Abstract
BACKGROUND:
Although both fetal and long-term growth restriction are well documented in fetal alcohol spectrum disorders, effects on pattern of growth trajectory have not been characterized. Furthermore, the degree to which growth trajectories are related to fetal alcohol-related neurocognitive deficits is unknown.
METHODS:
Ninety-three heavy drinking pregnant women and 64 controls were recruited at initiation of prenatal care in Cape Town, South Africa. Small for gestational age (SGA) was defined as birth weight <10th percentile. Length/height, weight, and head circumference were measured at 6.5 and 12 months and 5, 9, and 13 years. Four growth trajectories were identified: SGA with long-term postnatal growth restriction (length/height-for-age <10th percentile through 13 years); SGA with catch-up growth; no SGA or postnatal growth restriction; and late-onset postnatal stunting. IQ was assessed at 5 and 10 years, and learning, memory, and executive function at 10 years.
RESULTS:
Children born SGA with postnatal growth restriction were most heavily exposed. Exposure was intermediate for those born SGA with catch-up growth and lowest for those without prenatal or postnatal growth restriction. Effects on neurocognition were strongest in children with both prenatal and long-term growth restriction, more moderate in those with fetal growth restriction and postnatal catch-up, and weakest in those without growth restriction.
CONCLUSIONS:
These findings validate the use of growth restriction in the diagnosis of fetal alcohol spectrum disorders and identify growth trajectory as a biomarker of which heavily exposed children are at greatest risk for cognitive developmental deficits.
What’s Known on This Subject:
Animal and human studies have demonstrated fetal alcohol deficits in both pre- and postnatal growth. The diagnostic criteria for fetal alcohol syndrome treat prenatal and/or postnatal growth restriction similarly, requiring the presence of either.
What This Study Adds:
Fetal growth was more sensitive to prenatal alcohol exposure than postnatal growth. A child’s growth trajectory was a biological indicator of severity of alcohol-related neurocognitive effects, with the strongest effects seen in children with both fetal and postnatal growth restriction.
Fetal alcohol spectrum disorders (FASD) are the most common preventable cause of neurodevelopmental disabilities, with prevalence estimates of 20 to 50 per 1000 live births in the United States and >200 per 1000 in endemic communities, including the Western Cape Province of South Africa.1,2 In the United States, the median-adjusted annual cost of fetal alcohol syndrome (FAS), the most severe form of FASD, has been estimated at $4 billion, and costs associated with the full spectrum are much higher.3 Animal and human studies have consistently demonstrated intrauterine growth retardation among offspring exposed prenatally to alcohol, with smaller weight, length, and head circumference at birth.4–6 In 4 US prospective longitudinal cohort studies, prenatal alcohol exposure (PAE) has also been associated with postnatal growth restriction.7–11 Previous studies have not examined the degree to which some children with PAE exhibit postnatal catch-up growth, as seen with maternal cigarette smoking and malnutrition during pregnancy, whereas others exhibit persistent long-term growth restriction. In addition to a specific pattern of craniofacial dysmorphology, prenatal or postnatal growth retardation is required for a diagnosis of FAS and is a diagnostic criterion for partial FAS (PFAS).12,13 These diagnostic criteria do not distinguish between prenatal versus postnatal growth restriction, and, to our knowledge, no previous study has examined whether they are indicative of different degrees of severity of FASD damage.
The prevalence of heavy drinking during pregnancy and of FASD in the Cape Coloured (mixed ancestry) community in South Africa, is among the highest worldwide.2 We examined individual growth trajectories of children in our prospectively recruited Cape Town Longitudinal FASD Cohort14 with serial anthropometric measurements from infancy through 13 years and neurocognitive assessments at 5 and 10 years. We hypothesized that (1) PAE is associated with both fetal and postnatal growth restriction; (2) adverse effects of prenatal exposure on cognitive function in childhood are stronger among those born small for gestational age (SGA) than those born appropriate for gestational age (AGA); and (3) among those born SGA, effects on cognitive function are strongest among those who fail to catch up postnatally.
