There is growing concern about the current and future burden of noncommunicable lung disease (NCLD) in sub-Saharan Africa (sSA) (1). This is largely driven by recent data documenting the rise of several putative risk factors for NCLD in sSA, including uptake of tobacco and related products (2), exposure to worsening outdoor air pollution from vehicle and industrial emissions (3), and sustained indoor air pollution from the use of biomass fuels for cooking and heating (4). However, empirical evidence of the burden of NCLD, especially spirometry-based general population data, is lacking (Figure 1) (5, 6). This is not entirely surprising, given the limited pulmonary care expertise and clinical capacity in the region (7, 8). Further, most countries in sSA do not routinely collect health data or have vital registration systems (9, 10). As a result, with few exceptions, existing estimates of mortality and morbidity related to NCLD in sSA have been derived from infrequent national or scattered hospital and local surveys, demographic surveillance sites, and extrapolation from statistical models (9, 10). The few attempts to systematically review the burden of NCLD in sSA have primarily assessed chronic obstructive pulmonary disease and have been hampered by heterogeneity in study methods, sample selection, and diagnostic criteria (5, 6, 11, 12).
Figure 1.
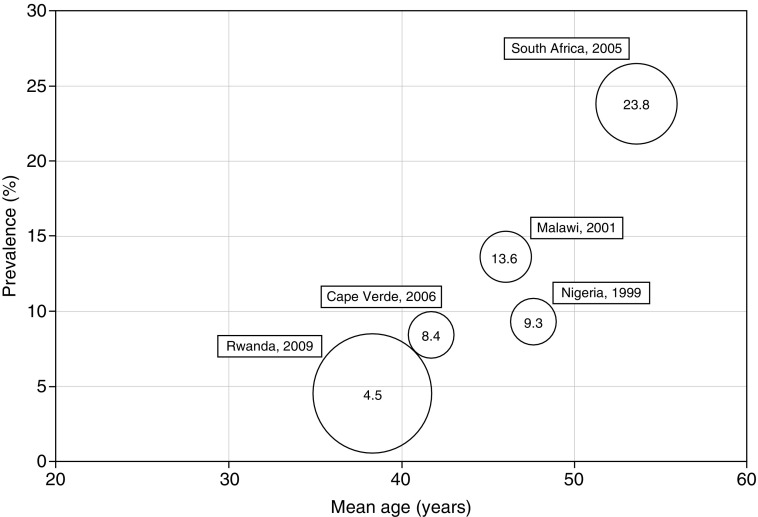
Distribution of spirometry-derived chronic obstructive pulmonary disease (COPD) prevalence rates in five sub-Saharan Africa (sSA) studies. This figure reports the mean age- and spirometry-derived COPD prevalence rates in five population-based studies in sSA identified in a systematic review covering the period from 1990 to 2014 (6). Information about the study sample and design of each study is summarized in the review. The size of the bubble corresponds to the relative sample size of the study. The year reported for each study is the time (or midpoint) of the study, not the year of publication.
In this context, the population-based survey of NCLD in Blantyre, Malawi, published in this issue of the Journal by Meghji and colleagues (pp. 67–76), provides an informative and needed contribution (13). The authors are to be commended for their efforts to recruit a large study sample from this resource-poor setting and to collect and report data that aligned with the standards of the Burden of Obstructive Lung Disease (BOLD) initiative (14). This work highlights the immense challenges of selecting appropriate sampling designs to facilitate NCLD research in urban sSA. It also raises important questions about the most clinically relevant and informative diagnostic criteria for NCLD in sSA, where participants may be distinctly different from the individuals for whom the gold standard diagnostic standards were derived.
The authors targeted enrollment of 2,000 randomly selected adults from a well-defined sampling district near the central referral hospital in Blantyre (13), enlisting several recruitment tools. These included the assistance of local partners, community engagement teams, and door-to-door enumeration of eligible adults. Yet fieldworkers were unable to locate 531 selected participants, and an additional 192 individuals identified for the study permanently left the area before the study onset. Ultimately, 53% of the initial target of 2,000 adults completed BOLD questionnaires and 37.5% completed acceptable spirometric testing. This lower-than-targeted enrollment, in addition to potentially affecting the authors’ statistical analyses, also raises the question of whether the sample that participated in the study is truly representative of the authors’ underlying target population of urban Malawian adults. These challenges likely reflect frequent migration and provisional housing situations found in most sSA settings and is a key logistical obstacle that deserves further attention. Developing and piloting local strategies to improve recruitment and retention of subjects, such as financial incentives for research participation, may help future researchers ensure study generalizability and sufficient statistical power.
Several results from the study by Meghji and colleagues warrant discussion in the context of existing data from other countries from sSA (13). Among urban Malawian participants who provided acceptable spirometric measurements, the prevalence of airway obstruction rates using a fixed ratio of FEV1/FVC < 70% was 4.3% (males) and 4.1% (females), with airway reversibility present in 4.2% of the cohort. These rates are notably among the lowest spirometry-derived chronic obstructive pulmonary disease estimates to be reported in studies and systematic reviews in sSA (5, 6, 11) (Figure 1). It is notable that there was no association between spirometric results and self-reported respiratory symptoms. Further, in contrast to previous reports, the authors did not find a measureable association between airway obstruction and exposure to biomass or tobacco use (5, 11, 12). More studies are needed to explain the observed variability in NCLD prevalence and risk between countries in sSA, including exploration of novel exposures and long-term health consequences of spirometric abnormalities.
