Abstract
The adverse effects of prenatal alcohol exposure constitute a continuum of disabilities (fetal alcohol spectrum disorders [FASD]). In 1996, the Institute of Medicine established diagnostic categories delineating the spectrum but not specifying clinical criteria by which diagnoses could be assigned. In 2005, the authors published practical guidelines operationalizing the Institute of Medicine categories, allowing for standardization of FASD diagnoses in clinical settings. The purpose of the current report is to present updated diagnostic guidelines based on a thorough review of the literature and the authors’ combined expertise based on the evaluation of >10 000 children for potential FASD in clinical settings and in epidemiologic studies in conjunction with National Institute on Alcohol Abuse and Alcoholism–funded studies, the Collaborative Initiative on Fetal Alcohol Spectrum Disorders, and the Collaboration on FASD Prevalence. The guidelines were formulated through conference calls and meetings held at National Institute on Alcohol Abuse and Alcoholism offices in Rockville, MD. Specific areas addressed include the following: precise definition of documented prenatal alcohol exposure; neurobehavioral criteria for diagnosis of fetal alcohol syndrome, partial fetal alcohol syndrome, and alcohol-related neurodevelopmental disorder; revised diagnostic criteria for alcohol-related birth defects; an updated comprehensive research dysmorphology scoring system; and a new lip/philtrum guide for the white population, incorporating a 45-degree view. The guidelines reflect consensus among a large and experienced cadre of FASD investigators in the fields of dysmorphology, epidemiology, neurology, psychology, developmental/behavioral pediatrics, and educational diagnostics. Their improved clarity and specificity will guide clinicians in accurate diagnosis of infants and children prenatally exposed to alcohol.
The adverse effects of alcohol on the developing fetus were described independently by Lemoine et al in 19681 and by Jones et al in 1973.2 As with most malformation syndromes, the most severely affected children were described first, with the associated pattern of malformation termed fetal alcohol syndrome (FAS).2 As pediatricians became more familiar with the clinical presentation of children prenatally exposed to alcohol, it became clear that the associated disabilities represent a spectrum, from mild to severe (fetal alcohol spectrum disorders or FASD). In 1996, the Institute of Medicine (IOM) described 4 distinct diagnostic categories within FASD: FAS, partial fetal alcohol syndrome (PFAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).3 However, the task force did not specify the clinical process by which individual children could be assigned to the groups. Since that time, a number of diagnostic systems have been proposed.4–10 In 2005, Hoyme et al4 described specific clinical guidelines that allowed for assigning diagnoses within the 1996 IOM classification.
Subsequently, the authors have evaluated >10 000 children for potential FASD in clinical settings and epidemiologic studies as part of National Institute on Alcohol Abuse and Alcoholism (NIAAA) supported studies, the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), and the Collaboration on FASD Prevalence (CoFASP). CIFASD was established by NIAAA in 2003 to investigate data-driven methods for complete diagnosis of the continuum of FASD, prevention of the adverse effects of prenatal alcohol exposure, and effective interventions for affected individuals.11,12 CoFASP seeks to establish the prevalence of FASD among school-age children in US communities by using active case ascertainment methodology.12 Based on this broad multidisciplinary experience, the purpose of this special article is to propose updated clinical guidelines for diagnosing FASD that clarify and expand on the original 2005 guidelines. These updated diagnostic criteria have been formulated, reviewed, and accepted by the investigators and collaborating sites of CoFASP and the administrative core of CIFASD. They do not necessarily represent the policy of the American Academy of Pediatrics.
Background and Scope of the Problem
FASDs are the leading cause of preventable developmental disabilities in the world. Recent school-based studies in the United States estimate the prevalence of FASD to be much higher than previously thought. May et al13 recently recorded combined rates of FAS and PFAS of 10.9 to 25.2 per 1000 (1.1%–2.5%) in a Rocky Mountain community, whereas the complete continuum of FASD (including ARND) was observed to be 24 to 48 per 1000 (2.4%–4.8%) in a community in the Northern Plains.14 In the mixed race population of the Western Cape Province in South Africa, the highest prevalence rates of FASD in the world have been documented, 135.1 to 207.5 per 1000 (13.5%–20.8%).15 The World Health Organization (WHO) is currently planning prevalence studies in several countries in Europe, Asia, Africa, and North America, which should lead to global data about the frequency of this continuum of disabilities.16
The high prevalence of FASD produces an immense burden to society in financial terms, unrealized productivity, and human suffering. In the United States, annual cost estimates have ranged from $74.6 million in 198417 to $4.0 billion in 1998.18 In 2007, the estimated annual cost of FASD in Canada was CAD $5.3 billion.19
The soaring prevalence and burden of FASD in children recently led the American Academy of Pediatrics to stress the following: no amount of alcohol intake during pregnancy can be considered safe; there is no safe trimester to drink alcohol; all forms of alcohol pose a similar risk; and binge drinking poses a dose-related risk to the fetus.20
Preparation of Updated Diagnostic Guidelines
These guidelines were formulated by the authors over a 12-month period, through a series of conference calls and face-to-face meetings at the offices of NIAAA in Rockville, MD. The following working subgroups of investigators were organized to revisit diagnostic criteria: dysmorphology evaluation, neurobehavioral assessment, and definition of significant documented prenatal alcohol exposure. Recommendations from the working committees were brought back to the larger group for discussion and revision. The guidelines presented herein are the result of a thorough review of the literature and the longstanding collective expertise of the authors. The updated clinical guidelines for diagnosis of FASD are set forth in Table 1.
TABLE 1.
Updated Criteria for the Diagnosis of FASD
| Diagnostic Categories |
|---|
| (See Table 2 for definition of documented prenatal alcohol exposure) |
| I. FAS |
| (With or without documented prenatal alcohol exposure) |
| A diagnosis of FAS requires all features, A–D: |
| A. A characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤10th centile) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| B. Prenatal and/or postnatal growth deficiency |
| 1. Height and/or weight ≤10th centile (plotted on a racially or ethnically appropriate growth curve, if available) |
| C. Deficient brain growth, abnormal morphogenesis, or abnormal neurophysiology, including ≥1 of the following: |
| 1. Head circumference ≤10th percentile |
| 2. Structural brain anomalies |
| 3. Recurrent nonfebrile seizures (other causes of seizures having been ruled out) |
| D. Neurobehavioral impairmenta |
| 1. For children ≥3 y of age (a or b): |
| a. WITH COGNITIVE IMPAIRMENT: |
| −Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥1.5 SD below the mean) |
| OR |
| −Cognitive deficit in at least 1 neurobehavioral domain ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. WITH BEHAVIORAL IMPAIRMENT WITHOUT COGNITIVE IMPAIRMENT: |
| −Evidence of behavioral deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioral regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 y of age: |
| −Evidence of developmental delay ≥1.5 SD below the mean |
| II. PFAS |
| -For children with documented prenatal alcohol exposure, a diagnosis of PFAS requires features A and B: |
| A. A characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤10th centile) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| B. Neurobehavioral impairmenta |
| 1. For children ≥3 y of age (a or b): |
| a. WITH COGNITIVE IMPAIRMENT: |
| −Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥1.5 SD below the mean) |
| OR |
| −Cognitive deficit in at least 1 neurobehavioral domain ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. WITH BEHAVIORAL IMPAIRMENT WITHOUT COGNITIVE IMPAIRMENT: |
| −Evidence of behavioral deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioral regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 y of age: |
| −Evidence of developmental delay ≥1.5 SD below the mean |
| -For children without documented prenatal alcohol exposure, a diagnosis of PFAS requires all features, A–C: |
| A. A characteristic pattern of minor facial anomalies, including ≥2 of the following: |
| 1. Short palpebral fissures (≤10th centile) |
| 2. Thin vermilion border of the upper lip (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| 3. Smooth philtrum (rank 4 or 5 on a racially normed lip/philtrum guide, if available) |
| B. Growth deficiency or deficient brain growth, abnormal morphogenesis, or abnormal neurophysiology |
| 1. Height and/or weight ≤10th centile (plotted on a racially or ethnically appropriate growth curve, if available), or: |
| 2. Deficient brain growth, abnormal morphogenesis or neurophysiology, including ≥1 of the following: |
| a. Head circumference ≤10th percentile |
| b. Structural brain anomalies |
| c. Recurrent nonfebrile seizures (other causes of seizures having been ruled out) |
| C. Neurobehavioral impairmenta |
| 1. For children ≥3 y of age (a or b): |
| a. WITH COGNITIVE IMPAIRMENT: |
| −Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥1.5 SD below the mean) |
| OR |
| −Cognitive deficit in at least 1 neurobehavioral domain ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment, or visual-spatial impairment) |
| b. WITH BEHAVIORAL IMPAIRMENT WITHOUT COGNITIVE IMPAIRMENT: |
| −Evidence of behavioral deficit in at least 1 domain ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioral regulation impairment, attention deficit, or impulse control) |
| 2. For children <3 y of age: |
| −Evidence of developmental delay ≥1.5 SD below the mean |
| III. ARND |
| Requires features A and B (this diagnosis cannot be made definitively in children <3 y of age): |
| A. Documented prenatal alcohol exposure |
| B. Neurobehavioral impairmenta |
| For children ≥3 y of age (a or b): |
| a. WITH COGNITIVE IMPAIRMENT: |
| −Evidence of global impairment (general conceptual ability ≥1.5 SD below the mean, or performance IQ or verbal IQ or spatial IQ ≥1.5 SD) |
| OR |
| −Cognitive deficit in at least 2 neurobehavioral domains ≥1.5 SD below the mean (executive functioning, specific learning impairment, memory impairment or visual-spatial impairment) |
| b. WITH BEHAVIORAL IMPAIRMENT WITHOUT COGNITIVE IMPAIRMENT: |
| −Evidence of behavioral deficit in at least 2 domains ≥1.5 SD below the mean in impairments of self-regulation (mood or behavioral regulation impairment, attention deficit, or impulse control) |
| IV. ARBD |
| Requires features A and B: |
| A. Documented prenatal alcohol exposure |
| B. One or more specific major malformations demonstrated in animal models and human studies to be the result of prenatal alcohol exposure: cardiac: atrial septal defects, aberrant great vessels, ventricular septal defects, conotruncal heart defects; skeletal: radioulnar synostosis, vertebral segmentation defects, large joint contractures, scoliosis; renal: aplastic/hypoplastic/dysplastic kidneys, “horseshoe” kidneys/ureteral duplications; eyes: strabismus, ptosis, retinal vascular anomalies, optic nerve hypoplasia; ears: conductive hearing loss, neurosensory hearing loss |
Diagnostic Caveats: The assignment of an FASD is a complex medical diagnostic process best accomplished through a multidisciplinary approach. As is the case with many medical conditions, sound clinical judgment must be used. Differential diagnoses should always include genetic disorders or conditions arising from other teratogens. Additionally, because head circumference, growth, and many cognitive and behavioral characteristics have moderate to high degrees of heritability, when information is available about the biological parents, these data should be considered in the final diagnostic decision.