Methods
Sample
Ninety-four heavy drinkers and 63 control subjects were recruited between July 1999 and January 2002 from an antenatal clinic that serves an economically disadvantaged, predominantly Cape Coloured population.14 Each mother was interviewed during her first antenatal visit regarding her alcohol consumption at conception and recruitment. Any woman averaging ≥1.0 oz absolute alcohol (AA) per day (∼2 standard drinks/day) or reporting at least 2 binge drinking episodes (5 drinks/occasion) during the first trimester was invited to participate. Women who drank <0.5 oz AA per day and did not binge drink were invited to participate as control subjects. Maternal exclusion criteria included age <18 years old, diabetes, epilepsy, or cardiac problems requiring treatment. Infant exclusion criteria were major chromosomal anomalies, seizures, neural tube defects, and multiple gestation pregnancy. Women who reported drinking during pregnancy were advised to stop or reduce their intake and were invited to participate in a home-visitor intervention program or referred for treatment. Informed consent, conducted in the mother’s preferred language (Afrikaans or English), was obtained from each mother. Approval was obtained from the Institutional Review Boards at Wayne State University and University of Cape Town.
In a timeline follow-back interview15 administered at recruitment, each mother was asked about her drinking on a day-by-day basis during a typical 2-week period around time of conception, with recall linked to specific daily activities. She was then asked about her drinking on a day-by-day basis during the past 2 weeks. Each mother was subsequently interviewed in midpregnancy about her alcohol, smoking, and drug use during the previous 2 weeks and at 1-month postpartum about a typical 2-week period during the latter part of pregnancy. Volume was recorded for each type of alcohol beverage consumed and converted to ounces of AA using weights proposed by Bowman et al.16 Three measures were constructed to summarize drinking at conception and across pregnancy: ounces AA per day, ounces AA per drinking occasion, and frequency of drinking. Maternal report of drinking during pregnancy was validated in relation to fatty acid ethyl esters levels, alcohol metabolites trapped in meconium specimens obtained from a subsample of newborns in this study,17 and, in our Detroit study, in relation to infant outcomes.15
In 2005, we held a diagnostic clinic in which the children were independently examined by expert FAS dysmorphologists (H.E. Hoyme, MD and L.K. Robinson, MD) for growth and FAS-related anomalies. There was substantial agreement between them on assessments of all dysmorphic features, including philtrum and vermilion ratings based on the lip/philtrum guide18 and palpebral fissure length and with a Cape Town-based expert FAS dysmorphologist (N. Khaole, MD), who evaluated 8 children who could not be scheduled for the clinic.14 Each case was reviewed in a case conference by HEH, LKR, SWJ, CDM, and JLJ, who jointly determined FASD diagnosis.12 Dr. Khaole’s diagnoses were confirmed independently by Dr. Hoyme at subsequent assessments in 2009 and/or 2013. All postnatal assessments were performed by examiners blind regarding PAE and FASD diagnosis.
Growth Trajectory
Birth weights were obtained from medical records. Child weight, length/height, and head circumference were measured at each postnatal visit (6.5 and 12 months and 5, 9, and 13 years) using standard protocols.7,19 Using the Centers for Disease Control and Prevention norms, weight-for-age, length/height-for-age, weight-for-length (for infancy visits), and BMI (for the 5- and 9-year visits), and head circumference and percentiles and z scores were calculated.19,20 Each child was classified as SGA (birth weight <10th percentile) or AGA, using ultrasound-based due date and the Oken et al21 percentiles. Three infants not seen after age 6 months were excluded from postnatal growth analyses. Postnatal growth restriction was defined as length/height-for-age <10th percentile; length/height was used because it is more stable over time than weight percentile and, except in cases of severe malnutrition and/or illness, less affected by postnatal nutrition and metabolic demands. Postnatal growth trajectories were classified as AGA with normal postnatal growth (length/height ≥10th percentile through 13 years), SGA with postnatal catch-up growth (postnatal length/height ≥10th percentile), SGA without catch-up growth (postnatal length/height <10th percentile through age 13 years), and late-onset stunting (length/height height <10th percentile after a period of normal growth).
Cognitive Outcomes
IQ was assessed at 5 years on the Junior South African Individual Scales22 and at 10 years on the Wechsler Intelligence Scales for Children IV.14 At 10 years, we also administered the California Verbal Learning Test-Children’s Version23 and the Delis-Kaplan Verbal Fluency test, an executive function measure of cognitive flexibility.24 Tests were administered in the language of instruction used in the child’s classroom.
