Abstract
Background & Aims
Vaccine efficacy against gastric Helicobacter pylori infection has been demonstrated in mice, but little is known about the effector mechanisms of bacterial clearance. Our aim was to investigate a possible T cell-neutrophil pathway of vaccine-induced protection.
Methods
Nonimmune and immunized mice were compared for their response to H. pylori challenge. T cell responses were assessed by recall assays. IL-17-induced chemokine production by leukocyte and nonleukocyte cells was evaluated by cytokine ELISA. In a kinetic study, biopsies were collected at multiple time points post-challenge and assessed for bacterial load and inflammation. Relative levels of T cells, IL-17, IFNγ, MIP-2, KC, and LIX were determined by quantitative PCR. The role of neutrophils was evaluated by antibody-mediated depletion of neutrophils following challenge.
Results
Immunization induced strong IFNγ and IL-17-producing T cell responses, and IL-17 was capable of inducing significant amounts of KC and MIP-2 from dendritic cells, macrophages, fibroblasts, and gastric epithelial cells. Challenge of immunized mice induced significantly greater gastritis than infected mice, preceding significantly lower bacterial loads by day 7. In immune mice, T cell recruitment to the gastric mucosa correlated with a continuous rise in IL-17 and IFNγ, followed by KC, MIP-2, and LIX production and the recruitment of significant numbers of neutrophils by day 5. Antibody-mediated depletion of neutrophils abrogated vaccine efficacy.
Conclusions
Vaccination of mice against H. pylori results in a significant Th-17 cell recall response associated with increases in chemokines that attract neutrophils to the stomach which are important for eradication of H. pylori.
Helicobacter pylori is a gram-negative bacteria that inhabits the gastric mucosa. Over half the world's population is currently infected.1 Although the majority of individuals remain asymptomatic, infection can lead to the development of peptic ulcer disease, gastric MALT lymphoma, and gastric cancer, which are associated with significant morbidity and mortality. No vaccine is available for use in humans and antimicrobial therapies are complex, expensive, and not practical for use in many parts of the world where infection is endemic. Without therapeutic intervention, infection with H. pylori is life-long.
Vaccination in mouse models of H. pylori can achieve protection, and this model provides an excellent tool for comparing the immune responses of unimmunized and immunized mice. Infected mice develop low-grade, active, chronic inflammation which fails to clear the bacteria from the gastric mucosa. The immune response following challenge of immunized mice is rapid and severe and leads to a significant reduction in bacterial load, and sometimes clearance, of the bacteria. It is well established that CD4+ T cells are necessary for facilitating this protection,2, 3 but it is not understood how these T cells exert their effects and which immune cells act downstream of T cell activation to eliminate the bacteria.
IL-17 exhibits proinflammatory effects and is known to mediate the recruitment of neutrophils by inducing chemokine secretion in various cell populations.4, 5 Little is currently known about the role of IL-17 in H. pylori infection and immunity, although this cytokine has been identified in biopsies of H. pylori-infected patients and IL-17 levels have been shown to increase over time in the stomachs of infected mice.6, 7 Neutrophils are abundant in post-immunization gastritis and are capable of translocating across the gastric epithelium into the lumen where they can phagocytose H. pylori.8 H. felis-infected IL-10−/− mice, which are normally capable of eliminating the bacteria, have delayed clearance when they are treated with a neutrophil-depleting antibody.9 A role for neutrophils in vaccine-induced protection against H. pylori has not been demonstrated, although a model of decreased immunity in mice that received a sialoadenectomy was also accompanied by reduced numbers of neutrophils in the gastric mucosa.10 Therefore, we performed a kinetic study of cellular recruitment and cytokine production along with a neutrophil depletion model to assess the contribution of neutrophils in immunized/challenged mice. We now demonstrate that the protective immune response includes the early recruitment of T cells and significant IL-17 production, followed by the elaboration of neutrophil-promoting chemokines and subsequent reduction in bacterial load. Additionally, depletion of neutrophils in immunized mice during challenge compromises the host's ability to eradicate H. pylori.
Our findings suggest that IL-17 plays a major role in the early immune response of immunized mice, contributing to the rapid, severe inflammation in which neutrophils are an important effector cell for clearance of H. pylori.
Methods
Bacteria
Helicobacter pylori Sydney strain 1 (Hp SS1) was grown on Columbia blood agar or in Brucella broth with supplements as previously described.3 Actively growing liquid broth cultures were used for infection and in vitro assays. Live Hp SS1 cultures from Columbia blood agar plates were sonicated in PBS and passed through a 0.2 μm filter to produce bacterial lysate used for immunization and in vitro assays.
