Abstract
Rationale: Galectin-3 (Gal-3) has been implicated in the development of pulmonary fibrosis in experimental studies, and Gal-3 levels have been found to be elevated in small studies of human pulmonary fibrosis.
Objectives: We sought to study whether circulating Gal-3 concentrations are elevated early in the course of pulmonary fibrosis.
Methods: We examined 2,596 Framingham Heart Study participants (mean age, 57 yr; 54% women; 14% current smokers) who underwent Gal-3 assessment using plasma samples and pulmonary function testing between 1995 and 1998. Of this sample, 1,148 underwent subsequent volumetric chest computed tomography.
Measurements and Main Results: Higher Gal-3 concentrations were associated with lower lung volumes (1.4% decrease in percentage of predicted FEV1 per 1 SD increase in log Gal-3; 95% confidence interval [CI], 0.8–2.0%; P < 0.001; 1.2% decrease in percentage of predicted FVC; 95% CI, 0.6–1.8%; P < 0.001) and decreased diffusing capacity of the lung for carbon monoxide (2.1% decrease; 95% CI, 1.3–2.9%; P < 0.001). These associations remained significant after multivariable adjustment (P ≤ 0.008 for all). Compared with the lowest quartile, participants in the highest Gal-3 quartile were more than twice as likely to have interstitial lung abnormalities visualized by computed tomography (multivariable-adjusted odds ratio, 2.67; 95% CI, 1.49–4.76; P < 0.001).
Conclusions: Elevated Gal-3 concentrations are associated with interstitial lung abnormalities coupled with a restrictive pattern, including decreased lung volumes and altered gas exchange. These findings suggest a potential role for Gal-3 in early stages of pulmonary fibrosis.
Keywords: biomarker, epidemiology, interstitial lung disease, pulmonary fibrosis
At a Glance Commentary
Scientific Knowledge on the Subject
Galectin-3 is thought to be a mediator of fibrosis and has been implicated in the development of pulmonary fibrosis in experimental studies. In small studies of human pulmonary fibrosis, circulating galectin-3 levels were found to be elevated.
What This Study Adds to the Field
In this study of a community-based sample without known pulmonary fibrosis, we found that elevated circulating galectin-3 concentrations were associated with interstitial lung abnormalities coupled with decreased lung volumes, altered gas exchange, and radiographic fibrosis. These findings suggest a potential role for galectin-3 in the early stages of pulmonary fibrosis.
Galectin-3 (Gal-3) is a β-galactoside–binding lectin that is expressed in a variety of cells and plays a central role in inflammation and fibrosis (1). It has previously been linked to solid organ fibrosis, including hepatic, renal, and cardiac disease (2–4). Although circulating Gal-3 concentrations predict prognosis in patients with clinically apparent heart failure (5, 6), Gal-3 levels are also elevated in individuals in the community who are at risk for the development of future heart failure (7). These findings suggest that Gal-3 measurement may be helpful in detecting early stages of an inflammatory and/or fibrotic process before the presentation of clinically apparent disease.
Prior studies evaluating the role of Gal-3 in human pulmonary fibrosis have noted elevated Gal-3 levels in the serum (8) and bronchoalveolar lavage fluid in some (8, 9) but not all (10) studies of patients with idiopathic pulmonary fibrosis (IPF). In small numbers of subjects, elevated Gal-3 levels were noted to precede declines in lung function and gas exchange that help to diagnose an acute exacerbation of IPF (8). Additionally, elevated Gal-3 levels have been noted in other forms of pulmonary fibrosis, such as Hermansky–Pudlak syndrome (10) and collagen vascular disease associated interstitial pneumonia (9). However, it is not known if elevated Gal-3 levels can be detected in stages of pulmonary fibrosis that precede clinical detection.
On the basis of these findings, we hypothesized that Gal-3 would be elevated early in the course of pulmonary fibrosis. To test this hypothesis, we initially evaluated the associations between Gal-3 levels and the presence of interstitial lung abnormalities (ILAs) in individuals in the Framingham Heart Study (FHS). Prior studies have demonstrated that research participants with ILA in general (11, 12), and in the FHS in particular (13), share imaging, physiologic, and genetic abnormalities observed in patients with clinically apparent pulmonary fibrosis. On the basis of our findings with regard to ILA, we additionally evaluated the associations between Gal-3 levels and both baseline and longitudinal change in measures of pulmonary function.