Adaptive skills should be assessed, but such deficits cannot stand alone for diagnosis.
Application of the Guidelines in the Diagnosis of FASD
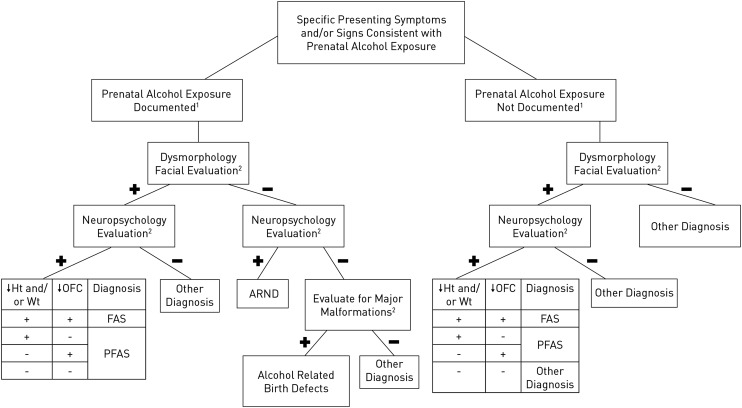
An FASD diagnostic algorithm incorporating the updated diagnostic guidelines is depicted in Fig 1.
FIGURE 1.
FASD diagnostic algorithm. See text for complete discussion. A positive dysmorphology facial evaluation requires 2 of the 3 cardinal facial features of FASD (short palpebral fissures, smooth philtrum, and this vermilion border of the upper lip). Cutoffs for neuropsychological testing are –1.5 SD. Cutoffs for stature, weight, and head circumference are at the 10th percentile.
Optimal Diagnostic Setting and the Role of the Pediatrician
Assignment of an FASD diagnosis is a complex medical diagnostic process best accomplished through a structured multidisciplinary approach by a clinical team comprising members with varied but complementary experience, qualifications, and skills. The assessment of individuals prenatally exposed to alcohol requires a medical assessment and team leadership by a pediatrician or clinical geneticist/dysmorphologist with expertise in the full range of human malformation syndromes and the dysmorphology evaluation of children with FASD. In addition, exposed children should have expert psychological/neuropsychological assessment, and a skilled interviewer should evaluate prenatal maternal alcohol intake. Other team members may include developmental behavioral pediatricians, psychiatrists, speech pathologists, occupational therapists, physical therapists, special educators, audiologists, and/or ophthalmologists.10,21–23
The essential role of the pediatrician in the identification and care of children with FASD cannot be overstated. Pediatricians are among the most likely practitioners to first encounter children with prenatal alcohol exposure who are potentially at risk for FASD. Jones et al24 demonstrated the accuracy of pediatricians in recognizing FAS on the basis of physical and other common associated features after a relatively short training session. In addition, once a diagnosis is assigned, pediatricians are called on to provide a medical home for affected children, coordinate mental health services, and manage other comorbid mental health disorders. Pediatricians also play an important role in the prevention of future alcohol-exposed pregnancies through counseling women with affected children.25
Documentation of Significant Prenatal Alcohol Exposure
Assessment of maternal prenatal alcohol intake is an essential part of the diagnostic process and is the first step in the diagnostic algorithm outlined in Fig 1. It is best measured by quantity of alcohol consumed per occasion (standard drinks per drinking day), frequency that it is consumed (eg, daily, times per week), and timing during gestation, because timing of significant exposure (even in the early weeks of pregnancy) can produce different physical and neurobehavioral phenotypes.26–30 Binge drinking (3–5 drinks or more per occasion) has been shown in animal and human studies to be the most detrimental to fetal development.26,31 Asking about use of other potential teratogens during pregnancy is also important because, in addition to their own potential teratogenicity, women who abuse drugs are more likely to use alcohol during pregnancy.13,14,32 Because in many populations it is likely that prenatal alcohol use will be denied completely or be significantly underreported,13,14,33–35 biomarkers can assist in documenting prenatal alcohol exposure. Most frequently, alcohol exposure information is collected retrospectively. It is well documented that accurate information on a particular pregnancy can be obtained from a willing respondent years after the birth of a child36–38 or from the medical or social service records or a collateral informant (eg, spouse, close relative, or friend) who had regular contact with the mother during pregnancy.15,26
In maternal interviews, because of potential stigmatization associated with prenatal alcohol use, and for accuracy, questions should be asked in a timeline followback manner,39,40 progressing from the broader context of health history (childbearing, general illness, nutrition, and dietary intake26,41,42) to the more sensitive alcohol use questions. It is important to also consider the overall drinking pattern immediately before pregnancy recognition, as it is common for the drinking pattern of 3 months before pregnancy to persist into early pregnancy.13,14,43–49
A consensus definition of significant prenatal alcohol exposure is set forth in Table 2. Note that although certain circumstances permit the diagnosis of FAS or PFAS without firm documentation of gestational alcohol use (Table 1), positive confirmation of alcohol exposure must be available for the diagnosis of ARND or ARBD to be assigned.
TABLE 2.
Definition of Documented Prenatal Alcohol Exposure (as Applied to the Diagnostic Categories Set Forth in Table 1)
| One or more of the following conditions must be met to constitute documented prenatal alcohol exposure during pregnancy (including drinking levels reported by the mother 3 mo before her report of pregnancy recognition or a positive pregnancy test documented in the medical record). The information must be obtained from the biological mother or a reliable collateral source (eg, family member, social service agency, or medical record): |
| − ≥6 drinks/wk for ≥2 wk during pregnancya |
| − ≥3 drinks per occasion on ≥2 occasions during pregnancya |
| − Documentation of alcohol-related social or legal problems in proximity to (before or during) the index pregnancy (eg, history of citation[s] for driving while intoxicated or history of treatment of an alcohol-related condition) |
| − Documentation of intoxication during pregnancy by blood, breath, or urine alcohol content testing |
| − Positive testing with established alcohol-exposure biomarker(s) during pregnancy or at birth (eg, analysis of fatty acid ethyl esters, phosphatidylethanol, and/or ethyl glucuronide in maternal hair, fingernails, urine, or blood, or placenta, or meconium)50–55 |
| − Increased prenatal risk associated with drinking during pregnancy as assessed by a validated screening tool of, for example, T-ACE (tolerance, annoyance, cut down, eye-opener) or AUDIT (alcohol use disorders identification test)56 |
Assignment of documented prenatal alcohol exposure to any individual case requires the sound judgment of an experienced clinician.