Mice
Wild-type C57BL/6 female and G-CSF knockout male and female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were housed under pathogen-free conditions in microisolator cages at Case Western Reserve University's Animal Resource Center (CWRU ARC). Mouse protocols were approved by the Institutional Animal Care and Use Committee. The CWRU ARC is accredited by the American Association for the Accreditation of Laboratory Animal Care International.
For immunization, mice received 100 μg Hp SS1 lysate and 5 μg cholera toxin adjuvant (Sigma, St. Louis, MO) in 20 μl PBS intranasally once a week for 4 weeks. For infection, 0.5 ml live Hp SS1 (approximately 107 CFU) in Brucella broth was administered by oral gavage on two consecutive days, unless otherwise noted.
in vivo Kinetic Study
Thirty five immunized and 35 unimmunized female C57BL/6 mice were infected on day 0 (approximately 1 month following the last immunization). Groups of five mice were sacrificed at days 1, 2, 3, 5, 7, 9, and 13. Five naïve mice were sacrificed at day 8. Mock-immunized (BSA and adjuvant) mice were infected with Hp SS1 and harvested at 2 weeks post-challenge. Stomachs were harvested for bacterial load determination, inflammation grading, and RNA isolation. Bacterial load (CFU/g tissue) was determined by homogenizing pre-weighed biopsies in Brucella broth and culturing serial dilutions on Columbia blood agar plates. Biopsies encompassing the outer curvature of the stomach were fixed in 10% buffered formalin, paraffin-embedded, and sectioned for histology. Gastritis and neutrophil levels were scored from H&E-stained sections by a pathologist (RWR) blinded to sample identities. The most inflamed area of each section was given a score of 0-5, as previously described.11 Gastric neutrophil counts were the number of neutrophils per high-power field of that most inflamed area. A third biopsy was stored in RNAlater (Ambion, Austin, TX) at −20°C, and then later used as a source of RNA for reverse transcription quantitative PCR for CD3, IFNγ, IL-17, KC, MIP-2, and LIX.
RT-quantitative PCR
Total RNA samples were quantified, and archive cDNA was generated using an ABI high-Capacity cDNA archive kit using similar amounts of total RNA as starting material in a 100ul RT reaction in an ABI 9700 PCR unit. 384-well plates were set up to accommodate triplicate reactions. Mouse beta actin was used as an endogenous control. The ABI assays for murine CD3, IFNγ, IL-17, KC, MIP-2, and LIX were purchased from ABI and run according to manufacturer's instructions for a 40 cycle run. Results were generated using ABI SDS 2.0 software and presented as relative fold changes versus a designated calibrator sample, which was a naïve mouse sample except for IFNγ and IL-17. For these samples, nothing was detected in the naïve controls, so an infected mouse from day one was used as a calibrator sample. Results include 95% confidence limits.
Cell Culture assays
Bone marrow (BM) was isolated from mouse hind leg femurs and tibias by standard technique. Macrophages were grown from BM cells in DMEM supplemented with 10% FBS, 1mM sodium pyruvate, 10mM HEPES, 20 μg/ml gentamicin, 50 μM β-mercaptoethanol, and 20% GM-CSF-conditioned media from Ladmac cell culture. Nonadherent cells were removed by washing. For DC cells, 106 BM cells were plated in 1 ml of RPMI (supplemented with 5% FBS, 10mM HEPES, 20 μg/ml gentamicin, 50 μM β-mercaptoethanol, and 10 ng/ml GM-CSF)/well in 24-well plates. Wells were gently washed on days 2 and 4. Loosely adherent cells were transferred on day 6 to petri dishes. Loosely adherent DC were collected from these dishes and used on day 7. Neutrophils were purified by percoll gradient centrifugation12 which yielded approximately 85% pure neutrophils by examination of H&E-stained cytospin slides. Fibroblasts were derived from mouse tail skin which was minced and incubated in DMEM containing 0.5 mg/ml dispase for 2 hours at 37°C. The cell-containing media was filtered and plated. Attached fibroblasts became confluent by day seven. The temperature sensitive GSM06 mouse gastric epithelial cell line originally cultured from SV40 T antigen-transgenic mice was purchased from the RIKEN Cell Bank (Tsukuba Science City, Japan). 13 They were grown to confluency in DMEM/F-12 (supplemented with 2% FBS and 1X ITES) at 33°C but used at 37°C in assays. All other cultures were maintained at 37°C. For assays, triplicate wells of 105 cells in 1.2 ml media were plated in 48-well plates and stimulated with 20 ng/ml recombinant mouse IL-17 or IFNγ (eBioscience, San Diego, CA).
in vitro Antigen Presenting Cell (APC)-T cell Co-cultures
Murine macrophages or DC were left untreated or stimulated with Hp SS1 lysate (10 μg/ml) for 4 hours, washed twice, and plated at 105 cells per well in 96-well, round-bottom plates in RPMI (supplemented with 10% FBS and 20 μg/ml gentamicin). For T cells, CD4+ cells were purified from bulk splenocytes using positive selection on MACS magnetic columns (Miltenyi Biotec, Auburn, CA). 106 CD4+ T cells from each mouse were co-cultured with APCs in triplicate. Supernatants were stored at −70°C until assessed by ELISA.
