Abstract
Rationale: Noncommunicable diseases are major causes of morbidity and mortality in sub-Saharan Africa (sSA). Valid burden of disease estimates are lacking for noncommunicable lung disease in sSA.
Objectives: We performed a community-based survey to determine the prevalence of chronic lung disease among adults 18 years or older in Malawi, using American Thoracic Society standard spirometry, internationally validated respiratory symptom and exposure questionnaires, and an assessment of HIV status.
Methods: An age- and sex-stratified random sample of 2,000 adults was taken from the population of the Chilomoni district of Blantyre, Malawi. Fieldworkers collected questionnaire data, conducted HIV testing, and performed pre- and post-bronchodilator spirometry on eligible participants. Survey-weighted population prevalence estimates of respiratory symptoms and spirometric abnormalities were computed, and bivariate and multivariable regression were used to identify associated variables.
Measurements and Main Results: Questionnaire data, HIV status, and standard spirometry were obtained from 1,059, 933, and 749 participants, respectively. Current respiratory symptoms, exposure to biomass, and ever-smoking were reported by 11.8, 85.2, and 10.4% of participants, respectively. HIV prevalence was 24.2%. Moderate to severe airway obstruction was seen in 3.6%. The prevalence of spirometric restriction was 38.6% using National Health and Nutrition Examination Survey III reference ranges and 9.0% using local reference ranges. Age was positively associated with obstruction, whereas low body mass index was associated with restriction.
Conclusions: More than 40% of the Malawian adults in our urban population sample had abnormal lung function (mostly restrictive) in the context of widespread exposure to biomass smoke and a high prevalence of HIV. These findings potentially have major public health implications for Malawi and the broader sSA region.
Keywords: noncommunicable disease, COPD, lung function, sub-Saharan Africa, biomass
At a Glance Commentary
Scientific Knowledge on the Subject
Although noncommunicable lung disease is believed to be an important determinant of morbidity and mortality in sub-Saharan Africa, valid burden of disease estimates are lacking.
What This Study Adds to the Field
We found that more than 40% of the Malawian adults in our urban population sample had abnormal lung function (mostly spirometric restriction) in the context of widespread exposure to biomass smoke and high HIV prevalence.
Noncommunicable diseases (NCDs) pose a major health and development challenge to low- and middle-income countries (LMICS) in the 21st century; 28 million NCD-related adult deaths occurred in LMICS in 2012, and cumulative economic losses of US$7 trillion due to NCDs are forecast for these regions by 2025 if better control and prevention measures are not instituted (1). The need for prioritization of NCDs was recognized in the World Health Organization NCD Global Action Plan, adopted in 2013 (2).
Chronic respiratory diseases are the fourth leading cause of NCD deaths globally, and burden of disease estimates place chronic obstructive pulmonary disease (COPD) as the 12th most common cause of years of life lost globally (3). Despite the likely high burden of chronic respiratory disease, there are limited data on its prevalence, natural history, and associated morbidity and mortality in LMICS (4). Information on chronic respiratory disease in sub-Saharan Africa (sSA) is especially scarce (5). Existing information suggests the burden in this setting may be high: The international BOLD (Burden of Obstructive Lung Disease) study (www.boldstudy.org) demonstrated a considerably higher prevalence of moderate to severe airway obstruction among adults aged 40 years or older in Cape Town, South Africa (19.1%) compared with that seen in Western Europe and North American settings (5.9–14.3%) (6). A higher prevalence of chronic respiratory disease in sSA is biologically plausible based on the intersection of several acknowledged risk factors for respiratory pathology in these settings, including poverty-related in utero and early childhood exposures, biomass fuel exposure, a rapidly increasing prevalence of smoking, lung damage caused by pulmonary tuberculosis (TB), and chronic HIV infection (1, 7–11). In addition, an association between restrictive lung diseases or low respiratory volumes and mortality has been observed in ecological and prospective cohort studies (12, 13).
We report the results of a community-based prevalence study of respiratory disease conducted according to BOLD study standards among adults 18 years or older in Blantyre, Malawi. The study includes information on respiratory symptoms, exposures, HIV status, and spirometry. Our aim was to define the burden of respiratory symptoms and patterns of abnormal spirometry in this setting, and to explore their risk factors.
Methods
An age- (18–39 and ≥40 yr) and sex-stratified population-representative sample of 2,000 adults was taken from an enumerated population of Chilomoni district of urban Blantyre, Malawi (14). Fieldworkers conducted home visits to assess eligibility and to seek informed consent between February 2013 and August 2014. Individuals were excluded if they were not permanent residents of the area, were pregnant, or were acutely unwell. Up to three repeat visits were conducted to locate initially absent residents (see the eMethods section in the online data supplement).
Standardized BOLD questionnaires about respiratory symptoms and exposures were administered in the local language, Chichewa (15). Pre- and post-bronchodilator spirometry was performed according to American Thoracic Society (ATS) standards using the ndd EasyOne Spirometer (ndd Medical Technologies; Zurich, Switzerland) (16). Anthropometric measurements and a blood sample for HIV and hematology assays were taken. Participants were given the results of the clinical observations and spirometry measurements at the point of testing. HIV test results were communicated to participants who wished to know their results. Minimal questionnaire data were collected from patients who declined to participate in the full study. Quality assurance of questionnaire and spirometry data was provided by the central BOLD coordinating center.
A target sample size of 1,200 adults completing the study, split equally between men and women, and those aged 18 to 39 years and 40 years or older, was chosen to allow stratified prevalence estimates of spirometric abnormalities with acceptable precision, in accordance with the BOLD protocol.
