Summary
MicroRNAs (miRNAs) play crucial roles in cancer development and progression. The purposes of this study were to explore the role of miR‐376c in cervical cancer and to clarify the regulation of BMI1 by miR‐376c. Quantitative RT‐PCR was used to measure miR‐376c expression in cervical cancer tissues and cell lines. The cell proliferation, cell cycle and Transwell invasion assays were performed. A luciferase reporter assay was conducted to confirm the target gene of miR‐376c, and the results were validated in cervical cancer cell lines and tissues. MiR‐376c was significantly downregulated in cervical cancer cell lines and clinical tissues. Upregulation of miR‐376c impaired cell proliferation, blocked G1/S checkpoint of cell cycle and suppressed cell invasion in vitro. BMI1 was verified as a direct target of miR‐376c, which was further confirmed by the inverse expression of miR‐376c and BMI1 in patient specimens. The newly identified miR‐376c/BMI1 pathway provides an insight into cervical cancer progression and may represent a novel therapeutic target.
Keywords: BMI1, Cervical cancer, HPV, miR‐376c
Cervical cancer is the third most common malignancy and the fourth leading cause of cancer mortality in women worldwide (Forouzanfar et al. 2011). Persistent infection with high‐risk human papillomavirus (HR‐HPV) is recognized as the most important risk factor causing cervical cancer (Naucler et al. 2011). However, a number of evidences show that the exposure to HPV alone is insufficient for cervical cancer development and that, besides HPV, other factors from host cells must be also critical in the process of cervical malignant transformation (Hildesheim & Wang 2002). Therefore, the identification of such factors would be important for the prevention, diagnosis and treatment of cervical cancer.
MicroRNAs (miRNAs) are small non‐coding RNAs that regulate gene expression by directly binding to the 3′‐untranslated regions (3′UTRs) of target mRNAs and causing the mRNA destabilization and protein downregulation (Filipowicz et al. 2008). miRNAs may act as either oncogenes or tumour suppressors in the development and progression of human malignancies (Calin & Croce 2006). An increasing number of miRNAs were shown to be involved in carcinogenesis and progression of cervical cancer, including miR‐31, miR‐29, miR‐1246 and miR‐20a (Li et al. 2011; Wang et al. 2014a; Yang et al. 2015; Zhao et al. 2015). A previous microRNA microarray analysis showed that miR‐376c expression was significantly decreased in cervical cancer cell lines compared with normal cervical tissue (Martinez et al. 2008), and several studies have shown that miR‐376c was frequently altered in a variety of cancers and that the functional role of this miRNA is extremely complex as it may act as an oncogenic or a tumour‐suppressive miRNA depending on the cellular contexts (Ye et al. 2011; Song et al. 2012; Zehavi et al. 2012; Jin et al. 2013; Formosa et al. 2014). Given the complexity of its functionality, it would be of interest to explore the functional roles of miR‐376c in cervical cancer development.
B‐cell‐specific Moloney murine leukaemia virus insertion site 1 (BMI1) is a member of the polycomb repressive complex 1 (PRC1) and acts as transcriptional repressors, which is highly expressed in several types of cancer, including cervical cancer (Tong et al. 2012; Paranjape et al. 2014; Wei et al. 2015). Moreover, the clinicopathological characteristics of BMI1 showed its significance in clinical diagnosis and potential therapy. However, the epigenetic regulatory mechanism of BMI1 in cervical cancer remains unknown.
In this study, we analysed the miR‐376c expression in cervical cancer tissues and cell lines and investigated its effects on proliferation and invasion of cervical cancer cells. Moreover, we demonstrated that BMI1 is a target for miR‐376c in cervical cancer cells and involved in the functional influence of miR‐376c on cervical cancer cell proliferation and invasion. These findings indicate a novel molecular mechanism involved in cervical cancer progression and may suggest novel clues for targeted treatment.
Materials and methods
Clinical samples, cell lines and transfection
Cervical cancer tissues and matched adjacent normal tissues were obtained from 29 patients with cervical cancer who received surgery at the Huangshi Tumor Hospital, Huangsi, China. Specimens were obtained with informed consent, and the study had been approved by local institutional review boards on human subject research and in accordance with the Declaration of Helsinki. All the histological diagnoses for cervical cancer and normal tissues were reviewed and recognized by two pathologists independently. Sample characteristics are described in Table 1.
Table 1.