Dysmorphology Evaluation
After assessing prenatal alcohol exposure, the presence or absence of the characteristic structural features of FASD must be evaluated. For the dysmorphology examination, height, weight, and head circumference should first be measured and plotted by using population-specific growth curves. In the United States, the authors advise following the Centers for Disease Control and Prevention (CDC) recommendations: use the WHO growth charts for children from birth to 2 years to assess height and weight. (The WHO growth standards for children younger than 2 years have been adapted for use in the United States.) Use the CDC growth charts for children and teenagers aged 2 to 19 years.61 In other countries, we recommend using more-specific population-normed charts, if available. If growth curves specific to the population studied are not available, we endorse the recommendations of the CDC for US children.61 Prenatal growth restriction can be determined from reference data published by Oken et al62 by gestational age. In these diagnostic guidelines, we define growth deficiency as ≤10th centile.4,8 Prenatal growth restriction should be exhibited, or a pattern of postnatal growth deficiency should be documented if possible (decreased height and/or weight on >1 occasion over 12 months, and unrelated to postnatal environmental deprivation). With respect to determination of head circumference centiles, we have used the head circumference growth charts from Nellhaus63 in all populations, in lieu of more-specific population-based norms. For the purposes of these guidelines, a small head circumference is defined as ≤10th centile.4,8
The presence or absence of the 3 cardinal facial characteristics of FASD must next be objectively assessed: short palpebral fissures, smooth philtrum, and thin vermilion border of the upper lip (Fig 2). Although other investigators have advocated for measurement of facial anthropometry from 2-dimensional photography, we feel that direct examinations are more practical in an office setting. Here we define short palpebral fissures as ≤10th centile.4,8 Palpebral fissure length centiles can be estimated from a number of published norms; we have used the curves derived from direct examination of children published by Thomas et al.64 If facial anthropometry is measured live, palpebral fissure norms derived from live examinations must be used. (If palpebral fissure lengths are measured from photographs, published norms obtained from 2-dimensional photography are available.65) Similar to the experience of the authors, Avner et al66 found palpebral fissure lengths measured from photographs to be consistently smaller than those measured live. Similarly, Astley67 found the norm for palpebral fissures measured from 2-dimensional photographic software to fall 1.6 SDs below the mean on a palpebral fissure chart derived from live examinations. Figure 3 A and B depicts the technique for direct measurement of palpebral fissure length, and Fig 3C demonstrates why, in our experience, 2-dimensional photographic assessment of palpebral fissure length is prone to inaccuracy because of individual variation in the zygomatic angle that cannot be corrected for by a single mathematical adjustment. However, it should be noted that investigators disagree on the method that results in the most accurate measurement of palpebral fissure length.67–69 The morphology of the philtrum and the vermilion border of the upper lip are objectively scored by comparison with a racially normed lip/philtrum guide (Fig 4).70,71 Scores of 4 or 5 are consistent with the effects of prenatal alcohol exposure. If 2 of the 3 cardinal facial characteristics are present (short palpebral fissures, smooth philtrum, and/or thin vermilion border of the upper lip) the child is classified as having a positive dysmorphology facial evaluation for FASD.
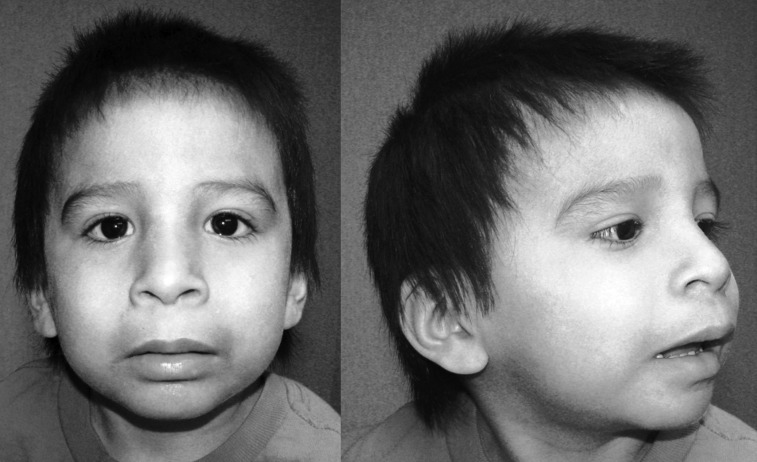
FIGURE 2.
Typical child with FAS. The 3 cardinal facial features are evident: short palpebral fissures, smooth philtrum, and relatively thin vermilion border of the upper lip. Midface hypoplasia is also apparent.
FIGURE 3.
A, Technique for measuring palpebral fissure length. A small plastic ruler is used to measure the distance between the endocanthion (where the eyelids meet medially) and the exocanthion (where the eyelids meet laterally). Subject and examiner should be seated at the same level opposite from one another. Keeping the chin level, the subject is asked to look up, allowing the examiner to bring the ruler as close to the eye as possible (without touching the lashes). The ruler can be rested on the cheek for stability while recording the measurement. B, Note that the ruler is angled slightly to follow the curve of the zygoma. C, The correct length of the palpebral fissure is depicted here as measurement “C.” This highlights the difficulty of 2-dimensional photographic measurement, because “B” is highly variable among individuals, leading to differences in the zygomatic angle (the angle between line segments B and C).
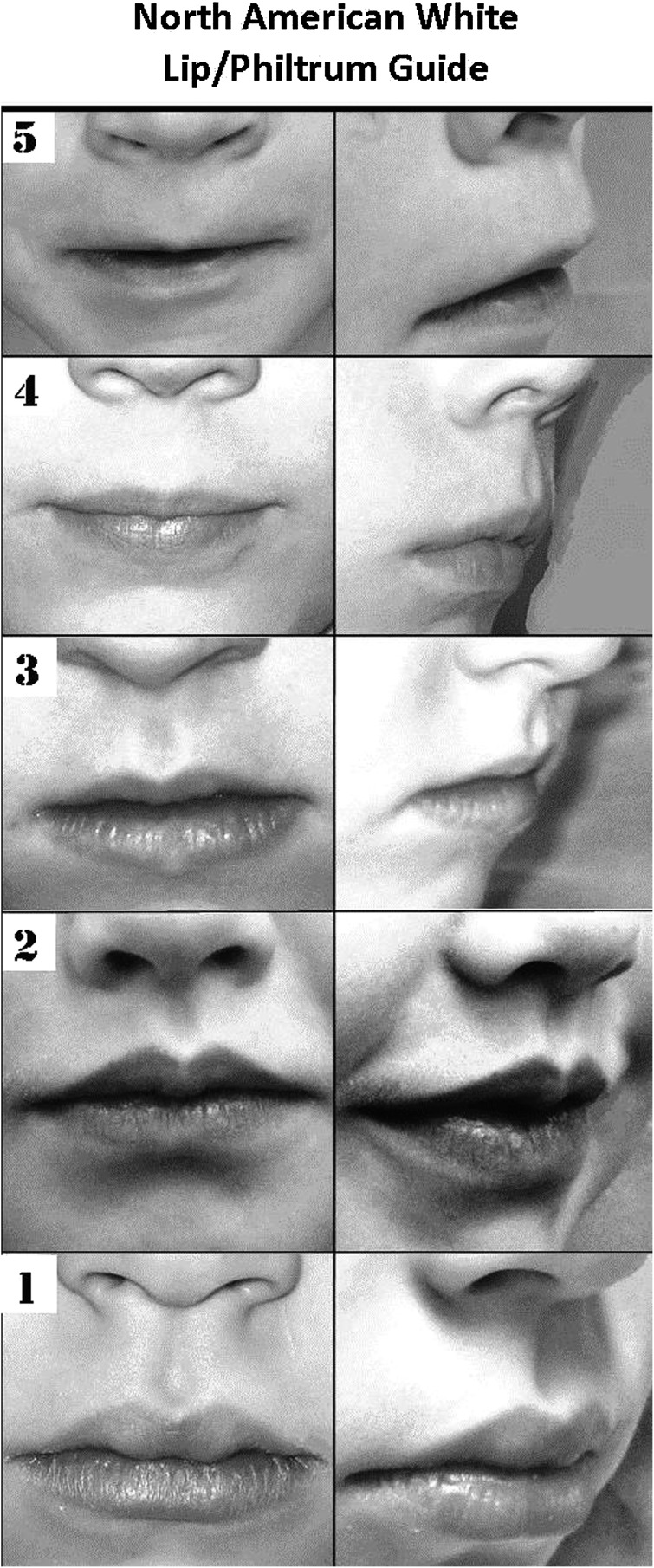
FIGURE 4.
Lip/philtrum guide for the white population, incorporating a 45-degree view. This guide was produced by analysis of photographs of >800 white children from school-based studies in the United States.13,14 Scores are assessed separately for the philtrum and vermilion border; scores of 4 or 5 are compatible with FAS or PFAS.
Neurodevelopmental Assessment and Neuropsychology Evaluation
Because the primary manifestations of the teratogenic effects of alcohol are demonstrated by changes in brain structure and/or function, comprehensive neurodevelopmental assessment is essential. Although the dysmorphology assessment of infants and small children for the growth and facial characteristics of FASD is feasible, a comprehensive cognitive/developmental evaluation may not be possible by using conventional assessment tools until after age 3 years.72 However, the cognitive and neurobehavioral phenotype of affected children evolves predictably over time73–76 and can be correlated with areas of brain vulnerability (Table 3).
TABLE 3.