Methods
Study Sample
Participants of the FHS offspring cohort were enrolled in 1971 and have been followed with serial examinations and questionnaires approximately every 4 years, as previously described (14). A total of 3,532 participants attended the “baseline” sixth examination cycle (1995–1998), which included a comprehensive medical history, physical examination, anthropometrics, fasting blood work, and pulmonary function testing. We excluded participants with missing Gal-3 measurements (n = 84), extreme Gal-3 outliers (>5 log SD above the log-transformed mean [n = 5], specified a priori), prevalent heart failure (n = 247), and stage IV kidney disease (n = 7), as well as those missing pulmonary function tests (n = 593), leaving 2,596 participants for the spirometry analysis. A total of 2,025 participants underwent subsequent assessment of diffusing capacity of the lung for carbon monoxide (DlCO) (2005–2008), and 1,148 underwent subsequent volumetric chest computed tomography (CT) between 2008 and 2011. The study was approved by the institutional review boards at Boston University Medical Campus, Massachusetts General Hospital, and Brigham and Women’s Hospital, and all participants provided written informed consent.
Biomarker Measurement
Fasting blood samples were collected and immediately processed and stored at −80°C until assayed. Plasma Gal-3 concentrations were measured using an enzyme-linked immunosorbent assay (BG Medicine, Waltham, MA) (15). Assay characteristics included a lower detection limit of 1.32 ng/ml and an upper detection limit of 96.6 ng/ml, with inter- and intraassay coefficients of variation of 1.2% and 4.1%, respectively.
Pulmonary Function Testing and Chest CT Analysis
Pulmonary function testing and measurement of DlCO were performed using the Collins Classic Pulmonary Function Laboratory system (Ferraris Respiratory, Ayer, MA). Spirometry was performed at the sixth (baseline) examination (1995–1998). DlCO was performed at the eighth examination (2005–2008).
Volumetric chest CT was performed using the 64-slice positron emission tomography–CT Discovery VCT scanner (GE Healthcare, Pittsburgh, PA). Images were analyzed by three readers (two chest radiologists, one pulmonologist) on a VirtualPlace workstation (AZE, Tokyo, Japan) using a sequential reading method as previously described (11, 13). ILAs were defined as nondependent ground-glass or reticular abnormalities, nonemphysematous cysts, diffuse centrilobular nodularity, honeycombing, or traction bronchiectasis involving more than 5% of any lung zone. Indeterminate scans were defined as focal or unilateral ground-glass abnormality, reticulation or patchy ground-glass attenuation interstitial attenuation involving less than 5% of the lung. Definite fibrosis was defined as parenchymal architectural distortion highly suggestive of fibrotic lung disease (1, 16). The diagnosis of definite fibrosis was obtained by consensus of the three readers, who were blinded to clinical characteristics of the participants. Quantitative measures of total lung capacity (TLC) were performed using Airway Inspector (www.airwayinspector.org) as described previously (2–4, 17).
Statistical Analysis
Baseline characteristics and pulmonary function measures are summarized by sex-specific Gal-3 quartiles. Gal-3 concentrations were log transformed due to a right-skewed distribution. FEV1, FVC, FEV1/FVC ratio, TLC, and DlCO were expressed as raw values, as well as percentage of predicted values, which were calculated using sex-specific regression models after accounting for age, age squared, and height squared among lifetime nonsmokers without known pulmonary disease (5, 6, 18).
All analyses evaluating the association of Gal-3 and pulmonary traits were performed using linear mixed-effects models implemented in the lmekin function in the R “kinship” package, with a covariance structure that was proportional to the kinship matrix. This allowed us to take into account known relatedness in our sample and to more accurately evaluate the statistical significance of our results. The cross-sectional associations of Gal-3 and percentage of predicted FEV1, FVC, FEV1/FVC ratio, TLC, and DlCO were evaluated, first adjusting for age, sex, and height and additionally adjusting for smoking history (never, former, or current), pack-years of tobacco use, body mass index, and diabetes mellitus (defined as fasting glucose level ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, or the use of antidiabetic medications). Effect sizes are expressed per 1-SD change in log-transformed Gal-3. The associations of Gal-3 and ILA and definite fibrosis were assessed, adjusting for the same covariates. Analyses were first performed by including indeterminate CT scans as “no ILA” and then by repeating analyses after excluding those with indeterminate scans.
In secondary analyses, we evaluated the association of Gal-3 with change in FEV1, FVC, and FEV1/FVC ratio between the sixth (1995–1998) and seventh (1998–2001) examination cycles. Linear mixed-effects models were used to account for familial correlation and to adjust for covariates as before, in addition to baseline pulmonary function parameters. We also conducted stratified analyses by smoking status. We tested for Gal-3 interactions with age, sex, and smoking status. Last, analyses for binary outcomes (ILA and fibrosis) were repeated using binomial regression to obtain risk ratios instead of odds ratios (ORs). A two-sided P value of 0.05 or less was considered significant. All analyses were conducted using R (version 2.15.3).