Clinicopathologic characteristics of patients with cervical cancer
| Clinicopathological parameters | Cases (n = 29) |
|---|---|
| Age (years) | |
| ≤35 | 13 |
| >35 | 16 |
| FIGO stage | |
| IB | 10 |
| IIA | 19 |
| Tumour size (cm) | |
| <4 | 12 |
| ≥4 | 17 |
| Differentiation | |
| Well | 11 |
| Moderate/poor | 18 |
| Lymph node metastasis | |
| Yes | 20 |
| No | 9 |
| Stromal invasion | |
| <2/3 | 7 |
| ≥2/3 | 22 |
HaCaT cells (an immortalized HPV‐negative skin keratinocyte line) and four human cervical cancer cell lines HeLa, SiHa, CasKi and C33A were obtained from Chinese Center for Type Culture Collection (Wuhan, China) and cultured according to the provider's instruction. Transfection was performed when cells were grown to 70% confluence, using the Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.
MicroRNA and plasmid construction
MiR‐376c mimics/inhibitor and corresponding controls were synthesized by RiboBio (Guangzhou, China). BMI1‐specific siRNA and negative control were designed by Ambion (Shanghai, China). The BMI1 cDNA was cloned into pcDNA3.1 to construct the BMI1 expression plasmid. For luciferase reporter, the 3′UTR of BMI1 containing the putative binding sites for miR‐376c was amplified by PCR and cloned into the pGL3 luciferase reporter plasmid (Promega, Madison, WI, USA). Mutations in the miR‐376c binding site of BMI1 3′UTR were introduced by the QuikChange Site‐Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA). Constructs were verified by sequencing.
RNA extraction and qRT‐PCR
Total RNA and miRNA were extracted from tissues and cells using RNeasy Mini and miRNeasy Mini Kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. For detection of miR‐376c expression, stem–loop RT‐PCR was performed as described (Wang et al. 2014a). Expression of U6 was used as an endogenous control. Real‐time PCR was performed using FastStart Universal SYBR Green Master kit (Roche Diagnostics, Mannhelm, Germany) and analysed with an Applied Biosystems 7900 Real‐Time PCR System. Primer sequences were as follows: BMI1, 5′‐GTGCTTTGTGGAGGGTACTTCAT‐3′ and 5′‐ TTGGACATCACAAATAGGACAATACTT‐3′; and GAPDH, 5′‐ ATGTCGTGGAGTCTACTGGC‐3′ and 5′‐ TGACCTTGCCCACAGCCTTG‐3′. Fold changes in expression were calculated. The qRT‐PCR data was analysed using the method of 2−ΔΔCt relative expression quantity as previously described (Wang et al. 2014b). All the qRT‐PCRs were run in triplicate.
Cell proliferation and cell cycle analysis
Transfected cells were seeded into 96‐well plates (2 × 103/well) and cultured for 0, 24, 48, 72 and 96 h. MTT (5 mg/ml) was added to each well for 4 h at 37°C. The reaction was stopped by 150 μl DMSO, and absorbance readings at 490 nm were obtained in triplicate using a spectrophotometric plate reader (Thermo Scientific, Waltham, MA, USA). For the cell cycle analysis, cells were harvested by trypsinization, washed twice using cold PBS and fixed in 70% ethanol overnight at 4°C. Then, cells were subsequently incubated with 20 μg/ml propidium iodide (Sigma, St. Louis, MO, USA) for 20 min at room temperature, and cell cycle analysis was performed with FACS flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Transwell invasion assay
The Matrigel invasion chamber was used to assess cell invasion ability. Aliquots of cells (1 × 105) were placed into upper chambers coated with 150 mg Matrigel (BD Biosciences, Bedford, MD, USA). The lower chambers were filled with DMEM containing 10% FBS. After incubation at 37°C for 24 h, cells remaining on the upper surface of the membrane were removed. Cells on the lower surface of the membrane were fixed and stained with crystal violet. Stained cells were visualized and counted under a light microscope. The assays were performed in triplicate and were repeated three times.
Dual‐luciferase assay
For the dual‐luciferase assay, HEK293 cells in a 96‐well plate were transfected with 50 nM miR‐376c or miR‐NC. The cells were then cotransfected with 0.2 mg/ml of vector with the wild‐type or mutant 3′UTR of BMI1 gene. After 48 h, luciferase activity was measured with the Dual‐Luciferase Reporter Assay System (Promega). Firefly luciferase activity was then normalized to the corresponding Renilla luciferase activity. Luciferase assays were performed in quadruplicate and repeated in three independent experiments.