Developmental Emergence of Neurocognitive and Behavioral Deficits Associated With FASD
| Infancy: 0–2 y | ||
| Areas of Brain Vulnerability in FASD | Neurocognitive/Behavioral Deficits Associated With Developmental Stage | |
| • Cortical synaptogenesis | Neurocognitive | • Delayed cognitive development or global developmental delay |
| • Development of cortical gray matter | ||
| • Myelination of sensory pathways | Self-Regulation | • Tremulousness, increased jitteriness |
| • Maturation of the limbic system | • Difficulty with self-soothing, and being soothed | |
| • Emotional withdrawal, decreased infant affective functioning | ||
| • Impaired stress reactivity; deficits in pain regulation | ||
| • Less complex play | ||
| • Myelination of motor pathways | Adaptive | • Delayed gross and fine motor milestones |
| • Poor feeding: poor sucking. Easily fatigued | ||
| Toddler/Preschool: 3–5 y | ||
| Areas of Brain Vulnerability in FASD | Neurocognitive/Behavioral Deficits Associated With Developmental Stage | |
| • Synaptogenesis | Neurocognitive | • Delayed cognitive development or global developmental delay |
| • Development of cortical gray matter | ||
| • Development of prefrontal cortex | Self-Regulation | • Attention: difficulties with attention regulation; hyperactivity and impulsivity; difficulty shifting attention; impaired visual and auditory attention; difficulty with sustained attention |
| • Executive function: difficulty encoding information; difficulty with multistep directions; difficulty with planning and organization; poor understanding of consequences | ||
| • Development of temporal lobes | • Sleep deficits: shortened sleep duration; increased sleep anxiety; parasomnias | |
| • Sensory processing: difficulty modulating sensory input; sensory seeking | ||
| • Development of dorsal motor cortex | Adaptive | • Delayed gross motor function: balance, coordination problems; “clumsiness” |
| • Poor fine motor skills: difficulty with writing/drawing; poor dexterity; visual-spatial deficits; impaired visual-motor coordination | ||
| • Development of temporal lobes | • Delayed auditory processing: central auditory delay | |
| • Speech and language deficits: difficulties with language acquisition; receptive, expressive language delays; deficits in word processing/word recognition; articulation errors; deficits in social pragmatics | ||
| • Memory deficits: difficulty remembering things previously learned | ||
| School-age: 6–12 y | ||
| Areas of Brain Vulnerability in FASD | Neurocognitive/Behavioral Deficits Associated With Developmental Stage | |
| • Decreased intracranial volume: | Neurocognitive | • Lower intellectual quotient |
| • Decreased volume of parietal and temporal lobes | • Learning disabilities | |
| • White matter abnormalities | • Deficits in mathematics (numerical operations/global mathematics skills) | |
| • Prefrontal cortex | Self-Regulation | • Executive function deficits: decreased working memory, decreased verbal fluency, poorer planning, sequencing, organization |
| • Attention deficits: hyperactivity; impulsivity | ||
| • Temporal lobe | Adaptive | • Language: deficits in higher order language processing |
| • Social pragmatics: deficits in social cognition: inappropriate social initiation/social interaction; inappropriate sexual behaviors | ||
| • Memory: difficulty encoding/consolidating new memory | ||
| • Parietal lobe | • Language processing: impaired gestural communication; deficits in social perception | |
| • Visual-spatial: deficits in spatial processing; poor handwriting; impaired visual-motor integration | ||
| Adolescence: 13–21 y | ||
| Areas of Brain Vulnerability in FASD | Neurocognitive/Behavioral Deficits Associated With Developmental Stage | |
| • Decreased intracranial volume: | Neurocognitive | • Lower intellectual quotient |
| • Decreased volume of parietal and temporal lobes | • Learning disabilities | |
| • White matter abnormalities | • Deficits in mathematics skills (numerical operations/global mathematics skills) | |
| • Myelination of prefrontal cortex (PFC) | Self-Regulation | • Executive function deficits: decreased verbal fluency, poorer planning, sequencing, organization; slow processing; deficits in judgment and metacognition |
| • Development of connections between PFC and basal ganglia | • Attention deficits: inattention | |
| • Temporal lobe | Adaptive | • Language: deficits in higher order language processing |
| • Social pragmatics: deficits in social cognition: inappropriate social initiation/social interaction; inappropriate sexual behaviors | ||
| • Working memory: difficulty encoding new memories; difficulty with memory recall | ||
| • Parietal lobe | • Language processing: deficits in social perception | |
| • Visual-spatial: deficits in spatial processing; poor handwriting; impaired visual-motor integration | ||
The authors promote the use of standardized tests that were developed by using normative groups that are representative of the population being tested. Therefore, in the updated guidelines, ≥1.5 SD below the mean refers to the mean of the normative group on which the tests were standardized. Therefore, both groups (alcohol-exposed children as well as unexposed children) are tested by using the same well-normed testing battery, thereby making the comparisons appropriate.
Multidisciplinary Case Conference
Once the prenatal exposure history, dysmorphology assessment, and neuropsychological testing have been obtained, a multidisciplinary case conference offers the best opportunity for full discussion of the case before assignment of an FASD or other diagnosis (Fig 1).
Phenocopies of FASD
Clinicians should be aware that the facial phenotype of FAS, although most commonly associated with prenatal alcohol exposure, is also observed in a variety of genetic and teratogenic conditions (Table 4). Therefore, physicians should use a low threshold for ordering additional genetic testing of children with potential FASD. A chromosome microarray has been shown to be the highest-yield diagnostic test when a genetic phenocopy of FASD is being considered.77,78
TABLE 4.
| Malformation Syndrome | Etiology |
|---|---|
| Cornelia deLange Syndrome OMIM 122470 | Autosomal dominant (Mutations in NIPBL, 60%) |
| Velocardiofacial Syndrome (del 22q11.2 Syndrome) OMIM #188400 | Chromosome microdeletion (del 22q11.2) |
| Duplication 15q Syndrome OMIM 608636 | Chromosome partial duplication (dup 15q) |
| Dubowitz Syndrome OMIM 223370 | Autosomal recessive |
| Noonan Syndrome OMIM 163950 | Autosomal dominant (Mutations in RAS-MAPK signal transduction pathway genes, PTPN11, SOS1, KRAS, NRAS, and others) |
| Williams Syndrome OMIM 194050 | Chromosome microdeletion (del 7q11.23, a contiguous gene syndrome incorporating the elastin gene) |
| Fetal Hydantoin Syndrome | Teratogenic effects of hydantoin exposure during gestation |
| Fetal Valproate Syndrome | Teratogenic effects of valproic acid exposure during gestation |
| Maternal Phenlyketonuria Effects | Teratogenic effects of high levels of phenylalanine, accompanying poorly controlled maternal phenylketonuria |
| Toluene Embryopathy | Teratogenic effects of maternal solvent exposure during pregnancy |
This list is not comprehensive. OMIM, Online Mendelian Inheritance in Man.56
Discussion
In the decade since the original operationalized IOM diagnostic criteria4 were published, extensive international research on the teratogenic effects of alcohol and the authors’ broad clinical experience have allowed for the development of further clarity and specificity in the diagnostic guidelines presented in this article. However, it should be noted that agreement on a universal diagnostic system for FASD is lacking among investigators in the field of FASD, especially concerning some of the features of the diagnostic guidelines set forth in Table 1. A discussion of the debated elements follows.
Diagnostic Categories Within the Continuum of FASD
It is the authors’ assertion that the 4 original IOM diagnostic categories3 within the continuum of FASD remain the most apt descriptors of the range of disabilities observed. These longstanding categories have heretofore been accepted by many of the diagnostic systems,4,8,9 and we see no need to introduce additional confusion into a field in which diagnostic consensus is critical. In addition, classification of individuals into 1 of the existing specific IOM categories allows for determination of prognosis and treatment planning. We also assert that the category of ARBDs, although uncommon, remains necessary, especially in epidemiologic studies.82,83 Our extensive database of alcohol-exposed children reveals many examples of affected children not fitting into 1 of the other categories who display 1 of the major malformations set forth in Table 1 and whose mothers binged during the embryonic stage critical to the developmental pathology of the malformation.
It should be noted that the Canadian diagnostic guideline for FASD recently was updated, collapsing the diagnostic categories under the diagnosis of “fetal alcohol spectrum disorder” to 2: FASD with sentinel facial features and FASD without sentinel facial features.10 Whether this simplified diagnostic scheme will result in practical improvements in the clinical care of affected individuals and more accurate epidemiologic studies estimating the prevalence of FASD remains to be demonstrated.
Sensitivity Versus Specificity in Clinical Diagnosis
Similar to others, our goals in the formulation of FASD diagnostic guidelines have been improved sensitivity and greater inclusion of children in the complete continuum of FASD4,8; thus, we have set cutoff levels for growth deficiency, head circumference, and palpebral fissure length at ≤10th centile and required 2, rather than 3, cardinal facial features for a diagnosis of FAS and PFAS. Because we advocate for a structured expert-led multidisciplinary diagnostic approach to the diagnosis of FASD, casting a broad net early in the diagnostic process and later using the case conference to carefully assign diagnoses has been our standard. Other diagnostic systems advocate for more stringent cutoffs: growth deficiency, head circumference, and palpebral fissure length less than or equal to the third centile and requiring all 3 of the cardinal facial features for alcohol-related diagnoses.5,9,10 Sensitivity and specificity are 2 sides of a diagnostic coin. Theoretically, the guidelines presented here demonstrating increased sensitivity could lead to overdiagnosis; thus, our advocacy for a structured expert multidisciplinary approach. On the other hand, strict diagnostic cutoffs associated with increased specificity could lead to underdiagnosis of affected children. Children with FASD are subject to a host of societal, educational, health, and judicial problems, all of which are affected by the time of diagnosis.84,85 Because early diagnosis and initiation of intervention should be of paramount importance, the authors assert that improved, sensitive, and inclusive diagnostic criteria for FASD should continue to be imperatives in the diagnostic process.