Participants who completed full and minimal questionnaires and adequate or inadequate spirometry were compared, with an assessment for selection bias using the chi-square or Student’s t test. To allow comparison with data from other BOLD study sites, age- and sex-stratified prevalence estimates of spirometric abnormalities (Table 1) were reported using reference ranges derived for white subjects from NHANES III (Third National Health and Nutrition Examination Survey, 1988–1994) and local reference ranges derived within this study from the spirometry of nonsmoking adults with no history of respiratory disease or symptoms (15).
Table 1.
Spirometric Definitions (34)
| Finding | Spirometric Definition |
|---|---|
| Post-bronchodilator obstruction | FEV1/FVC ratio <0.7 |
| Post-bronchodilator moderate to severe obstruction | FEV1/FVC ratio <0.7 and FEV1 <80% predicted* |
| Spirometric restriction | FEV1/FVC ratio >0.7 and FVC <80% predicted* |
| Airway reversibility | FEV1 increase ≥200 ml and ≥12% following bronchodilator |
Age-, sex-, and height-standardized predicted values obtained from National Health and Nutrition Examination Survey III or local reference ranges derived from spirometry results of healthy, never-smoking Malawian adults.
Bivariate associations between spirometric abnormalities and exposure variables including age, sex, participant education, HIV status, self-reported previous TB, hemoglobin, eosinophilia, body mass index (BMI), smoking status, smoking pack-year exposure, indoor biomass exposure, and occupational exposures were examined. Home ownership and household water and sanitation were used as proxy markers of socioeconomic status (SES). No adjustments were made for multiple tests in exploratory analyses. Multivariable logistic regression models were constructed, including age and sex a priori, and variables with a P value <0.2 on bivariate analysis. A manual forward stepwise regression technique was used to develop multivariable models. Missing data were imputed using simple univariate procedures, and sensitivity analyses were used to compare results with complete case analyses. Prevalences of respiratory symptoms were described, and regression analyses were used to identify associated variables. The associations between abnormal spirometry and respiratory symptoms were described. Analyses were conducted using STATA (Stata Statistical Software: release 13; StataCorp LP, College Station, TX). Survey weighting was used to calculate population representative prevalence estimates and develop regression models using the Svy package in Stata (15).
Ethical approval was given by the National Research Ethics Committee of Malawi and the Liverpool School of Tropical Medicine Research Ethics Committee (Protocol 12.08).
Results
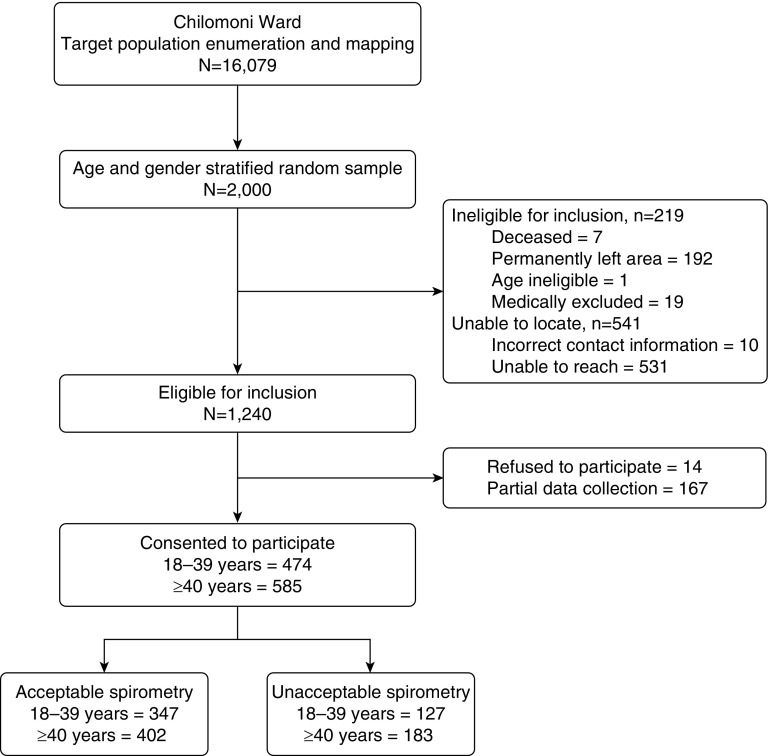
Of the 2,000 randomly selected adults, 1,469 (73.5%) were located by fieldworkers, and 1,240 (62.0%) were eligible for inclusion. Of eligible adults, 85.4% (1,059 of 1,240) consented to participate and completed the full BOLD questionnaire, with 70.7% (749 of 1,059) performing ATS standard spirometry (Figure 1).
Figure 1.
Participant recruitment flow diagram.
Participant Characteristics
Mean participant age was 41.9 years (SD, 15.3), and 57.9% were female. Overall, 37.4% were educated to primary school level only. Although 59.3% of participants were from households that owned their own home, only 25.1% had access to flush toilets, and 46.6% had a private indoor or outdoor water supply (Table 2). Eligible individuals who declined to participate in the study but who provided minimal data were more likely to be men (56.3% vs. 43.7%) and current smokers (9.0% vs. 4.3%) than those who contributed full data (see Table E1 in the online supplement).
Table 2.