Western blot
Proteins were extracted by RIPA lysis buffer (Beyongtime, China). Protein concentrations were quantified by the BCA protein assay kit (Beyotime, Haimen, China). Equal amounts of protein were separated by SDS‐PAGE, transferred onto PVDF membranes (Bio‐Rad, Hercules, CA, USA) and blocked for 0.5 h at room temperature. Membranes were probed with primary antibodies against BMI1 and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight followed by incubation with HRP‐conjugated secondary antibodies. Blots were detected using an ECL detection system.
Statistical analysis
SPSS16.0 statistical software package (SPSS, Chicago, IL, USA) was used for statistical analysis. Experiments were repeated independently at least three times, and the results are expressed as mean ± SD. The correlation between miR‐376c and BMI1 was analysed using Spearman's correlation test. Statistical differences between groups were evaluated using Student's paired two‐tailed t‐test. P < 0.05 was considered statistically significant.
Ethical approval statement
The study had been approved by the Ethical and Scientific Committees of Wuhan University and in accordance with the Declaration of Helsinki.
Results
miR‐376c is significantly downregulated in cervical cancer tissues and cell lines
Expression of miR‐376c was first examined in the cervical cancer tissues and cell lines by real‐time PCR. Consistent with the microarray‐based results, miR‐376c expression was significantly lower in human cervical cancer tissues than that of adjacent normal cervical tissues (n = 29, 1.437 ± 0.654 vs. 2.549 ± 1.083, P < 0.01, Figure 1a and b). Moreover, miR‐376c expression was also significantly decreased in four cervical cancer cell lines compared with that of HaCaT cells (P < 0.05, Figure 1c).
Figure 1.
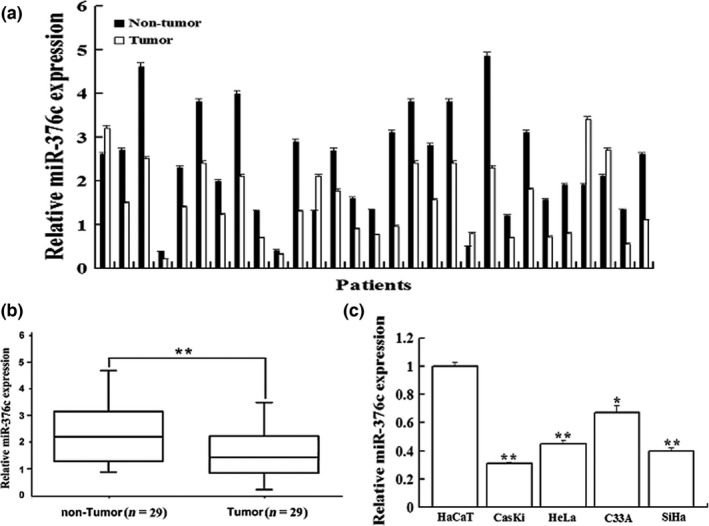
miR‐376c was decreased in cervical cancer tissues and cell lines. (a) A paired bar chart shows the expression level of miR‐376c in 29 cervical cancer tissues and their pair‐matched adjacent noncancerous cervical tissues. (b) The mean expression level of miR‐376c in cervical cancer tissues was significantly lower than that in pair‐matched adjacent noncancerous cervical tissues (P < 0.01). (c) miR‐376c was significantly decreased in four cervical cancer cell lines compared with that in HaCaT cell. *P < 0.05, **P < 0.01 compared with control group.