Deficient Brain Growth, Abnormal Morphogenesis, or Abnormal Neurophysiology
In the updated criteria, we have added documentation of recurrent nonfebrile seizures to the potential assignment of children to the diagnostic categories of FAS or PFAS. A child with FAS must now exhibit deficient brain growth, structure, or neurophysiology. This modification was prompted by a growing body of research that indicates that epilepsy is a frequent accompaniment of FASD.86,87 More commonly observed in children with FASD, a small head circumference is a reliable, easily obtained proxy for decreased brain volume.88,89 Finally, a number of structural brain anomalies have been observed in imaging studies in animals and human subjects with FASD. Although no specific anatomic region of the brain is preferentially affected, malformations resulting from migration abnormalities, changes in size and shape of the corpus callosum, cerebellar vermis hypoplasia, and hypoplasia of the basal ganglia and hippocampus have been documented.90,91
The 4-digit diagnostic code5 assesses these features as “structural evidence of central nervous system damage,” and the updated Canadian guideline for diagnosis of FASD10 includes a similar category (abnormal neuroanatomy/neurophysiology) as 1 of the 10 central nervous system domains that may be impaired, although this category is not a universal part of other diagnostic systems.6–8
Other Minor Anomalies in Children With FASD
In dysmorphology, clinical diagnoses are based on recognizable patterns of major and minor anomalies. Although the dysmorphology contribution to FASD diagnoses is derived from objective evaluation of the face, a number of other minor anomalies have been observed consistently and more commonly in children prenatally exposed to alcohol than in nonexposed controls.4,13,14,92,93 The clinical assessment of the presence or absence of these features should be part of the dysmorphology evaluation of children with potential FASD. The overall dysmorphic variation in any individual child can be quantified by calculation of a dysmorphology score (an updated dysmorphology scoring system based on objective observations of growth and minor anomalies in 370 children with FAS is presented in Table 5). The dysmorphology score allows for objective comparison among groups of children with FASD and has proven to be a valuable research tool. It is also a useful instrument to review as part of the differential diagnostic process when assessing features of genetic or other teratogenic disorders that may mimic FASD (Table 4). The score has been observed to correlate significantly with prenatal maternal alcohol intake, as well as with the cognitive and neurobehavioral characteristics of the affected child.26,94
TABLE 5.
Revised Dysmorphology Scoring System (Based on Quantitative Analysis of Growth Restriction and Minor Anomalies in 370 Children With FAS)
| Feature | No. Affected | Score |
|---|---|---|
| OFC ≤10% | 354 | 3 |
| Growth deficiency | ||
| Height ≤10% | 327 | 2 |
| Weight ≤10% | 322 | 1 |
| Short PFL (≤10%) | 313 | 3 |
| Smooth philtrum | 307 | 3 |
| Thin vermilion | 293 | 3 |
| Hypoplastic midface | 216 | 2 |
| Epicanthal folds | 204 | 2 |
| Decreased IPD/ICD (≤25%) | 202/104 | 2 |
| Flat nasal bridge | 179 | 2 |
| Altered palmar crease | 173 | 2 |
| 5th finger clinodactyly | 149 | 2 |
| Long philtrum (≥90%) | 122 | 2 |
| Anteverted nares | 118 | 2 |
| Camptodactyly | 114 | 2 |
| Ptosis | 64 | 1 |
| “Railroad track” ears | 57 | 1 |
| Heart murmur/confirmed CHD | 50/6 | 1 |
| Strabismus | 35 | 1 |
| Limited elbow supination | 31 | 1 |
| Hypoplastic nails | 23 | 1 |
| Prognathism | 21 | 1 |
| Hypertrichosis | 19 | 1 |
| Total possible score | 41 |
CHD, congenital heart disease; ICD, intercanthal distance; IPD, interpupillary distance; OFC, occipitofrontal (head) circumference; PFL, palpebral fissure length.
The Revised Dysmorphology Score was derived from analysis of growth and structural data from 370 children with full-blown FAS. The subjects were among the international cohort of children examined by the dysmorphology experts (HEH, MAM, LKR, MPA, OAR, TJ, KLJ) involved in NIAAA-supported CIFASD and CoFASP studies. The children were examined blindly by the investigators as part of school-based epidemiology studies of children in grade 1 (ages 5–8). Interexaminer agreement on anthropometric measures was high (Cronbach’s α scores ranged from 0.975 to 0.855 for craniofacial assessment items).
The cardinal diagnostic features (small head circumference, growth restriction [height and weight combined], short palpebral fissures, smooth philtrum, and thin vermilion border of the upper lip) were assigned a score of 3. Other features observed in ≥100 children were assigned a score of 2. Features observed in <100 children received a score of 1. The score provides an objective method of quantifying dysmorphic features and comparing the structural phenotype of FASD among affected children; it is not used in assigning FASD diagnoses. However, compilation of the minor anomalies cataloged in the score is useful in differentiating children with FASD from genetic and teratogenic phenocopies (Table 5).
This supplants the original scoring system that was based on the authors’ subjective analysis of the frequency of minor anomalies associated with FASD.4
Specificity of Neurobehavioral Impairment
The updated guidelines now require that all children assigned FASD diagnoses (with the exception of those with ARBD) must display neurobehavioral impairment (cognitive impairment or behavioral impairment without cognitive impairment). The original guidelines allowed for children with the requisite facial features, growth restriction, and/or microcephaly to be assigned an FASD diagnosis in the absence of significant neurobehavioral impairment. However, because neurocognitive impairment and abnormal behavior are the principal sources of disability in FASD, assignment of children with prenatal alcohol exposure into an FASD category without neurobehavioral impairment has no practical utility for either the child or the child’s family.
The definition of neurobehavioral impairment in FASD has become more specific over the past decade.36,72–76 The original 1996 IOM criteria and the 2005 guidelines defined neurobehavioral impairment as “evidence of a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level that cannot be explained by genetic predisposition, family background, or environment alone.”3,4 Although the 2005 criteria outlined areas of marked neurobehavioral impairment, levels of deficit and affected functional domains were not clearly articulated. The guidelines set forth in Table 1 clearly delineate domains of functioning to be assessed and levels of deficit to be reached to meet the diagnostic criteria for FAS, PFAS, and ARND.
The domains of function outlined in the updated criteria encompass the following: (1) global intellectual ability (full-scale, verbal, performance, or spatial IQ), (2) cognition (executive functioning, learning, memory, and visual-spatial skills), (3) behavior and self-regulation (mood, behavioral regulation, attention, and impulse control), and (4) adaptive skills. These functional domains were selected based on the empirical evidence of deficits in children prenatally exposed to alcohol and/or who have been given a diagnosis of FASD.32,95–107
For children >3 years of age, diagnoses of FAS or PFAS require evidence of global cognitive impairment (reflected in a deficit of ≥1.5 SDs below the mean on a measure of global intelligence [full-scale IQ score] or performance, verbal, or visual/spatial IQ) or evidence of behavioral deficit ≥1.5 SDs below the mean in ≥1 domain in impairments of self-regulation (mood or behavioral regulation impairment, attention deficit, or impulse control).
A diagnosis of ARND can be made only if there is confirmed prenatal alcohol exposure and global cognitive impairment, reflected in a deficit of ≥1.5 SDs below the mean on a measure of global intelligence (full-scale IQ score) or performance, verbal, or visual/spatial IQ. If cognitive impairment is not present (often the case with individuals prenatally exposed to alcohol), cognitive deficits in at least 2 additional neurobehavioral domains (executive functioning, specific learning, memory, or visual-spatial) are required at ≥1.5 SDs below the mean. Additionally, the new guidelines provide for an ARND diagnosis based on behavioral impairment without cognitive impairment, as evidenced by deficits at ≥1.5 SDs below the mean in at least 2 behavioral domains: mood or behavioral regulation, attention deficit, or impulse control. Adaptive skills also should be assessed.108–110 The adaptive scores can be used to assist with the diagnosis; however, specific cutoffs and adaptive behavior requirements are not included in the diagnostic criteria.
For children who are ≤3 years of age, a diagnosis of FAS and PFAS can be made if there is evidence of developmental delay ≥1.5 SDs below the mean on a standardized measure of developmental trajectory. However, for ARND, a definitive diagnosis cannot be made before 3 years of age.