Enzyme-linked Immunosorbent Assays
Culture supernatants were analyzed for IL-17, IFNγ, KC, MIP-2, and LIX levels using DuoSet ELISA Development Systems according to the manufacture's protocols (R&D Systems, Minneapolis, MN). Concentrations were determined by comparison to a standard curve included on each plate.
Neutrophil Depletion study
The neutrophil-specific NIMP-R14 hybridoma was a generous gift from Dr. Eric Pearlman (CWRU SOM, Cleveland, OH). 14 Monoclonal rat IgG antibody was purified from hybridoma supernatants by protein G affinity chromatography. Male and female G-CSF KO mice were used at approximately six weeks of age and distributed equally among groups. Hp SS1-immunized G-CSF KO mice were given 1 mg NIMP-R14 in PBS i.p. beginning one day prior to Hp SS1 challenge and continuing every other day until harvest (2 weeks post-challenge). Another group of immunized/challenged G-CSF KO mice were injected with control rat IgG monoclonal antibody (BioExpress, West Lebanon, NH). Immunized/challenged G-CSF KO and WT mice without antibody treatment were also included. Venous blood collected at harvest was analyzed for all white blood cell levels on a Hemavet 950 (Drew Scientific, Oxford, CT). Stomach biopsies were harvested and analyzed for bacterial load and inflammation as described above. Gastric histology was also analyzed for neutrophil and mast cell levels by H&E and toluidine blue staining, respectively.
Statistics
Statistics were determined using the Student's t test. Differences between groups were considered significant at an interval level of p<0.05.
Results
CD4+ T cells from immunized mice exhibit a robust IL-17 recall response in vitro
Recall assays were performed to investigate potential differences in the proinflammatory properties of splenic CD4+ T cells taken from immunized (unchallenged) mice a week after the final boost, compared to cells from chronically infected mice taken at least three weeks after infection. H. pylori-pulsed BM-derived macrophages or DC were used to activate CD4+ cells from naïve, infected, and immunized mice. IFNγ was produced by CD4+ cells from immunized mice stimulated with pulsed DC by 48 hours, but not by cells from non-immunized infected mice (Figure 1A). When macrophages were used, IFNγ was also produced by the CD4+ cells from immunized mice, but at approximately half the levels observed in DC-stimulated cultures (Figure 1B). IL-17 was also detected at higher concentrations than IFNγ (Figure 1C and D). Similar to IFNγ production, the use of DC resulted in approximately twice the amount of IL-17 compared to macrophages. Little, if any, IL-17 was produced by cells from unimmunized, infected mice. No cytokine was detected from pulsed APC monocultures (data not shown), cultures of CD4+ cells in the absence of APC, or from any cultures using cells from naïve mice.
Figure 1.
Cytokine recall response of T cells from H. pylori-infected and immune mice. Purified CD4+ cells were isolated from the spleens of naïve mice, immunized unchallenged mice, or mice experimentally infected with H. pylori and co-cultured for 48 hours with antigen-pulsed APCs. The culture supernatants were assessed by quantitative ELISA for the presence of IFNγ or IL-17. (A) IFNγ production in the presence of pulsed dendritic cells (*P = .004 at 48 hr). (B) IFNγ production in the presence of pulsed macrophages (*P < .03 at 48 hr). (C) IL-17 production in the presence of pulsed DC (*P ≤ .005 at 24 and 48 hr). (D) IL-17 production in the presence of pulsed macrophages (*P = .02 at 24 and 48 hr). U/C = Unimmunized/challenged. I = Immunized. DC = dendritic cells. Macs = macrophages.