The suppressive effect of miR‐376c on cervical cancer cell proliferation and invasion in vitro
To investigate the role of miR‐376c in cervical cancer cell proliferation and invasion, C33A cells expressing relatively high level of miR‐376c and CasKi cells expressing relatively low level of miR‐376c were transfected with miR‐376c inhibitor and miR‐376c mimics, respectively, which decreased the level of miR‐376c in C33A by 3.2‐fold and increased that in CasKi by 15.4‐fold, as compared to corresponding negative control (Figure 2a). As expected, downregulation of miR‐376c significantly promoted the proliferation of C33A cells, whereas upregulation of miR‐376c significantly reduced the proliferation of CasKi cells, as demonstrated by the MTT assay (Figure 2b). To further determine the mechanisms by which miR‐376c inhibited cervical cancer cell proliferation, we subsequently investigated whether miR‐376c has an effect on cell cycle progression of cervical cancer cells using flow cytometry. The results showed that downregulation of miR‐376c in C33A cells led to a significant decrease in the cellular population in G0/G1 phase but a sharp increase in S phase (G1 = 58.9% ± 3.7% vs. 47.2 ± 1.9%, S = 30.2% ± 2.6% vs. 42.3 ± 1.7%, P < 0.01), while upregulation of miR‐376c in CasKi cells noticeably induced G1 phase arrest (G1 = 65.8% ± 4.8% vs. 76.7 ± 5.4%, S = 24.6% ± 1.6% vs. 13.9 ± 3.2%, P < 0.01, Figure 2c). Thus, the growth‐inhibiting function of miR‐376c may attribute to suppression of cell cycle progression at G1/S transition in cervical cancer cells. Moreover, Matrigel Transwell assays showed that miR‐376c markedly decreased the invasive ability of CasKi cells (124.6 ± 7.9 vs. 58.5 ± 10.6, P < 0.01), while anti‐miR‐376c promoted this activity of C33A cells (91.3 ± 8.6 vs. 176.4 ± 3.7, P < 0.01, Figure 2d).
Figure 2.
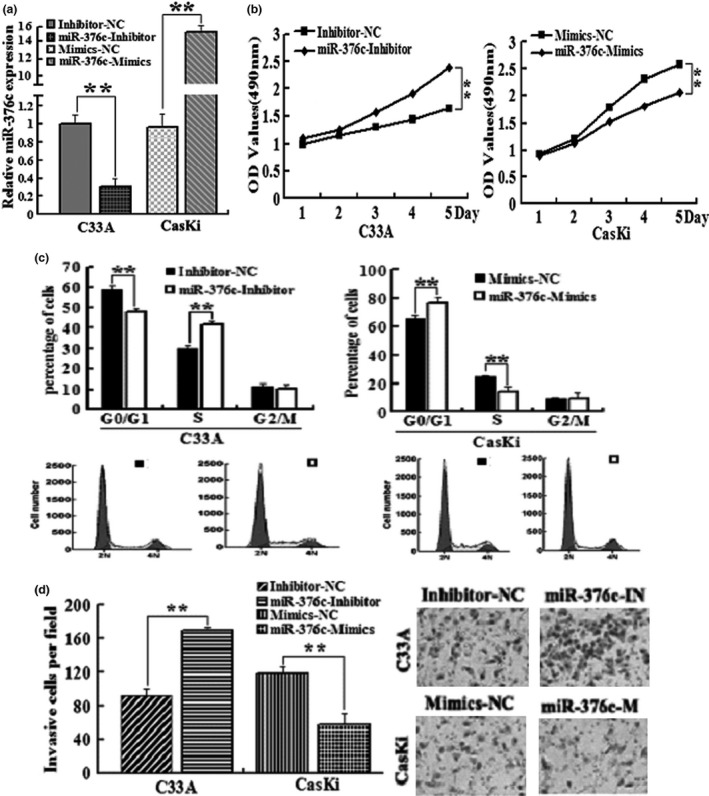
miR‐376c suppresses cervical cancer cell proliferation and invasion. (a) qRT‐PCR analysis of miR‐376c levels in C33A and CasKi cells, transfected with miR‐376c inhibitor and miR‐376c mimics, respectively. (b) Determination of C33A and CasKi cells proliferation with MTT assay. (c) Determination of C33A and CasKi cell cycle with flow cytometry. (d) Determination of C33A and CasKi cells invasion with Transwell invasion assay. *P < 0.05, **P < 0.01 compared with control group.
BMI1 is a direct target of miR‐376c and inversely correlated with it in cervical cancer tissues
To investigate the underlying molecular mechanisms of miR‐376c in cervical cancer growth and invasion, we searched for the putative target genes of miR‐376c using bioinformatic tools, such as TargetScan, miRanda and E1MMo2, which identified that BMI1 might be a putative target gene of miR‐376c and the 3′UTR of BMI1 mRNA contains a highly conserved binding site from position 466 to 473 for miR‐376c seed sequence (the core sequence that encompasses the first 2–8 bases of the mature miRNA, Figure 3a). To examine whether BMI1 was the target of miR‐376c,we performed Western blot analysis. The level of BMI1 protein was markedly decreased by miR‐376c overexpression in CasKi cells but significantly increased by silencing of miR‐376c in C33A cells (Figure 3b). Moreover, the levels of BMI1 mRNA were further tested in 29 pairs of cervical cancer and their adjacent normal tissues using qRT‐PCR. We found significantly higher expression level of BMI1 in cervical cancer tissues as compared with the matched normal tissues (3.21 ± 1.6 vs. 1.67 ± 1.3, P < 0.01). Furthermore, the expression pattern of BMI1 was inversely correlated with miR‐376c level in cervical cancer tissues (Figure 3c and d).