The neurobehavioral criteria for diagnoses within the FASD continuum differ from those proposed by other investigators5,9,10 (our guidelines require: cutoffs of –1.5 SDs rather than –2 SDs, for neurobehavioral assessment and less stringent neurobehavioral criteria for those affected children who demonstrate the requisite dysmorphology allowing classification into the categories of FAS and PFAS). Our previously published data confirm that because the dysmorphology score has the highest correlation with confirmed diagnoses in the FASD continuum, confidence in an FAS or PFAS diagnosis can be ensured with impairment in fewer neurobehavioral domains.26,94
Differentiation Between ARND and Neurobehavioral Disorder With Prenatal Alcohol Exposure
These updated criteria continue to include ARND as a necessary diagnostic category. With the introduction of Neurobehavioral Disorder with Prenatal Alcohol Exposure (ND-PAE) into the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition as a “condition in need of further study,”7 there has been significant confusion about the necessity of retaining both ARND and ND-PAE as diagnostic entities. To be clear, ARND is a complex medical diagnosis, best assigned as part of a multidisciplinary team evaluation for FASD. It has been widely applied in epidemiologic studies14,93 and in clinical settings and has been found to accurately describe the end of the continuum of FASD without dysmorphology.111,112 In contrast, ND-PAE is an experimental mental health diagnostic code that is intended to be used in clinical settings by clinicians from a variety of theoretical orientations, including psychiatrists (and other physicians), psychologists, social workers, nurses, occupational and rehabilitation therapists, and counselors. This code triggers payment for services related to the condition as well as helps individuals access needed interventions and treatments.113 According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, ND-PAE requires ≥1 deficits in neurocognition and in self-regulation plus ≥2 deficits in adaptive functioning (with at least 1 in communication or social communication and interaction).99,114 An ARND diagnosis can be made based on global cognitive deficits alone without the behavioral issues that fall into the psychiatric realm. ARND also can be diagnosed if there is evidence of behavioral deficits in at least 2 behavioral domains in the absence of cognitive deficits. Whether in the long run they will merge into a single entity will depend on further study and refinement of both ARND and ND-PAE as they are applied in practice.
Future Directions
The guidelines presented here are based on the most recent FASD research and clinical data. However, their accuracy will need to be reevaluated over time as their validity is more extensively assessed. Among areas in need of further study are the following: potential use of improved and more practical 3-dimensional photographic imaging as an accurate proxy for live facial anthropometric measurements115; improved noninvasive biomarkers for alcohol exposure throughout pregnancy and postnatally50–55; postnatal epigenetic markers as a proxy for documentation of prenatal maternal alcohol intake116–118; improved definition of which fetal and postnatal growth patterns are most consistent with the teratogenic effects of alcohol; and a more precise definition of what constitutes minimal criteria for adverse fetal alcohol exposure during gestation. Finally, other diagnostic approaches to FASD that can be readily applied in resource-poor settings should be explored.
Conclusions
FASD continues to represent a pressing global public health challenge. The first step in addressing this dilemma is to recognize the magnitude of the problem through careful case definition. Since the authors’ diagnostic guidelines were published in 2005, considerable progress has been made in further specifying the anatomic and neurobehavioral characteristics of FASD. These updated guidelines reflect consensus among a large and experienced cadre of FASD investigators in the fields of dysmorphology, epidemiology, neurology, psychology, developmental/behavioral pediatrics, and educational diagnostics. They do not necessarily represent the policy of the American Academy of Pediatrics. The improved specificity of these guidelines will aid clinicians in assignment of more accurate diagnoses of alcohol-exposed infants and children, thereby leading to more widespread early intervention and improved prevention efforts.
Glossary
- ARBD
alcohol-related birth defects
- ARND
alcohol-related neurodevelopmental disorder
- CDC
Centers for Disease Control and Prevention
- CIFASD
Collaborative Initiative on Fetal Alcohol Spectrum Disorders
- CoFASP
Collaboration on Fetal Alcohol Spectrum Disorders Prevalence
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorders
- IOM
Institute of Medicine
- ND-PAE
Neurobehavioral Disorder with Prenatal Alcohol Exposure
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PFAS
partial fetal alcohol syndrome
- WHO
World Health Organization
Footnotes
Dr Hoyme was the first author of the original diagnostic guidelines for fetal alcohol spectrum disorders (FASD) published in Pediatrics in 2005, conceptualized and designed the study, and drafted the initial document; Ms Kalberg with Dr Coles formulated the psychological testing battery used for the subjects and assisted in assignment of FASD diagnoses, along with Drs Elliott and Coles she was charged with clarifying the definition of alcohol-related neurodevelopmental disorder, and she revised the document; Dr Elliott assisted with assignment of FASD diagnoses, along with Ms Kalberg and Dr Coles she was charged with clarifying the definition of alcohol-related neurodevelopmental disorder, and she revised the document; Dr Blankenship and Mr Buckley comprised the data analysis group for the FASD team collaboration, they oversaw the gathering, storing, and analysis of sensitive subject data, and produced tables and figures for the manuscript; Dr Blankenship died before submission of the paper; Mr Buckley revised the document; Ms Marais coordinated the multidisciplinary diagnostic case conferences on the children from whom the data for this report were collected and revised the document; Drs Manning, Robinson, Adam, Abdul-Rahman, Jewett, and Jones examined all of the children in the studies on which the updated criteria are based, assigned diagnostic categories to the subjects, aided in crafting the definitions of the updated diagnostic criteria, and revised the document; Dr Coles contributed significantly to the clarification of alcohol-related neurodevelopmental disorder and revised the manuscript; Dr Chambers contributed significantly to the development of the overall diagnostic scheme for FASD and revised the manuscript; Dr Adnams developed the psychological and neuropsychological testing battery and oversaw the testing of the large South African cohort of children examined over the past decade, participated in developing the neuropsychological criteria set forth in the article, and revised the document; Dr Shah authored the sections on the natural history of the neurobehavioral phenotype of FASD over the life span, and revised the document; Drs Riley and Charness added significantly to the formulation of the specific revised diagnostic guidelines and revised the document; Dr Warren is the driving force behind the epidemiological investigations that form the basis of the revised guidelines, participated in formulating the specific guidelines, and edited the document; Dr May is principal investigator of the school-based population studies in South Africa, Italy, and the United States on which this manuscript is based, and revised the document; and all authors agree to be accountable for all aspects of the work and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This project was funded by the National Institutes of Health (NIH): National Institute on Alcohol Abuse and Alcoholism grants R01 AA11685, RO1/UO1 AA01115134, and U01 AA019879-01/NIH-NIAAA (Collaboration on Fetal Alcohol Spectrum Disorders Prevalence); and by the Oxnard Foundation, Newport Beach, CA. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
The guidelines/recommendations in this article are not American Academy of Pediatrics policy, and publication herein does not imply endorsement.
References
- 1.Lemoine P, Harousseau H, Borteyru JP, Menuet JC. Les enfants des parents alcoholiques: anomolies observees a propos de 127 cas [The children of alcoholic parents: anomalies observed in 127 cases]. Quest Medical. 1968;25:476–482 [Google Scholar]
- 2.Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271 [DOI] [PubMed] [Google Scholar]
- 3.Stratton K, Howe C, Battaglia F, eds. Institute of Medicine. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academies Press; 1996 [Google Scholar]
- 4.Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115(1):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-digit diagnostic code. Alcohol. 2000;35(4):400–410 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992 [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing; 2013:798–801 [Google Scholar]
- 8.Centers for Disease Control and Prevention Fetal Alcohol Spectrum Disorders: Guidelines for Referral and Diagnosis. Atlanta, GA: Centers for Disease Control and Prevention; 2004 [Google Scholar]
- 9.Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N; Public Health Agency of Canada’s National Advisory Committee on Fetal Alcohol Spectrum Disorder . Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ. 2005;172(suppl 5):S1–S21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook JL, Green CR, Lilley CM, et al. . Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ. 2015;188(3):191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson SN, Foroud T, Sowell ER, et al. ; CIFASD . Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44(7-8):635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute on Alcohol Abuse and Alcoholism Major initiatives. Fetal alcohol spectrum disorders. Available at: www.niaaa.nih.gov/research/major-initiatives/fetal-alcohol-spectrum-disorders. Accessed March 29, 2016
