Abstract
Rationale: Asthma is one of the most common chronic diseases worldwide, and individuals with severe asthma experience recurrent exacerbations. Exacerbations are predominantly viral associated and have been linked to defective airway IFN responses. Ascertaining the molecular mechanisms underlying this deficiency is a major research goal to identify new therapeutic targets.
Objectives: We investigated the hypothesis that reduced Toll-like receptor 7 (TLR7)–derived signaling drove the impaired IFN responses to rhinovirus by asthmatic alveolar macrophages (AMs); the molecular mechanisms underlying this deficiency were explored.
Methods: AMs were recovered from bronchoalveolar lavage from healthy subjects and patients with severe asthma. Expression of pattern-recognition receptors and microRNAs was evaluated by quantitative polymerase chain reaction and Western blotting. A TLR7–luciferase reporter construct was created to evaluate binding of microRNAs to the 3′ untranslated region of TLR7. IFN production was measured by quantitative polymerase chain reaction and ELISA.
Measurements and Main Results: The expression of TLR7 was significantly reduced in severe asthma AMs and was associated with reduced rhinovirus and imiquimod-induced IFN responses by these cells compared with healthy AMs. Severe asthma AMs also expressed increased levels of three microRNAs, which we showed were able to directly reduce TLR7 expression. Ex vivo knockdown of these microRNAs restored TLR7 expression with concomitant augmentation of virus-induced IFN production.
Conclusions: In severe asthma, TLR7 deficiency drives impaired innate immune responses to virus by AMs. Blocking a group of microRNAs that are up-regulated in these cells can restore antiviral innate responses, providing a novel approach for therapy in asthma.
Key words: interferon, rhinovirus, alveolar macrophage
At a Glance Commentary
Scientific Knowledge on the Subject
Airway cells from patients with asthma have impaired innate immune responses to virus infection, which leads to recurrent lower respiratory tract virus infection and disease exacerbation. However, the mechanisms underlying defective innate immune responses to virus in asthma are unclear and have not been investigated in airway cells from patients with severe, persistent asthma.
What This Study Adds to the Field
We show that the expression of Toll-like receptor (TLR) 7, a pattern-recognition receptor that is crucial for responses to single-stranded RNA viruses, is significantly deficient in alveolar macrophages from patients with severe asthma. This deficiency negatively impacts IFN responses to rhinovirus infection. We also show that TLR7 deficiency is due to aberrant expression of three microRNAs—miR-150, miR-152, and miR-375—and that by blocking the expression of these miRNAs, TLR7 expression can be restored and, more importantly, IFN responses to rhinovirus augmented, representing a viable therapeutic approach in this setting.
Asthma is one of the most common chronic diseases worldwide. Most of the morbidity, mortality, and economic burden associated with asthma is related to disease exacerbations (1). Approximately 80% of exacerbations are associated with respiratory tract viral infection (2, 3), and in adults, rhinovirus (RV) infection is responsible for more than 60% of these cases (2, 4). The exacerbation frequency is greatest in patients with severe treatment–resistant asthma (SA). These patients have poorly controlled disease despite being on maximal treatment. Although they represent approximately 10% of the overall asthma population, they account for more than 50% of asthma-related costs (5). Ascertaining the precise mechanisms underlying asthma exacerbations in SA is a major research goal to identify new therapeutic targets.
RV infection causes more frequent and longer-lasting lower respiratory tract (LRT) infections in patients with asthma compared with healthy subjects (6). This vulnerability is believed to be caused by an impaired IFN response to virus (7–10). IFN is an antiviral cytokine, and its production is triggered when pattern recognition receptors (PRRs) are activated by viral genomic material (11). These PRRs include Toll-like receptors (TLRs) 7 and 8, which are activated by the single-stranded RV genome, and TLR3, the retinoic acid–inducible gene 1 (RIG-1), and melanoma differentiation–associated gene 5 (MDA5), which are activated by the double-stranded RNA intermediary formed during viral replication (12, 13).
Alveolar macrophages (AMs) are the most numerous leukocyte in the lower airways (14) and internalize RV to rapidly release antiviral cytokines (15). These create an antiviral state in nearby epithelial cells to protect them against RV infection and boost epithelial cell cytokine production up to 40-fold (15). In mouse models, AMs are an important initial source of IFN following virus infection (16). Following RV infection in humans, RV has been found to co-localize with AMs (17). These findings suggest that AMs are vital in the response to RV infections. Because AMs do no fully support RV replication (18, 19), the initiation of the antiviral response would rely on adequate signaling via the PRRs responsible for responses to single-stranded RNA, namely, TLR7 and 8.
Previous work has shown that bronchoalveolar lavage (BAL) cells (which are predominantly AMs) from individuals with nonsevere asthma have a deficient IFN response to RV infection (7, 9); TLR7 function has been shown to be impaired in the peripheral blood mononuclear cells of patients with asthma (20). Therefore, we hypothesized that reduced TLR7-derived signaling drove the impaired IFN responses to RV by the AMs in patients with asthma. We show that the expression of TLR7 is significantly reduced in AMs from patients with SA (SA-AM).
To elucidate the molecular mechanisms underlying this TLR7 deficiency, we investigated the expression of microRNAs (miRNAs) in SA and healthy AMs. MiRNAs are noncoding RNA molecules that regulate gene expression by binding to the 3′ untranslated region (3′UTR) of target mRNAs to either cause mRNA degradation or inhibit protein translation (21). We also show that by blocking a group of miRNAs that are up-regulated in SA-AM, TLR7 expression and the antiviral response mounted by these cells can be significantly augmented, providing a novel target for therapy in asthma.
Methods
Full details are available in the online supplement.
