Summary
p120 catenin is the better studied member of a subfamily of proteins that associate with the cadherin juxtamembrane domain to suppress cadherin endocytosis. p120 also recruits the minus ends of microtubules to the cadherin complex leading to junction maturation. In addition, p120 regulates the activity of Rho family GTPases through multiple interactions with Rho GEFs, GAPs, Rho GTPases, and their effectors. Nuclear signaling is affected by the interaction of p120 with Kaiso, which regulates Wnt-responsive genes, as well as transcriptional repression of methylated promoters. Multiple alternative spliced p120 isoforms and complex phosphorylation events affect these p120 functions. In cancer, reduced p120 expression correlates with reduced E-cadherin function and tumor progression. In contrast, in tumor cells that have lost E-cadherin expression p120 promotes cell invasion and anchorage-independent growth. Furthermore, p120 is required for Src induced oncogenic transformation and provides a potential target for future therapeutic interventions.
Keywords: RhoA, Rac1, cell-cell junctions, Kaiso, EMT, E-cadherin, microtubules, endocytosis, Src, RTK
1. Introduction - History of p120
The p120 catenin (p120ctn; p120 herein) protein was originally identified in a screen for substrates of the Src tyrosine kinase as a 120kDa protein whose phosphorylation on tyrosine residues correlated with cellular transformation ([1], reviewed in [2]). A number of additional Src substrates, including Focal Adhesion Kinase (FAK), p130cas, talin and cortactin were also identified in the screen and subsequently shown to regulate cytoskeletal remodeling and focal adhesion signaling [3]. Unlike the other Src substrates, p120 was shown to interact with classical cadherins at the adherens junctions (AJs) [4–6], where it suppresses cadherin endocytosis [7–9]. p120 regulates cytoskeletal re-organization by affecting the activities of Rho GTPases [10–12], or by mediating the association of microtubules to the cell junctions [13–16]. Additionally, p120 interacts with the transcription factor Kaiso to regulate nuclear signaling events [17, 18]. Recent studies have re-evaluated the role of p120 in cellular transformation, indicating that p120 is required for the anchorage-independent growth of tumor cells lacking E-cadherin, or of cells overexpressing Src [19, 20].
2. Structure
2.1 Family members
p120 is the better studied member of a subfamily of armadillo repeat containing proteins, that share a common genetic and protein structure; the other members are also found at cell-cell junctions and can participate in Rho signaling [2]: ARVCF and δ-catenin are found at AJs, like p120; plakophilins at desmosomes; and p0071 at both (reviewed in [21–23]). p120 is ubiquitously expressed, while the expression of other family members is more restricted, suggesting specialized cellular functions. One difference between p120 and the other three family members found at AJs is that p120 lacks a C-terminal PDZ binding motif with high affinity for Erbin and hScrib [24, 25].
p120 is also superficially similar to β-catenin, both in structure, being a member of the armadillo repeat (ARM) family of proteins, and in function, interacting with cadherins at AJs and regulating transcription. p120’s functions however, are substantially different from those of β-catenin [2]. p120 and β-catenin associate with the cadherin juxtamembrane (JMD) and the catenin binding domains (CBD), respectively. Surprisingly, the mechanism is similar, with both proteins utilizing basic grooves within their ARM domains to recognize charged and hydrophobic residues in the JMD or CBD [26]. A distinct set of surface exposed residues is responsible for the differential ability of these proteins to bind either the JMD or the CBD. Through it’s binding to the JMD, p120 selectively regulates cadherin endocytosis and stability. Another important difference is that p120 has a well-established role in regulating members of the Rho family of small GTPases, which are key mediators of cytoskeletal dynamics and cadherin-mediated cell-cell adhesion (reviewed in [27]).
2.2. Isoforms
Cloning and analysis of the p120 gene revealed 4 alternately spliced exons and 4 transcriptional start sites that together can potentially produce 64 different isoforms of p120 ([28, 29], reviewed in [21, 30]. p120 isoforms are named after the transcriptional start site used (1–4), and the alternatively spliced exons they express (A–D). The complexity of p120’s exon-intron structure and regulation and potential competition for cadherin binding from other family members [31, 32] suggest that p120 and the JMD play a key regulatory function in the cadherin complex. This hypothesis is further supported by evidence that p120 can be phosphorylated in multiple serine, threonine and tyrosine residues that affect its function [33–36]. The potential of this variation in protein sequence and phosphorylation status makes p120 an interesting but also a complex member of AJs.
The functions of particular p120 isoforms are largely unexplored. However, a change in the ratio of p120 isoforms is evident in epithelial vs. mesenchymal cells [5, 29, 37]. During epithelial-to-mesenchymal transition (EMT), or after ectopic Snail expression, the larger p120 isoform 1 is induced [38–40] via a process that involves Epithelial Splicing Regulatory Proteins 1 and 2 [41].
2.3 Evolution
Analysis of proteomes from 14 different vertebrate and metazoan species shows that the p120 protein family evolved from a common δ-catenin-like ancestor present in all metazoans [42, 43]. Successive rounds of gene duplication and diversification are responsible for the seven p120 family members present in the human genome. Ablation of the single δ-catenin-like p120 family member in invertebrate species (e.g., Droshophila melanogaster, Caenorhabditis elegans) produced minor adhesion phenotypes and did not affect survival [44–46]. In contrast, p120 knockdown or depletion in vertebrates is embryonic lethal [47–51]. Paradoxically, p120 has evolved in vertebrates into a ubiquitously expressed protein that is essential for life, whereas δ-catenin, ARVCF and p0071 are more restricted in expression and their loss better tolerated.
3. Function
3.1 Cadherin stability and function
It is well established that p120 binding to the JMD of the cadherin cytoplasmic tail is directly responsible for stabilizing cadherin expression at the cell surface, as well as for inducing cadherin clustering that results in the formation of AJs (reviewed in [21, 52]). The dissociation of p120 from the cadherin complex results in endocytic internalization of cadherins [8, 9, 53] (Figure 1). Endocytosed cadherins can be either degraded in the lysosomes, or they can be recycled to the plasma membrane via a process that may involve p120 binding and the Rap1 GTPase [53, 54]. Thus p120 is a master regulator (a rheostat) that controls cadherin stabilization and assembly into AJs.