- 13.May PA, Keaster C, Bozeman R, et al. Prevalence and characteristics of fetal alcohol syndrome and partial fetal alcohol syndrome in a Rocky Mountain Region City. Drug Alcohol Depend. 2015;155:118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May PA, Baete A, Russo J, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014;134(5):855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.May PA, Blankenship J, Marais AS, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37(5):818–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centre for Addiction and Mental Health. Fetal alcohol spectrum disorders: How widespread are they in Canada? Available at: www.camh.ca/en/hospital/about_camh/newsroom/news_releases_media_advisories_and_backgrounders/current_year/Pages/Fetal-Alcohol-Spectrum-Disorders-How-Widespread-Are-They-in-Canada.aspx. Accessed March 29, 2016
- 17.Abel EL, Sokol RJ. A revised conservative estimate of the incidence of FAS and its economic impact. Alcohol Clin Exp Res. 1991;15(3):514–524 [DOI] [PubMed] [Google Scholar]
- 18.Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Updated Methods and Data. Report Prepared by the Lewin Group. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000 [Google Scholar]
- 19.Stade B, Ali A, Bennett D, et al. The burden of prenatal exposure to alcohol: revised measurement of cost. Can J Clin Pharmacol. 2009;16(1):e91–e102 [PubMed] [Google Scholar]
- 20.Williams JF, Smith VC; Committee on Substance Abuse . Fetal alcohol spectrum disorders. Pediatrics. 2015;136(5). Available at: www.pediatrics.org/cgi/content/full/136/5/e1395 [DOI] [PubMed] [Google Scholar]
- 21.Clarren SK, Lutke J, Sherbuck M. The Canadian guidelines and the interdisciplinary clinical capacity of Canada to diagnose fetal alcohol spectrum disorder. J Popul Ther Clin Pharmacol. 2011;18(3):e494–e499 [PubMed] [Google Scholar]
- 22.Grundfast KM. The role of the audiologist and otologist in the identification of the dysmorphic child. Ear Hear. 1983;4(1):24–30 [DOI] [PubMed] [Google Scholar]
- 23.Strömland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addict Biol. 2004;9(2):153–157, discussion 159–160 [DOI] [PubMed] [Google Scholar]
- 24.Jones KL, Robinson LK, Bakhireva LN, et al. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118(6). Available at: www.pediatrics.org/cgi/content/full/118/6/e1734 [DOI] [PubMed] [Google Scholar]
- 25.Gahagan S, Sharpe TT, Brimacombe M, et al. Pediatricians’ knowledge, training, and experience in the care of children with fetal alcohol syndrome. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e657 [DOI] [PubMed] [Google Scholar]
- 26.May PA, Blankenship J, Marais AS, et al. Maternal alcohol consumption producing fetal alcohol spectrum disorders (FASD): quantity, frequency, and timing of drinking. Drug Alcohol Depend. 2013;133(2):502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulik KK. Fetal alcohol spectrum disorder: pathogenesis and mechanisms. Handb Clin Neurol. 2014;125:463–475 [DOI] [PubMed] [Google Scholar]
- 28.Lipinski RJ, Hammond P, O’Leary-Moore SK, et al. Ethanol-induced face-brain dysmorphology patterns are correlative and exposure-stage dependent. PLoS One. 2012;7(8):e43067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parnell SE, Holloway HE, Baker LK, Styner MA, Sulik KK. Dysmorphogenic effects of first trimester-equivalent ethanol exposure in mice: a magnetic resonance microscopy-based study. Alcohol Clin Exp Res. 2014;38(7):2008–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schambra UB, Goldsmith J, Nunley K, Liu Y, Harirforoosh S, Schambra HM. Low and moderate prenatal ethanol exposures of mice during gastrulation or neurulation delays neurobehavioral development. Neurotoxicol Teratol. 2015;51:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Res Health. 2001;25(3):168–174 [PMC free article] [PubMed] [Google Scholar]
- 32.Ceccanti M, Fiorentino D, Coriale G, et al. Maternal risk factors for fetal alcohol spectrum disorders in a province in Italy. Drug Alcohol Depend. 2014;145:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manich A, Velasco M, Joya X, et al. Validity of a maternal alcohol consumption questionnaire in detecting prenatal exposure [in Spanish]. An Pediatr (Barc). 2012;76(6):324–328 [DOI] [PubMed] [Google Scholar]
- 34.Wurst FM, Kelso E, Weinmann W, Pragst F, Yegles M, Sundström Poromaa I. Measurement of direct ethanol metabolites suggests higher rate of alcohol use among pregnant women than found with the AUDIT—a pilot study in a population-based sample of Swedish women. Am J Obstet Gynecol. 2008;198(4):407.e1–407.e5 [DOI] [PubMed] [Google Scholar]
- 35.Bryanton J, Gareri J, Boswall D, et al. Incidence of prenatal alcohol exposure in Prince Edward Island: a population-based descriptive study. CMAJ Open. 2014;2(2):E121–E126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hannigan JH, Chiodo LM, Sokol RJ, et al. A 14-year retrospective maternal report of alcohol consumption in pregnancy predicts pregnancy and teen outcomes. Alcohol. 2010;44(7-8):583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Czarnecki DM, Russell M, Cooper ML, Salter D. Five-year reliability of self-reported alcohol consumption. J Stud Alcohol. 1990;51(1):68–76 [DOI] [PubMed] [Google Scholar]
- 38.Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006;30(3):510–515 [DOI] [PubMed] [Google Scholar]
- 39.Sobell LC, Agrawal S, Annis H, et al. Cross-cultural evaluation of two drinking assessment instruments: alcohol timeline followback and inventory of drinking situations. Subst Use Misuse. 2001;36(3):313–331 [DOI] [PubMed] [Google Scholar]
- 40.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402 [DOI] [PubMed] [Google Scholar]
- 41.King AC. Enhancing the self-report of alcohol consumption in the community: two questionnaire formats. Am J Public Health. 1994;84(2):294–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May PA, Gossage JP, Brooke LE, et al. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 2005;95(7):1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Floyd RL, Decouflé P, Hungerford DW. Alcohol use prior to pregnancy recognition. Am J Prev Med. 1999;17(2):101–107 [DOI] [PubMed] [Google Scholar]
- 44.Tan CH, Denny CH, Cheal NE, Sniezek JE, Kanny D. Alcohol use and binge drinking among women of childbearing age - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2015;64(37):1042–1046 [DOI] [PubMed] [Google Scholar]
- 45.Mallard SR, Connor JL, Houghton LA. Maternal factors associated with heavy periconceptional alcohol intake and drinking following pregnancy recognition: a post-partum survey of New Zealand women. Drug Alcohol Rev. 2013;32(4):389–397 [DOI] [PubMed] [Google Scholar]
- 46.Parackal SM, Parackal MK, Harraway JA. Prevalence and correlates of drinking in early pregnancy among women who stopped drinking on pregnancy recognition. Matern Child Health J. 2013;17(3):520–529 [DOI] [PubMed] [Google Scholar]
- 47.Russell M, Czarnecki DM, Cowan R, McPherson E, Mudar PJ. Measures of maternal alcohol use as predictors of development in early childhood. Alcohol Clin Exp Res. 1991;15(6):991–1000 [DOI] [PubMed] [Google Scholar]
- 48.Skagerström J, Alehagen S, Häggström-Nordin E, Årestedt K, Nilsen P. Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: a cross sectional study in Sweden. BMC Public Health. 2013;13:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvik A, Haldorsen T, Lindemann R. Consistency of reported alcohol use by pregnant women: anonymous versus confidential questionnaires with item nonresponse differences. Alcohol Clin Exp Res. 2005;29(8):1444–1449 [DOI] [PubMed] [Google Scholar]
- 50.Ostrea EM, Jr. Testing for exposure to illicit drugs and other agents in the neonate: a review of laboratory methods and the role of meconium analysis. Curr Probl Pediatr. 1999;29(2):37–56 [PubMed] [Google Scholar]
- 51.Bearer CF, Jacobson JL, Jacobson SW, et al. Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143(4):463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soderberg BL, Salem RO, Best CA, Cluette-Brown JE, Laposata M. Fatty acid ethyl esters. Ethanol metabolites that reflect ethanol intake. Am J Clin Pathol. 2003;119(suppl):S94–S99 [DOI] [PubMed] [Google Scholar]
- 53.Kulaga V, Pragst F, Fulga N, Koren G. Hair analysis of fatty acid ethyl esters in the detection of excessive drinking in the context of fetal alcohol spectrum disorders. Ther Drug Monit. 2009;31(2):261–266 [DOI] [PubMed] [Google Scholar]
- 54.Pichini S, Marchei E, Vagnarelli F, et al. Assessment of prenatal exposure to ethanol by meconium analysis: results of an Italian multicenter study. Alcohol Clin Exp Res. 2012;36(3):417–424 [DOI] [PubMed] [Google Scholar]
- 55.Morini L, Marchei E, Tarani L, et al. Testing ethylglucuronide in maternal hair and nails for the assessment of fetal exposure to alcohol: comparison with meconium testing. Ther Drug Monit. 2013;35(3):402–407 [DOI] [PubMed] [Google Scholar]
- 56.Allen JP, Wilson VB Assessing alcohol problems: a guide for clinicians and researchers. 2nd ed. NIH publication No. 03-3745; revised 2003. Available at: http://pubs.niaaa.nih.gov/publications/AssessingAlcohol/index.htm. Accessed March 29, 2016
- 57.May PA, Gossage JP, Marais AS, et al. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 2008;32(5):738–753 [DOI] [PubMed] [Google Scholar]
- 58.Day NL, Robles N, Richardson G, et al. The effects of prenatal alcohol use on the growth of children at three years of age. Alcohol Clin Exp Res. 1991;15(1):67–71 [DOI] [PubMed] [Google Scholar]
- 59.Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 1990;51(5):403–407 [DOI] [PubMed] [Google Scholar]
- 60.Larkby CA, Goldschmidt L, Hanusa BH, Day NL. Prenatal alcohol exposure is associated with conduct disorder in adolescence: findings from a birth cohort. J Am Acad Child Adolesc Psychiatry. 2011;50(3):262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Use and interpretation of the WHO and CDC growth charts for children from birth to 20 years in the United States. Available at: www.cdc.gov/nccdphp/dnpa/growthcharts/resources/growthchart.pdf. Accessed March 29, 2016