IL-17 and H. pylori induce the production of chemokines for neutrophil recruitment in vitro
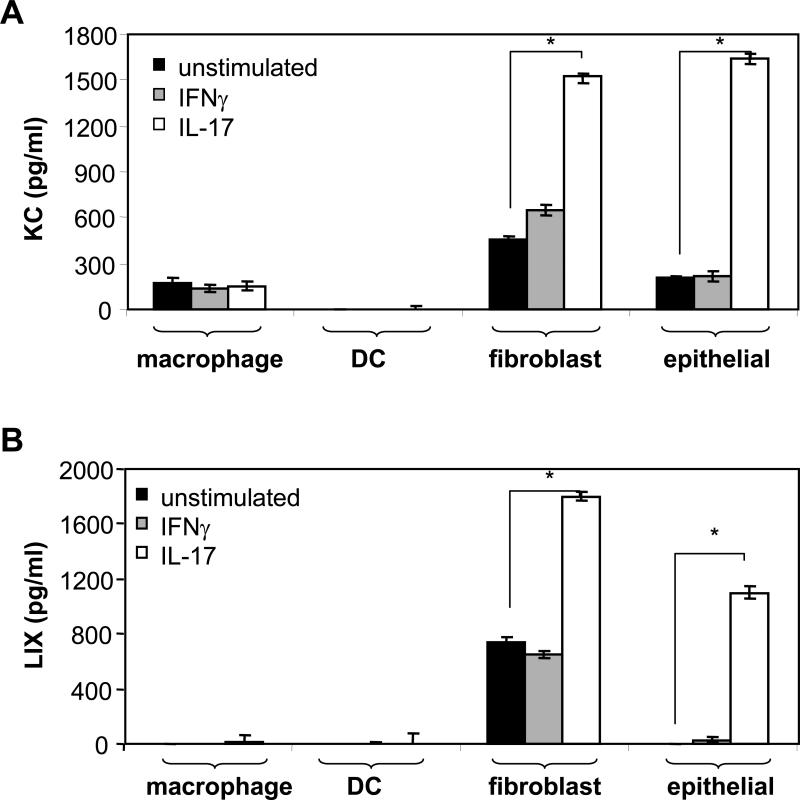
In vitro culture models of cell types likely to be present in the gastric mucosa, including macrophages, DC, fibroblasts, and epithelial cells, were stimulated with IFNγ or IL-17, and the supernatants were evaluated for KC, MIP-2, and LIX. IFNγ failed to induce the production of chemokines beyond the levels observed for unstimulated cells (Figure 2A and B). Recombinant IL-17, however, induced fibroblasts and epithelial cells to produce significant amounts of both KC (Figure 2A) and LIX (Figure 2B) by 24 hours. No MIP-2 could be detected in any cultures stimulated with IL-17 or IFNγ. Direct stimulation of cells with live H. pylori or H. pylori lysate antigen induced differing patterns of secretion. DC and fibroblasts produced KC, MIP-2, and LIX, macrophages produced KC and MIP-2, and epithelial cells produced some KC and LIX. Neutrophils were shown to produce MIP-2 only (data not shown).
Figure 2.
Chemokine secretion induced by IL-17 and IFNγ in vitro. Bone marrow-derived macrophages and DC, primary cultures of fibroblasts, and the gastric epithelial line GSM06 were cultured in the presence of 20 ng/ml of either IFNγ or IL-17 for 24 hours, and the culture supernatants were assessed for (A) KC or (B) LIX by quantitative ELISA. *P < .0005 when compared to unstimulated cells. No MIP-2 was produced.
T cells and IL-17 are detected at day 3 post-challenge in immunized mice, prior to increase in inflammation
Immunized mice were assessed at days 1, 2, 3, 5, 7, 9, and 13 post-challenge to characterize the early immune response. Significant gastritis developed by day five and inflammation continued to increase through day 13 (Figure 3A). In contrast, unimmunized mice did not develop statistically significant inflammation until day 13. The increase in inflammation in immunized mice at day five preceded a subsequent decrease in bacterial load that was evident by day seven (Figure 3B). Unlike the immunized mice, unimmunized mice displayed levels of bacteria that continued to increase over time.
Figure 3.
Kinetic evaluation of the gastric mucosa following challenge of immunized and unimmunized mice with H. pylori. Mice were immunized with H. pylori lysate antigen plus cholera toxin adjuvant and then challenged with H. pylori. (A) Subgroups of mice were harvested and evaluated at multiple timepoints through day 13 post-challenge for the degree histologic gastritis. (B) Longitudinal biopsies encompassing the entire length of the gastric mucosa were homogenized and plated on nutrient agar for CFU determination. *P < 0.05 for U/C versus I/C groups. U/C = Unimmunized/challenged. I/C = Immunized/challenged.