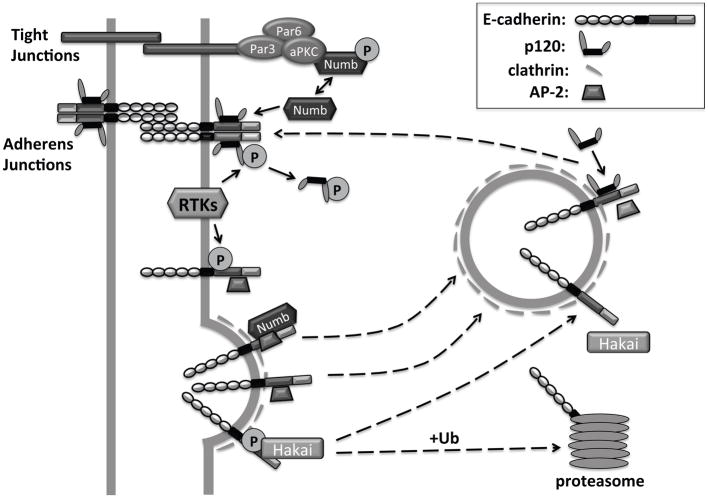
Figure 1. Cadherin stabilization by p120 catenin.
p120 binding to the cadherin juxtamembrane domain stabilizes cadherins at the cell membrane and promotes the formation of adherens junctions. Removal of p120 from the cadherin complex can proceed via multiple mechanisms, including phosphorylation of p120 and/or E-cadherin downstream of receptor tyrosine kinases (RTKs), or association with non-phosphorylated Numb, a protein normally phosphorylated by aPKC. p120 dissociation uncovers an adaptor protein 2 (AP-2) binding motif, as well as a phosphorylation-dependent motif for the recruitment of the E3 ligase Hakai. AP-2 binding promotes clathrin-dependent endocytosis of E-cadherin. The endocytosed E-cadherin can be recycled back to the membrane, upon binding to p120. Alternatively, Hakai-induced ubiquitination of E-cadherin can lead to cadherin degradation in the proteasome.
Insight into the mechanism by which p120 binding prevents cadherin endocytosis was provided recently by the elucidation of the p120 structure in association to E-cadherin [26]. Both “dynamic” and “static” interactions contribute to the binding of p120’s ARM domain with the cadherin JMD. Importantly, these interactions include JMD residues that are implicated in clathrin-mediated endocytosis and Hakai-dependent ubiquitination of E-cadherin [26] (Figure 1). Based on the structural evidence, p120 binding physically impedes the interaction of cadherin molecules with either E3 ligases or the endocytic machinery.
Interestingly, the N-terminal domain of p120 is in close proximity to the CBD and β-catenin. This p120 domain is highly regulated by alternative splicing and by phosphorylation. Serine/threonine phosphorylation of p120’s N-terminus is thought to control E-cadherin dynamics at the cell membrane [35]. The data argue that both cadherin binding, which is mediated by p120’s central ARM domain, and signaling events mediated by p120’s N-terminus are required for cadherin stabilization.
Surprisingly, early studies argued that the JMD has both positive and negative functions in cadherin clustering and cell-cell adhesion (reviewed in [21]). A model whereby p120 is “activated” upon cadherin trans dimerization was proposed, whereas intracellular signaling events were suspected to “de-activate” p120 and block its function in promoting cell adhesion [21]. This model was supported by the observation that cadherin activating antibodies induce cadherin conformational changes and promote cell-cell adhesion via the de-phosphorylation of selective p120 residues in the N-terminus [55]. The positive effect of dephosphorylated “activated” p120 may be due to increased cadherin clustering, possibly via direct p120 dimerization [26]. Alternatively, p120 may serve as a scaffold for additional proteins, enabling them to directly affect adhesion or to regulate the function of other cadherin/catenin complex proteins. One candidate is PLEKHA7, a novel p120 N-terminal interacting protein that is required for mature junction formation [16, 56]. Additionally, p120 can recruit a number of kinases and phosphatases that could influence adhesion by affecting the entire cadherin-catenin complex. p120 “de-activation” may also relate to signaling events leading to cadherin endocytosis. Recently, p120 was shown to mediate the interaction of E-cadherin complexes with a protein called Numb. Numb is normally phosphorylated by aPKC, a main component of the tight junctions. However, upon dephosphorylation, Numb can bind to either the C-terminus of p120 or to non-p120-bound E-cadherin, mediating in both cases clathrin-dependent endocytosis of E-cadherin [57] (Figure 1).
Finally, the signaling that regulates the interaction of p120 with cadherins likely includes dynamic phosphorylation of p120 and cadherin (reviewed in [58]). Tyrosine phosphorylation of E-cadherin at Y755/756 or VE-cadherin at Y658 disrupts p120 binding [26, 59, 60] (Figure 1). Additional kinases, like PAK (p21 Associated Kinase) or CK1 epsilon can reportedly also phosphorylate cadherins and disrupt p120 association [61, 62]. Clearly, the JMD plays a critical role in cadherin stability and cell adhesion, and its function is highly regulated by phosphorylation and by interaction with a host of p120-related proteins (including isoforms and family members).
3.2 Regulation of Rho GTPase signaling
The Rho GTPases RhoA, Rac1, and Cdc42, are molecular switches that regulate cell migration, as well as cadherin-mediated adhesion and cell-cell junction formation through the manipulation of the actin cytoskeleton [63–67]. Interestingly, cadherin ligation can reciprocally modulate the signaling of Rho GTPases, and such regulation is thought to require p120 (reviewed in [27]). Experiments to date suggest the existence of several different mechanisms by which p120 can affect Rho GTPases:
The first involves the direct association of p120 with Rho GTPases. When not bound to cadherins, p120 acts as a guanine-nucleotide dissociation inhibitor (GDI) and directly binds to and suppresses RhoA activity [36, 68]. At least two p120 domains are essential for a stable interaction with RhoA and inhibition of its activity; a central polybasic region termed “ΔRho” (amino acids 622-628) [36, 69] and an N-terminal region (including amino acids 131-156) [70]. The interaction of p120 with RhoA is further stabilized by tyrosine phosphorylation of p120 at Y217 and Y228 by Src, or destabilized by phosphorylation at Y112 by Fyn [36]. In addition to RhoA, p120 can also associate with Rac1 and especially, Rac1b, an alternatively spliced constitutively active Rac1 isoform. This interaction, however, does not interfere with the intrinsic GTPase activity of either Rac1 or Rac1b [71].
The second mechanism involves the association of p120 with Rho GEFs or GAPs, proteins that regulate the activation or inactivation of downstream Rho GTPases. At nascent AJs, cadherin-associated p120 interacts with Vav2 locally to activate Rac1 and Cdc42 to promote reorganization of the actin cytoskeleton [11, 50]. It is now clear that association with Rho regulatory proteins in addition to Rho GTPases is a general feature of the p120 family of proteins. The p120 family member p0071 has been shown to associate with the Rho GEF Ect-2 to regulate cytokinesis [72], while δ-catenin binds p190 RhoGEF [73]. p120 is also responsible for the cortical localization of p190 RhoGAP, a negative regulator of RhoA, which is activated by Src signaling and mediates Rac-mediated suppression of RhoA [74].
A third mechanism of action is p120 interaction with Rho GTPase effector proteins. p120 can associate with and recruit Rho-associated protein kinase 1 (ROCK1) to sites of cell-cell contact [75]. Additionally, cytosolic p120 associates directly with and inhibits the activity of myosin phosphatase Rho–interacting protein (Mrip), an antagonist of Rho/Rock function and ROCK activation [76]. While questions remain, the data suggest that p120 family members act as scaffolds to promote the association of positive or negative regulators with Rho GTPases and their subsequent effector proteins at particular subcellular sites depending on contextual signals. Consistent with this hypothesis, the recruitment of Rac1 to newly formed cell-cell contacts and the accumulation of the Rac1 effector PAK to these sites require the cadherin JMD and p120 association [77].