Study Subjects
Forty-four healthy subjects and 43 patients with asthma were recruited. Healthy subjects were recruited through local advertising and were nonatopic; they had no previous relevant medical history and no bronchial hyperresponsiveness. Patients with SA (n = 35) had a history of recurrent disease exacerbations and inadequate disease control despite being on Step 4/5 asthma management of the Global Initiative for Asthma (22). Asthma control was evaluated using exacerbation history and the Asthma Control Questionnaire (ACQ) (23), with an ACQ score ≤1 reflecting good control. Atopy was defined as one or more positive skin prick test responses. The study was approved by the South West Hampshire Local Research Ethics Committee. All subjects gave written informed consent. Further details are given in the online supplement.
Alveolar Macrophages
Subjects underwent flexible bronchoscopy in accordance with established guidelines (24). AMs were obtained using the adherence to plastic technique, an established method for purifying AMs (25–30). Functional and transfection studies are described in the online supplement.
RNA Work
RNA was isolated using TRI-Reagent (Life Technologies Ltd., Paisley, UK). Reverse transcription and quantitative polymerase chain reactions (qPCR) was performed as previously described (31).
Protein Expression Analysis
TLR7 protein expression was analyzed by Western blotting as previously described (32). Cytokine production was measured using Meso Scale Discovery (Rockville, MD) singleplex kits (described in the online supplement).
Luciferase Assays
Vector generation and transfections are described in the online supplement.
Statistical Analysis
Data were analyzed using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA). Clinical characteristics were compared using the Mann-Whitney test, except for comparison of sex and atopy, for which Fisher’s exact test was used. Depending on normality of data, the Mann-Whitney test or unpaired t test was used to compare miRNA and gene expression between clinical groups. Cytokine induction by AMs was compared using the Kruskal-Wallis test, followed by between-group testing with the Mann-Whitney test (for unpaired data) or the Wilcoxon test (for paired data), if the results were significant. Correlations were analyzed using the Spearman test. In vitro luciferase assays and the effects of dexamethasone and cytokines were analyzed using analysis of variance. P < 0.05 was considered significant.
Results
Clinical characteristics of study participants are shown in Table 1. Compared with healthy subjects, patients with SA were older, had poorer lung function, and had a higher body mass index (BMI). Overall, BAL cells were 86% macrophages, and this differed significantly between healthy subjects and patients with SA. Patients with SA had significantly more neutrophils in their BALs compared with healthy subjects, whereas the proportion of eosinophils was not different, which is consistent with the suppressive effect of their inhaled corticosteroid (ICS) therapy.
Table 1.
Clinical Characteristics of Study Participants
| Healthy | Severe Asthma | P Value | |
|---|---|---|---|
| Number | 44 | 35 | |
| Age | 21.0 (18–54) | 45.0 (21–68) | <0.0001 |
| Sex, M/F | 23/21 | 11/24 | 0.072 |
| FEV1 | 3.9 (2.5–5.5) | 1.9 (0.7–3.9) | <0.0001 |
| FEV1, % predicted | 103.5 (77–141) | 72.0 (27–123) | <0.0001 |
| FVC | 4.6 (3.1–7.3) | 2.8 (1.6–5.0) | <0.0001 |
| FVC, % predicted | 106.0 (67–145) | 84.0 (58–124) | <0.0001 |
| BMI | 24.3 (19–32) | 33.1 (20-49) | <0.0001 |
| Atopy, n (%) | 0 (0) | 23 (68)* | N/A |
| GINA treatment step | N/A | 4/5 | — |
| ACQ score | N/A | 2.9 (0.8–4.9) | — |
| ICS dose, BDP equivalent, μg | N/A | 2,400 (1,000–4,000) | — |
| Number on daily oral steroids | N/A | 10 | — |
| Number of exacerbations in previous year | N/A | 6 (1–12) | — |
| BAL cell differential | |||
| % macrophages | 87.0 (73–97) | 76.5 (3–97) | 0.012 |
| % neutrophils | 2.7 (0–14) | 4.8 (0.5–95) | 0.002 |
| % eosinophils | 0.3 (0–2.3) | 0.5 (0–30) | 0.266 |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; BAL = bronchoalveolar lavage; BDP = beclomethasone; BMI = body mass index; GINA = Global Initiative for Asthma; ICS = inhaled corticosteroid; N/A = not applicable.
Values are medians with range in parentheses unless otherwise noted.
Data unavailable for one subject.
TLR7 Expression and Function Is Reduced in SA-AM
BAL cells from patients with nonsevere asthma have been shown to have impaired RV-induced IFN responses (7, 9), and we first set out to confirm that SA-AMs have a similar deficiency. The expression of IFN-β and IFN-α mRNA and protein levels were measured 24 hours after ex vivo stimulation of healthy subjects and patients with SA-AM with RV16, UV-RV (ultraviolet-inactivated RV16), or medium alone. Despite comparable amounts of RV RNA within healthy subjects and patients with SA-AM (see Figure E2 in the online supplement), the latter produced significantly lower amounts of IFN-β and IFN-α mRNA and protein compared with healthy AMs (Figure 1A). Based on our hypothesis, we then evaluated the expression of TLR7 in SA-AMs and found significantly reduced expression of TLR7 mRNA and protein (Figure 1B) in SA-AMs compared with healthy AMs. The difference in TLR7 expression remained significant after adjusting for age and BMI using multiple linear regression analyses (P = 0.002). Subgroup analysis showed that within SA-AMs there were no differences in TLR7 expression between atopic and nonatopic subjects (see Figure E3).
Figure 1.