Recruitment of T cells in these mice correlated with the development of inflammation. Significant levels of CD3 mRNA were detectable in the stomachs of immunized mice by three days post-challenge, and similar to the overall gastritis scores in these mice, continued to increase throughout the entire 13 days (Figure 4A). This suggests T cell recruitment commences immediately prior to the development of significant gastritis on day five (Figure 4A). Unimmunized mice did not develop levels of CD3 that were significantly greater than naïve mice until day 13. Increased expression of CD3 was accompanied by the production of IL-17 that was significantly greater than unimmunized mice by day three (Figure 4B). IFNγ levels also increased by day five but were significantly lower than IL-17 expression on days five through 13. Unimmunized mice produced very low levels of both cytokines beginning at day 13 post-challenge. Because mock immunization with CT alone has occasionally been shown to elicit some degree of Helicobacter immunity, in a separate experiment we assessed the efficacy of mock immunization and the amount of IL-17 in the gastric mucosa. Although the CT immunized mice had less bacteria than unimmunized/challeneged mice, they had a significantly elevated bacterial load (P = 0.01) compared to mice immunized by the standard vaccine. Gastric IL-17 and IFNγ mRNA expression in CT-immunized mice was comparable to unimmunized/challenged mice and significantly less than mice immunized by our standard protocol (P < 0.004; data not shown).
Figure 4.
T cell response in the gastric mucosa following challenge of immunized mice with H. pylori. Longitudinal gastric biopsies from both nonimmune and immune mice were collected at multiple time points following challenge with 1 × 107 H. pylori, and RNA was isolated for quantitative PCR. A single group of naïve mice were evaluated for use as T = 0. Tissues were assessed for relative expression of (A) CD3 mRNA as a marker for total T cells, (B) IL-17 mRNA, and (C) IFNγ mRNA. *P ≤ .03 for immunized/challenged mice compared to nonimmune/challenged mice. U/C = Unimmunized/challenged. I/C = Immunized/challenged. RU = Relative Units.
Immune mice recruit neutrophils to the gastric mucosa prior to reductions in bacterial load
To determine whether the IL-17 observed above promoted neutrophil recruitment, KC, MIP-2, and LIX mRNA was also measured. Although the relative quantity of LIX mRNA was significantly elevated by day three in immunized mice (P < 0.02; Figure 5A), notably elevated mRNA for all three chemokines were observed by day five (P ≤ 0.02; Figure 5A, B, and C). Little or no expression of KC, MIP-2, or LIX was detected in the stomachs of nonimmunized mice. Histologic scoring of sections from these stomach tissues revealed significant increases in the number of neutrophils by day five post-challenge in immune mice. Neutrophil levels remained elevated throughout the time course, although levels did decline following significant decreases in bacterial load (Figure 5D – 5F).
Figure 5.
Local chemokine production in the gastric mucosa following challenge of immunized mice with H. pylori. Longitudinal gastric biopsies from both nonimmune and immune mice were collected at multiple time points following challenge with H. pylori, and RNA was isolated for quantitative PCR. A single group of naïve mice were evaluated for use as T = 0. Tissues were assessed for relative expression of (A) LIX mRNA, (B) KC mRNA, and (C) MIP-2 mRNA (*P ≤ .02 for immunized/challenged mice compared to nonimmune/challenged mice). (D) Neutrophils were quantified by histologic evaluation for days 1 through 13 (*P ≤ .04 for immunized/challenged mice compared to nonimmune/challenged mice). (E) H & E stained section of mouse gastric mucosa of immunized mouse three days post-challenge (magnification 20X). (F) H&E stained section of mouse gastric mucosa five days post-challenge illustrating significant neutrophil recruitment (Magnification 20X). U/C = Unimmunized/challenged. I/C = Immunized/challenged. RU = Relative Units.
Neutrophil depletion of immunized G-CSF−/− mice results in reduced protective immunity against H. pylori challenge
Neutrophils were depleted to determine if they represent an important effector mechanism in the clearance of H. pylori from the gastric mucosa of immunized mice. G-CSF−/− mice were used to decrease the baseline amount of neutrophils. NIMP-R14 antibody was administered to immunized mice throughout the challenge phase and peripheral blood cell counts were performed at harvest to assess the efficacy of antibody treatment. NIMP-R14-treated immunized mice had significantly lower peripheral blood neutrophils than either control untreated groups or mice treated with control rat monoclonal antibody (Figure 6A; P<0.01). Immunized mice treated with control rat IgG or left untreated had comparable blood neutrophil levels. The majority of NIMP-R14-treated immunized mice also had reduced neutrophil counts in the gastric mucosa compared to control antibody treated mice (Figure 6B). Mast cell levels in the gastric mucosa of these mice were low and did not vary among groups (data not shown).
Figure 6.
Vaccine efficacy against H. pylori challenge in neutrophil-depleted mice. One day prior to challenge and then every other day until harvest at day 14, immunized mice were given NIMP-R14 monoclonal antibody or control rat IgG monoclonal antibody (1 mg/dose). (A) Peripheral blood neutrophil levels were assessed at the time of harvest using whole blood (*P < .01). (B) Gastric neutrophil levels at the time of harvest were enumerated by histologic analysis. (C) Inflammation scores were determined by histologic analysis of longitudinal sections. (D) Bacterial load was derived by CFU determination of homogenized gastric biopsies (*P < .04). U/C = Unimmunized/challenged. I/C = Immunized/challenged.