Figure 3.
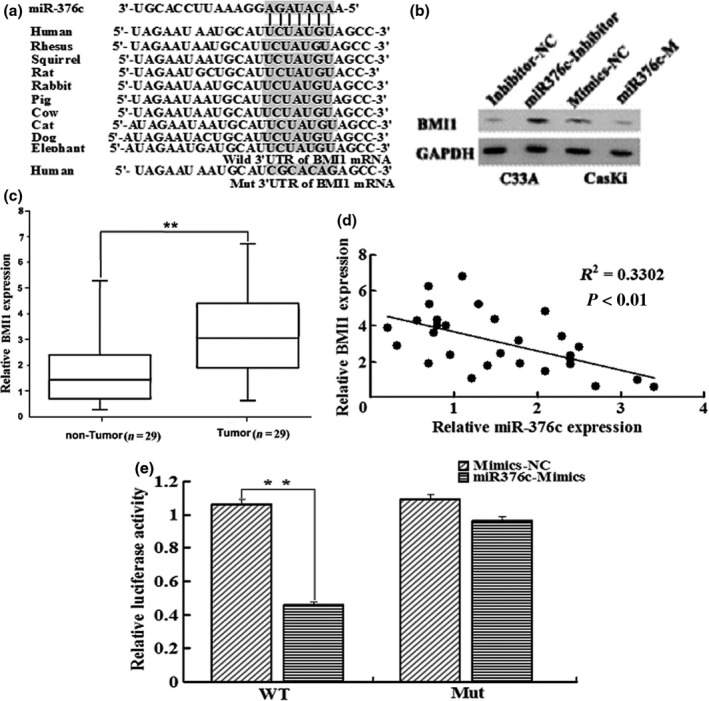
BMI1 is a direct target of miR‐367c. (a) putative miR‐376c binding site in 3′UTR region of BMI1 and interspecies conservation of seed matching sequences (grey box). Mutation was generated in the BMI1 3′UTR by mutating seed matching sequence. (b) Protein level of BMI1 was detected by Western blot in C33A and CasKi cells transfected with miR‐376c inhibitor and miR‐376c mimics along with corresponding controls, respectively. (c) BMI1 mRNA level was examined by qRT‐PCR, and it was remarkably increased in cervical cancer tissues. (d) BMI1 mRNA level was inversely correlated with miR‐376c level in cervical cancer tissues (Spearman's correlation analysis). (e) HEK293 cells were cotransfected with miR‐376c and WT or Mut BMI1 3′UTR luciferase reporter construct. *P < 0.05, **P < 0.01 compared with control group.
To assess whether BMI1 is a direct target of miR‐376c, we constructed luciferase reporter vector containing wild‐type BMI1 3′UTR with miR‐376c binding site (WT) or containing the mutant 3′UTR (mutation of the putative miR‐376c target site, MUT, Figure 3a). Luciferase activity assay indicated that miR‐376c significantly suppressed the luciferase activity of BMI1‐3′UTR‐WT reporter, compared with control, but did not affect that of the mutant reporter in HEK293 cells (Figure 3e).
BMI1 is a functional target of miR‐376c in cervical cancer cells
We further determined whether BMI1 is involved in the effects of miR‐376c on cervical cancer cells. MTT and in vitro invasion assays showed that silencing of BMI1 significantly suppressed anti‐miR‐376c‐induced proliferation and invasion of C33A cells, while overexpression of BMI1 significantly reversed the tumour‐suppressive effects of miR‐376c on the proliferation and invasion of CasKi cells (Figure 4a, b and c). Furthermore, knockdown of BMI1 markedly abrogated the enhancement of cell cycle progression at G1/S transition induced by anti‐miR‐376c in C33A cells (G1 = 56.4% ± 4.8% vs. 72.1% ± 6.3% vs. 58.2% ± 2.4%, S = 29.7% ± 5.4% vs. 17.4% ± 4.3% vs. 27.5% ± 1.9%, P < 0.01), whereas upregulation of BMI1 clearly rescued the G1 phase arrest induced by miR‐376c in CasKi cells (G1 = 59.1% ± 5.2% vs. 43.2% ± 2.3% vs. 61.3% ± 3.8%, S = 28.4% ± 4.9% vs. 39.2% ± 2.1% vs. 25.7% ± 4.5%, P < 0.01, Figure 4d). These results suggested that miR‐376c inhibited the proliferation and invasion of cervical cancer cell via targeting BMI1.