Toll-like receptor-7 (TLR7) expression and function is reduced in severe asthma alveolar macrophages (SA-AM). (A) Rhinovirus-dependent induction of IFN-β and IFN-α mRNA and protein occurs in both SA-AMs (n = 10) and healthy AMs (n = 10), but the magnitude of the response is significantly lower in SA-AMs compared with healthy AMs. mRNA is quantified by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) and is normalized to glyceraldehyde phosphate dehydrogenase (GAPDH). Protein levels are quantified using a Meso Scale Discovery electrochemiluminescence platform. (B) TLR7 mRNA is reduced in SA-AMs (n = 23) compared with healthy AMs (n = 23), quantified using qRT-PCR, and normalized to GAPDH. TLR7 protein is reduced in SA-AMs compared with healthy AMs using Western blot, relative to β-actin (mean + SEM). The TLR7/β-actin ratio was arbitrarily adjusted to one in the healthy control subjects. The panel below is a representative Western blot image of TLR7 and β-actin in healthy (HC) and SA-AMs. (C) TLR8, TLR3, retinoic acid–inducible gene 1 (RIG-1) and melanoma differentiation–associated gene 5 (MDA5) mRNA expression is similar in SA-AMs (n = 23) and healthy AMs (n = 23), quantified using qRT-PCR, and normalized to GAPDH. (D) Imiquimod-induced production of IFNβ, IFNα, 2′5′ oligoadenylate synthetase (OAS), and myxovirus resistance protein (MxA) mRNA is reduced in SA-AMs (n = 11) compared with healthy AMs (n = 10), quantified using qRT-PCR, and normalized to GAPDH. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ns = not significant; RV = rhinovirus; UV = ultraviolet.
Because TLR7 signals via the adaptor protein Myd88, we evaluated whether this cytoplasmic signal transducer was altered in SA; we found no difference in its cellular expression comparing SA-AMs with healthy AMs (see Figure E4). No difference in the mRNA expression of other RV relevant PRRs (TLR8, TLR3, RIG-1, and MDA5) was found in SA-AMs compared with healthy AMs (Figure 1C).
SA and healthy AMs were then treated with imiquimod (5 μg/ml), a synthetic specific TLR7 agonist (33), and IFN responses were measured at 24 hours. After imiquimod treatment, production of IFN-β and IFN-α mRNA was significantly reduced in SA-AMs compared with healthy AMs (Figure 1D). As part of the cellular response to virus, IFNs induce the production of IFN-stimulated genes (ISGs), such as 2′5′ oligoadenylate synthetase (OAS) and myxovirus resistance protein (MxA), which are important effectors of the antiviral response (34). We found that imiquimod-induced expression of OAS and MxA mRNA was also significantly reduced in SA-AMs compared with healthy AMs (Figure 1D). These results suggest that reduced TLR7 expression negatively affects the induction of the IFN pathway in SA-AMs. Healthy and SA-AMs had comparable IFN-β responses to Poly:IC (10 μg/ml), which is a double-stranded RNA that activates TLR3, RIG-1, and MDA5, suggesting that IFN production via this pathway was intact (see Figure E5).
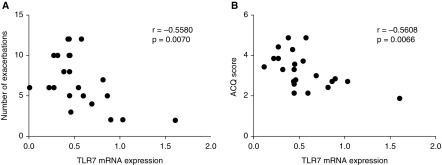
TLR7 mRNA expression in SA-AMs correlated inversely with the number of exacerbations the patient had experienced in the previous year (Figure 2; r = −0.5580; P = 0.0070). Poor disease control increases the tendency for disease exacerbation. Current control was thus assessed with the ACQ. We found that TLR7 mRNA expression correlated inversely with the ACQ scores of the patients (Figure 2; r = −0.5608; P = 0.0066). Based on these two clinical correlations, we propose that TLR7 deficiency and the associated impaired IFN response to virus drives the vulnerability of patients with SA to recurrent LRT viral infection. TLR7 expression did not correlate with the age of patients, BMI, lung function, dose of ICS treatment, BAL macrophages, neutrophils, or eosinophils (see Table E1).
Figure 2.
Expression of Toll-like receptor 7 (TLR7) correlates with clinical parameters of asthma control. Expression of TLR7 mRNA in severe asthma alveolar macrophages are inversely correlated with the (A) number of exacerbations experienced in the previous 12 months and with the (B) patients’ Asthma Control Questionnaire (ACQ) score.
miR-150, miR-152, and miR-375 Are Up-regulated in SA-AMs and Directly Regulate TLR7 Expression
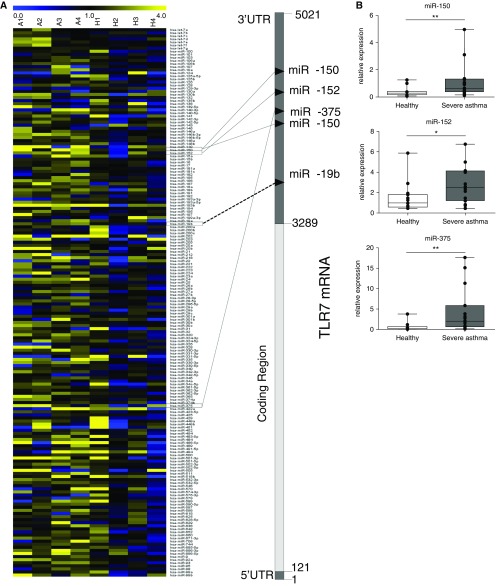
To investigate if reduced TLR7 expression in SA-AM was due to aberrant miRNA expression, we performed miRNA microarrays on AMs from healthy volunteers and volunteers with asthma. This identified that, of the 745 miRNAs evaluated, 16 miRNAs were differentially up-regulated in AMs from those with asthma (Figure 3A; see Table E2). In silico analysis showed that three of the up-regulated miRNAs, miR-150, miR-152, and miR-375, potentially targeted TLR7 (see Table E2 and Figure E6). qPCR on these three miRNAs confirmed that their expression was significantly increased in SA-AMs (Figure 3B). We also evaluated the expression of other miRNAs (miR-15a, miR-19b, miR-101, miR-201, and miR-144), which were predicted by bioinformatics analysis to be of relevance to TLR7, but they did not show differential expression in the original array, and we found no significant increase in SA-AMs (see Figure E7).