Complicating matters, 1) p120 isoforms differ in their ability to regulate Rho GTPases. Expression of the mesenchymal p120 isoform 1 inhibits Rho activity, while expression of the epithelial p120 isoforms 3 and 4, has no effect, or activates RhoA, respectively; 2) Since the stability of microtubules can impact the activities of Rho GTPases via microtubule-binding proteins such as GEF-H1, the ability of p120 to associate with and stabilize microtubules can indirectly influence Rho GTPases; and 3) The effects of p120 on Rho GTPase activation/inhibition may depend on cell context, and specifically the type of cadherins that a cell expresses.
3.3 Nuclear signaling
Another signaling function of p120 is the regulation of gene transcription, particularly the convergent regulation of canonical Wnt/β-catenin target genes such as Cyclin D1 and Wnt-11. Regulation of transcription by p120 occurs through its interaction with the transcription factor Kaiso (reviewed in [78, 79]). Kaiso recognizes two DNA sites: a sequence-specific DNA consensus site (5′CTGCNA3′) [80] and methylated CpG dinucleotides [18, 80]. Many Wnt/β-catenin target genes contain the sequence-specific Kaiso binding site in their promoters and Kaiso acts as a transcriptional repressor toward these genes, antagonizing β-catenin-mediated transcriptional activation [81, 82]. p120 interacts with Kaiso’s zinc finger domain to prevent Kaiso-DNA binding; thus p120 antagonizes Kaiso’s transcriptional repression, facilitating Wnt/β-catenin signaling.
The signals that govern the p120-Kaiso interaction and their combined regulation of transcription are largely unclear. However, a recent study in Xenopus indicates that p120 is stabilized by Frodo, a downstream target of Wnt/Dsh signaling [82]. This stabilization resulted in the inhibition of Kaiso-mediated repression of Wnt/β-catenin target genes. Consistent with this, Wnt3a signaling controls the transcriptional activity of Kaiso, via the CK1ε-mediated phosphorylation of p120 [83]. Thus p120’s transcriptional activity may be regulated by Wnt signaling: another point of signaling convergence between p120 and β-catenin. The signals that govern the cytoplasmic vs. nuclear localization of Kaiso are also not well characterized, although it is suggested that the cell’s microenvironment plays a role. As with p120’s other roles in mediating cadherin stabilization and Rho GTPase signaling, cell context undoubtedly affects the ability of p120 to regulate transcription; the details regarding how remain to be elucidated. Finally, an interaction of nuclear p120 with the transcriptional repressor Gli-similar 2 (Glis2) was also reported, although its functional significance is still unclear [84].
3.4 Regulation of cell motility by p120-Rho GTPase signaling
Low-level p120 overexpression via a retroviral delivery system promotes cell motility [12]. This effect is mediated by Rho GTPases, and possibly their subsequent effects on integrin-mediated signaling after adhesion to the extracellular matrix (ECM) [85–87]. The effects of p120 on cell migration depend on the specific cadherins expressed in the cells [88]. How mesenchymal cadherins promote cell migration is currently unclear, but it may involve the p120-mediated activation of Rac1 signaling and subsequent actin reorganization [88–90]. Inhibition of RhoA by cytoplasmic p120 in cells expressing mesenchymal cadherins may also promote migration by allowing the re-organization of the actin cytoskeleton [88, 91]. In contrast, in cells expressing E-cadherin, p120 is important for the pro-migratory signaling of either EGF or HGF, via a mechanism that involves p120’s N-terminus and RhoA activation [92]. In the case of breast cancer cells expressing E-cadherin, p120-mediated Rac1 activation was required for HER2/ErbB2 induced cell migration [89]. Therefore, it seems that p120 and Rho GTPase signaling are involved in the motile behavior of cells expressing mesenchymal cadherins or activated receptor tyrosine kinases, but the specific mechanisms are not well defined.
3.5. p120 and microtubules
It has been shown that p120 interacts with the microtubule network in several ways, both directly and indirectly. This interaction supports a bi-directional functional role, by regulating a) p120 trafficking in the cell, and b) microtubule bundling and tethering to the junctions. p120 interacts with microtubules directly via the ARM repeats and this interaction is mutually exclusive to E-cadherin binding [13]. p120 can also bind to microtubules indirectly via it’s N-terminus [13, 14, 16]. An interaction with conventional kinesin heavy chain controls p120 trafficking [13, 14, 93] and nuclear accumulation [13, 94]. Dynein-mediated tethering of microtubules to the junctions enables delivery of p120 to these sites [93].
Additionally, p120 promotes microtubule stabilization, bundling, and tethering to the junctions [13, 15, 95], via a novel binding partner called PLEKHA7 [16, 56]. This interaction is again mediated by the N-terminus of the p120 molecule. p120 and PLEKHA7 form a complex at the AJs that connects to the microtubule network through the microtubule minus end capping protein Nezha and the KIFC3 motor [16]. The p120-PLEKHA7-Nezha interaction links E-cadherin-p120 complexes with the minus ends of non-centrosomal microtubules, stabilizing AJs [16]. Importantly, prior studies have shown that non-centrosomal microtubule organization is essential for AJ symmetry and planar polarity in Drosophila [96], while vertically-aligned non-centrosomal polyglutamylated microtubules are required for polarized vesicle transport and the establishment of epithelial cell polarity [97]. Interestingly, the interaction of p120 with PLEKHA7 occurs selectively at mature AJs in polarized Caco2 cells, and not at perijunctional cadherin puncta [16]. Overall, p120-microtubule cross talk is essential in mediating both the intracellular localization of p120 and in stabilizing the microtubule-junctional architecture.
4. p120 in Cancer
Normal cells inhibit their growth and migration when they adhere to each other. These properties are progressively lost in tumor cells, contributing to increased rates of cell proliferation and migration. The processes imply that adhesion-triggered signaling events regulate both cell growth and motility. Based on its ability to regulate E-cadherin function, p120 is expected to act as a tumor suppressor, by stabilizing junctions. Surprisingly, recent studies show that signaling events downstream of p120 and cadherins are crucial for the anchorage-independent growth of tumor cells, as well as for Src-mediated transformation [19, 20]. Therefore, p120 exerts both pro-tumorigenic as well as anti-tumorigenic functions, an intriguing behavior (Figure 2). Despite many remaining questions that will provide the basis for future investigations, several important factors that have been so far identified to affect p120 behavior in cancer are discussed here.
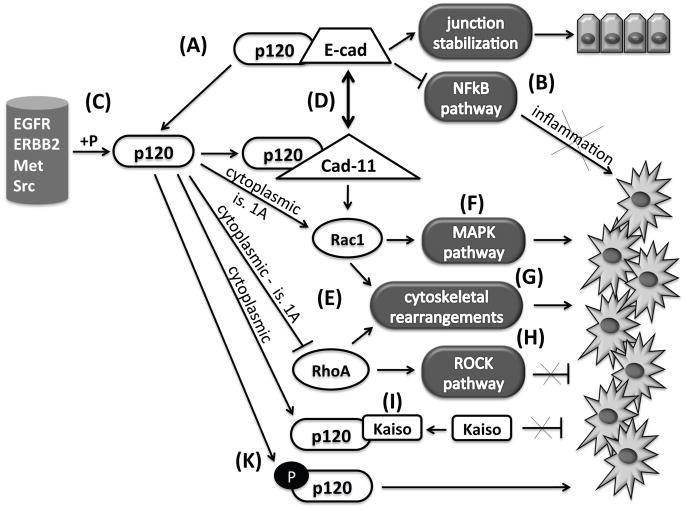
Figure 2. p120 catenin in cancer.