Gastric inflammation scores in the G-CSF−/− mice were equivalent in all three immunized groups 14 days post-challenge suggesting that reduction in neutrophil counts did not affect the overall intensity of inflammation (Figure 6C). However, although gastritis levels were comparable among these groups of mice, only the NIMP-R14-treated mice had a reduced capacity to eradicate the H. pylori from the gastric mucosa (Figure 6D). Untreated and control IgG-treated immunized mice achieved protective immunity, while the bacterial load in neutrophil-depleted mice was equivalent to unimmunized mice.
Discussion
The ability to achieve protective immunity against H. pylori in mice has been well documented. The immunologic events responsible for reduced bacterial loads in immunized mice, however, are still being elucidated. CD4+ T cells are necessary for the development of protective immunity2, 3 and the p40 subunit of both IL-12 and IL-23 is also important as immunized mice are not fully protected in its absence.15 More recently mast cells have also been implicated as an important mediator of protection in an H. felis immunization model.16 We now demonstrate that depletion of neutrophils in immunized mice compromises the host's ability to eradicate H. pylori following challenge.
The capacity of neutrophils to kill H. pylori in vitro 17 and in vivo 8 has been described, and the abundance of these phagocytes combined with their ability to cross the epithelium make them attractive candidates as effectors of bacterial clearance. Additional evidence for the importance of neutrophils in Helicobacter eradication comes from a study in IL-10−/− mice.9 These mice develop severe inflammation capable of clearing H. felis from the gastric mucosa. However, when neutrophils were depleted, the mice failed to significantly reduce the bacterial load.
Data from our kinetic study suggest that challenge of immunized mice results in a temporally-ordered sequence of events involving proinflammatory T cell cytokines, resident nonleukocyte cells, and the production of chemokines that favor neutrophil recruitment. T cells were present by day three post-challenge and the production of IFNγ, and, to a greater extent, IL-17 occurred concomitantly with recruitment of the T cells. The production of these cytokines was rapidly followed by significant increases in KC, MIP-2, and LIX and a significant increase in general inflammation. The recruitment of significant numbers of neutrophils did not occur until the relevant chemokines were evident on day five. As the levels of neutrophils continued to increase on day seven, the bacterial load began to significantly decrease.
The rapid induction of inflammation post-challenge in immunized mice reported here are consistent with previous reports that included early timepoints.11, 18 Also similar to our analysis, Walduck et al. reported that gastric CD4+ and CD8+ cells were detectable in immune mice by day three post-challenge followed by a significant decrease in bacterial load by day seven.18 Of particular interest is the link between the CD4+ T cell response and recruitment and activation of neutrophils. In the absence of CD4+ T cells, mice fail to develop significant gastritis when infected with Helicobacter strains.19 Thus, neutrophils do not inherently participate in the acute phase response to H. pylori infection and become prevalent only following appropriate T cell help signals. During natural and experimental infection T regulatory cells can suppress the activity of proinflammatory T cells.20, 21 In immunized mice, however, T helper cells are sufficiently activated to drive a proinflammatory response that ultimately reduces or eliminates H. pylori. The amount of IL-17 produced in vitro by isolated T cells from immune mice, and the prevalence of IL-17 in the gastric mucosa following challenge of immune mice compared to nonimmune mice indicate that IL-17-producing T cells promote this response.
Th-17 cells have recently been described as a distinct population of proinflammatory T cells that play an important role in the host response to infectious disease.4, 22 Cells expressing receptors for IL-17 include fibroblasts and epithelial cells.4, 23 Thus our experiments in which IL-17 induced the production of KC, MIP-2, and LIX primarily from murine fibroblasts and gastric epithelial cells are consistent with the known activity of IL-17 and supports our tissue-based observations that these three chemokines show a rapid and significant increase following the local production of IL-17.