Figure 3.
MicroRNAs (miRNAs) targeting Toll-like receptor 7 (TLR7) are increased in severe asthma alveolar macrophages (SA-AMs). (A) Heat map representing Taqman low-density array expression of miRNAs comparing AMs from four patients with mild steroid–naive asthma (A1–4) and four healthy (H1–4) subjects. The 3′ untranslated region (UTR) of TLR7 is also shown with predicted targeting by miRNAs that are increased in patients with asthma. (B) Expression of miR-150, miR-152, and miR-375 is elevated in SA-AMs (n = 15) compared with healthy AMs (n = 15) and quantified by quantitative reverse-transcriptase polymerase chain reaction and normalized to RNU44. *P < 0.05; **P < 0.01.
To investigate if steroid treatment or cytokines in the lung environment could affect miRNA levels, we evaluated for any change in the expression of miR-150, miR-152, and miR-375 in healthy AMs exposed to dexamethasone (at concentrations of 10, 100, and 1000 nM), prototypical Th1 (tumor necrosis factor-α and IFN-γ) and Th2 (IL-4 and IL-13) cytokines (each at 10 ng/ml) ex vivo. This analysis showed that after 24 hours, the expression of miR-150, miR-152, and miR-375 was not significantly altered by steroids (Figure 4A) or by cytokines associated with asthmatic airway inflammation (Figure 4B). Exposure to dexamethasone for a further 24 hours showed that even after 48 hours of steroid exposure, levels of the three miRNAs were not significantly altered (Figure 4A).
Figure 4.
Ex vivo steroids and prototypical T helper 1 (Th1) and Th2 cytokines do not increase expression of microRNA-150 (miR-150), miR-152, and miR-375. (A) Expression of miR-150, miR-152, and miR-375 in alveolar macrophages (AMs) from healthy subjects is not altered by dexamethasone treatment at concentrations of 10, 100, and 1,000 nM at 24 or 48 hours. (B) Expression of miR-150, miR-152, and miR-375 in healthy AMs is not altered by treatment with IL-4, IL-13, tumor necrosis factor-α (TNFα), or IFN-γ for 24 hours (all cytokines used at 10 ng/ml). Data are from seven subjects, quantified by quantitative reverse-transcriptase polymerase chain reaction, and normalized to RNU44.
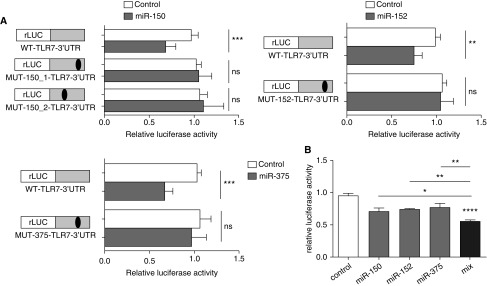
MiRNAs exert inhibitory effects on protein expression by binding to partially complementary sites within the 3′ UTR of the target mRNA. To confirm that miR-150, miR-152, and miR-375 directly bind to the 3′ UTR of TLR7, we generated luciferase reporter constructs containing the 3′ UTR sequence of TLR7 and found that the overexpression of miR-150, miR-152, and miR-375 led to a significant decrease in luciferase activity (Figure 5A). This response was abrogated when the binding sites for the 3 miRNAs was mutated (Figure 5A and see Figure E6). When all three miRNAs were overexpressed together, there was a significantly greater decrease in luciferase activity, suggesting that the miRNAs cooperated to reduce TLR7 expression TLR7 (Figure 5B). These results provide evidence to support our hypothesis that reduced TLR7 expression in SA-AMs is caused by aberrant miRNA expression.
Figure 5.
miR-150, miR-152, and miR-375 directly target Toll-like receptor 7 (TLR7) to reduce its expression. (A) Transfection of miR-150, miR-152, or miR-375 into HeLa cells reduces renilla-luciferase (rLUC) activity of the TLR7-reporter (WT-TLR7-3′ untranslated region [UTR]). This effect is abrogated when the predicted binding sequence for miR-150 (MUT-150_1-TLR7-3′UTR and MUT-150_2-TLR7-3′UTR), miR-152 (MUT-152-TLR7-3′UTR), or miR-375 (MUT-152-TLR7-3′UTR) are mutated. (B) Expression of all three microRNAs shows further reduced expression of rLUC in WT-TLR7-3′UTR compared with individual microRNAs (mean + SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. miR = microRNA; ns = not significant; WT = wild type.
Blocking of miR-150, miR-152, and miR-375 Increases Virus-induced IFN Production by AMs
We next hypothesized that knockdown of miR-150, miR-152, and miR-375 would restore TLR7 expression and function, thereby ameliorating the defective virus-induced IFN production in AMs. Using anti-miRNA oligonucleotides, we were able to cause a reduction in the level of functional miR-150, miR-152, and miR-375 by at least 50% (see Figures E1 and E8). By blocking miR-150, miR-152, and miR-375 in AMs, TLR7 expression could be significantly augmented (Figure 6A). Importantly, when these AMs were exposed to RV16, they showed significantly augmented production of IFN-α and IFN-β mRNA and secreted proteins compared with control transfected cells (Figure 6B). Furthermore, when these transfected AMs were challenged with imiquimod, a similar significant increase in mRNA production of IFNs and ISGs was seen (Figure 6C). To confirm that the augmentation in IFN responses was due to the effects of these miRNAs on TLR7, we also stimulated the transfected cells with Poly:IC. Inhibition of miR-150, miR-152, and miR-375 had no effect on Poly:IC-induced IFN-β responses (see Figure E9). Together, these data suggest that blocking these three miRNAs restores the antiviral IFN response in SA-AMs by increasing the expression of TLR7.