Under normal conditions, p120 binds E-cadherin, stabilizes junctions (A), and suppresses inflammatory signaling pathways (B). However, upon phosphorylation by several kinases (C) or after cadherin switch (D), p120 induces pro-tumorigenic pathways, mainly by modulating Rho GTPase activities (E). These pro-tumorigenic events include activation of the MAPK pathway (F), cytoskeletal rearrangements (G) and suppression of the ROCK pathway (H). In addition, p120 cytoplasmic localization results in Kaiso sequestration to the cytoplasm and inactivation (I), also relieving transcriptional suppression of oncogenic signals. p120 phosphorylation is well correlated to tumorigenesis (K).
4.1 p120 and cadherin switch in tumor growth and progression
During gastrulation, ingressing epithelial cells in the ectoderm undergo EMT, which allows them to adopt new cell fates, migrate through the basement membrane away from the epithelial monolayer, and establish new tissues. This normal developmental process overcomes the structural requirements for epithelial cell survival, and is characterized by a switch in the expression of epithelial versus mesenchymal markers, including cell-cell adhesion receptors of the cadherin family. During tumor progression though, differentiated epithelial cells undergo a similar transition, lose their characteristic phenotype and acquire mesenchymal properties, becoming motile and invasive. A major event that influences this transformation is an essential change in the type of junctions that these cells form and the subsequent signaling events that are triggered. This change is mainly due to a switch in the type of cadherins expressed. In particular, while epithelial cells primarily express E-cadherin, which is a well-established tumor-suppressor, expression of E-cadherin is decreased or diminished during EMT, whereas expression of other cadherins, such as N-cadherin or Cadherin-11 is induced. These cadherins are collectively described as “mesenchymal” cadherins. This switch results in membrane ruffling, immature junctions, induced cell motility and invasiveness, all hallmarks of tumorigenic transformation. Indeed, it is well established in a variety of epithelial cancers, such as invasive lobular breast carcinoma and diffuse-type gastric adenocarcinoma, that loss of E-cadherin expression is correlated with increased infiltrative growth and tumor cell motility (reviewed in [98, 99]). Several signaling pathways and mediators of this process have been described so far. For example, E-cadherin engagement during cell-cell adhesion regulates the levels of the cyclin kinase inhibitor p27 [100, 101] and suppresses signaling from a variety of receptor tyrosine kinases (RTKs), including EGF receptor (EGFR) and c-Met [102], resulting in decreased rates of cell growth. In addition, cadherin-mediated cell-cell adhesion suppresses cell migration by regulating the activity of Rho family GTPases via p120 [88].
Since p120 stabilizes E-cadherin complexes, it is expected that it should exert anti-tumorigenic activities (Figure 2). In several cases of p120 deletion in vivo, the main effect was severe epithelial barrier malfunction and induction of inflammation, via RhoA and NFkB activation in the skin [103], or by accumulation of COX-2 expressing neutrophils in the colon [47, 104]. Solid evidence of the tumor-suppressor function of p120 in vivo was given in the salivary gland, where p120 deletion resulted in neoplastic lesions, as a result of severe adhesion defects and E-cadherin destabilization [48]. The tumor suppressor function of p120 was further established recently, where in vivo deletion of p120 resulted in invasive squamous neoplastic lesions in the oral cavity, esophagus, and forestomach [105]. p120 deletion was again accompanied by induction of pro-inflammatory events (Figure 2), such as activation of NFkB, Akt, Stat-3 and production of the granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), monocyte chemotactic protein-1 (MCP-1), and of the tumor necrosis factor a (TNFa) from tumor-derived cells. These events were followed by desmoplasia and accumulation of immature myeloid cells, both hallmarks of the development of a tumorigenic microenvironment. The above findings demonstrate the anti-tumorigenic and anti-inflammatory properties of p120, in vivo, and are associated with E-cadherin expression in the cells.
In agreement with the animal data, several immunohistochemical studies argue that p120 expression is downregulated in certain tumors [106–110]. Also, p120 is transcriptionally downregulated by FOXC2 in non-small cell lung cancer (NSCLC) [111]. Downregulation of p120 would be expected to decrease E-cadherin-mediated adhesion, and possibly relieve inhibition of RTKs. The full ramifications of this condition are not yet understood, however, the combined loss of cell adhesiveness, increased RTK signaling, and altered Rho signaling may have profound effects on the aggressiveness of these tumors. Consistent with this, p120 knockdown in lung cancer cell lines enhanced migration and invasiveness [112]. The correlation of p120 loss with increased tumor aggressiveness [108] also suggests that in a background of other cancer-related mutations, p120 loss may promote metastasis.
On the other hand, p120 is also affected by cadherin switch during EMT and is an essential effector of the downstream signaling events induced by growth factor receptors, which lead to tumorigenesis (Figure 2). Early studies argued that p120 mislocalizes to the cytoplasm as a result of E-cadherin loss during tumor progression [113, 114]. For example, during EMT in colon cancer cells, E-cadherin is gradually lost and p120 gets mislocalized to the cytoplasm, where it inhibits Rho activity [91]. In this case, cytoplasmic p120 correlated strongly with later-stage tumors, lymph node metastasis and reduced survival in a cohort of colorectal cancer cases [91]. Cytoplasmic p120 was also strongly correlated with late-stage pancreatic cancer [115]. Abnormal overexpression and cytoplasmic localization of p120 was associated with poor prognosis in lung squamous cell carcinoma and adenocarcinoma [109]. In ovarian cancer, gonadotropin-releasing hormone (GnRH) receptor expression promotes an E- to P-cadherin switch, p120 mislocalization, induction of Rac1 and Cdc42 activities, and tumor invasiveness [90]. A potential mechanism by which p120 can directly affect the cadherin switch was also proposed. Cells that were forced to overexpress R-cadherin had reduced levels of E- and P-cadherin. This phenomenon was the result of competition between the different cadherins for p120 binding. R-cadherin overexpression resulted in sequestration of p120 to R-cadherin, which led to E-cadherin de-stabilization, endocytosis and degradation [116].
p120 is not only affected by a cadherin switch and E-cadherin loss, but is also essential in influencing downstream pathways towards induction of cell motility and invasiveness [19, 88]. p120-dependent regulation of Rho GTPase signaling is one example (Figure 2). Upon loss of E-cadherin, cytoplasmic p120 was implicated in a murine model of infiltrating lobular carcinoma of the breast (ILC) via interaction and inhibition of Mrip, an antagonist of Rho/Rock function and ROCK activation [76]. Experiments in breast and kidney cancer cell lines have revealed that in addition to loss of E-cadherin, an accompanying increase in mesenchymal cadherin expression, and p120/mesenchymal cadherin-associated Rac1 signaling is required for the invasiveness of E-cadherin deficient tumor cells [88]. Therefore, cadherin switching during EMT and deregulated Rho GTPase signaling promote cell migration and tumor growth.