Characteristics of Participants Completing Full BOLD Core Questionnaire, Including Those with and without ATS Standard Spirometry
| Variable (n) | Number (%)* |
|---|---|
| Age group, yr (n = 1,058) | |
| 18–29 | 283 (26.8) |
| 30–39 | 190 (18.0) |
| 40–49 | 253 (23.9) |
| 50–59 | 182 (17.2) |
| 60–69 | 98 (9.3) |
| ≥ 70 | 52 (4.9) |
| Sex (n = 1059) | |
| Male | 446 (42.1) |
| Female | 613 (57.9) |
| Level of education (n = 1,054) | |
| None | 59 (5.6) |
| Primary | 394 (37.4) |
| Middle | 391 (37.1) |
| High school or college | 210 (19.9) |
| Years of education, mean (SD) (n = 1,057) | 9.29 (4.35) |
| HIV status (n = 933) | |
| Negative | 707 (75.8) |
| Positive | 226 (24.2) |
| Self-reported previous TB (n = 1,057) | |
| No | 1026 (97.1) |
| Yes | 31 (2.9) |
| Hemoglobin, g/dl, mean (SD) (n = 936) | 13.9 (1.80) |
| Eosinophil blood count >2% (n = 927) | |
| No | 291 (31.4) |
| Yes | 636 (68.6) |
| BMI group, kg/m2 (n = 972) | |
| Underweight (BMI < 18.5) | 77 (7.9) |
| Normal (18.5 ≤ BMI < 25) | 568 (58.4) |
| Overweight (25 ≤ BMI < 30) | 202 (20.8) |
| Obese (BMI ≥ 30) | 125 (12.9) |
| Home ownership (n = 1,057) | |
| Yes | 627 (59.3) |
| No | 430 (40.7) |
| Access to private water supply (indoor or outdoor tap) (n = 1,057) | |
| Yes | 492 (46.6) |
| No | 565 (53.4) |
| Household has flush toilet (n = 1,057) | |
| Yes | 265 (25.1) |
| No | 792 (74.9) |
| Smoking status (n = 1,057) | |
| Ever | 110 (10.4) |
| Never | 947 (89.6) |
| Pack-years of smoking (n = 1,057) | |
| 0 | 947 (89.6) |
| >0 and <10 | 89 (8.4) |
| ≥10 | 21 (2.0) |
| Biomass exposure† (n = 1,057) | |
| No | 157 (14.8) |
| Yes | 900 (85.2) |
| Working in farming >3 mo (n = 1,056) | |
| No | 748 (70.8) |
| Yes | 308 (29.2) |
Definition of abbreviations: ATS = American Thoracic Society; BMI = body mass index; BOLD = Burden of Obstructive Lung Disease; TB = tuberculosis.
Unless otherwise indicated.
Use of charcoal, coal, or coke, or burning of wood, dung, or crop residue for more than 6 months for cooking, or burning of wood, dung, or crop residue for heating water.
Respiratory Exposures
Smoking exposure was more frequently reported in men; 9.2% of men and 0.7% of women were current smokers, and 12.8% of men and 1.3% of women were ex-smokers (Table 2). Of those who smoked, 80.9% reported less than 10 pack-years of exposure. Self-reported biomass exposure was more common than cigarette smoking: 85.2% reported use of a biomass fuel (mostly charcoal) for cooking on an open fire for ≥6 months, and 31.9% reported using an open fire burning wood, dung, or crop residues for heating water. Farming was the reported occupation of 29.2% of participants.
Comorbidities
A total of 7.9% of the population had a low BMI (<18.5 kg/m2); 20.8% were overweight (BMI 25–30 kg/m2), and 12.9% were obese (BMI >30 kg/m2). Only 2.9% reported previous TB.
Blood Results
Data on HIV status were available for 88.1% of those who completed the core questionnaire, of whom 24.2% were infected with HIV. Hemoglobin levels were normally distributed with a mean of 13.9 g/dl (SD, 1.8) and a range of 6.7 to 21.4 g/dl. The median white cell count was 5.0 (interquartile range [IQR], 4.1–5.9); 68.6% of participants had blood eosinophil counts exceeding 2.0% of the total white cell count.
Respiratory Symptoms
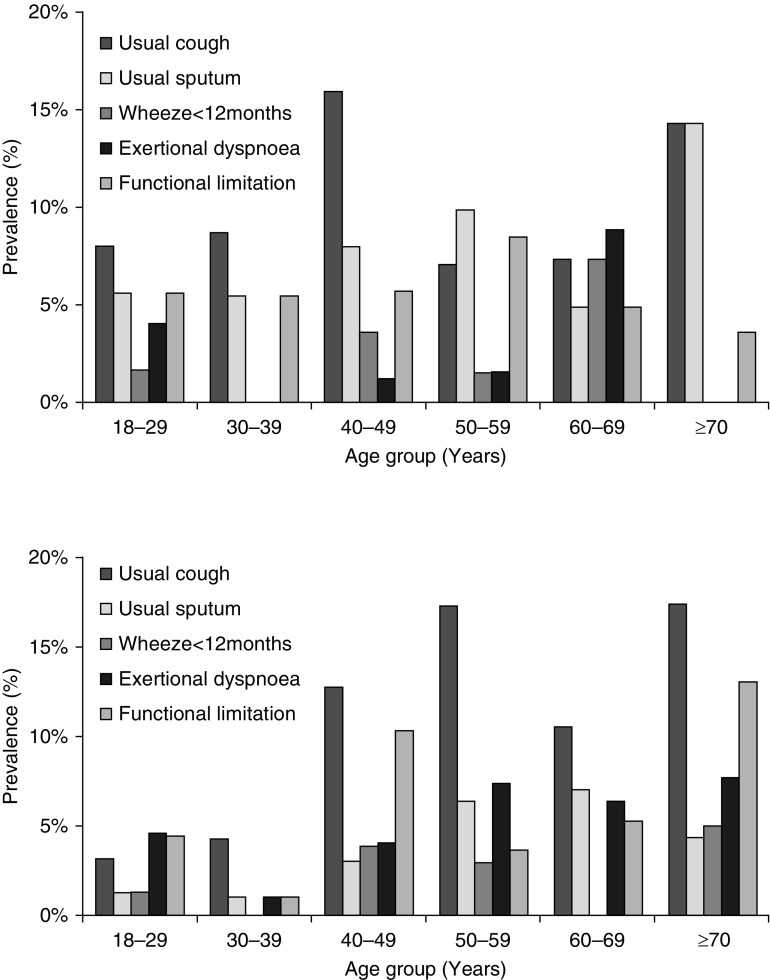
Overall, 11.8% (SE, 1.2) of participants had at least one respiratory symptom, and 5.0% (SE, 0.8) reported respiratory problems interfering with their daily activities. Cough was reported by 7.5% (SE, 0.9) of participants, but chronic cough (cough present on most days for ≥3 mo/yr) was reported by only 0.5% (SE, 0.2). Only 0.2% (SE, 0.2) of all adults reported chronic sputum production (phlegm on most days for ≥3 mo/yr). Breathlessness was described by 3.6% (SE, 0.7) of participants; 21.2% (SE, 8.5) of this group had severe functional impairment and reported stopping for breath after walking 100 yards on a flat surface (modified Medical Research Council breathlessness score = 3). Wheeze within the past year in the absence of an upper respiratory tract infection was reported by 1.4% (SE, 0.4) (Figure 2; see Table E2).