Figure 6.
Blocking miR-150, miR-152, and miR-375 in alveolar macrophages (AMs) augments Toll-like receptor 7 (TLR7) expression and increases IFN responses to rhinovirus (RV). (A) Knockdown of mir-150, miR-152, and miR-375 (anti-miR Mix) increases TLR7 mRNA expression as quantified by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) and normalized to glyceraldehyde phosphate dehydrogenase (GAPDH). Data are from 12 independent experiments. (B) Knockdown of miR-150, miR-152, and mir375 (anti-miR Mix) increases RV-induced production of IFN-β and IFN-α mRNA and protein compared with cells transfected with scrambled anti-miR (Control). mRNA are quantified by qRT-PCR and normalized to GAPDH, and protein is quantified using a Meso Scale Discovery electrochemiluminescence platform. AMs are from three healthy subjects and four patients with severe asthma. (C) Knockdown of miR-150, miR-152, and miR-375 (anti-miR Mix) increases imiquimod induction of IFN-β, IFN-α, 2′5′ oligoadenylate synthetase (OAS), and myxovirus resistance protein (MxA) mRNA, quantified by qRT-PCR and normalized to GAPDH. AMs are from two healthy subjects and three patients with severe asthma. Anti-miR Mix = alveolar macrophages transfected with anti-miR-150, anti-miR-152, and anti-miR-375, Control = alveolar macrophages transfected with an anti-miR scrambled control. Nonparametric test. *P < 0.05; **P < 0.01. miR = microRNA; UV = ultraviolet.
Discussion
Our results demonstrated that AMs from patients with SA have a deficient IFN response to RV. We also identified that the mechanism underlying this defect is reduced expression of TLR7 caused by aberrant expression of miR-150, miR-152, and miR-375 in SA-AM. The reduction in TLR7 expression correlates with clinical parameters of asthma control and can be ameliorated by blocking miR-150, miR-152, and miR-375. This leads to augmented IFN responses to RV by AMs.
Studies have shown that structural and BAL cells from patients with asthma have an impaired IFN-dependent antiviral response to RV ex vivo (7–9, 35). However, there is a lack of consistency in the literature, with reports that IFN production by asthmatic fibroblasts (36), peripheral blood mononuclear cells (9), and epithelial cells from patients with well-controlled asthma (37) is adequate. We demonstrate that in patients with SA, AMs have impaired RV-induced IFN responses. Although SA-AMs have been shown to have impaired phagocytosis (30), RV enters AMs via receptor-mediated endocytosis (38). The expression of the receptor it uses, intercellular adhesion molecule-1, is increased by RV infection (39), making it unlikely that impairment of the phagocytic pathway affects RV detection and subsequent IFN production.
Because the AM is not supportive of RV replication (18), we hypothesized that reduced expression of TLR7 is responsible for the innate immune defect we observed in SA-AMs. Consistent with this, we identified defective expression of TLR7 at both the mRNA and protein level. This was demonstrated to be functionally relevant, because the induction of IFNs by imiquimod, a specific TLR7 agonist (33), was similarly attenuated. Our results are in keeping with a recent publication that showed reduced IFN responses in TLR7 knockout mice exposed to RV (40). Interestingly, this publication implicated Th2-induced eosinophilia as suppressing TLR7 expression. Our results would not support a straightforward relationship between luminal eosinophils, as reflected by BAL eosinophils, and the expression of TLR7 by AMs, because there was no significant correlation between these two variables. This may reflect the different site of study, small airways for BAL, and larger airways for sputum and biopsy, and the more complex underlying inflammatory process in severe asthma, which is not purely a Th2-driven disease.
The importance of reduced TLR7 expression is underlined by the normality in the expression of other PRRs and intermediaries involved in responses to replicating viruses (MyD88, TLR3, RIG-1, and MDA5). TLR8 has not been very well studied in humans, and our data suggests a trend toward reduced expression in SA-AMs (P = 0.058). This warrants further exploration because reduction in TLR8 could further hinder viral recognition and subsequent IFN responses by SA-AMs. Our study focused on SA. Previous work has been unable to detect a deficiency in TLR7 expression in peripheral blood mononuclear cells or BAL cells from patients with mild-to-moderate asthma (9, 20). Based on our results and these findings, we propose that TLR7 deficiency is related to asthma severity. To support this, we found that TLR7 expression in AMs from patients with mild asthma (who were treated with low-dose inhaled steroids or who were steroid-naive) was not deficient compared with healthy AMs (see Figure E10). In addition, bronchial biopsies from patients with SA (but not mild) showed reduced TLR7 expression (40, 41).
AM production of IFNs occurs early and probably before epithelial IFN production, because the latter requires viral replication (42). Although epithelial cells are the predominant source of cytokines during RV infection, because less than 10% of epithelial cells actually become infected with RV (42, 43), the paracrine release of cytokines from virus-activated AMs are paramount in activating epithelial cells (15) and promoting an antiviral state in the lower airways. We propose that reduced IFN responses from SA-AMs drives the vulnerability of these patients to recurrent LRT viral infection. Failure to clear virus is associated with more severe symptoms (44), poorer lung function (44), increased proinflammatory cell recruitment (45), and an exaggerated local inflammatory state, which are features that would all contribute to the immunopathology and symptoms of severe asthma. To support this theory, we found a clear correlation between the level of TLR7 expression in SA-AM and the number of exacerbations the patient had experienced in the previous year and their ACQ score.