Surprisingly, in some cases p120 exerts tumorigenic activities even when E-cadherin is present. For example, ERBB2 overexpression in normal mammary epithelial MCF10A cells activates Rac1 and Cdc42 and induces cell migration and invasion in a p120-dependent manner, without any changes in E-cadherin expression [89]. In this study, p120 overexpression in an ERBB2-overxpressing but weakly metastatic breast cancer cell line, the BT474 cells, dramatically induced its ability to metastasize in vivo [89]. Rac1b, a constitutively active isoform of Rac1, required p120 binding in order to promote cell motility, in the E-cadherin positive mouse mammary epithelial cells [71]. In another recent example, p120 was shown to be essential in the progression of a highly lethal form of cancer, the inflammatory breast cancer (IBC) [117]. In IBC, overexpression of the translation initiation factor eIF4GI promotes p120 overexpression via specific IRES. As a result, E-cadherin is stabilized and favors IBC progression, likely by supporting the formation of the characteristic emboli of IBC. Therefore, in IBC, p120-E-cadherin complexes are overexpressed and pro-tumorigenic. Furthermore, aberrantly cytoplasmic p120 in invasive breast carcinomas that express P-cadherin, correlated with poor survival, especially when E-cadherin was also present [118]. In agreement, in cases of ILC where E-cadherin is still expressed, p120 is cytoplasmic, suggesting mis-regulation of the cadherin-p120 complex [119]. p120 is also required for the collective migration of A431 cells in a three-dimensional medium (matrigel or collagen) upon EGFR stimulation. This function of p120 is attributed to cadherin stabilization required for the cells to maintain their junctions in order to collectively migrate and invade [120].
Overall, although there is solid evidence that p120 exerts both pro-tumorigenic and anti-tumorigenic functions depending of the type of cadherin present, there are a number of cases where this relationship is not linear, indicating that the full mechanistic details of these functions remain to be elucidated.
4.2 p120 isoform-specific tumorigenic events
An extra layer of complexity in identifying the role of p120 in cancer progression involves the relative expression of p120 isoforms (Figure 2). We have reported that full-length p120 and N-terminally truncated p120 isoforms differentially affect Rho GTPase activities, cell migration and tumor cell invasion, while a p120 isoform switch during tumor progression predicts metastatic disease [69]. More specifically, the long isoform 1A is capable of promoting invasiveness, whereas isoform 4A cannot, and its expression was found to correlate with renal cancer micrometastasis. Both isoforms are able to bind RhoA downstream of HGF and Met signaling, however, only the cooperative binding of RhoA to a central binding domain and to the N-terminus that is contained in the long isoform 1A stabilizes RhoA binding and effectively inhibits its activity [69]. By efficiently suppressing the RhoA-ROCK pathway and by activating Rac1, isoform 1A induces migration and invasiveness of breast cancer cells. Furthermore, examination of p120 isoform expression across a series of breast cancer cell lines showed that the luminal, more invasive cells show high expression of the large isoforms 1 and 2, whereas isoform 3 is almost universally expressed [121]. Overexpression of isoform 3A attenuated proliferation of colon cancer HT-29 cells, in agreement with the aforementioned observations, although a role of this isoform in aberrant mitosis and induction of polyploidy was also proposed [122].
A new transcription factor named Zeppo 1 was shown to deregulate junction formation and promote lung metastases of breast cancer, by inducing the expression of the pro-tumorigenic isoform 1 of p120 [123]. Other studies also showed that overexpression and mis-localization of p120 isoform 1 strongly correlates with poor outcome in lung cancer [124].
Interestingly, both isoforms 1 and 3 are able to induce cell proliferation, via different pathways that ultimately affect Cyclin D1. In particular, p120 isoform 1A can induce Cyclin D1 via β-catenin increased expression and signaling, whereas isoform 3A does it by decreasing the levels of Kaiso in the nucleus, thereby relieving Cyclin D1 repression [125]. This study highlights the complexity in attributing pro-tumorigenic functions to particular p120 isoforms, indicating that these functions may be context-dependent, and clearly not yet fully delineated.
4.3 p120 phosphorylation correlates with cancer occurrence
p120 is phosphorylated by Src family kinases at a number of tyrosines within its N-terminus, such as Y96, Y112, Y228, Y257, Y280, Y291, Y296, and Y302 [33]. EGFR also phosphorylates p120 at Y228, without Src being the necessary intermediate [126] (Figure 2). A number of studies have showed that p120 phosphorylation correlates with tumorigenic events. In particular, phosphorylation of p120 at pY228 correlates positively with oral squamous cancer [127]. Phosphorylation of p120 in several tyrosine and serine sites is inversely related to cadherin activation and adhesion strengthening [55].
In addition to tyrosine, serine phosphorylation of p120, which is mainly induced by PKC [58], has been also associated with pro-tumorigenic events. Phosphorylation of p120 isoform 3 at S288 increased Kaiso binding and promoted lung cancer cell invasion [75]. Furthermore, Wnt signaling induced phosphorylation of p120 at S268 and S269, dissociating it from E-cadherin and subsequently promoting Kaiso sequestration and activation of downstream Wnt signaling events [83]. Overall, the evidence points towards a pro-tumorigenic role for p120 phosphorylation, however, the mechanistic details of this action are still unclear.
4.4 Regulation of anchorage independent growth by p120
A hallmark property of cells that are transformed with oncogenes is their capacity to grow under anchorage-independent conditions. The acquisition of anchorage independent growth (AIG) correlates strongly with tumorigenicity in vivo, and is thought to be a key transition in tumor progression associated with aggressive disease. In essence, AIG allows epithelial cells to grow in the wrong environmental context. Loss of dependence to the basement membrane for survival and progression through the cell cycle are properties associated with AIG that are closely related to the induction of tumor growth associated with carcinoma in situ, a key transition during human tumor progression.
Normal epithelial cells undergo apoptosis when suspended, in a process termed anoikis. There is evidence that suggest a link between cadherin-mediated adhesion and anoikis. In the context of the colonic epithelium, cadherin-mediated cell-cell junctions prevented anoikis via the activation of Src and PI3K-dependent signaling pathways [128, 129]. Two separate studies argue that 1) anoikis can be prevented by maintaining cell-cell junctions, who are often lost upon cell suspension, and 2) that anoikis is induced by the EGF-stimulated disruption of enterocytic cell-cell contacts upon detachment from the ECM [130]. However, cellular context and epigenetic modifications may play a critical role in the overall effect of E-cadherin depletion on anoikis. For example, in the context of p53 mutations, loss of E-cadherin in mammary epithelial cells leads to ILC, due in part to anoikis resistance [76]. Overall, it is likely that intercellular adhesion-related signaling events can substitute for focal adhesion signaling and drive the transformed growth of tumor cells. Following up on the original observation that p120 is a transformation-relevant Src substrate, recent work has established that p120 is an essential mediator of the Src and Rac1 induced AIG [20] (Figure 2). p120 mediated suppression of the Rho-ROCK pathway was necessary for AIG induction driven by the overexpression of either Rac1 or Src [20]. The observation that ROCK is recruited to E-cadherin complexes by binding directly p120 [75], further establishes the connection between p120, ROCK and AIG.