Figure 2.
Age- and sex-stratified prevalence of symptoms among study participants. Upper panel depicts symptom prevalence among men; lower panel depicts symptom prevalence among women. The questions asked were as follows: Do you usually have a cough when you don’t have a cold? (n = 1,056); Do you usually bring up phlegm from your chest? (n = 1,056); Have you had wheezing/whistling in your chest at any point in in the past 12 months, in the absence of a cold? (n = 1,007); Do you have shortness of breath when hurrying on the level or walking up a slight hill? (n = 970); and Have breathing problems interfered with your usual daily activities? (n = 1,056).
Spirometry
Three factors were statistically significantly associated with completion of ATS standard spirometry: lower median age (41.0 yr; IQR, 28–51 yr vs. 44.0 yr; IQR, 31–56 yr), higher mean hemoglobin (14.07 [SD, 1.80] vs. 13.62 [SD, 1.76]), and a greater average number of years of education (9.47 [SD, 4.20] vs. 8.85 [SD, 4.65]). No other statistically significant differences were identified at the α = 0.05 level between groups who did and did not complete spirometry (see Table E3).
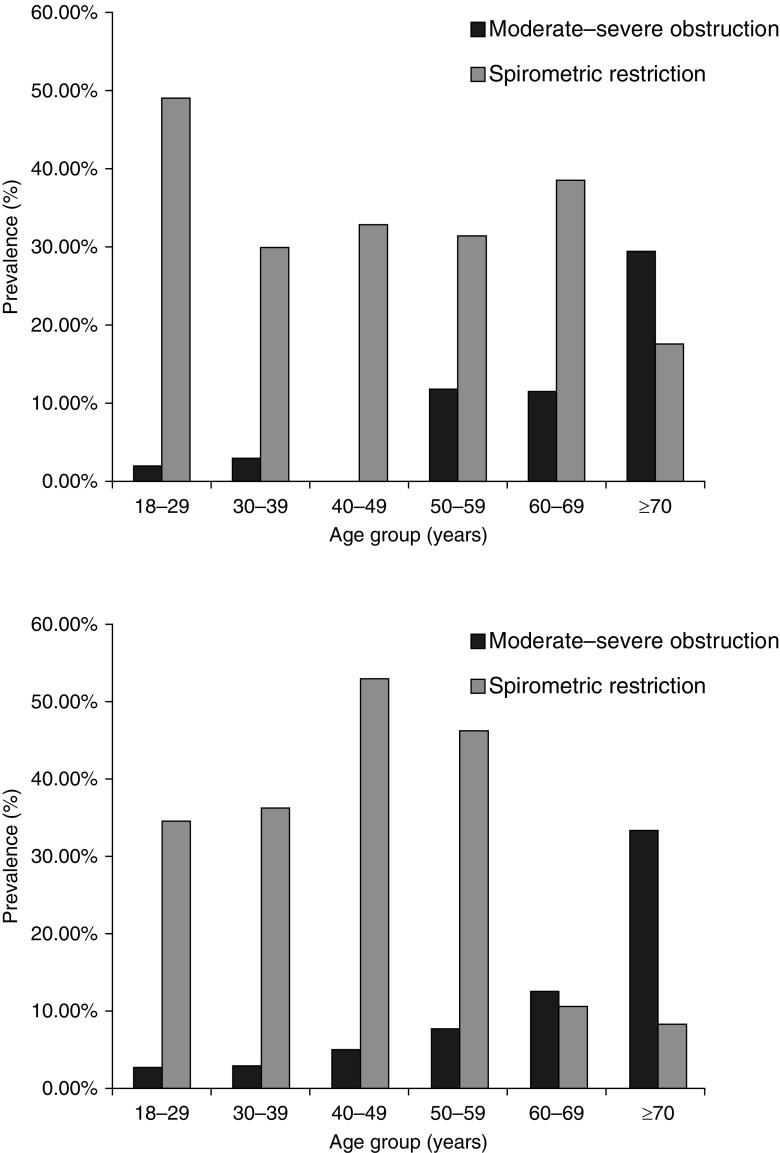
A total of 4.3% (SE, 1.1) of men and 4.1% (SE, 1.1) of women had post-bronchodilator obstruction (FEV1/FVC <0.7) on spirometry. A high proportion of this was at least moderate in severity; 3.2% (SE, 1.0) of men and 3.9% (SE, 1.0) of women had an FEV1 <80% predicted using the NHANES reference ranges, and 2.0% (SE, 0.7) of men and 2.7% (SE, 0.9) of women using local reference ranges (Figure 3; see Table E4). Any obstruction and moderate to severe obstruction were seen in 2.9% (SE, 0.9) and 2.6% (SE, 0.9) of 18 to 39 year olds, and in 9.0% (SE, 1.4) and 7.0% (SE, 1.2) of those aged 40 years or older.