To estimate the prevalence of moderate-to-severe obstruction and spirometric restriction, Meghji and colleagues used and compared two distinct populations as reference values (13). In the first analysis, they applied age-, sex-, and height-standardized predicted values from the third survey wave of the National Health and Nutrition Examination Survey (NHANES III), derived from Caucasians in the United States (13). In the second analysis, they used locally derived reference ranges from nonsmoking, asymptomatic participants in the study (13). The use of different reference ranges limitedly altered the prevalence estimates of moderate-to-severe obstruction (NHANES = 3.6%; locally derived = 2.3%). In contrast, the prevalence of restriction differed by 29.6%, depending on the reference ranges used (NHANES = 38.6% vs. locally derived = 9.0%). The authors propose reasonable explanations for the difference in estimates, such as ethnic variation in lung size and anthropometry, the latter of which is well documented in analyses of NHANES III data (15). As the “correct” reference population remains debatable, it may be informative to also compare the Malawian population with the African-American participants in NHANES III (15). Given the human and financial costs of misdiagnosis, development of a case definition of obstructive and restrictive lung disease that is adaptable to diverse global populations is a public health priority (6, 16, 17).
In conclusion, the work by Meghji and colleagues demonstrates the operational challenges to conducting high-quality NCLD research in a low-resource urban sSA setting (13). The authors’ success in conducting a standardized BOLD study protocol highlights the positive and continued influence of the BOLD initiative in guiding comparable and population-based research data in sSA (14, 16). The findings of this research reinforce the need for future longitudinal studies that can validate spirometry metrics in diverse populations, provide greater clarity about modifiable exposures for NCLD, and explore the long-term health outcomes of NCLD in resource-poor settings (18). As we await data from these future studies, consortiums such as the BOLD initiative will continue to improve local health infrastructures and medical provider knowledge and remain critical to advancing our knowledge of the global burden of NCLD (7, 8, 14, 16, 19).
Footnotes
M.O.H. was supported by the National Institutes of Health (F31HL127947).
Author Contributions: Conception and design: M.O.H.; analysis and interpretation: M.O.H. and D.A.; and drafting the manuscript for important intellectual content: M.O.H. and D.A.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kontis V, Mathers CD, Bonita R, Stevens GA, Rehm J, Shield KD, Riley LM, Poznyak V, Jabbour S, Garg RM, et al. Regional contributions of six preventable risk factors to achieving the 25 × 25 non-communicable disease mortality reduction target: a modelling study. Lancet Glob Health. 2015;3:e746–e757. doi: 10.1016/S2214-109X(15)00179-5. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 3.Dieter S.Review of urban air quality in Sub-Saharan Africa region - air quality profile of SSA countries Washington, DC; World Bank; 2012 [accessed 2016 Jan 26]. Available at: http://documents.worldbank.org/curated/en/2012/03/16193871/review-urban-air-quality-sub-saharan-africa-region-air-quality-profile-ssa-countries [Google Scholar]
- 4.Gordon SB, Bruce NG, Grigg J, Hibberd PL, Kurmi OP, Lam KB, Mortimer K, Asante KP, Balakrishnan K, Balmes J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adeloye D, Basquill C, Papana A, Chan KY, Rudan I, Campbell H. An estimate of the prevalence of COPD in Africa: a systematic analysis. COPD. 2015;12:71–81. doi: 10.3109/15412555.2014.908834. [DOI] [PubMed] [Google Scholar]
- 6.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, et al. Global Health Epidemiology Reference Group (GHERG) Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5:020415. doi: 10.7189/jogh.05-020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obaseki D, Adeniyi B, Kolawole T, Onyedum C, Erhabor G. Gaps in capacity for respiratory care in developing countries: Nigeria as a case study. Ann Am Thorac Soc. 2015;12:591–598. doi: 10.1513/AnnalsATS.201410-443AR. [DOI] [PubMed] [Google Scholar]
- 8.Chakaya JM, Carter EJ, Hopewell PC. Pulmonary specialty training to improve respiratory health in low- and middle-income countries: needs and challenges. Ann Am Thorac Soc. 2015;12:486–490. doi: 10.1513/AnnalsATS.201502-071PS. [DOI] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finney LJ, Feary JR, Leonardi-Bee J, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013;17:583–589. doi: 10.5588/ijtld.12.0619. [DOI] [PubMed] [Google Scholar]
- 12.van Gemert F, van der Molen T, Jones R, Chavannes N. The impact of asthma and COPD in sub-Saharan Africa. Prim Care Respir J. 2011;20:240–248. doi: 10.4104/pcrj.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meghji J, Nadeau G, Davis KJ, Wang D, Nyirenda MJ, Gordon SB, Mortimer K. Noncommunicable lung disease in sub-Saharan Africa: a community-based cross-sectional study of adults in urban Malawi. Am J Respir Crit Care Med. 2016;194:67–76. doi: 10.1164/rccm.201509-1807OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, Crapo RO, Jensen RL, Burney PG. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2:277–283. [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, Burney P, Miravitlles M, García-Rio F, Akbari K, et al. BOLD Collaborative Research Group; EPI-SCAN Team; PLATINO Team; PREPOCOL Study Group. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971–985. doi: 10.1378/chest.14-2535. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk WD, Gupta N, Tan WC, Bourbeau J. Clinical relevance of diagnosing COPD by fixed ratio or lower limit of normal: a systematic review. COPD. 2014;11:113–120. doi: 10.3109/15412555.2013.781996. [DOI] [PubMed] [Google Scholar]
- 18.Dalal S, Beunza JJ, Volmink J, Adebamowo C, Bajunirwe F, Njelekela M, Mozaffarian D, Fawzi W, Willett W, Adami HO, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. Int J Epidemiol. 2011;40:885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 19.World Health OrganizationGlobal Alliance against Chronic Respiratory Diseases 2014 General Meeting Report. Geneva, Switzerland: WHO Press; 2014