To investigate the molecular mechanisms leading to reduced TLR7 expression in SA-AMs, we chose to study miRNAs. MiRNAs may regulate up to one-third of all protein coding genes in the human genome (46), and the relevance of miRNAs in asthma has been previously implied in studies of their expression in human asthma and animal models of allergic airway inflammation (47–52). We performed miRNA microarrays on AMs from healthy subjects and patients with asthma, and performed bioinformatics analyses followed by qPCR confirmation of the in silico results. These showed, for the first time, that the expression of miR-150, miR-152, and miR-375 is significantly increased in SA-AMs, and that they all target TLR7 to reduce its expression. Using in vitro luciferase assays with cloned sequences of the three miRNAs, we demonstrated that all three miRNAs had a stronger effect on TLR7 3′ UTR than when tested individually. These findings are consistent with the concept that miRNAs frequently operate in a coordinated fashion to have a considerably greater biological effect than individual miRNAs acting alone (53).
A confounding factor in the interpretation of our findings is the steroid therapy that the patients with SA were receiving. Although in vitro studies have identified that steroids hinder the overall cellular production of miRNAs (54), in vivo studies have identified no effect or a very limited effect of steroids on pulmonary miRNA expression, with changes favoring normalization (48, 55). Such changes would promote an increase rather than a decrease in protein expression, which is the opposite of what we saw for TLR7 in SA-AMs. Our results did not show a significant effect of dexamethasone on the expression of miR-150, miR-152, and miR-375 in the AMs studied ex vivo. However, it was a limited study, and we could not fully exclude a steroid effect on these miRNAs. Furthermore, we could not identify any change in the expression of these miRNAs after exposure to the cytokines typically associated with asthmatic airway inflammation. Thus, the precise mechanisms involved in the dysregulation of miR-150, miR-152, and miR-375 in SA-AMs remain to be determined.
Other confounding factors were the older age and higher BMIs of the patients with SA. We adjusted for these confounding factors using multiple linear regression analysis and confirmed that the expression of TLR7 was significantly lower in SA-AMs compared with healthy AMs despite these group responses. Although the functional responses of TLRs are believed to decrease with age, this immunosenescence has been shown to occur in much older individuals (≥65 yr) (56), and only two patients in our SA group were older than 65 years.
Studies have shown that exogenous IFN, even in very small amounts, enables the asthmatic epithelium to elicit a normal antiviral response and limit viral replication (8, 57, 58). Therefore, if AMs are able to show a more robust IFN response to RV, the protective antiviral state induced in nearby bronchial epithelial cells could lead to avoidance of the pathophysiological events leading to disease exacerbation. Our results demonstrate that the AMs in which we blocked miR-150, miR-152, and miR-375 showed increased TLR7 expression and augmented IFN responses to RV16 compared with control AMs. More specifically, this effect was seen when the transfected cells were stimulated with imiquimod, which exclusively signals through TLR7 (33), and this effect was not seen when transfected AMs were stimulated with Poly:IC (which signals via TLR3, RIG-1, and MDA5), suggesting that the increase in IFN responses is due to miRNA-mediated changes in TLR7 expression and function. This indicates a potential for the translation of antagomir-based therapy as a novel approach to correct the impaired innate antiviral response in SA-AM and thereby prevent disease exacerbation.
Although antagomir transfection significantly affects AM responses to RV, the impact of this within a clinical setting is at present undetermined. In that situation, the longer term exposure to antagomirs may elicit a stronger response and rescue of TLR7 function with a clear therapeutic benefit. The next step would be to follow with in vivo studies in animal models to determine safety and optimize delivery and dosage to support the future extension of this work toward human administration. MiRNA-targeted therapy is already being addressed in other disease areas (59), and tolerance to antagomirs, administered via the inhalational route, is supported by on-going trials with inhaled siRNAs, oligonucleotides of similar biochemical nature to the antagomirs used in our study (60). Compared with the administration of nebulized IFN (61), targeting miRNAs would have the advantage of using the cell’s own machinery to produce IFN in a spatiotemporally desirable manner.
In summary, our findings provide strong evidence that miR-150, miR-152, and miR-375 mediated deficiency in the expression of TLR7 is responsible for reduced expression of protective IFN after viral infection in SA-AM; these defective IFN responses can be ameliorated by manipulating the expression of the three miRNAs. International guidelines recognize that prevention of disease exacerbations is a major goal of asthma treatment, and we propose that successful translation of our work will provide a novel approach to prevent and treat virus-induced asthma exacerbations. In addition, our findings may have relevance to other respiratory diseases in which impaired virus-induced IFN production has been described (62).
Acknowledgments
Acknowledgment
The clinical component of the study was conducted in the Southampton Centre for Biomedical Research (SCBR) and NIHR Respiratory Biomedical Research Unit (RBRU). The authors are grateful for the support of the SCBR and RBRU staff and Jon Ward, who assisted with bronchoscopies. The authors are grateful to all the patients and volunteers who participated in the study. The authors wish to thank Paul Bassett for assistance with statistical analysis and Professor Peter Friedmann and Dr. Jane Collins for their critical reading of the manuscript. Array data will be deposited in Gene Expression Omnibus (GEO) at acceptance.
Footnotes
Supported by Medical Research Council (MRC) Clinical Research Fellowship (H.R.), MRC grant G08000649 (Wessex Severe Asthma Cohort) and MRC grant MR/K001035/1.