Figure 3.
Age- and sex-stratified prevalence of moderate to severe airway obstruction (FEV1/FVC <0.7 and FEV1 <80% predicted) and spirometric restriction (FEV1/FV >0.7 and FEV1 <80%), defined using National Health and Nutrition Examination Survey reference ranges, among those completing American Thoracic Society standard spirometry (n = 749). Upper panel depicts the prevalence of spirometric abnormalities among men; lower panel depicts the prevalence of spirometric abnormalities among women.
Spirometric restriction was more common than obstruction in both genders and across age-group strata. The estimated prevalence was considerably higher when NHANES reference ranges were used (38.6%; SE, 2.1) compared with locally derived reference ranges (9.0%; SE, 1.2). Airway reversibility was present in 4.2% (0.8) of the cohort, but only 17.3% (SE, 7.7) of those with reversibility had airway obstruction after bronchodilator use.
Factors Associated with Respiratory Symptoms
Ever-smoking was positively associated with both cough and sputum production in bivariate analysis (see Table E5), and in the multivariable analysis, the odds of usual cough were 2.37 higher in current smokers versus never-smokers (95% confidence interval [CI], 1.12–5.02) (Table 3). The odds of regular sputum expectoration were 7.05 times higher (95% CI, 2.28–21.80) in those with previous biomass exposure compared with those without. Employment in farming was positively associated with all symptoms in the bivariate analyses, and the odds of exertional breathlessness remained significantly higher (odds ratio [OR], 6.32; 95% CI, 1.87–21.32) in those employed in farming for more than 3 months compared with nonfarmers in the multivariable analysis.
Table 3.
Multivariable Associations with Respiratory Symptoms* in All Age Groups (n = 1,056)
| Variable | Usual Cough (n = 103) |
Usual Sputum (n = 51) |
Exertional Dyspnea (n = 35) |
Wheeze (n = 21) |
||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age group, yr | ||||||||
| 18–29 | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| 30–39 | 0.97 | 0.44–2.17 | 1.12 | 0.38–3.12 | 0.09 | 0.01–0.80† | Empty | — |
| 40–49 | 2.37 | 1.19–4.71† | 1.97 | 0.75–5.16 | 0.28 | 0.08–1.03 | 1.97 | 0.48–8.06 |
| 50–59 | 1.82 | 0.87–3.81 | 3.64 | 1.43–9.26† | 0.40 | 0.11–1.42 | 0.99 | 0.17–5.73 |
| ≥60 | 1.81 | 0.85–3.86 | 2.32 | 0.82–6.55 | 0.56 | 0.14–2.17 | 1.39 | 0.24–7.93 |
| Sex | ||||||||
| Male | 1.0 | — | 1.0 | — | 1.0 | — | ||
| Female | 0.72 | 0.41–1.27 | 0.31 | 0.15–0.65† | 1.78 | 0.68–4.68 | 0.41 | 0.13–1.27 |
| Smoking status | ||||||||
| Never | 1.0 | — | ||||||
| Ever | 2.37 | 1.12–5.02† | ||||||
| HIV status‡ | ||||||||
| Negative | 1.0 | — | ||||||
| Positive | 1.94 | 1.10–3.44† | ||||||
| Hemoglobin‡, g/dl | 0.74 | 0.58–0.94† | ||||||
| Working in farming >3 mo | ||||||||
| No | 1.0 | — | ||||||
| Yes | 6.32 | 1.87–21.32† | ||||||
| Any biomass exposure§ | ||||||||
| No | 1.0 | — | ||||||
| Yes | 7.05 | 2.28–21.80† | ||||||
| BMI, kg/m2 | ||||||||
| Underweight (BMI < 18.5) | 3.26 | 1.32–8.07† | 5.38 | 1.23–23.50† | ||||
| Normal (18.5 ≤ BMI < 25) | 1.0 | — | 1.0 | — | ||||
| Overweight (25 ≤ BMI < 30) | 0.37 | 0.15–0.91† | 1.16 | 0.26–5.16 | ||||
| Obese (BMI ≥ 30) | 1.15 | 0.31–4.30 | 5.47 | 0.93–32.38 | ||||
| Household has flush toilet | ||||||||
| Yes | 1.0 | — | ||||||
| No | 0.42 | 0.19–0.91† | ||||||
Definition of abbreviations: BMI = body mass index; BOLD = Burden of Obstructive Lung Disease; CI = confidence interval.
Presence of respiratory symptoms were ascertained using the following questions, derived from the BOLD study core questionnaire: Usual cough: Do you usually cough when you don’t have a cold? Usual sputum: Do you usually bring up phlegm from your chest, or do you usually have phlegm in your chest that is difficult to bring up, when you don’t have a cold? Exertional dyspnea: Are you troubled by breathlessness when hurrying on the level or walking up a slight hill? Wheeze: Have you had wheeze/whistling in your chest at any time in the past 12 months? In the last 12 months have you had this wheeze or whistling only when you have had a cold? (exclude if yes to the latter question).
P < 0.05.
Imputation of data required for 11.9% of HIV values, and 11.8% of hemoglobin values. Imputation of all other values ≤0.1%.
Use of charcoal, coal, or coke, or burning of wood, dung, or crop residue for more than 6 months for cooking, or burning of wood, dung, or crop residue for heating water.