Results
Among 2,596 participants, the mean age was 57 years and 54% were women. Fourteen percent were current smokers, and 50% were past smokers. The mean FEV1 was 2.8 ± 0.8 L, mean FVC was 3.8 ± 1.0 L, and mean FEV1/FVC ratio was 0.73 ± 0.07. The median Gal-3 concentration was 13.5 ng/ml (25th–75th percentile range, 11.5–15.9). Clinical characteristics by sex-specific Gal-3 quartiles are presented in Table 1. Participants in higher Gal-3 quartiles were older, with a higher prevalence of diabetes and higher body mass index.
Table 1.
Clinical Characteristics of Sample by Sex-Specific Galectin-3 Quartiles
| Quartile 1 (n = 695) | Quartile 2 (n = 668) | Quartile 3 (n = 677) | Quartile 4 (n = 556) | |
|---|---|---|---|---|
| Age, yr | 54 (9) | 56 (9) | 59 (9) | 61 (9) |
| Women, n (%) | 379 (55) | 352 (53) | 364 (54) | 295 (53) |
| Diabetes mellitus, n (%) | 41 (6) | 42 (6) | 55 (8) | 64 (12) |
| Body mass index, kg/m2 | 26.8 (4.6) | 27.5 (4.9) | 28.4 (5.2) | 28.6 (5.2) |
| Current smoker, n (%) | 93 (13) | 102 (15) | 113 (17) | 66 (12) |
| Past smoker, n (%) | 333 (48) | 343 (51) | 330 (49) | 294 (53) |
| Pack-years | 13 (20) | 16 (21) | 17 (22) | 18 (23) |
Definition of abbreviation: Q = quartile.
Values are means (SD) unless otherwise noted. Sex-specific quartile ranges for galectin-3 concentrations are as follows: for men, Q1 = 3.9–11.1 ng/ml, Q2 = 11.2–13.1 ng/ml, Q3 = 13.2–15.4 ng/ml, Q4 = 15.5–54.4 ng/ml; for women, Q1 = 5.0–12.0 ng/ml, Q2 = 12.1–14.3 ng/ml, Q3 = 14.4–16.8 ng/ml, Q4 = 16.9–52.1 ng/ml.
Higher Gal-3 Is Associated with Lower Lung Volumes and DlCO
The percentage of predicted FEV1, FVC, and TLC declined across Gal-3 quartiles (predicted FEV1 quartile 1 [Q1], 95%; Q4, 91%; predicted FVC Q1, 99%; Q4, 96%; predicted TLC Q1, 86%; Q4, 84%), whereas the FEV1/FVC ratio did not change across Gal-3 quartiles (Table 2). Specifically, in age-, sex-, and height-adjusted analyses, each 1-SD increase in log Gal-3 was associated with a 1.4% decrease in percentage of predicted FEV1 (95% confidence interval [CI], 0.8–2.0%; P < 0.001), a 1.2% decrease in percentage of predicted FVC (95% CI, 0.6–1.8%; P < 0.001), and a 1% decrease in percentage of predicted TLC (95% CI, 0.02–2.0%; P = 0.04), with no difference in the FEV1/FVC ratio (P = 0.21).
Table 2.
Pulmonary Function and Chest Computed Tomography Measures by Sex-Specific Galectin-3 Quartiles
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Pulmonary function measures | ||||
| n | 665 | 631 | 646 | 530 |
| FEV1, % predicted | 95 (15) | 94 (15) | 94 (16) | 91 (17) |
| FVC, % predicted | 99 (12) | 98 (13) | 98 (14) | 96 (14) |
| FEV1/FVC, % predicted | 95 (9) | 95 (9) | 95 (9) | 95 (10) |
| TLC, % predicted* | 86 (15) | 84 (14) | 85 (16) | 84 (14) |
| DlCO, % predicted* | 99 (17) | 95 (16) | 92 (18) | 90 (18) |
| Chest CT measures |
||||
| n | 352 | 314 | 287 | 195 |
| ILA, n (%) | 22 (6.3) | 28 (8.9) | 36 (12.5) | 39 (20) |
| Definite fibrosis, n (%) | 3 (0.9) | 11 (3.5) | 15 (5.2) | 10 (5.1) |
Definition of abbreviations: CT = computed tomography; DlCO = diffusing capacity of the lung for carbon monoxide; ILA = interstitial lung abnormality; Q = quartile; TLC = total lung capacity.
Values are means (SD) unless otherwise noted. Sex-specific quartile ranges for galectin-3 concentrations are as follows: for men, Q1 = 3.9–11.1 ng/ml, Q2 = 11.2–13.1 ng/ml, Q3 = 13.2–15.4 ng/ml, Q4 = 15.5–54.4 ng/ml; for women, Q1 = 5.0–12.0 ng/ml, Q2 = 12.1–14.3 ng/ml, Q3 = 14.4–16.8 ng/ml, Q4 = 16.9–52.1 ng/ml.