Signaling downstream from ECM and focal adhesions cooperates with growth factors to induce a sustained activation of ERK/MAP kinase, increased expression of Cyclin D1, and cell cycle progression [131]. Interestingly, p120 is essential in bypassing the requirement for ECM induction of the ERK/MAP kinase pathway, and for Cyclin D1 activation of tumor cells grown in soft agar. This activation occurred in the presence of cadherin 11 but not of E-cadherin and was mediated by Rac1 activation [19]. The data supported a model where in the presence of E-cadherin p120 promotes the stabilization of E-cadherin complexes and their ability to suppress Ras and Rac1 signaling, thus blocking cell growth. However, upon E-cadherin loss during EMT or tumor progression, the negative regulation of Ras is relieved, endogenous p120 induces Rac1 activation, constitutive activation of the ERK MAP kinase signaling pathway, cell cycle progression, and anchorage-independent growth [19].
Therefore, it is important to note that anchorage independence in epithelial cells is a complicated process, requiring both suppression of apoptosis and progression through the cell cycle via signaling events triggered by cell-cell contact. Despite many remaining questions that will provide the basis for future investigations, the current data argue that intercellular adhesion and p120 can regulate the actin cytoskeleton and the activity of mitogenic pathways under conditions of anchorage independence.
References
- 1.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–638. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanner SB, Reynolds AB, Vines RR, Parsons JT. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100), and the cadherin/catenin complex. J Cell Biol. 1995;130:369–381. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, et al. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 11.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512–9521. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–557. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–6594. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 16.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokhortchouk A, Hendrich B, Jorgensen H, Ruzov A, Wilm M, Georgiev G, Bird A, Prokhortchouk E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001;15:1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soto E, Yanagisawa M, Marlow LA, Copland JA, Perez EA, Anastasiadis PZ. p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression. Journal of Cell Biology. 2008;183:737–749. doi: 10.1083/jcb.200805113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohn MR, Brown MV, Reynolds AB. An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol. 2009;184:437–450. doi: 10.1083/jcb.200807096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Science. 2000;113:1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- 22.Hatzfeld M. The armadillo family of structural proteins. Int Rev Cytol. 1999;186:179–224. doi: 10.1016/s0074-7696(08)61054-2. [DOI] [PubMed] [Google Scholar]
- 23.Hatzfeld M. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Laura RP, Witt AS, Held HA, Gerstner R, Deshayes K, Koehler MF, Kosik KS, Sidhu SS, Lasky LA. The Erbin PDZ domain binds with high affinity and specificity to the carboxyl termini of delta-catenin and ARVCF. J Biol Chem. 2002;277:12906–12914. doi: 10.1074/jbc.M200818200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Yeh S, Appleton BA, Held HA, Kausalya PJ, Phua DC, Wong WL, Lasky LA, Wiesmann C, Hunziker W, Sidhu SS. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J Biol Chem. 2006;281:22299–22311. doi: 10.1074/jbc.M602902200. [DOI] [PubMed] [Google Scholar]
- 26.Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Anastasiadis PZ. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 28.Aho S, Rothenberger K, Uitto J. Human p120ctn catenin: tissue-specific expression of isoforms and molecular interactions with BP180/type XVII collagen. J Cell Biochem. 1999;73:390–399. [PubMed] [Google Scholar]
- 29.Keirsebilck A, Bonne S, Staes K, van Hengel J, Nollet F, Reynolds A, van Roy F. Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics. 1998;50:129–146. doi: 10.1006/geno.1998.5325. [DOI] [PubMed] [Google Scholar]
- 30.Pieters T, van Roy F, van Hengel J. Functions of p120ctn isoforms in cell-cell adhesion and intracellular signaling. Front Biosci. 2012;17:1669–1694. doi: 10.2741/4012. [DOI] [PubMed] [Google Scholar]
- 31.Mariner DJ, Wang J, Reynolds AB. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120(ctn) in E-cadherin complexes [In Process Citation] J Cell Sci. 2000;113:1481–1490. doi: 10.1242/jcs.113.8.1481. [DOI] [PubMed] [Google Scholar]
- 32.Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS. delta-catenin, an Adhesive Junction-associated Protein Which Promotes Cell Scattering. J Cell Biol. 1999;144:519–532. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- 34.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry. 2003;42:9195–9204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 35.Fukumoto Y, Shintani Y, Reynolds AB, Johnson KR, Wheelock MJ. The regulatory or phosphorylation domain of p120 catenin controls E-cadherin dynamics at the plasma membrane. Exp Cell Res. 2008;314:52–67. doi: 10.1016/j.yexcr.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castano J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, Garcia de Herreros A, Dunach M. Specific Phosphorylation of p120-Catenin Regulatory Domain Differently Modulates Its Binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo YY, Reynolds AB. Identification of murine p120 isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996;56:2633–2640. [PubMed] [Google Scholar]
- 38.Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carnahan RH, Rokas A, Gaucher EA, Reynolds AB. The molecular evolution of the p120-catenin subfamily and its functional associations. PLoS One. 2010;5:e15747. doi: 10.1371/journal.pone.0015747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao ZM, Reynolds AB, Gaucher EA. The evolutionary history of the catenin gene family during metazoan evolution. BMC Evol Biol. 2011;11:198. doi: 10.1186/1471-2148-11-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myster SH, Cavallo R, Anderson CT, Fox DT, Peifer M. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J Cell Biol. 2003;160:433–449. doi: 10.1083/jcb.200211083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol. 2003;162:15–22. doi: 10.1083/jcb.200212136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magie CR, Pinto-Santini D, Parkhurst SM. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development. 2002;129:3771–3782. doi: 10.1242/dev.129.16.3771. [DOI] [PubMed] [Google Scholar]