Women were less likely to report cough or sputum production in the bivariate analyses, and their odds of sputum production remained lower (OR, 0.31; 95% CI, 0.15–0.65) than men in the multivariable analysis. Low BMI was positively associated with wheeze and sputum production in the bivariate analysis, and the odds of usual sputum production remained 3.26 (95% CI, 1.32–8.07) times higher in those with a BMI of less than 18.5 kg/m2 compared with normal weight in the multivariable model. One-third of those with cough were HIV-infected, and a positive association was seen between cough and HIV infection (OR, 1.94; 95% CI 1.10–3.44). HIV infection was not associated with other respiratory symptoms. Self-reported previous TB was not statistically associated with any symptoms. Moderate to severe obstruction, restriction, or reversibility, defined using the NHANES III reference ranges, were not associated with respiratory symptoms (see Table E6).
Factors Associated with Post-bronchodilator Airway Obstruction
Participant age was the factor most clearly associated with airway obstruction in bivariate and multivariable analyses, increasing in a nonlinear fashion with 10.12 times higher odds of moderate to severe obstruction (95% CI, 3.54–28.93) in those aged 60 years or older compared with 18- to 29-year-olds (Table 4; see Tables E7 and E8). This finding was also seen in the sensitivity analysis restricted to complete-case data. No association was seen between HIV infection or biomass exposure and airway obstruction. Individuals without access to private water supply had a 2.44 times higher risk of moderate to severe obstruction in the multivariable analysis (95% CI, 1.01–5.88).
Table 4.
Multivariable Associations of Risk Factors with National Health and Nutrition Examination Survey Defined Moderate to Severe Post-bronchodilator Airway Obstruction (FEV1/FVC ratio <0.7 and FEV1 < 80% predicted) (n = 749)
| Variable | Multivariable Association* |
|
|---|---|---|
| Odds Ratio | 95% CI | |
| Age group, yr | ||
| 18–29 | 1.0 | — |
| 30–39 | 1.38 | 0.36–5.25 |
| 40–49 | 0.93 | 0.27–3.15 |
| 50–59 | 4.66 | 1.58–13.79† |
| ≥60 | 10.12 | 3.54–28.93† |
| Sex | ||
| Male | 1.0 | — |
| Female | 1.34 | 0.57–3.17 |
| Access to private water supply (indoor or outdoor tap) | ||
| Yes | 1.0 | — |
| No | 2.44 | 1.01–5.88† |
Definition of abbreviation: CI = confidence interval.
Multivariable model developed using variables correlated at P < 0.2 level in the bivariate analysis, and age group and sex as a priori risk factors, with imputation for missing data.
P < 0.05.
Factors Associated with Spirometric Restriction
BMI was strongly associated with restrictive deficit defined using NHANES criteria: Those who were underweight had 4.09 (95% CI, 2.04–8.22) times higher odds of restriction compared with those with normal weight in the multivariable model. Older age was negatively associated with restriction, and those who were older than 60 years had 0.53 (95% CI, 0.29–0.96) times the odds of restriction of those in the 18- to 29-year-old group. No other risk factors for restrictive deficit were identified in the multivariable model (Table 5; see Table E9).
Table 5.
Multivariable Associations of Risk Factors with National Health and Nutrition Examination Survey Defined Spirometric Restriction (FEV1/FVC ratio >0.7 and FVC >80% predicted) (n = 756)
| Variable | Multivariable Associations* |
|
|---|---|---|
| Odds Ratio | 95% CI | |
| Age group, yr | ||
| 18–29 | 1.0 | — |
| 30–39 | 0.64 | 0.40–1.02 |
| 40–49 | 1.00 | 0.66–1.53 |
| 50–59 | 0.85 | 0.52–1.38 |
| ≥60 | 0.53 | 0.29–0.96† |
| Sex | ||
| Male | 1.0 | — |
| Female | 0.95 | 0.65–1.40 |
| BMI, kg/m2 | ||
| Underweight (BMI < 18.5) | 4.09 | 2.04–8.22† |
| Normal (18.5 ≤ BMI < 25) | 1.0 | — |
| Overweight (25 ≤ BMI < 30) | 1.07 | 0.66–1.73 |
| Obese (BMI ≥ 30) | 1.58 | 0.85–2.96 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval.
Multivariable model developed using variables correlated at P < 0.2 level in bivariate analysis, and age group and sex as a priori risk factors, with imputation for missing data.
P < 0.05.
When spirometric restriction was defined using local reference ranges (see Table E10), no clear trend in the relationship between age and restriction emerged. The odds of restriction were higher, if underweight (OR, 2.80; 95%, CI 1.19–6.55) in the bivariate analysis only. In the multivariable analysis, previous TB was associated with higher odds of restriction (OR, 3.01; 95% CI, 1.07–8.50).
Discussion
We conducted a population-based, cross-sectional study of the prevalence of and risk factors for noncommunicable respiratory disease conducted to BOLD standards among adults aged 18 years or older in Malawi. We found a high burden of chronic respiratory symptoms and abnormal spirometry in the population, with an especially high burden of spirometric restriction, and large differences in the estimated prevalence of restriction using NHANES and locally defined reference ranges.
Abnormal spirometry was defined in relation to age-, height-, and sex-standardized predicted values. Two different reference ranges were used in this analysis: the NHANES reference range is drawn from a healthy white population in the United States and is believed to represent the best possible lung function in the absence of any known detrimental respiratory exposures; and the locally derived reference range reflects the spirometry of nonsmoking, symptom-free Malawian adults within this study. Although values may be ethnically more appropriate in the latter, they are also constrained by common exposures within in this setting (17). The marked difference in the burden of restriction as defined using NHANES (38.6%) versus reference ranges determined from our study population (9.0%) suggests that on average, Malawian adults have smaller lungs than U.S. white populations. This finding is consistent with previous studies that demonstrated lower FVC ranges in populations from resource-poor settings (12).