- 62.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nellhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41(1):106–114 [PubMed] [Google Scholar]
- 64.Thomas IT, Gaitantzis YA, Frias JL. Palpebral fissure length from 29 weeks gestation to 14 years. J Pediatr. 1987;111(2):267–268 [DOI] [PubMed] [Google Scholar]
- 65.Clarren SK, Chudley AE, Wong L, Friesen J, Brant R. Normal distribution of palpebral fissure lengths in Canadian school age children. Can J Clin Pharmacol. 2010;17(1):e67–e78 [PubMed] [Google Scholar]
- 66.Avner M, Henning P, Koren G, Nulman I. Validation of the facial photographic in fetal alcohol spectrum disorder screening and diagnosis. J Popul Ther Clin Pharmacol. 2014;21(1):e106–e113 [PubMed] [Google Scholar]
- 67.Astley SJ. Canadian palpebral fissure length growth charts reflect a good fit for two school and FASD clinic-based U.S. populations. J Popul Ther Clin Pharmacol. 2011;18(2):e231–e241 [PubMed] [Google Scholar]
- 68.Cranston ME, Mhanni AA, Marles SL, Chudley AE. Concordance of three methods for palpebral fissure length measurement in the assessment of fetal alcohol spectrum disorder. Can J Clin Pharmacol. 2009;16(1):e234–e241 [PubMed] [Google Scholar]
- 69.Astley SJ. Palpebral fissure length measurement: accuracy of the FAS facial photographic analysis software and inaccuracy of the ruler. J Popul Ther Clin Pharmacol. 2015;22(1):e9–e26 [PubMed] [Google Scholar]
- 70.Astley S. FAS Diagnostic and Prevention Network. Available at: http://depts.washington.edu/fasdpn/htmls/lip-philtrum-guides.htm. Accessed March 29, 2016
- 71.Hoyme HE, Hoyme DB, Elliott AJ, et al. A South African mixed race lip/philtrum guide for diagnosis of fetal alcohol spectrum disorders. Am J Med Genet A. 2015;167A(4):752–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olson HC, Jirikowic T, Kartin D, Astley SJ. Responding to the challenge of early intervention for fetal alcohol spectrum disorders. Infants Young Child. 2007;20(2):172–189 [Google Scholar]
- 73.Coles CD, Smith I, Fernhoff PM, Falek A. Neonatal neurobehavioral characteristics as correlates of maternal alcohol use during gestation. Alcohol Clin Exp Res. 1985;9(5):454–460 [DOI] [PubMed] [Google Scholar]
- 74.Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13(4):357–367 [DOI] [PubMed] [Google Scholar]
- 75.Brown RT, Coles CD, Smith IE, et al. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicol Teratol. 1991;13(4):369–376 [DOI] [PubMed] [Google Scholar]
- 76.Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31(1):116–126 [DOI] [PubMed] [Google Scholar]
- 77.Douzgou S, Breen C, Crow YJ, et al. Diagnosing fetal alcohol syndrome: new insights from newer genetic technologies. Arch Dis Child. 2012;97(9):812–817 [DOI] [PubMed] [Google Scholar]
- 78.Abdelmalik N, van Haelst M, Mancini G, et al. Diagnostic outcomes of 27 children referred by pediatricians to a genetics clinic in the Netherlands with suspicion of fetal alcohol spectrum disorders. Am J Med Genet A. 2013;161A(2):254–260 [DOI] [PubMed] [Google Scholar]
- 79.Leibson T, Neuman G, Chudley AE, Koren G. The differential diagnosis of fetal alcohol spectrum disorder. J Popul Ther Clin Pharmacol. 2014;21(1):e1–e30 [PubMed] [Google Scholar]
- 80.Jones KL, Jones MC, del Campo M. Smith’s Recognizable Patterns of Human Malformation. 7th ed. Philadelphia, PA: Elsevier Saunders; 2013 [Google Scholar]
- 81.OMIM (Online Mendelian Inheritance in Man). An online catalog of human genes and genetic disorders. Updated November 13, 2015. Available at: www.omim.org/. Accessed March 29, 2016
- 82.Hofer R, Burd L. Review of published studies of kidney, liver, and gastrointestinal birth defects in fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. 2009;85(3):179–183 [DOI] [PubMed] [Google Scholar]
- 83.O’Leary CM, Nassar N, Kurinczuk JJ, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126(4). Available at: www.pediatrics.org/cgi/content/full/126/4/e843 [DOI] [PubMed] [Google Scholar]
- 84.Salmon J. Fetal alcohol spectrum disorder: New Zealand birth mothers’ experiences. Can J Clin Pharmacol. 2008;15(2):e191–e213 [PubMed] [Google Scholar]
- 85.Salmon JV, Buetow SA. An exploration of the experiences and perspectives of New Zealanders with fetal alcohol spectrum disorder. J Popul Ther Clin Pharmacol. 2012;19(1):e41–e50 [PubMed] [Google Scholar]
- 86.Bell SH, Stade B, Reynolds JN, et al. The remarkably high prevalence of epilepsy and seizure history in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(6):1084–1089 [DOI] [PubMed] [Google Scholar]
- 87.Nicita F, Verrotti A, Pruna D, et al. Seizures in fetal alcohol spectrum disorders: evaluation of clinical, electroencephalographic, and neuroradiologic features in a pediatric case series. Epilepsia. 2014;55(6):e60–e66 [DOI] [PubMed] [Google Scholar]
- 88.Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33(5):239–241 [DOI] [PubMed] [Google Scholar]
- 89.Treit S, Zhou D, Andrew G, Chudley A, Beaulieu C. Abstract. Is head circumference an accurate proxy for brain volume in individuals with fetal alcohol spectrum disorders? Int J Dev Neurosci. 2015;47:84–85 [Google Scholar]
- 90.Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25(3):185–191 [PMC free article] [PubMed] [Google Scholar]
- 91.Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31(2):239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones KL, Hoyme HE, Robinson LK, et al. Fetal alcohol spectrum disorders: extending the range of structural defects. Am J Med Genet A. 2010;152A(11):2731–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.May PA, Gossage JP, Marais AS, et al. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88(2-3):259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.May PA, Tabachnick BG, Gossage JP, et al. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend. 2011;119(1-2):18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Streissguth AP. The behavioral teratology of alcohol: performance, behavioral and intellectual deficits in prenatally exposed children. In: West J, ed. Alcohol and Brain Development. New York, NY: Oxford University Press; 1986:3–44 [Google Scholar]
- 96.Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(5):810–816 [DOI] [PubMed] [Google Scholar]
- 97.Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14(6):1022–1033 [DOI] [PubMed] [Google Scholar]
- 98.Coles CD, Lynch ME, Kable JA, Johnson KC, Goldstein FC. Verbal and nonverbal memory in adults prenatally exposed to alcohol. Alcohol Clin Exp Res. 2010;34(5):897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18(3):331–354 [DOI] [PubMed] [Google Scholar]
- 100.Kodituwakku PW, Kalberg W, May PA. The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health. 2001;25(3):192–198 [PMC free article] [PubMed] [Google Scholar]
- 101.Aragón AS, Kalberg WO, Buckley D, Barela-Scott LM, Tabachnick BG, May PA. Neuropsychological study of FASD in a sample of American Indian children: processing simple versus complex information. Alcohol Clin Exp Res. 2008;32(12):2136–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26(2):263–271 [PubMed] [Google Scholar]
- 103.Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21(1):150–161 [PubMed] [Google Scholar]
- 104.Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15(3):218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ware AL, O’Brien JW, Crocker N, et al. ; CIFASD . The effects of prenatal alcohol exposure and attention-deficit/hyperactivity disorder on psychopathology and behavior. Alcohol Clin Exp Res. 2013;37(3):507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mattson SN, Roesch SC, Fagerlund A, et al. ; Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) . Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(9):1640–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mattson SN, Roesch SC, Glass L, et al. ; CIFASD . Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Whaley SE, O’Connor And MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res. 2001;25(7):1018–1024 [PubMed] [Google Scholar]
- 109.Crocker N, Vaurio L, Riley EP, Mattson SN. Comparison of adaptive behavior in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. Alcohol Clin Exp Res. 2009;33(11):2015–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jirikowic T, Kartin D, Olson HC. Children with fetal alcohol spectrum disorders: a descriptive profile of adaptive function. Can J Occup Ther. 2008;75(4):238–248 [DOI] [PubMed] [Google Scholar]
- 111.Consensus statement on recognizing alcohol-related neurodevelopmental disorder (ARND) in primary health care of children. Interagency Coordinating Committee on Fetal Alcohol Spectrum Disorders (ICCFASD). Joseph F. Hagan, Jr., M.D., Conference Panel Chair. Available at: http://niaaa.nih.gov/sites/default/files/ARNDConferenceConsensusStatementBooklet_Complete.pdf. Accessed March 29, 2016
- 112.AAP Division of Children with Special Needs AAP endorses statement on alcohol-related neurodevelopmental disabilities. AAP News. 2012;33:18 [Google Scholar]
- 113.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM). Available at: www.psychiatry.org/psychiatrists/practice/dsm. Accessed March 29, 2016
- 114.Doyle LR, Mattson SN. Neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE): review of evidence and guidelines for assessment. Curr Dev Disord Rep. 2015;2(3):175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suttie M, Foroud T, Wetherill L, et al. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Veazey KJ, Parnell SE, Miranda RC, Golding MC. Dose-dependent alcohol-induced alterations in chromatin structure persist beyond the window of exposure and correlate with fetal alcohol syndrome birth defects. Epigenetics Chromatin. 2015;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleiber ML, Diehl EJ, Laufer BI, et al. Long-term genomic and epigenomic dysregulation as a consequence of prenatal alcohol exposure: a model for fetal alcohol spectrum disorders. Front Genet. 2014;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mead EA, Sarkar DK. Fetal alcohol spectrum disorders and their transmission through genetic and epigenetic mechanisms. Front Genet. 2014;5:154. [DOI] [PMC free article] [PubMed] [Google Scholar]