Data Analysis
Statistical analyses were performed using SPSS (v.22; IBM, Armonk, NY). All variables were examined for normality of distribution; 1 variable (ounces AA per day across pregnancy) was positively skewed (>3.0) and subjected to natural log (X + 1) transformation. χ2 analyses were used to compare the prevalence of each growth trajectory category between children with PAE and controls. t tests and analyses of variance (with least significant difference post hoc analysis) were used to compare levels of PAE between children with different growth trajectories.
The relation of AA/day to neurocognitive outcomes was assessed using Pearson correlation. To examine whether growth trajectory category was related to severity of alcohol-related neurocognitive deficits, the correlation of AA/day to each neurocognitive outcome was examined, stratified by growth trajectory category. Each outcome was then regressed on AA/day, SGA (yes/no), and an interaction term for AA/day by SGA to test the hypothesis that cognitive performance is most strongly affected by PAE in children born SGA. The outcomes were also regressed on AA/day, SGA with postnatal growth restriction (yes/no), and an AA/day by SGA with postnatal growth restriction interaction term to test the hypothesis that cognitive performance is most strongly affected in children born SGA who fail to catch-up postnatally. Children with late-onset postnatal stunting, a pattern indicative of malnutrition and/or severe illness, were excluded from these analyses. Given the low power of interaction terms in observational studies,25 it has been suggested that the critical value for P should be relaxed for testing statistical interactions. Although Selvin et al26 have advocated a critical value of .20, we used the somewhat more conservative cutoff of P < .10.
Results
Sample
Children of heavy drinkers and control subjects did not differ in participation rates (see ns in Table 1). Heavy drinkers presented 2.5 weeks later for prenatal care and were less educated and more likely to be unmarried. Although heavy drinkers reduced their frequency of drinking after conception (P < .001), they continued to average 8 standard drinks/occasion across pregnancy. Among the control women, all but 2 (96.8%) abstained during pregnancy, and the 2 drank ≤2 drinks per occasion. Heavy drinkers were more likely to smoke than control subjects, but average cigarettes per day was similar among smokers in both groups (<0.5 packs per day, range 4–40 cigarettes per day). Drug use during pregnancy was rare. Among heavy drinkers, 12 reported using marijuana (mean = 2.4 occasions per month); 4 methaqualone (mean = 1.6 occasions per month); 1 cocaine (2.6 occasions per month). Two control subjects used marijuana (mean = 2.2 occasions per month); none, cocaine or methaqualone. Almost half the children born to the heavy drinkers were diagnosed with FAS or PFAS.
TABLE 1.
Sample Characteristics
| n | Control Subjects | n | Heavy Exposure | t(df) | |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Weeks’ gestation at recruitment | 63 | 17.9 ± 5.9 | 92 | 20.4 ± 5.9 | −2.59 (153)* |
| Age at delivery | 63 | 26.3 ± 5.6 | 94 | 27.2 ± 6.5 | −0.90 (155) |
| Years of school completed | 63 | 9.8 ± 1.8 | 94 | 7.9 ± 2.6 | 5.38 (155)*** |
| Married (%) | 63 | 29 (46.0) | 94 | 21 (22.3) | 9.75 (1)** |