- 47.Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. p120-Catenin is required for mouse vascular development. Circ Res. 2010;106:941–951. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang X, Ji H, Kim SW, Park JI, Vaught TG, Anastasiadis PZ, Ciesiolka M, McCrea PD. Vertebrate development requires ARVCF and p120 catenins and their interplay with RhoA and Rac. J Cell Biol. 2004;165:87–98. doi: 10.1083/jcb.200307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 54.Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–4783. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 55.Petrova YI, Spano MM, Gumbiner BM. Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol Biol Cell. 2012;23:2092–2108. doi: 10.1091/mbc.E11-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pulimeno P, Bauer C, Stutz J, Citi S. PLEKHA7 is an adherens junction protein with a tissue distribution and subcellular localization distinct from ZO-1 and E-cadherin. PloS one. 2010;5:e12207. doi: 10.1371/journal.pone.0012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, Kaibuchi K. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell. 2011;22:3103–3119. doi: 10.1091/mbc.E11-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alema S, Salvatore AM. p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 60.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 61.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Casagolda D, Del Valle-Perez B, Valls G, Lugilde E, Vinyoles M, Casado-Vela J, Solanas G, Batlle E, Reynolds AB, Casal JI, de Herreros AG, Dunach M. A p120-catenin-CK1epsilon complex regulates Wnt signaling. Journal of cell science. 2010;123:2621–2631. doi: 10.1242/jcs.067512. [DOI] [PubMed] [Google Scholar]
- 63.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 64.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kodama A, Takaishi K, Nakano K, Nishioka H, Takai Y. Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells. Oncogene. 1999;18:3996–4006. doi: 10.1038/sj.onc.1202773. [DOI] [PubMed] [Google Scholar]
- 68.Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001;13:604–610. doi: 10.1016/s0955-0674(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 69.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, Copland JA, Anastasiadis PZ. A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastatic disease. J Biol Chem. 2008;283:18344–18354. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SH, Peng IF, Ng YG, Yanagisawa M, Bamji SX, Elia LP, Balsamo J, Lilien J, Anastasiadis PZ, Ullian EM, Reichardt LF. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and beta-catenin. J Cell Biol. 2008;183:893–908. doi: 10.1083/jcb.200807188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orlichenko L, Geyer R, Yanagisawa M, Khauv D, Radisky ES, Anastasiadis PZ, Radisky DC. The 19-amino acid insertion in the tumor-associated splice isoform Rac1b confers specific binding to p120 catenin. The Journal of biological chemistry. 2010;285:19153–19161. doi: 10.1074/jbc.M109.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf A, Keil R, Gotzl O, Mun A, Schwarze K, Lederer M, Huttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nature Cell Biology. 2006;8:1432–U1478. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 73.Kim H, Han JR, Park J, Oh M, James SE, Chang S, Lu Q, Lee KY, Ki H, Song WJ, Kim K. Delta-catenin-induced dendritic morphogenesis. An essential role of p190RhoGEF interaction through Akt1-mediated phosphorylation. J Biol Chem. 2008;283:977–987. doi: 10.1074/jbc.M707158200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 75.Smith AL, Dohn MR, Brown MV, Reynolds AB. Association of Rho-associated protein kinase 1 with E-cadherin complexes is mediated by p120-catenin. Mol Biol Cell. 2012;23:99–110. doi: 10.1091/mbc.E11-06-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schackmann RC, van Amersfoort M, Haarhuis JH, Vlug EJ, Halim VA, Roodhart JM, Vermaat JS, Voest EE, van der Groep P, van Diest PJ, Jonkers J, Derksen PW. Cytosolic p120-catenin regulates growth of metastatic lobular carcinoma through Rock1-mediated anoikis resistance. The Journal of clinical investigation. 2011;121:3176–3188. doi: 10.1172/JCI41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodwin M, Kovacs EM, Thoreson MA, Reynolds AB, Yap AS. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J Biol Chem. 2003;278:20533–20539. doi: 10.1074/jbc.M213171200. [DOI] [PubMed] [Google Scholar]
- 78.Daniel JM. Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso. Biochim Biophys Acta. 2007;1773:59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 79.van Roy FM, McCrea PD. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer. 2005;5:956–964. doi: 10.1038/nrc1752. [DOI] [PubMed] [Google Scholar]
- 80.Daniel JM, Spring CM, Crawford HC, Reynolds AB, Baig A. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30:2911–2919. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, Daniel JM, McCrea PD. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol. 2004 doi: 10.1038/ncb1191. [DOI] [PubMed] [Google Scholar]
- 82.Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: distinct catenin subfamilies promote Wnt signals. Dev Cell. 2006;11:683–695. doi: 10.1016/j.devcel.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Del Valle-Perez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, de Herreros AG, Dunach M. Wnt controls the transcriptional activity of Kaiso through CK1epsilon-dependent phosphorylation of p120-catenin. Journal of cell science. 2011;124:2298–2309. doi: 10.1242/jcs.082693. [DOI] [PubMed] [Google Scholar]
- 84.Hosking CR, Ulloa F, Hogan C, Ferber EC, Figueroa A, Gevaert K, Birchmeier W, Briscoe J, Fujita Y. The transcriptional repressor Glis2 is a novel binding partner for p120 catenin. Mol Biol Cell. 2007;18:1918–1927. doi: 10.1091/mbc.E06-10-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz MA, Shattil SJ. Signaling networks linking integrins and rho family GTPases. Trends Biochem Sci. 2000;25:388–391. doi: 10.1016/s0968-0004(00)01605-4. 388_00001605 00001608_00001605. [DOI] [PubMed] [Google Scholar]
- 87.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 88.Yanagisawa M, Anastasiadis PZ. p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. J Cell Biol. 2006;174:1087–1096. doi: 10.1083/jcb.200605022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson E, Seachrist DD, DeLeon-Rodriguez CM, Lozada KL, Miedler J, Abdul-Karim FW, Keri RA. HER2/ErbB2-induced breast cancer cell migration and invasion require p120 catenin activation of Rac1 and Cdc42. The Journal of biological chemistry. 2010;285:29491–29501. doi: 10.1074/jbc.M110.136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheung LW, Leung PC, Wong AS. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene. 2010;29:2427–2440. doi: 10.1038/onc.2009.523. [DOI] [PubMed] [Google Scholar]
- 91.Bellovin DI, Bates RC, Muzikansky A, Rimm DL, Mercurio AM. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 2005;65:10938–10945. doi: 10.1158/0008-5472.CAN-05-1947. [DOI] [PubMed] [Google Scholar]
- 92.Cozzolino M, Stagni V, Spinardi L, Campioni N, Fiorentini C, Salvati E, Alema S, Salvatore AM. p120 Catenin Is Required for Growth Factor-dependent Cell Motility and Scattering in Epithelial Cells. Mol Biol Cell. 2003;14:1964–1977. doi: 10.1091/mbc.E02-08-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–819. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 94.Roczniak-Ferguson A, Reynolds AB. Regulation of p120-catenin nucleocytoplasmic shuttling activity. J Cell Sci. 2003;116:4201–4212. doi: 10.1242/jcs.00724. [DOI] [PubMed] [Google Scholar]
- 95.Ichii T, Takeichi M. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12:827–839. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 96.Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 99.Nollet F, Berx G, Roy Fv. The Role of the E-Cadherin/Catenin Adhesion Complex in the Development and Progression of Cancer. Molecular Cell Biology research Communications. 1999;2:77–85. doi: 10.1006/mcbr.1999.0155. [DOI] [PubMed] [Google Scholar]