Figure 4.
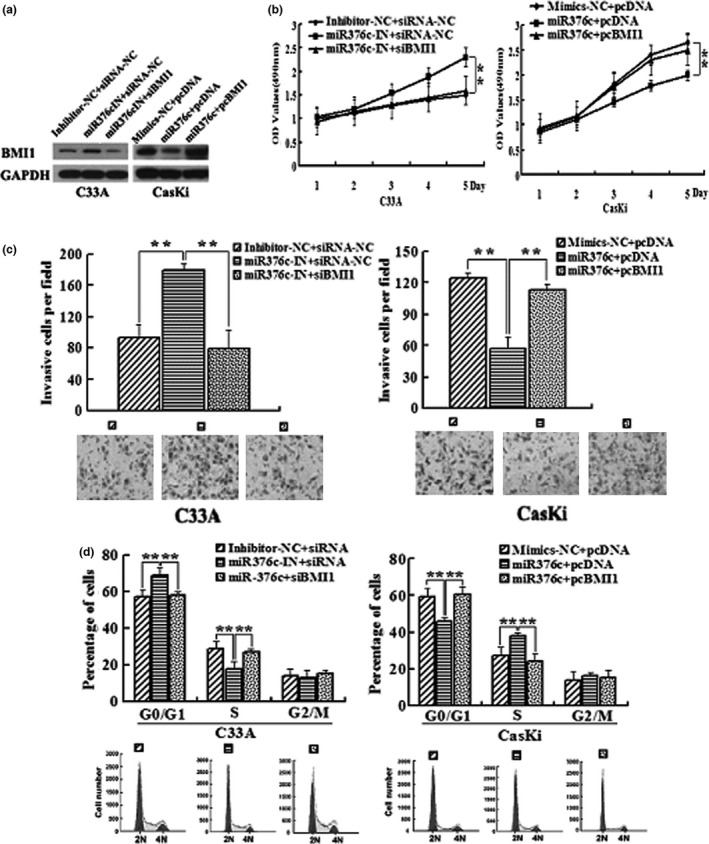
BMI1 is involved in miR‐376c regulation of cervical cancer cell proliferation and invasion. C33A cells were transfected with miR‐376c inhibitor or cotransfected with miR‐376c inhibitor and BMI1‐specific siRNAs(siBMI1), while CasKi cells were transfected with miR‐376c mimics or cotransfected with miR‐376c mimics and BMI1 expression vector(pcBMI1). (a) Western blotting analysis of BMI1 expression in C33A and CasKi cells. (b) Determination of cell proliferation with the MTT assay. (c) Determination of cell invasion ability with the Transwell assay. (d) Flow cytometry analysis of the cell cycle.*P < 0.05, **P < 0.01 compared with control group.
Discussion
Emerging data have shown that the aberrant expression of miRNAs contributes to tumorigenesis by inhibiting the expression of their target genes and potentially serves as biomarkers for prediction and prognosis in various cancers including cervical cancer (Schickel et al. 2008; Park et al. 2014). Hence, the identification of specific miRNAs and their targets involved in tumorigenesis would provide critical clues for the diagnosis and therapy of patients with malignancies. In the present study, we found that the expression of miR‐376c was significantly decreased in cervical cancer tissue samples and cell lines, consistent with data from previous microarray analyses (Martinez et al. 2008). We also showed that miR‐376c could suppress the growth and invasion of cervical cancer cells, which were accompanied by inhibiting cell cycle transition from G1 phase to S phase, and miR‐376c could inhibit BMI1 expression by directly targeting its 3′UTR region. Moreover, BMI1 could counteract the functional influences of miR‐376c on cervical cancer cells. These results suggest that miR‐376c functions as a tumour suppressor and plays a critical role in the proliferation and invasion of cervical cancer. However, its roles in vivo and the relationship between deregulation of its expression and clinical outcomes await further studies.