IL-17 is elevated in biopsies from infected patients6 but since infection persists in humans, little can be concluded about the role of IL-17 in the host response. Our results in the mouse model show that immunized mice exhibit a robust IL-17 recall response not seen with cells or tissue from unimmunized/challenged mice. IL-17 expression occurred within a few days of bacterial challenge and remained elevated throughout the effector phase of bacterial eradication. A central role for Th-17 cells as mediators of protective Helicobacter immunity may explain why several IFNγ-deficient mice can still be protected by vaccination.15, 24 Such results suggest that it is IL-17 and not IFNγ that drives the protective inflammation, or that in the absence of IFNγ, IL-17 serves as a surrogate pathway for developing protective gastritis. A crucial role for IL-17 is also consistent with the inability to protectively immunize p40 knockout mice since IL-23 may be required for the proper development of Th-17 cells.15
Our results contrast with a report by Velin et al. in which antibody-mediated depletion of neutrophils failed to ameliorate vaccine-induced protective immunity against H. felis.16 Differences in results may be due to differences between the H. pylori and H. felis models of infection, or the different time points used for evaluation. However, as noted above, Ismail et al. demonstrated a substantial contribution of neutrophils to non-vaccination based bacterial clearance in IL-10−/− mice using H. felis.9 It is more likely the different results are due to the effectiveness of neutrophil depletion. Although Velin et al. reported significant reductions of neutrophil levels in peripheral blood, such observations cannot necessarily be extrapolated to the tissue in which inflammation is induced. Ismail et al. observed a decrease in bacterial load as neutrophil levels began to rebound in the stomach, despite the continued significant reduction of peripheral neutrophils. H. felis typically induces robust inflammation, so it is possible that whatever neutrophil levels remained following antibody treatment were concentrated in the gastric mucosa where they could effectively kill the bacteria. Since gastric neutrophil levels were not assessed by Velin et al, it is impossible to know whether in fact the neutrophil depletion obtained in the periphery was apparent in the gastric mucosa as well.
In our study, to better evaluate the contribution of neutrophils in the protective response, we used G-CSF−/− mice combined with treatment with the anti-neutrophil antibody NIMP-R14. Our preliminary studies in C57BL/6 wild type mice showed that antibody treatment becomes less effective over time (data not shown), possibly due to the host's eventual immune response to the presence of foreign antibody. The inability to suppress neutrophil levels for prolonged periods is problematic when studying models of chronic infection like H. pylori where treatment regimens last for weeks. The use of G-CSF−/− mice in addition to antibody-mediated depletion achieved sufficiently low neutrophil levels for most mice for the entire two week study. The importance of maintaining low levels of neutrophils for the duration is illustrated by our observation that two of the NIMP-R14-treated mice with higher gastric neutrophil counts than average were still able to clear the bacteria.
One concern with the use of G-CSF−/− mice is the possibility that the lack of G-CSF might affect the development of other cell populations. While these mice do tend to have fewer circulating monocytes, the deficiency becomes significant only in older mice. 25 Additionally, peripheral blood analyses at harvest confirmed that no differences in erythrocyte, lymphocyte, or eosinophil levels were observed compared to wild type mice. Interestingly, there were no differences in gastric mast cell levels among any of the groups of G-CSF−/− mice in our study (data not shown). Since Velin et al. demonstrated that mast cells may be a mediator of H. felis eradication in immunized mice, it was important to verify that the failure of the immunized NIMP-R14-treated G-CSF−/− mice to reduce their bacterial load was not due to reduced mast cell numbers.
Our study does not negate a role for mast cells in vaccine-induced protective Helicobacter immunity, but suggests that neutrophils are also important and play an independent role from mast cells. It is possible that mast cells are required for the activation of neutrophils. Indeed mast cells have been shown to recruit neutrophils in other models, including allergic gastric inflammation.26 Additionally, others have reported that mast cell-mediated neutrophil recruitment via IL-8 was dependent on the heterotypic adhesion of mast cells and activated T cells in vitro.27 Such findings may explain the necessity of CD4+ T cells, mast cells, and neutrophils for the clearance of H. pylori in immunized mice.
In conclusion, our study demonstrates that neutrophils are required for the clearance of H. pylori in a model of vaccine-induced immunity. We also suggest that unlike unimmunzed mice, immune mice are able to eliminate the bacteria due to the rapid activation of Th-17 cells which act on resident nonleukocyte cell populations to produce chemokines that subsequently recruit and activate the neutrophils. The means by which immunization predominantly promotes Th-17 cells rather than IFNγ-producing T cells and the relationship between the Th-17 cells and nonlymphocyte effector cells will be the subject of future investigation.
Acknowledgments
Grant support: This research was supported by NIH grant AI055710 (T.G.B.) and a grant to the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (P30 CA43703).
