorship has been excluded.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported by the programme PRVOUK P37/02. The authors wish to thank R. Fingrova and D. Jezkova for their technical assistance.
References
- Ben Mosbah I., Alfany‐Fernández I., Martel C. et al (2010) Endoplasmic reticulum stress inhibition protects steatotic and non‐steatotic livers in partial hepatectomy under ischemia‐reperfusion. Cell Death Dis. 1, e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden J.E., Peart M.J. & Johnstone R.W. (2006) Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discov. 5, 769–784. [DOI] [PubMed] [Google Scholar]
- Brunetti‐Pierri N., Lanpher B., Erez A. et al (2011) Phenylbutyrate therapy for maple syrup urine disease. Hum. Mol. Genet. 20, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusilow S.W. (1991) Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr. Res. 29, 147–150. [DOI] [PubMed] [Google Scholar]
- Bucher N.L.R. (1967) Experimental aspects of hepatic regeneration. N. Engl. J. Med. 277, 686–696. [DOI] [PubMed] [Google Scholar]
- Bucher N.L.R. & Swaffield M.N. (1964) The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res. 24, 1611–1625. [PubMed] [Google Scholar]
- Burrage L.C., Jain M., Gandolfo L., Lee B.H., Members of the Urea Cycle Disorders Consortium , Nagamani S.C. (2014) Sodium phenylbutyrate decreases plasma branched‐chain amino acids in patients with urea cycle disorders. Mol. Genet. Metab. 113, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. (1956) A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci M.A., Nelson J.B., Chan‐Tack K.M. et al (1996) Phenylbutyrate induces apoptosis in human prostate cancer and is more potent than phenylacetate. Clin. Cancer Res. 2, 379–387. [PubMed] [Google Scholar]
- Davies N.A., Wright G., Ytrebø L.M. et al (2009) L‐ornithine and phenylacetate synergistically produce sustained reduction in ammonia and brain water in cirrhotic rats. Hepatology 50, 155–164. [DOI] [PubMed] [Google Scholar]
- Enns G.M., Berry S.A., Berry G.T., Rhead W.J., Brusilow S.W. & Hamosh A. (2007) Survival after treatment with phenylacetate and benzoate for urea‐cycle disorders. N. Engl. J. Med. 356, 2282–2292. [DOI] [PubMed] [Google Scholar]
- Fischer J.E. & Baldessarini R.J. (1971) False neurotransmitters and hepatic failure. Lancet 2(7715), 75–80. [DOI] [PubMed] [Google Scholar]
- Garlick P.J., McNurlan M.A. & Preedy V.R. (1980) A rapid and convenient technique for measuring the rate of protein synthesis in tissue by injection of [3H]phenylalanine. Biochem. J. 192, 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes‐Marcondes M.C.C. & Tisdale M.J. (2002) Induction of protein catabolism and the ubiquitin‐proteasome pathway by mild oxidative stress. Cancer Lett. 180, 69–74. [DOI] [PubMed] [Google Scholar]
- Higgins G.M. & Anderson R.M. (1931) Experimental pathology of the liver. I. Restoration of liver of white rat following partial surgical removal. Arch. Pathol. 12, 186–202. [Google Scholar]
- Holecek M. (2014) Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy‐therapeutic perspectives. Metab. Brain Dis. 29, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holecek M. & Kovarik M. (2011) Alterations in protein metabolism and amino acid concentrations in rats fed by a high‐protein (casein‐enriched) diet ‐ effect of starvation. Food Chem. Toxicol. 49, 3336–3342. [DOI] [PubMed] [Google Scholar]
- Holecek M., Simek J., Kruf M. & Zadak Z. (1985) Effect of branched chain amino acids on liver regeneration after partial hepatectomy. Physiol. Bohemoslov. 34, 359–366. [PubMed] [Google Scholar]
- Holecek M., Simek J., Dvorackova I., Subrtova D. & Palicka V. (1986) Spontaneous ingestion of different types of carbohydrates in rats with liver damage and their effect on liver repair. Cs. Gastroenterol. Vyziva 40, 268–275. [Google Scholar]
- Holecek M., Simek J., Palicka V. & Zadak Z. (1991) Effect of glucose and branched chain amino acid (BCAA) infusion on onset of liver regeneration and plasma amino acid pattern in partially hepatectomized rats. J. Hepatol. 13, 14–20. [DOI] [PubMed] [Google Scholar]
- Huang J., Barr E. & Rudnick D.A. (2013) Characterization of the regulation and function of zinc‐dependent histone deacetylases during rodent liver regeneration. Hepatology 57, 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q., Yang R.N., Ye F. et al (2012) Impairment of liver regeneration by the histone deacetylase inhibitor valproic acid in mice. J. Zhejiang Univ. Sci. B 13, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohmaraie M. & Kretchmar D.H. (1990) Comparisons of four methods for quantification of lysosomal cysteine proteinase activities. J. Anim. Sci. 68, 2362–2370. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L. & Randall R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- McGuire B.M., Zupanets I.A., Lowe M.E. et al (2010) Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology 51, 2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNurlan M.A., Tomkins A.M. & Garlick P.J. (1979) The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem. J. 178, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K.S. & Short K.R. (2005) Hormonal and signaling role of branched‐chain amino acids. J. Nutr. 135, 1547S–1552S. [DOI] [PubMed] [Google Scholar]
- Rockey D.C., Vierling J.M., Mantry P. et al (2014) Randomized, double‐blind, controlled study of glycerol phenylbutyrate in hepatic encephalopathy. Hepatology 59, 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglia F., Carter S., O'Brien W.E. & Lee B. (2004) Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol. Genet. Metab. 81, S79–S85. [DOI] [PubMed] [Google Scholar]
- Welle S. (1999) Methods for studying protein metabolism in humans Welle S, pp. 29–71. New‐York: Human protein metabolism. Springer‐Verlag. [Google Scholar]
- Wilcken B. (2004) Problems in the management of urea cycle disorders. Mol. Genet. Metab. 81, S86–S91. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Yunoki T., Aoyagi K. et al (1995) Effect of glutamine supplement and hepatectomy on DNA and protein synthesis in the remnant liver. J. Surg. Res. 59, 475–481. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wei L., Yang Y. & Yu Q. (2004) Sodium 4‐phenylbutyrate induces apoptosis of human lung carcinoma cells through activating JNK pathway. J. Cell. Biochem. 93, 819–829. [DOI] [PubMed] [Google Scholar]