Sample sizes are n = 2,596 for FEV1, FVC, and FEV1/FVC, n = 1,137 for TLC, n = 2,025 for DlCO, and n = 1,148 for chest CT measures.
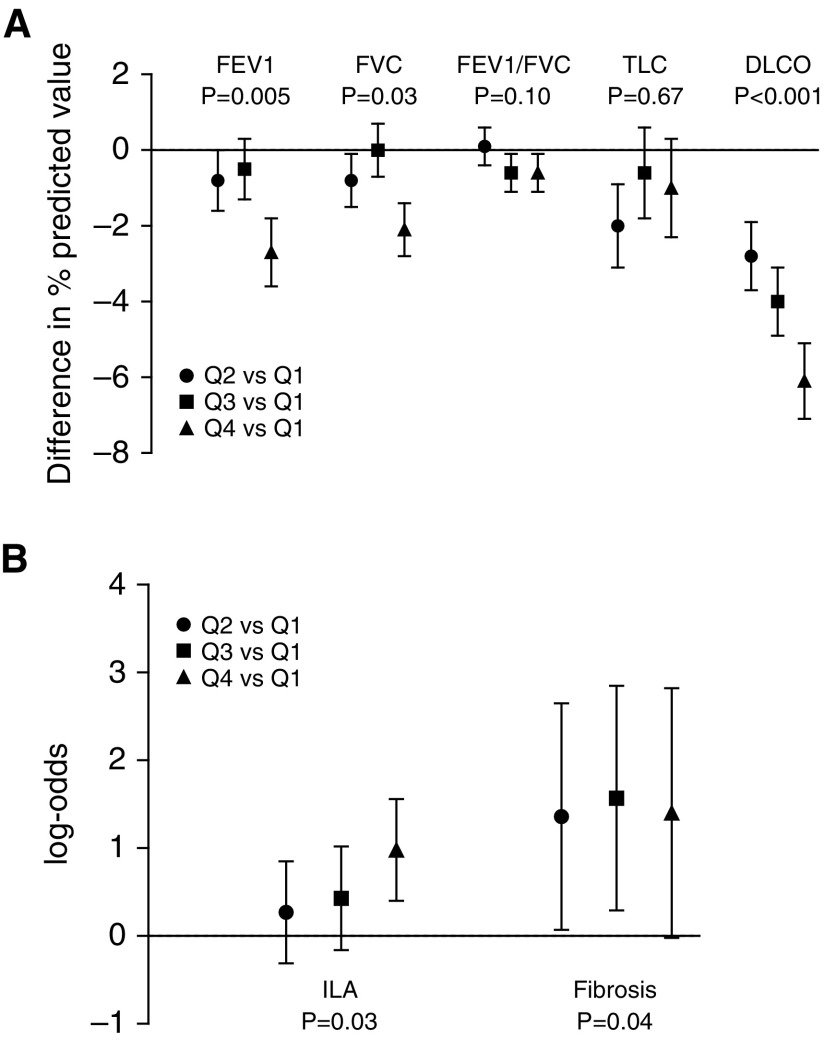
The association of higher Gal-3 with lower FEV1 and FVC remained significant after multivariable adjustment (P = 0.001 and P = 0.008, respectively) (Table 3). There was no significant association of Gal-3 with FEV1/FVC ratio in multivariable analyses (P = 0.08). In quartile-based analyses, participants in the highest Gal-3 quartile had a 2.7% lower percentage of predicted FEV1 and a 2.1% lower percentage of predicted FVC compared with those in the lowest Gal-3 quartile (P for trend = 0.005 and P for trend = 0.03 in multivariable-adjusted analyses, respectively) (Figure 1A; for details, see Table E1 in the online supplement).
Table 3.
Galectin-3 Is Associated with Measures of Pulmonary Structure and Function
| PFT Measures | Age, Sex, and Height Adjusted |
Multivariable Adjusted* |
||
|---|---|---|---|---|
| β Value (SE) | P Value | β Value (SE) | P Value | |
| FEV1, % predicted | −1.4 (0.3) | <0.001 | −1.0 (0.3) | 0.001 |
| FVC, % predicted | −1.2 (0.3) | <0.001 | −0.7 (0.3) | 0.008 |
| FEV1/FVC, % predicted | −0.2 (0.2) | 0.21 | −0.3 (0.2) | 0.07 |
| TLC, % predicted | −1.0 (0.5) | 0.04 | −0.7 (0.5) | 0.16 |
| DlCO, % predicted | −2.1 (0.4) | <0.001 | −2.2 (0.4) | <0.001 |
| Chest CT Measures | OR (95% CI) | P Value | OR (95% CI) | P Value |
|---|---|---|---|---|
| ILA | 1.57 (1.27–1.94) | <0.001 | 1.59 (1.28–1.97) | <0.001 |
| Fibrosis | 1.68 (1.14–2.45) | 0.008 | 1.65 (1.11–2.44) | 0.01 |
Definition of abbreviations: CI = confidence interval; CT = computed tomography; DlCO = diffusing capacity of the lung for carbon monoxide; ILA = interstitial lung abnormality; OR = odds ratio; PFT = pulmonary function test; TLC = total lung capacity.