Author Contributions: Involved in the conception, hypothesis delineation, design of the study, analysis of data, and writing of the manuscript: H.R., T.S.-E, and P.H.H. Acquisition of the data: H.R., A.S.F.-G., R.T.M.-N., P.D., C.G., N.J., T.H., and L.C.K.L.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201502-0280OC on January 27, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Custovic A, Johnston SL, Pavord I, Gaga M, Fabbri L, Bel EH, Le Souëf P, Lötvall J, Demoly P, Akdis CA, et al. EAACI position statement on asthma exacerbations and severe asthma. Allergy. 2013;68:1520–1531. doi: 10.1111/all.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med. 2005;172:433–439. doi: 10.1164/rccm.200412-1621OC. [DOI] [PubMed] [Google Scholar]
- 3.Wark PA, Johnston SL, Moric I, Simpson JL, Hensley MJ, Gibson PG. Neutrophil degranulation and cell lysis is associated with clinical severity in virus-induced asthma. Eur Respir J. 2002;19:68–75. doi: 10.1183/09031936.02.00226302. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–67. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 6.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831–834. doi: 10.1016/S0140-6736(02)07953-9. [DOI] [PubMed] [Google Scholar]
- 7.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 8.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sykes A, Edwards MR, Macintyre J, del Rosario A, Bakhsoliani E, Trujillo-Torralbo MB, Kon OM, Mallia P, McHale M, Johnston SL.Rhinovirus 16-induced IFN-alpha and IFN-beta are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol 2012. 129:1506–1514.e6 [DOI] [PubMed]
- 10.Iikura K, Katsunuma T, Saika S, Saito S, Ichinohe S, Ida H, Saito H, Matsumoto K. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int Arch Allergy Immunol. 2011;155:27–33. doi: 10.1159/000327262. [DOI] [PubMed] [Google Scholar]
- 11.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435–445. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 13.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 14.Ancochea J, González A, Sánchez MJ, Aspa J, López-Botet M. Expression of lymphocyte activation surface antigens in bronchoalveolar lavage and peripheral blood cells from young healthy subjects. Chest. 1993;104:32–37. doi: 10.1378/chest.104.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Korpi-Steiner NL, Valkenaar SM, Bates ME, Evans MD, Gern JE, Bertics PJ. Human monocytic cells direct the robust release of CXCL10 by bronchial epithelial cells during rhinovirus infection. Clin Exp Allergy. 2010;40:1203–1213. doi: 10.1111/j.1365-2222.2010.03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Bentley JK, Sajjan US, Dzaman MB, Jarjour NN, Lee WM, Gern JE, Hershenson MB.Rhinovirus colocalizes with CD68- and CD11b-positive macrophages following experimental infection in humans. J Allergy Clin Immunol 2013. 132:758–761.e3 [DOI] [PMC free article] [PubMed]
- 18.Gern JE, Dick EC, Lee WM, Murray S, Meyer K, Handzel ZT, Busse WW. Rhinovirus enters but does not replicate inside monocytes and airway macrophages. J Immunol. 1996;156:621–627. [PubMed] [Google Scholar]
- 19.Laza-Stanca V, Stanciu LA, Message SD, Edwards MR, Gern JE, Johnston SL. Rhinovirus replication in human macrophages induces NF-kappaB-dependent tumor necrosis factor alpha production. J Virol. 2006;80:8248–8258. doi: 10.1128/JVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roponen M, Yerkovich ST, Hollams E, Sly PD, Holt PG, Upham JW. Toll-like receptor 7 function is reduced in adolescents with asthma. Eur Respir J. 2010;35:64–71. doi: 10.1183/09031936.00172008. [DOI] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Global Initiative for AsthmaGlobal strategy for asthma management and prevention. Vancouver, WA: GINA; 2014[accessed 2014 Oct 2]. Available from www.ginasthma.org
- 23.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 24.British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56:i1–i21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenson CS, Wrona CT, Grove LJ, Maloney J, Garlipp MA, Wallace PK, Stewart CC, Sethi S. Impaired alveolar macrophage response to Haemophilus antigens in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;174:31–40. doi: 10.1164/rccm.200509-1461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senft AP, Taylor RH, Lei W, Campbell SA, Tipper JL, Martinez MJ, Witt TL, Clay CC, Harrod KS. Respiratory syncytial virus impairs macrophage IFN-alpha/beta- and IFN-gamma-stimulated transcription by distinct mechanisms. Am J Respir Cell Mol Biol. 2010;42:404–414. doi: 10.1165/rcmb.2008-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 28.Oliver BG, Lim S, Wark P, Laza-Stanca V, King N, Black JL, Burgess JK, Roth M, Johnston SL. Rhinovirus exposure impairs immune responses to bacterial products in human alveolar macrophages. Thorax. 2008;63:519–525. doi: 10.1136/thx.2007.081752. [DOI] [PubMed] [Google Scholar]
- 29.Karta MR, Wickert LE, Curran CS, Gavala ML, Denlinger LC, Gern JE, Bertics PJ. Allergen challenge in vivo alters rhinovirus-induced chemokine secretion from human airway macrophages. J Allergy Clin Immunol. 2014;133:1227–1230. doi: 10.1016/j.jaci.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Z, Zhang Q, Thomas CM, Chana KK, Gibeon D, Barnes PJ, Chung KF, Bhavsar PK, Donnelly LE. Impaired macrophage phagocytosis of bacteria in severe asthma. Respir Res. 2014;15:72. doi: 10.1186/1465-9921-15-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Nunez RT, Louafi F, Sanchez-Elsner T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1) J Biol Chem. 2011;286:1786–1794. doi: 10.1074/jbc.M110.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) J Biol Chem. 2009;284:16334–16342. doi: 10.1074/jbc.M109.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, Cottam HB. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsons KS, Hsu AC, Wark PA. TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection. Clin Exp Allergy. 2014;44:91–101. doi: 10.1111/cea.12218. [DOI] [PubMed] [Google Scholar]