Footnotes
No conflicts of interest exist
References
- 1.Marshall BJ. The 1995 Albert Lasker Medical Research Award. [i]Helicobacter pylori[/i]. The etiologic agent for peptic ulcer. Jama. 1995;274:1064–6. doi: 10.1001/jama.274.13.1064. [DOI] [PubMed] [Google Scholar]
- 2.Ermak TH, Giannasca PJ, Nichols R, Myers GA, Nedrud J, Weltzin R, Lee CK, Kleanthous H, Monath TP. Immunization of mice with urease vaccine affords protection against [i]Helicobacter pylori[/i] infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottwein JM, Blanchard TG, Targoni OS, Eisenberg JC, Zagorski BM, Redline RW, Nedrud JG, Tary-Lehmann M, Lehmann PV, Czinn SJ. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Inf Dis. 2001;184:308–14. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 4.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–6. [PubMed] [Google Scholar]
- 6.Luzza F, Parrello T, Monteleone G, Sebkova L, Romano M, Zarrilli R, Imeneo M, Pallone F. Up-regulation of IL-17 is associated with bioactive IL-8 expression in [i]Helicobacter pylori[/i]-infected human gastric mucosa. J Immunol. 2000;165:5332–7. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 7.Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr., Cover TL. Host response to [i]Helicobacter pylori[/i] infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51:577–86. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 8.Zu Y, Cassai ND, Sidhu GS. Light microscopic and ultrastructural evidence of in vivo phagocytosis of [i]Helicobacter pylori[/i] by neutrophils. Ultrastruct Pathol. 2000;24:319–23. doi: 10.1080/019131200750035049. [DOI] [PubMed] [Google Scholar]
- 9.Ismail HF, Fick P, Zhang J, Lynch RG, Berg DJ. Depletion of neutrophils in IL-10(−/−) mice delays clearance of gastric Helicobacter infection and decreases the Th1 immune response to Helicobacter. J Immunol. 2003;170:3782–9. doi: 10.4049/jimmunol.170.7.3782. [DOI] [PubMed] [Google Scholar]
- 10.Shirai Y, Wakatsuki Y, Kusumoto T, Nakata M, Yoshida M, Usui T, Iizuka T, Kita T. Induction and maintenance of immune effector cells in the gastric tissue of mice orally immunized to [i]Helicobacter pylori[/i] requires salivary glands. Gastroenterology. 2000;118:749–59. doi: 10.1016/s0016-5085(00)70144-2. [DOI] [PubMed] [Google Scholar]
- 11.Garhart CA, Redline RW, Nedrud JG, Czinn SJ. Clearance of [i]Helicobacter pylori[/i] infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect Immun. 2002;70:3529–3538. doi: 10.1128/IAI.70.7.3529-3538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiyama N, Tabuchi Y, Horiuchi T, Obinata M, Furusawa M. Establishment of gastric surface mucous cell lines from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Exp Cell Res. 1993;209:382–7. doi: 10.1006/excr.1993.1324. [DOI] [PubMed] [Google Scholar]
- 14.Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–94. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 15.Garhart CA, Heinzel FP, Czinn SJ, Nedrud JG. Vaccine-induced reduction of [i]Helicobacter pylori[/i] colonization in mice is interleukin-12 dependent but gamma interferon and inducible nitric oxide synthase independent. Infect Immun. 2003;71:910–21. doi: 10.1128/IAI.71.2.910-921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velin D, Bachmann D, Bouzourene H, Michetti P. Mast cells are critical mediators of vaccine-induced Helicobacter clearance in the mouse model. Gastroenterology. 2005;129:142–55. doi: 10.1053/j.gastro.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Andersen LP, Blom J, Nielsen H. Survival and ultrastructural changes of [i]Helicobacter pylori[/i] after phagocytosis by human polymorphonuclear leukocytes and monocytes. Apmis. 1993;101:61–72. [PubMed] [Google Scholar]
- 18.Walduck A, Schmitt A, Lucas B, Aebischer T, Meyer TF. Transcription profiling analysis of the mechanisms of vaccine-induced protection against [i]H. pylori.[/i] Faseb J. 2004;18:1955–7. doi: 10.1096/fj.04-2321fje. [DOI] [PubMed] [Google Scholar]
- 19.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of [i]Helicobacter felis[/i]-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 20.Anderson KM, Czinn SJ, Redline RW, Blanchard TG. Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric [i]Helicobacter pylori[/i] infection. J Immunol. 2006;176:5306–13. doi: 10.4049/jimmunol.176.9.5306. [DOI] [PubMed] [Google Scholar]
- 21.Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, Reindl W, Dossumbekova A, Friedrich M, Saur D, Wagner H, Schmid RM, Prinz C. CD25+/Foxp3+ T cells regulate gastric inflammation and [i]Helicobacter pylori[/i] colonization in vivo. Gastroenterology. 2006;131:525–37. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–51. [PubMed] [Google Scholar]
- 23.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 24.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y, Iwakura Y, Imanishi J. Role of gamma interferon in [i]Helicobacter pylori[/i]-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–46. [PubMed] [Google Scholar]
- 26.Wershil BK, Furuta GT, Wang ZS, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–90. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- 27.Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human mast cells release Interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells. Allergy. 2005;60:1316–9. doi: 10.1111/j.1398-9995.2005.00886.x. [DOI] [PubMed] [Google Scholar]