β-Coefficient and odds ratio are change in pulmonary trait per 1 SD of log-transformed galectin-3.
Adjusted for age, sex, height, body mass index, current or former smoking status, pack-years of smoking, and diabetes.
Figure 1.
Association of galectin-3 (Gal-3) quartiles and lung function and structure. (A) Difference in spirometry, lung volume, and diffusing capacity of the lung for carbon monoxide (DlCO) measures between Gal-3 quartiles after adjustment for age, sex, and height. Error bars represent SE. (B) Log odds for interstitial lung abnormalities (ILAs) and definite fibrosis across Gal-3 quartiles, with Q1 serving as the referent. P values are for trend. Q = quartile; TLC = total lung capacity.
Higher Gal-3 concentrations were also associated with lower DlCO. Every 1- SD increase in log Gal-3 was associated with a 2.1% lower percentage of predicted DlCO in age- and sex-adjusted analyses (95% CI, 1.3–2.9%; P < 0.001). This association was not attenuated after multivariable adjustment (P < 0.001) (Table 3).
Higher Gal-3 Is Associated with ILA and Pulmonary Fibrosis Visualized by Chest CT
Of 1,148 participants who underwent chest CT, 11% had ILA and 47% had indeterminate scans. In primary analyses, indeterminate scans were grouped with normal scans. The mean Gal-3 concentration in those with ILA was 15.2 ± 4.2 ng/ml compared with 13.2 ± 3.9 ng/ml in those without ILA. The proportion of participants with ILA increased across Gal-3 quartiles, with 6% in Q1, 9% in Q2, 13% in Q3, and 20% in Q4 (Table 2). In age- and sex-adjusted analyses, each 1-SD increase in log Gal-3 was associated with a 57% increased odds of having ILA (OR, 1.57; 95% CI, 1.27–1.94; P < 0.001) (Table 3). The association of higher Gal-3 and ILA persisted after multivariable adjustment (OR, 1.59; 95% CI, 1.28–1.97; P < 0.001). In multivariable-adjusted analyses, those in the highest Gal-3 quartile had a 2.67-fold increased odds of having ILA compared with those in the lowest quartile (OR, 2.67; 95% CI, 1.49–4.76; P for trend = 0.001) (Figure 1B). After excluding indeterminate scans from the analysis, Gal-3 remained associated with ILA (multivariable-adjusted OR, 1.54 per 1-SD increase in log Gal-3; 95% CI, 1.17–2.01; P = 0.002).
Three percent of participants who underwent chest CT had definite fibrosis, with 1.0% in Q1, 3.5% in Q2, 5.2% in Q3, and 5.1% in Q4 (Table 1). Each 1-SD increase in log Gal-3 was associated with a 68% increased odds of having definite fibrosis in age- and sex-adjusted analyses (OR, 1.68; 95% CI, 1.14–2.45; P = 0.008) and remained significant after multivariable adjustment (OR, 1.65; 95% CI, 1.11–2.44; P = 0.01) (Table 3).
Secondary Analyses
In a secondary analysis, we examined the association of Gal-3 and pulmonary measures stratified by ever smokers (n = 1,671) and never smokers (n = 922) (Table E2). In multivariable-adjusted analyses, Gal-3 was associated with DlCO, ILA, and fibrosis in ever smokers (P < 0.05 for all). Among never smokers, Gal-3 was associated with FEV1, FVC, and DlCO, whereas the association with ILA and fibrosis was no longer significant, albeit in a modest sample (n = 400). We formally tested for the interaction of Gal-3 and smoking status and found no significant effect modification by smoking (P ≥ 0.05 for all).
In analyses in which we examined longitudinal changes in pulmonary measures over approximately 3 years, we did not find any significant association of Gal-3 concentrations and longitudinal changes in FEV1, FVC, or FEV1/FVC ratio (Table E3). In a post hoc power calculation using the software QUANTO (19) and assuming a two-sided α of 0.05, we had 80% power to detect an annual decline of 6 ml in FEV1 and FVC, an effect size representing approximately 15% of the annual decline in lung volume conferred by smoking. Last, risk ratios calculated for the association of Gal-3 and binary outcomes (ILA and fibrosis visualized by chest CT) were similar to ORs obtained in primary analyses (Table E4).