- 36.Bedke N, Haitchi HM, Xatzipsalti M, Holgate ST, Davies DE. Contribution of bronchial fibroblasts to the antiviral response in asthma. J Immunol. 2009;182:3660–3667. doi: 10.4049/jimmunol.0802471. [DOI] [PubMed] [Google Scholar]
- 37.Sykes A, Macintyre J, Edwards MR, Del Rosario A, Haas J, Gielen V, Kon OM, McHale M, Johnston SL. Rhinovirus-induced interferon production is not deficient in well controlled asthma. Thorax. 2014;69:240–246. doi: 10.1136/thoraxjnl-2012-202909. [DOI] [PubMed] [Google Scholar]
- 38.Rossmann MG, Bella J, Kolatkar PR, He Y, Wimmer E, Kuhn RJ, Baker TS. Cell recognition and entry by rhino- and enteroviruses. Virology. 2000;269:239–247. doi: 10.1006/viro.2000.0258. [DOI] [PubMed] [Google Scholar]
- 39.Papi A, Johnston SL. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 40.Hatchwell L, Collison A, Girkin J, Parsons K, Li J, Zhang J, Phipps S, Knight D, Bartlett NW, Johnston SL, et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax. 2015;70:854–861. doi: 10.1136/thoraxjnl-2014-205465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shikhagaie MM, Andersson CK, Mori M, Kortekaas Krohn I, Bergqvist A, Dahl R, Ekblad E, Hoffmann HJ, Bjermer L, Erjefält JS. Mapping of TLR5 and TLR7 in central and distal human airways and identification of reduced TLR expression in severe asthma. Clin Exp Allergy. 2014;44:184–196. doi: 10.1111/cea.12176. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, et al. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol. 2006;34:192–203. doi: 10.1165/rcmb.2004-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosser AG, Brockman-Schneider R, Amineva S, Burchell L, Sedgwick JB, Busse WW, Gern JE. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 44.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu J, Message SD, Qiu Y, Mallia P, Kebadze T, Contoli M, Ward CK, Barnathan ES, Mascelli MA, Kon OM, et al. Airway inflammation and illness severity in response to experimental rhinovirus infection in asthma. Chest. 2014;145:1219–1229. doi: 10.1378/chest.13-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Jardim MJ, Dailey L, Silbajoris R, Diaz-Sanchez D. Distinct microRNA expression in human airway cells of asthmatic donors identifies a novel asthma-associated gene. Am J Respir Cell Mol Biol. 2012;47:536–542. doi: 10.1165/rcmb.2011-0160OC. [DOI] [PubMed] [Google Scholar]
- 48.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–974. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collison A, Herbert C, Siegle JS, Mattes J, Foster PS, Kumar RK. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm Med. 2011;11:29. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiba Y, Misawa M. MicroRNAs and their therapeutic potential for human diseases: MiR-133a and bronchial smooth muscle hyperresponsiveness in asthma. J Pharmacol Sci. 2010;114:264–268. doi: 10.1254/jphs.10r10fm. [DOI] [PubMed] [Google Scholar]
- 51.Perry MM, Baker JE, Gibeon DS, Adcock IM, Chung KF. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol. 2014;50:7–17. doi: 10.1165/rcmb.2013-0067OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rupani H, Sanchez-Elsner T, Howarth P. MicroRNAs and respiratory diseases. Eur Respir J. 2013;41:695–705. doi: 10.1183/09031936.00212011. [DOI] [PubMed] [Google Scholar]
- 53.Peter ME. Targeting of mRNAs by multiple miRNAs: the next step. Oncogene. 2010;29:2161–2164. doi: 10.1038/onc.2010.59. [DOI] [PubMed] [Google Scholar]
- 54.Smith LK, Shah RR, Cidlowski JA. Glucocorticoids modulate microRNA expression and processing during lymphocyte apoptosis. J Biol Chem. 2010;285:36698–36708. doi: 10.1074/jbc.M110.162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams AE, Larner-Svensson H, Perry MM, Campbell GA, Herrick SE, Adcock IM, Erjefalt JS, Chung KF, Lindsay MA. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4:e5889. doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cakebread JA, Xu Y, Grainge C, Kehagia V, Howarth PH, Holgate ST, Davies DE.Exogenous IFN-beta has antiviral and anti-inflammatory properties in primary bronchial epithelial cells from asthmatic subjects exposed to rhinovirus. J Allergy Clin Immunol 2011. 127:1148–1154.e9 [DOI] [PubMed]
- 58.Gaajetaan GR, Geelen TH, Vernooy JH, Dentener MA, Reynaert NL, Rohde GG, Beuken EV, Grauls GE, Bruggeman CA, Stassen FR. Interferon-β induces a long-lasting antiviral state in human respiratory epithelial cells. J Infect. 2013;66:163–169. doi: 10.1016/j.jinf.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 60.DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Djukanović R, Harrison T, Johnston SL, Gabbay F, Wark P, Thomson NC, Niven R, Singh D, Reddel HK, Davies DE, et al. INTERCIA Study Group. The effect of inhaled IFN-β on worsening of asthma symptoms caused by viral infections. A randomized trial. Am J Respir Crit Care Med. 2014;190:145–154. doi: 10.1164/rccm.201312-2235OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, Aniscenko J, Laza-Stanca V, Edwards MR, Slater L, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]