MiR‐376c has been reported to be dysregulated in several cancers and plays a vital role in cancer development. For example, Formosa et al. indicated that miR‐376c was downregulated in prostate cancer and correlated with a higher incidence of metastatic events and higher prostate‐specific antigen (PSA) levels (Formosa et al. 2014). Zehavi et al. showed that in melanoma, miR‐376c was significantly downregulated in melanoma cell lines, benign nevi and melanoma samples relative to normal melanocytes and modulated the growth and migration of melanoma cells via targeting IGF1R (Zehavi et al. 2012). Jin et al. suggested that miR‐376c inhibited osteosarcoma cell proliferation and invasion by targeting TGF‐α (Jin et al. 2013). However, Ye et al. showed that miR‐376c enhanced ovarian cancer cell survival by inhibiting ALK7 (Ye et al. 2011). Song et al. found that miR‐376c was markedly upregulated in patients with gastric cancer compared with controls and demonstrated significantly positive correlation with poor differentiation of gastric cancer (Song et al. 2012). These dual roles of miR‐376c could attribute to organ‐specific actions and different cellular contexts. The present study expanded the function of miR‐376c in cervical cancer and suggested that miR‐376c mainly functions as a tumour suppressor miRNA in cervical cancer.
To investigate the underlying mechanisms of miR‐376c in cervical cancer, we used three bioinformatics algorithms to predict gene targets for miR‐376c. BMI1 was one of the cancer‐associated genes predicted by all of these algorithms, whose mRNA contains a highly conserved miR‐376c binding site on the 3′UTR. Our experimental data further showed that the expression level of BMI1 was significantly higher in cervical cancer tissues as compared with that of normal cervical tissues, which were inversely associated with miR‐376c expression patterns. In addition, downregulation of miR‐376c increased, while its overexpression inhibited BMI1 expression in cervical cancer cells. Moreover, our luciferase reporter assay showed that miR‐376c caused a significant decrease in the luciferase activity of a wild‐type BMI1 3′UTR reporter but did not influence that of a ‘seed region’ mutant BMI1 3′UTR reporter. These data indicate that miR‐376c directly regulates BMI1 gene expression via binding to the 3′UTR of its mRNA.
Recent studies have shown that BMI1 is overexpressed in various human cancers and associated with poor prognosis. Song et al. indicated that BMI1 was significantly overexpressed in pancreatic cancer cell lines and tissues and that suppression of BMI1 inhibited cell growth, delayed the G1/S transition and induced cell apoptosis (Song et al. 2010). Li et al. found that BMI1 promoted migration and invasion of breast cancer cells by regulating EMT (Li et al. 2014). Tong et al. showed that BMI1 was upregulated in cervical cancer and correlated with a poorer prognosis, suggesting that it plays a vital role in cervical cancer development (Tong et al. 2012). This result was consistent with our findings that BMI1 was a functional target of miR‐376c. We showed that overexpression of BMI1 abrogated tumour‐suppressive effects of miR‐376c on cervical cancer cell proliferation and invasion. Our results for the first time establish a functional link between miR‐376c and BMI1 and confirm that miR‐376c‐inhibited cervical cancer cell growth and invasion is mediated, in part, by suppressing BMI1.
Collectively, we found that miR‐376c was significantly downregulated in cervical cancer and affected tumour phenotypes. Upregulation of miR‐376c inhibited cervical cancer cell proliferation and invasion in vitro. Further experiments revealed that BMI1 was a direct and functional target of miR‐367c in cervical cancer cells. These data demonstrate that the miR‐376c‐BMI1 axis may be a potential therapeutic target for patients with cervical cancer.
Author contribution
YP Deng contributed to project development, data collection, data analysis and writing of the manuscript. Y Xiong performed data collection and data analysis. YJ Liu contributed to project development and data analysis.
Conflicts of interest
The authors declare that they do not have any conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81101306).