The cause of this difference is unclear. The role of ethnicity must be considered, as body frame differences related to ethnicity are known to affect lung function (18). However, it has been suggested that in utero and early childhood exposures, including suboptimal in utero conditions, low birth weight, respiratory tract infections, and nutritional deficiencies can predict adult lung health and are more prevalent in resource poor settings (7, 19, 20). Although the use of height standardized spirometry reference ranges may have “controlled” for stunting related to childhood nutritional deficits, a low BMI was strongly associated with NHANES-defined spirometric restriction. This is consistent with an ongoing nutritional influence on FVC, and the finding of small lungs in adults may be explained by poor nutrition causing impaired lung growth at vulnerable stages of lung development. Interest in the link between acute and chronic malnutrition and long-term disability is growing (21), and prospective studies into the effect on lung function in later life would fit well within this agenda.
The morbidity and mortality associated with the lower FVC that we identified in the Malawian setting remain poorly described. Spirometric restriction has been shown to be associated with increased mortality, even in the absence of respiratory symptoms and a diagnosis of respiratory disease, in both the U.S. setting and ecological studies (12, 13). The negative correlation seen here between spirometric restriction and age may represent a birth cohort effect, whereby the incidence of restriction is decreasing over time, but it would also fit with earlier mortality among those with restriction. Prospective studies are required to investigate the prognostic implications of restriction in sSA settings, and to determine the public health implications of our findings.
Cough was the most common respiratory symptom reported by participants. The association with HIV raises the possibility that undiagnosed pulmonary TB may be responsible for part of this presentation (22–26). However, cough was less prevalent among younger age groups in whom the TB incidence would be expected to be highest. In addition, the association of chronic cough with ever-smoking and the strong association between sputum production and biomass smoke exposure also suggest that bronchial irritation from smoke inhalation with chronic bronchitis may underlie these symptoms. Of note, no association was seen between spirometric results and respiratory symptoms. Further investigation of the relationship among structural lung pathology, abnormal airway physiology, and respiratory symptoms in this setting is required.
No association was seen in our data between moderate to severe chronic airflow obstruction and exposure to tobacco or biomass, although both are recognized risk factors for obstruction (27–30). The absence of a relationship between biomass exposure and airway obstruction may be explained by limited study power related to the low prevalence of moderate to severe obstruction (3.6%), or misclassification of self-reported rather than objectively quantified biomass exposure, but it is consistent with findings of a recent population-based study in Nigeria (31, 32). In the case of tobacco, it may also reflect a low intensity of exposure in this setting, which was insufficient to produce significant levels of clinically important COPD; smoking prevalence was limited and pack-year exposure was low. This is in contrast to the findings of the BOLD study conducted in Cape Town, South Africa, in which the prevalence of moderate to severe airway obstruction among adults aged 40 years or older was much higher (19.1%) and in whom ever-smoking was more common among those with COPD (6). This illustrates important differences in the epidemiology of respiratory disease within sSA; the patterns of exposure and pathology in Cape Town, South Africa may be closer to those of a high income and more industrialized setting than those seen in Malawi and other low income countries across the region.
Increasing age was the only measured factor significantly associated with airflow obstruction in our study. The persistence of this association for moderately severe obstruction suggests that this is not simply the result of expected changes in the FEV1/FVC ratio seen with aging, but constitutes abnormal pathology (33). The small sample size in the context of a low prevalence condition was likely partly responsible for the lack of other significant findings. We noted the non–statistically significant positive association between peripheral blood eosinophil level and airflow obstruction that was not seen with restriction.
A major strength of our study is the inclusion of all adults from the age of 18 years and older and measurement of HIV status. Spirometry was conducted to ATS standards, with careful quality control. The enumeration of the target population at the start of the study, followed by age- and sex-stratified sampling, allowed for population-weighted prevalence estimates to be drawn and associations to be measured.
Limitations of this study include the challenges posed by working with a mobile urban population; it was not possible to locate more than 25% of the initial random sample, and 9.6% had permanently left the area between enumeration and fieldworker home visits. In addition, only 71% of those who were included were able to complete adequate spirometry, with fewer older individuals. As a result, we did not reach the target sample size of 600 adults older than 40 years and 600 adults younger than 40 years of age with high quality spirometry. This reduced our power to detect significant associations in the exploratory analyses. The study was cross-sectional in nature, and although efforts were made to assess the impact of respiratory pathology on patients’ lives using self-reported symptoms and health-related quality of life, prospective data are required to determine the impact of respiratory pathology on morbidity and mortality over time. Measurement of total lung capacity, in addition to FVC, would allow us to better understand the nature and impact of the spirometric restriction seen here. Symptomatic individuals were not screened for pulmonary TB, and the proportion of abnormalities attributable to this more acute pathology are therefore unclear. In addition, the observational nature of this study means that correlations identified may not be causal in nature and could be explained by unidentified or unmeasured confounding factors. Lastly, our data were drawn from a single urban site study, making it difficult to identify strong risk factors for respiratory disease that are ubiquitous within the sample population. Ecological studies comparing data from diverse populations within sSA and with Western cohorts will be required to explore these determinants. Comparable data from rural settings, and areas with different environmental and/or occupational exposures are required to determine the generalizability of our results.
The key finding of our study is a high burden of restrictive spirometry among Malawian adults, with a large difference in prevalence using NHANES and locally defined reference ranges. There is a clear and pressing need to better understand the etiology, pathology, epidemiology and prognosis of spirometric restriction in sSA to inform the development of prevention and management approaches for this condition. If, as has been seen elsewhere, spirometric restriction in this setting is associated with increased mortality, this will have major public health implications for Malawi and potentially the broader sSA region.