- 100.Motti ML, Califano D, Baldassarre G, Celetti A, Merolla F, Forzati F, Napolitano M, Tavernise B, Fusco A, Viglietto G. Reduced E-cadherin expression contributes to the loss of p27kip1-mediated mechanism of contact inhibition in thyroid anaplastic carcinomas. Carcinogenesis. 2005;26:1021–1034. doi: 10.1093/carcin/bgi050. [DOI] [PubMed] [Google Scholar]
- 101.StCroix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smalley-Freed WG, Efimov A, Short SP, Jia P, Zhao Z, Washington MK, Robine S, Coffey RJ, Reynolds AB. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PloS one. 2011;6:e19880. doi: 10.1371/journal.pone.0019880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, Rustgi AK. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dillon DA, D’Aquila T, Reynolds AB, Fearon ER, Rimm DL. The expression of p120ctn protein in breast cancer is independent of alpha- and beta-catenin and E-cadherin. Am J Pathol. 1998;152:75–82. [PMC free article] [PubMed] [Google Scholar]
- 107.Karayiannakis AJ, Syrigos KN, Efstathiou J, Valizadeh A, Noda M, Playford RJ, Kmiot W, Pignatelli M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J Pathol. 1998;185:413–418. doi: 10.1002/(SICI)1096-9896(199808)185:4<413::AID-PATH125>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 108.Kallakury BV, Sheehan CE, Winn-Deen E, Oliver J, Fisher HA, Kaufman RP, Jr, Ross JS. Decreased expression of catenins (alpha and beta), p120 CTN, and E-cadherin cell adhesion proteins and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer. 2001;92:2786–2795. doi: 10.1002/1097-0142(20011201)92:11<2786::aid-cncr10128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 109.Wang EH, Liu Y, Xu HT, Dai SD, Liu N, Xie CY, Yuan XM. Abnormal expression and clinicopathologic significance of p120-catenin in lung cancer. Histol Histopathol. 2006;21:841–847. doi: 10.14670/HH-21.841. [DOI] [PubMed] [Google Scholar]
- 110.Bremnes RM, Veve R, Gabrielson E, Hirsch FR, Baron A, Bemis L, Gemmill RM, Drabkin HA, Franklin WA. High-throughput tissue microarray analysis used to evaluate biology and prognostic significance of the E-cadherin pathway in non-small-cell lung cancer. J Clin Oncol. 2002;20:2417–2428. doi: 10.1200/JCO.2002.08.159. [DOI] [PubMed] [Google Scholar]
- 111.Mortazavi F, An J, Dubinett S, Rettig M. p120-catenin is transcriptionally downregulated by FOXC2 in non-small cell lung cancer cells. Molecular cancer research : MCR. 2010;8:762–774. doi: 10.1158/1541-7786.MCR-10-0004. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y, Li QC, Miao Y, Xu HT, Dai SD, Wei Q, Dong QZ, Dong XJ, Zhao Y, Zhao C, Wang EH. Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thoreson MA, Reynolds AB. Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation. 2002;70:583–589. doi: 10.1046/j.1432-0436.2002.700911.x. [DOI] [PubMed] [Google Scholar]
- 114.Sarrio D, Perez-Mies B, Hardisson D, Moreno-Bueno G, Suarez A, Cano A, Martin-Perez J, Gamallo C, Palacios J. Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene. 2004;23:3272–3283. doi: 10.1038/sj.onc.1207439. [DOI] [PubMed] [Google Scholar]
- 115.Mayerle J, Friess H, Buchler MW, Schnekenburger J, Weiss FU, Zimmer KP, Domschke W, Lerch MM. Up-regulation, nuclear import, and tumor growth stimulation of the adhesion protein p120 in pancreatic cancer. Gastroenterology. 2003;124:949–960. doi: 10.1053/gast.2003.50142. [DOI] [PubMed] [Google Scholar]
- 116.Maeda M, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, Caplan S, Wahl JK, 3rd, Wheelock MJ, Johnson KR. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120(ctn) Oncogene. 2006;25:4595–4604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- 117.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol. 2009;11:903–908. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 118.Paredes J, Correia AL, Ribeiro AS, Milanezi F, Cameselle-Teijeiro J, Schmitt FC. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. Journal of clinical pathology. 2008;61:856–862. doi: 10.1136/jcp.2007.052704. [DOI] [PubMed] [Google Scholar]
- 119.Rakha EA, Patel A, Powe DG, Benhasouna A, Green AR, Lambros MB, Reis-Filho JS, Ellis IO. Clinical and biological significance of E-cadherin protein expression in invasive lobular carcinoma of the breast. The American journal of surgical pathology. 2010;34:1472–1479. doi: 10.1097/PAS.0b013e3181f01916. [DOI] [PubMed] [Google Scholar]
- 120.Macpherson IR, Hooper S, Serrels A, McGarry L, Ozanne BW, Harrington K, Frame MC, Sahai E, Brunton VG. p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene. 2007;26:5214–5228. doi: 10.1038/sj.onc.1210334. [DOI] [PubMed] [Google Scholar]
- 121.Paredes J, Correia AL, Ribeiro AS, Schmitt F. Expression of p120-catenin isoforms correlates with genomic and transcriptional phenotype of breast cancer cell lines. Cellular oncology : the official journal of the International Society for Cellular Oncology. 2007;29:467–476. doi: 10.1155/2007/395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chartier NT, Oddou CI, Laine MG, Ducarouge B, Marie CA, Block MR, Jacquier-Sarlin MR. Cyclin-dependent kinase 2/cyclin E complex is involved in p120 catenin (p120ctn)-dependent cell growth control: a new role for p120ctn in cancer. Cancer Res. 2007;67:9781–9790. doi: 10.1158/0008-5472.CAN-07-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Slorach EM, Chou J, Werb Z. Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin isoform expression and localization. Genes & development. 2011;25:471–484. doi: 10.1101/gad.1998111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miao Y, Liu N, Zhang Y, Liu Y, Yu JH, Dai SD, Xu HT, Wang EH. p120ctn isoform 1 expression significantly correlates with abnormal expression of E-cadherin and poor survival of lung cancer patients. Medical oncology. 2010;27:880–886. doi: 10.1007/s12032-009-9300-2. [DOI] [PubMed] [Google Scholar]
- 125.Jiang G, Wang Y, Dai S, Liu Y, Stoecker M, Wang E. P120-catenin isoforms 1 and 3 regulate proliferation and cell cycle of lung cancer cells via beta-catenin and Kaiso respectively. PloS one. 2012;7:e30303. doi: 10.1371/journal.pone.0030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mariner DJ, Davis MA, Reynolds AB. EGFR signaling to p120-catenin through phosphorylation at Y228. Journal of cell science. 2004;117:1339–1350. doi: 10.1242/jcs.01001. [DOI] [PubMed] [Google Scholar]
- 127.Ma LW, Zhou ZT, He QB, Jiang WW. Phosphorylated p120-catenin expression has predictive value for oral cancer progression. Journal of clinical pathology. 2012;65:315–319. doi: 10.1136/jclinpath-2011-200516. [DOI] [PubMed] [Google Scholar]
- 128.Hofmann C, Lippert E, Falk W, Scholmerich J, Rogler G, Obermeier F. Primary human colonic epithelial cells are transiently protected from anoikis by a Src-dependent mechanism. Biochemical and biophysical research communications. 2009;390:908–914. doi: 10.1016/j.bbrc.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 129.Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, Rogler G, Grossmann J. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587–600. doi: 10.1053/j.gastro.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 130.Lugo-Martinez VH, Petit CS, Fouquet S, Le Beyec J, Chambaz J, Pincon-Raymond M, Cardot P, Thenet S. Epidermal growth factor receptor is involved in enterocyte anoikis through the dismantling of E-cadherin-mediated junctions. Am J Physiol Gastrointest Liver Physiol. 2009;296:G235–244. doi: 10.1152/ajpgi.90313.2008. [DOI] [PubMed] [Google Scholar]
- 131.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–6033. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]