Discussion
Higher circulating Gal-3 concentrations are associated with ILAs, a restrictive pattern on pulmonary function testing, and reduced measures of gas exchange in participants in the FHS. Specifically, higher Gal-3 was associated with lower lung volumes, lower DlCO, and preserved FEV1/FVC ratio. Compared with the lowest quartile, participants in the highest Gal-3 quartile were more than twice as likely to have ILAs, even after accounting for potential clinical confounders.
Prior human studies of Gal-3 in pulmonary fibrosis are limited to case–control series in specific disease conditions, with mixed results. Gal-3 was elevated in lung biopsy and bronchoalveolar lavage fluid in 10 patients with biopsy-proven usual interstitial pneumonia, the pathologic hallmark of IPF, when compared with 10 healthy control subjects (7, 8). Moreover, circulating Gal-3 serum concentrations were higher in usual interstitial pneumonia, and increased during acute exacerbations in this study. Gal-3 in bronchoalveolar lavage fluid was increased in 8 patients with IPF and 17 patients with collagen vascular disease-associated interstitial pneumonia when compared with patients with other pulmonary conditions and healthy control subjects (8, 9). In contrast, Gal-3 was not elevated in bronchoalveolar lavage fluid among 19 patients with IPF compared with healthy control subjects in another study, though Gal-3 was elevated in 8 patients with pulmonary fibrosis associated with Hermansky–Pudlak syndrome, where it appeared to correlate with pulmonary disease severity (8, 10).
Although researchers in studies of Gal-3 and pulmonary fibrosis have reached differing conclusions, our study demonstrates that circulating Gal-3 concentrations are elevated in undiagnosed research participants with imaging abnormalities suggestive of an early stage of pulmonary fibrosis as well as reductions in physiologic measures suggesting the development of a restrictive lung deficit with reduced gas exchange. Notably, Gal-3 was measured more than 10 years before chest CT assessment, suggesting that high Gal-3 concentrations may precede radiographic abnormalities. While simultaneous CT measures were not available to confirm an association with true incident interstitial abnormalities, our findings nevertheless suggest that elevated serum biomarkers, similarly to genetic markers (9, 13), may be helpful in detecting early stages of pulmonary fibrosis before a disorder is apparent clinically.
Experimental studies suggest that Gal-3 plays a key role in mediating inflammation and fibrosis (1, 10). Gal-3 has previously been implicated in solid organ disease, including hepatic (2, 8), renal (3, 10), and cardiac (4, 9) fibrosis. Gal-3 is highly expressed in normal lung tissue and localizes to alveolar macrophages, bronchial epithelium, and lung fibroblasts (8, 11, 12, 20). Gal-3 expression is upregulated in the setting of lung injuries such as irradiation (13, 21), ozone exposure (14, 22), and cigarette smoke (15, 23). In animal models of pulmonary fibrosis, genetic disruption of Gal-3 resulted in attenuation of fibrosis in response to transforming growth factor-β1 compared with control subjects (8, 11, 13). This may in part be due to Gal-3’s effects on the alveolar epithelial-to-mesenchymal transition, a key step in fibrosis (24). These studies suggest that modulation of the Gal-3 pathway may be a potential therapeutic target in pulmonary fibrosis.
Pharmacologic inhibition of Gal-3 has been shown to improve cardiac remodeling and renal fibrosis in animal models (25, 26). In a bleomycin mouse model of pulmonary fibrosis, administration of TD139, a high-affinity inhibitor of the Gal-3 carbohydrate-binding domain, resulted in a marked reduction in fibrosis and blocked β-catenin translocation and phosphorylation in response to transforming growth factor-β1 (8). Inhaled TD139 (Galecto Biotech AB, Copenhagen, Denmark) is currently being tested in a phase I clinical trial (ClinicalTrials.gov identifier NCT02257177) (27) and recently was approved by the U.S. Food and Drug Administration as an investigational new drug with plans for a phase II clinical trial focused on patients with IPF (28). It is important to note that pulmonary effects of Gal-3 likely extend beyond fibrosis. Gal-3 is thought to activate pulmonary neutrophils (29), which in turn appear to play an integral role in IPF (30). Further, Gal-3 may have direct bacteriostatic effects in mouse models of Streptococcus pneumonia infection (31) and may also play a role in allergic asthma (32, 33). Future studies are needed to determine if Gal-3 inhibition can help to prevent the development or propagation of pulmonary fibrosis in humans.