| Parity | 63 | 2.0 ± 1.1 | 94 | 2.4 ± 1.5 | −1.75 (155)† |
| SESa | 60 | 123.0 ± 47.6 | 88 | 94.5 ± 53.4 | −3.33 (146)*** |
| Alcohol consumption at conception | |||||
| Average ounces AA/d | 63 | 0.0 ± 0.0 | 94 | 1.4 ± 1.5 | −7.39 (155)*** |
| Average ounces AA/drinking occasion | 63 | 0.1 ± 0.3 | 94 | 4.2 ± 2.7 | −12.18 (155)*** |
| Number of drinking d/wk | 63 | 0.0 ± 0.1 | 94 | 2.2 ± 1.4 | −11.67 (155)*** |
| Alcohol consumption across pregnancy | |||||
| Average ounces AA/d | 63 | 0.0 ± 0.0 | 94 | 0.9 ± 1.0 | −7.44 (155)*** |
| Average ounces AA/drinking occasion | 63 | 0.0 ± 0.2 | 94 | 4.0 ± 2.4 | −13.27 (155)*** |
| Number of drinking d/wk | 63 | 0.0 ± 0.0 | 94 | 1.5 ± 1.1 | −10.78 (155)*** |
| Cigarette users, n | 63 | 29 (46.0) | 94 | 82 (87.2) | 30.91 (1)*** |
| Cigarettes/d among users | 8.6 ± 8.3 | 7.6 ± 5.0 | 0.77 (109) | ||
| Marijuana users, n | 63 | 2 (3.2) | 94 | 12 (12.8) | 4.27 (1)* |
| Occasions/mo among users | 2.2 ± 0.7 | 2.4 ± 2.2 | −0.11 (12) | ||
| Child characteristics | |||||
| Sex (% male) | 63 | 29 (46.0) | 94 | 53 (56.4) | 1.62 (1) |
| Gestational age at birth (wk) | 63 | 39.1 ± 1.8 | 94 | 38.5 ± 2.7 | 1.65 (155)† |
| Birth weight (g) | 63 | 3150.8 ± 466.4 | 94 | 2707.9 ± 604.2 | 4.92 (155)*** |
| Birth weight percentile | 63 | 33.1 ± 25.3 | 94 | 18.1 ± 22.1 | 3.94 (155)** |
| Birth weight <2500 g (%) | 63 | 4 (6.3) | 94 | 27 (28.7) | 11.92 (1)*** |
| Age at visits | |||||
| 6.5 mo | 57 | 7.1 (0.6) | 77 | 7.2 (0.7) | −0.55 (132) |
| 12 mo | 50 | 12.3 (0.6) | 74 | 12.3 (0.7) | −0.57 (122) |
| 5 y | 57 | 4.7 (0.7) | 84 | 5.1 (0.8) | −3.27 (139)*** |
| 9 y | 52 | 8.6 (0.8) | 77 | 9.1 (0.8) | −3.34 (127)*** |
| 13 y | 49 | 12.7 (0.7) | 73 | 13.1 (0.8) | −2.93 (120)** |
| FASD diagnosis (age 5 y) | 57 | 83 | 37.13 (2)*** | ||
| FAS, % | 0.0 (0.0) | 17 (20.5) | |||
| PFAS, % | 0.0 (0.0) | 22 (26.5) |
Values are mean ± SD or n (%). 1 ounce AA ∼2 standard drinks. Comparisons of ns for the exposed and control groups at each visit using t tests show no group differences at P < .10.
Hollingshead Four Factor Index of Social Status Scale.27
P ≤ .05.
P ≤ .001.
P ≤ .01.
P ≤ .10.
Fetal and Postnatal Growth Trajectory Patterns
More than half of heavily exposed children were SGA at birth, compared with fewer than one-fifth of control subjects (Table 2). Among controls, all but 1 (1.7%) child born SGA exhibited postnatal catch-up growth. Among the 41 heavily-exposed children born SGA who did not exhibit late-onset postnatal stunting, 17 (41.5%) continued to exhibit growth restriction through 13 years. Among exposed children who exhibited catch-up growth, 84.3% had caught up by 12 months. Late-onset postnatal stunting was not related to PAE (P = .517). Socioeconomic status (SES)27 was similar among growth trajectory categories (P = .270; post hoc Ps > .10).
TABLE 2.
Prevalence of Growth Trajectory Patterns Through Age 13 Years in Children With and Without Heavy Exposure
| Fetal Growtha | Postnatal Growth Trajectorya | |||||
|---|---|---|---|---|---|---|
| AGA (n = 93) | SGA (n = 64) | AGA With Normal Postnatal Growth (n = 71) | SGA With Catch-up (n = 32) | SGA Without Catch-up (n = 18) | Late-Onset Postnatal Stunting (n = 24) | |
| Control subjects | 51 (81.0) | 12 (19.0) | 43 (71.7) | 8 (13.3) | 1 (1.7) | 8 (13.3) |
| Heavy alcohol exposure | 42 (44.7) | 52 (55.3) | 28 (32.9) | 24 (28.2) | 17 (20.0) | 16 (18.8) |
Values are n (%).