References
- Calin G.A. & Croce C.M. (2006) MicroRNA signatures in human cancers. Nat. Rev. Cancer 6, 857–866. [DOI] [PubMed] [Google Scholar]
- Filipowicz W., Bhattacharyya S.N. & Sonenberg N. (2008) Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9, 102–114. [DOI] [PubMed] [Google Scholar]
- Formosa A., Markert E.K., Lena A.M. et al (2014) MicroRNAs, miR‐154, miR‐299‐5p, miR‐376a, miR‐376c, miR‐377, miR‐381, miR‐487b, miR‐485‐3p, miR‐495 and miR‐654‐3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 33, 5173–5182. [DOI] [PubMed] [Google Scholar]
- Forouzanfar M.H., Foreman K.J., Delossantos A.M. et al (2011) Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet 378, 1461–1484. [DOI] [PubMed] [Google Scholar]
- Hildesheim A. & Wang S.S. (2002) Host and viral genetics and risk of cervical cancer: a review. Virus Res. 89, 229–240. [DOI] [PubMed] [Google Scholar]
- Jin Y., Peng D., Shen Y. et al (2013) MicroRNA‐376c inhibits cell proliferation and invasion in osteosarcoma by targeting to transforming growth factor‐alpha. DNA Cell Biol. 32, 302–309. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang F., Xu J. et al (2011) Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV‐related target genes for miR‐29. J. Pathol. 224, 484–495. [DOI] [PubMed] [Google Scholar]
- Li H., Song F., Chen X., Li Y., Fan J. & Wu X. (2014) Bmi‐1 regulates epithelial‐to‐mesenchymal transition to promote migration and invasion of breast cancer cells. Int. J. Clin. Exp. Pathol. 7, 3057–3064. [PMC free article] [PubMed] [Google Scholar]
- Martinez I., Gardiner A.S., Board K.F. et al (2008) Human papillomavirus type 16 reduces the expression of microRNA‐218 in cervical carcinoma cells. Oncogene 27, 2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naucler P., Mabota da Costa F., da Costa J.L., Ljungberg O., Bugalho A. & Dillner J. (2011) Human papillomavirus type‐specific risk of cervical cancer in a population with high human immunodeficiency virus prevalence: Case‐control study. J. Gen. Virol. 92, 2784–2791. [DOI] [PubMed] [Google Scholar]
- Paranjape A.N., Balaji S.A., Mandal T. et al (2014) Bmi1 regulates self‐renewal and epithelial to mesenchymal transition in breast cancer cells through Nanog. BMC Cancer 14, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Lee M.J., Jeong J.Y. et al (2014) Dysregulated microRNA expression in adenocarcinoma of the uterine cervix: clinical impact of miR‐363‐3p. Gynecol. Oncol. 13, 5565–5572. [DOI] [PubMed] [Google Scholar]
- Schickel R., Boyerinas B., Park S.M. & Peter M.E. (2008) MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 27, 5959–5974. [DOI] [PubMed] [Google Scholar]
- Song W., Tao K., Li H. et al (2010) Bmi‐1 is related to proliferation, survival and poor prognosis in pancreatic cancer. Cancer Sci. 101, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.Y., Pan K.F., Su H.J. et al (2012) Identification of serum microRNAs as novel non‐invasive biomarkers for early detection of gastric cancer. PLoS ONE 7, e33608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y.Q., Liu B., Zheng H.Y. et al (2012) Overexpression of BMI‐1 is associated with poor prognosis in cervical cancer. Asia. Pac. J. Clin. Oncol. 8, e55–e62. [DOI] [PubMed] [Google Scholar]
- Wang N., Zhou Y., Zheng L. & Li H. (2014a) MiR‐31 is an independent prognostic factor and functions as an oncomir in cervical cancer via targeting ARID1A. Gynecol. Oncol. 134, 129–137. [DOI] [PubMed] [Google Scholar]
- Wang N., Zhan T., Ke T. et al (2014b) Increased expression of RRM2 by human papillomavirus E7 oncoprotein promotes angiogenesis in cervical cancer. Br. J. Cancer 110, 1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Ojo D., Lin X. et al (2015) BMI1 attenuates etoposide‐induced G2/M checkpoints via reducing ATM activation. Oncogene 34, 3063–3075. [DOI] [PubMed] [Google Scholar]
- Yang Y., Xie Y.J., Xu Q., Chen J.X., Shan N.C. & Zhang Y. (2015) Down‐regulation of miR‐1246 in cervical cancer tissues and its clinical significance. Gynecol. Oncol. 138, 683–688. doi:10.1016/j.ygyno.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Ye G., Fu G., Cui S. et al (2011) MicroRNA 376c enhances ovarian cancer cell survival by targeting activin receptor‐like kinase 7: implications for chemoresistance. J. Cell Sci. 124, 359–368. [DOI] [PubMed] [Google Scholar]
- Zehavi L., Avraham R., Barzilai A. et al (2012) Silencing of a large microRNA cluster on human chromosome 14q32 in melanoma: biological effects of mir‐376a and mir‐376c on insulin growth factor 1 receptor. Mol. Cancer. 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Yao D., Chen J., Ding N. & Ren F. (2015) MiR‐20a promotes cervical cancer proliferation and metastasis in vitro and in vivo. PLoS ONE 10, e0120905. [DOI] [PMC free article] [PubMed] [Google Scholar]