Beyond serving as a potential therapeutic target, Gal-3 may represent a new biomarker associated with subclinical pulmonary fibrosis. The use of biomarkers for diagnosis or prognosis in IPF is currently experimental and has been identified as an important future area of research (34, 35). Prior studies have shown several biomarkers to predict prognosis, including Krebs von den Lungen 6 (KL-6) and surface protein A (36, 37). Our findings suggest that Gal-3 levels are elevated even at preclinical stages of pulmonary fibrosis. Future studies are needed to elucidate potential clinical applicability as a screening marker as well as prognostic value for pulmonary fibrosis.
There are several limitations of our study to consider. First, it is important to note that circulating Gal-3 concentrations are likely not specific to pulmonary fibrosis. We have previously shown that elevated Gal-3 predicts all-cause mortality, incident heart failure, atrial fibrillation, and chronic kidney disease in FHS participants (7, 38, 39). The association of Gal-3 and cause-specific mortality related to lung disease is unknown. Second, Gal-3 was measured more than 10 years before thoracic chest CT scans were obtained in the FHS participants. We also acknowledge limited power, particularly in testing the association of Gal-3 and fibrosis on the basis of chest CT. Specifically, Gal-3 was not associated with ILA or fibrosis among never smokers, although we did not detect significant effect modification by smoking status. We did not find an association of Gal-3 and longitudinal changes in lung function. This finding may be due to competing risk of death. It may also be that changes in lung function do not manifest until much later in the disease process. Therefore, while our findings suggest that elevated circulating Gal-3 concentrations may precede the development of the abnormal imaging findings, further longitudinal studies with repeated imaging and Gal-3 measurements, as well as experimental observations, are likely required to determine if elevated Gal-3 levels are an important cause or marker of pulmonary fibrosis.
It is important to note that the outcomes of research participants with ILA are not currently known. In our sample, none of the individuals with ILAs had known chronic lung disease; however, the incidence of IPF was not systematically adjudicated. Other studies have shown that ILAs are associated with abnormalities in lung function, gas exchange, exercise capacity, and progressive worsening of radiographic lung findings in follow-up (40). Thus, future longitudinal studies are required to determine if those with ILAs are at risk of developing a progressive and fatal lung disease (34).
In summary, our study shows that elevated Gal-3 concentrations are associated with imaging, spirometric, and gas exchange abnormalities. Participants in the upper Gal-3 quartile had a greater than twofold increased odds of having ILAs, coupled with decreased lung volumes, altered gas exchange, and greater odds of definite fibrosis. These findings suggest a potential role for Gal-3 in early stages of pulmonary fibrosis. Future studies are needed to elucidate underlying biological pathways and to further examine whether Gal-3 might represent a potential therapeutic target for pulmonary fibrosis, even in early stages of the disease.
Footnotes
This work was partially supported by the NHLBI’s Framingham Heart Study (contract numbers N01-HC-25195 and HHSN268201500001I, and National Institutes of Health [NIH] grant R01 HL111024); NIH grants K23-HL116780 (J.E.H.), 5K23-CA157631 (M.N.), R01 HL107246 (G.R.W.), R01 HL116473 (G.R.W.), R01 HL122464 (G.R.W.), P01 HL114501 (I.O.R. and G.M.H.), and R01 HL111024 (G.M.H.); and a Boston University School of Medicine, Department of Medicine Career Investment Award (J.E.H.). Galectin-3 assays were provided by BG Medicine (Waltham, MA).