Acknowledgments
Acknowledgment
The authors thank the volunteers who took part in the study, The Malawi-Liverpool-Wellcome Trust, through which this study was administered, GlaxoSmithKline for funding this work, and The Burden of Obstructive Lung Disease study team.
Footnotes
Supported by GlaxoSmithKline (study number EPI116520), The Wellcome Trust grants 085790/Z/08/Z and 106065/Z/14/A, and The Burden of Obstructive Lung Disease team.
Author Contributions: Analyzed the data and led the writing of the manuscript with senior authorship leadership from K.M.: J.M. Conceptualized and developed the study protocol: G.N., K.J.D., M.J.N., S.B.G., and K.M. Shared principal investigator responsibilities for the implementation of the study: K.M., S.B.G., and M.J.N. Provided senior statistical support for the data analysis: D.W. Reviewed and wrote the manuscript: all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201509-1807OC on January 20, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Global status report on noncommunicable diseases, 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.World Health Organization. Global action plan for the prevention and control of non-communicable diseases, 2013-2020. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beran D, Zar HJ, Perrin C, Menezes AM, Burney P Forum of International Respiratory Societies Working Group Collaboration. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3:159–170. doi: 10.1016/S2213-2600(15)00004-1. [DOI] [PubMed] [Google Scholar]
- 5.Finney LJ, Feary JR, Leonardi-Bee J, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013;17:583–589. doi: 10.5588/ijtld.12.0619. [DOI] [PubMed] [Google Scholar]
- 6.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AMB, Sullivan SD, Lee TA, Weiss KB, et al. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 7.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 8.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 10.Ehrlich RI, Adams S, Baatjies R, Jeebhay MF. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis. 2011;15:886–891. doi: 10.5588/ijtld.10.0526. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick M, Crothers K, Morris A. Future directions: lung aging, inflammation, and human immunodeficiency virus. Clin Chest Med. 2013;34:325–331. doi: 10.1016/j.ccm.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burney P, Jithoo A, Kato B, Janson C, Mannino D, Nizankowska-Mogilnicka E, Studnicka M, Tan W, Bateman E, Koçabas A, et al. Burden of Obstructive Lung Disease (BOLD) Study. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty--a BOLD analysis. Thorax. 2014;69:465–473. doi: 10.1136/thoraxjnl-2013-204460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66:49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- 14.Piddock KC, Gordon SB, Ngwira A, Msukwa M, Nadeau G, Davis KJ, Nyirenda MJ, Mortimer K. A cross-sectional study of household biomass fuel use among a periurban population in Malawi. Ann Am Thorac Soc. 2014;11:915–924. doi: 10.1513/AnnalsATS.201311-413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, Crapo RO, Jensen RL, Burney PG. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2:277–283. [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quanjer PH. Lung function, genetics and socioeconomic conditions. Eur Respir J. 2015;45:1529–1533. doi: 10.1183/09031936.00053115. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJP, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaheen SO, Barker DJ, Holgate ST. Do lower respiratory tract infections in early childhood cause chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 1995;151:1649–1651. [Discussion 1651–1652.]. doi: 10.1164/ajrccm/151.5_Pt_1.1649. [DOI] [PubMed] [Google Scholar]
- 21.Groce N, Challenger E, Berman-Bieler R, Farkas A, Yilmaz N, Schultink W, Clark D, Kaplan C, Kerac M. Malnutrition and disability: unexplored opportunities for collaboration. Paediatr Int Child Health. 2014;34:308–314. doi: 10.1179/2046905514Y.0000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbett EL, MacPherson P. Tuberculosis screening in high human immunodeficiency virus prevalence settings: turning promise into reality. Int J Tuberc Lung Dis. 2013;17:1125–1138. doi: 10.5588/ijtld.13.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, Simwinga M, Shanaube K, Chishinga N, Bond V, Dunbar R, et al. ZAMSTAR team. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–1194. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 25.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, Williams BG, Munyati SS, Butterworth AE, Mason PR, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munyati SS, Dhoba T, Makanza ED, Mungofa S, Wellington M, Mutsvangwa J, Gwanzura L, Hakim J, Nyakabau M, Mason PR, et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin Infect Dis. 2005;40:1818–1827. doi: 10.1086/429912. [DOI] [PubMed] [Google Scholar]
- 27.Hooper R, Burney P, Vollmer WM, McBurnie MA, Gislason T, Tan WC, Jithoo A, Kocabas A, Welte T, Buist AS. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J. 2012;39:1343–1353. doi: 10.1183/09031936.00002711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelkonen M, Notkola IL, Tukiainen H, Tervahauta M, Tuomilehto J, Nissinen A. Smoking cessation, decline in pulmonary function and total mortality: a 30 year follow up study among the Finnish cohorts of the Seven Countries Study. Thorax. 2001;56:703–707. doi: 10.1136/thorax.56.9.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu G, Zhou Y, Tian J, Yao W, Li J, Li B, Ran P. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 30.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 31.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obaseki DO, Erhabor GE, Gnatiuc L, Adewole OO, Buist SA, Burney PG. Chronic airflow obstruction in a black African population: results of BOLD study, Ile-Ife, Nigeria. COPD. 2016;13:42–49. doi: 10.3109/15412555.2015.1041102. [DOI] [PubMed] [Google Scholar]
- 33.Roberts SD, Farber MO, Knox KS, Phillips GS, Bhatt NY, Mastronarde JG, Wood KL. FEV1/FVC ratio of 70% misclassifies patients with obstruction at the extremes of age. Chest. 2006;130:200–206. doi: 10.1378/chest.130.1.200. [DOI] [PubMed] [Google Scholar]
- 34.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of COPD. 2016 [accessed 2016 Jan 4]. Available from: http://goldcopd.org.