Author Contributions: Conception and design: J.E.H., R.S., G.T.O’C., and G.M.H.; acquisition and analysis of data: J.E.H., W.G., D.L., T.A., I.O.R., H.H., J.C.L., M.N., J.D., G.R.W., G.T.O’C., and G.M.H.; interpretation of data: J.E.H., W.G., R.S., J.D., G.T.O’C., and G.M.H.; drafting, critical revisions, and final approval: all authors; and accountable for all aspects of the work: J.E.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201509-1753OC on January 18, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 2.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ, Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JPM, Schroen B, André S, Crijns HJGM, Gabius HJ, Maessen J, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 5.Lok DJ, Van Der Meer P, de la Porte PW, Lipsic E, Van Wijngaarden J, Hillege HL, van Veldhuisen DJ. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323–328. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijers WC, Januzzi JL, deFilippi C, Adourian AS, Shah SJ, van Veldhuisen DJ, de Boer RA. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: a pooled analysis of 3 clinical trials. Am Heart J. 2014;167:853–860.e4. doi: 10.1016/j.ahj.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, Simpson AJ, Forbes SJ, Hirani N, Gauldie J, et al. Regulation of transforming growth factor-β1–driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishi Y, Sano H, Kawashima T, Okada T, Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y, et al. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 10.Cullinane AR, Yeager C, Dorward H, Carmona-Rivera C, Wu HP, Moss J, O’Brien KJ, Nathan SD, Meyer KC, Rosas IO, et al. Dysregulation of galectin-3: implications for Hermansky-Pudlak syndrome pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;50:605–613. doi: 10.1165/rcmb.2013-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RSJ, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, et al. COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 15.Christenson RH, Duh SH, Wu AHB, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P. Multi-center determination of galectin-3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43:683–690. doi: 10.1016/j.clinbiochem.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee HY, Seo JB, Steele MP, Schwarz MI, Brown KK, Loyd JE, Talbert JL, Schwartz DA, Lynch DA. High-resolution CT scan findings in familial interstitial pneumonia do not conform to those of idiopathic interstitial pneumonia. Chest. 2012;142:1577–1583. doi: 10.1378/chest.11-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross JC, Estépar RSJ, Díaz A, Westin CF, Kikinis R, Silverman EK, Washko GR. Lung extraction, lobe segmentation and hierarchical region assessment for quantitative analysis on high resolution computed tomography images. Med Image Comput Comput Assist Interv. 2009;12:690–698. doi: 10.1007/978-3-642-04271-3_84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Gauderman WJ. Sample size requirements for matched case-control studies of gene–environment interaction. Stat Med. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Lee J, Hyun JW, Park JW, Joo HG, Shin T. Expression and immunohistochemical localization of galectin-3 in various mouse tissues. Cell Biol Int. 2007;31:655–662. doi: 10.1016/j.cellbi.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Kasper M, Hughes RC. Immunocytochemical evidence for a modulation of galectin 3 (Mac-2), a carbohydrate binding protein, in pulmonary fibrosis. J Pathol. 1996;179:309–316. doi: 10.1002/(SICI)1096-9896(199607)179:3<309::AID-PATH572>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Sunil VR, Francis M, Vayas KN, Cervelli JA, Choi H, Laskin JD, Laskin DL. Regulation of ozone-induced lung inflammation and injury by the β-galactoside-binding lectin galectin-3. Toxicol Appl Pharmacol. 2015;284:236–245. doi: 10.1016/j.taap.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebel S, Gerstmayer B, Kuhl P, Borlak J, Meurrens K, Müller T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicol Sci. 2006;93:422–431. doi: 10.1093/toxsci/kfl071. [DOI] [PubMed] [Google Scholar]
- 24.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu L, Ruifrok WPT, Meissner M, Bos EM, van Goor H, Sanjabi B, van der Harst P, Pitt B, Goldstein IJ, Koerts JA, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 26.Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6:e18683. doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garber K. Galecto Biotech. Nat Biotechnol. 2013;31:481. doi: 10.1038/nbt0613-481. [Published erratum appears in Nat Biotechnol 2013;31:773.] [DOI] [PubMed] [Google Scholar]
- 28.FDA approves an IND from Galecto Biotech for TD139 treatment of IPF patients. Phase Ib/IIa trial to start in the UK. Galecto Biotech; 2015 March 1 [accessed 2015 Jul 23]. Available from: http:// www.galecto.com
- 29.Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, Hayashi K, Simpson AJ, Rossi AG, Haslett C, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch JP, III, Standiford TJ, Rolfe MW, Kunkel SL, Strieter RM. Neutrophilic alveolitis in idiopathic pulmonary fibrosis: the role of interleukin-8. Am Rev Respir Dis. 1992;145:1433–1439. doi: 10.1164/ajrccm/145.6.1433. [DOI] [PubMed] [Google Scholar]
- 31.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 32.del Pozo V, Rojo M, Rubio ML, Cortegano I, Cárdaba B, Gallardo S, Ortega M, Civantos E, López E, Martín-Mosquero C, et al. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through interleukin-5 gene downregulation. Am J Respir Crit Care Med. 2002;166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]
- 33.López E, Zafra MP, Sastre B, Gámez C, Lahoz C, del Pozo V. Gene expression profiling in lungs of chronic asthmatic mice treated with galectin-3: downregulation of inflammatory and regulatory genes. Mediators Inflamm. 2011;2011:823279. doi: 10.1155/2011/823279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier J-F, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle TJ, Pinto-Plata V, Morse D, Celli BR, Rosas IO. The expanding role of biomarkers in the assessment of smoking-related parenchymal lung diseases. Chest. 2012;142:1027–1034. doi: 10.1378/chest.12-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 37.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest. 2009;135:1557–1563. doi: 10.1378/chest.08-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, McManus DD, Lubitz SA, Larson MG, Benjamin EJ. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014;167:729–734.e1. doi: 10.1016/j.ahj.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol. 2013;24:1470–1477. doi: 10.1681/ASN.2012090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189:770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]