P < .001
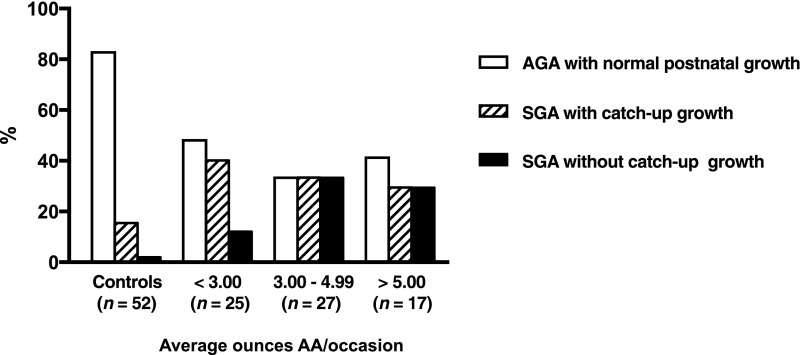
Table 3 compares PAE levels across growth trajectory categories. Children born SGA were exposed to higher levels of alcohol than children born AGA. A linear relation was seen between PAE and growth trajectory: those born AGA with normal postnatal growth had the lowest levels of exposure; those born SGA followed by catch-up growth, intermediate levels; those born SGA without catch-up growth had the highest. In post hoc analyses, all 3 groups differed from each other on AA/day at P < .05. Alcohol exposure among children with late-onset stunting did not differ from that of children born AGA with normal postnatal growth (post hoc P > .10). Figure 1 displays the linear relation between exposure and severity of growth restriction. Moreover, among children born SGA, the prevalence of catch-up growth decreased with increasing exposure, indicating that fetal growth is more sensitive to PAE than postnatal growth.
TABLE 3.
Prenatal Alcohol Exposure by Growth Trajectory Pattern
| Fetal Growth | Postnatal Growth Trajectory | |||||||
|---|---|---|---|---|---|---|---|---|
| AGA (n = 93) | SGA (n = 64) | tdf | AGA With Normal Postnatal Growth (n = 71) | SGA With Catch-up (n = 32) | SGA Without Catch-up (n = 18) | Late-Onset Postnatal Stunting (n = 24) | Fdf | |
| Daily alcohol consumption (ounces AA/d) | 0.3 | 0.9 | 4.24155*** | 0.3 | 0.8 | 1.2 | 0.4 | 7.383,141*** |
| Ounces AA/drinking occasion | 1.9 | 3.2 | 3.06155** | 1.7 | 2.9 | 4.4 | 2.3 | 5.493,141*** |
| Drinking frequency (d/wk) | 0.6 | 1.4 | 5.04155*** | 0.5 | 1.3 | 1.7 | 0.9 | 8.033,141*** |
Values are group means.
P ≤ .001.
P ≤ .01.
FIGURE 1.
Growth trajectories across levels of alcohol exposure. Excludes children with late-onset postnatal stunting.
Growth Trajectories as Predictors of FASD-Related Neurocognitive Deficits
As we have previously reported, average daily PAE was associated with lower IQ scores, poorer learning and recognition discrimination, and poorer category switching on the Verbal Fluency test (Table 4).23 In stratified analyses comparing children born SGA to those born AGA, the effects of alcohol exposure on neurocognitive outcomes in this sample were seen only in the children born SGA. In regression analyses, P values for the alcohol × SGA (yes/no) interaction term met our P < .10 critical value for interaction effects on 5- and 10-year IQ, the Wechsler Intelligence Scales for Children IV Perceptual Reasoning, Working Memory, and Processing Speed Indices, and the Verbal Fluency Letters and Category Switching tasks.
TABLE 4.
Relation of Prenatal Alcohol Exposure (Ounces AA/Day) to Neurocognitive Outcomes Stratified by Growth Trajectory
| Whole Cohort | Stratified by Fetal Growth | Stratified by Fetal and Postnatal Growtha | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AGA | SGA | Interaction Pb | AGA With Normal Postnatal Growth | SGA With Catch-up Growth | SGA Without Catch-up Growth | Interaction Pc | SGA Without Catch-up Growth Excluding Children With FAS | ||
| 5-y visit | (n = 85) | (n = 56) | (n = 67) | (n = 31) | (n = 18) | (n = 7) | |||
| JSAIS IQ | –0.19* | 0.07 | –0.37** | .027 | 0.04 | –0.21 | –0.48* | .180 | –0.55 |
| 10-y visit | |||||||||
| WISC-IV | (n = 77) | (n = 51) | (n = 64) | (n = 29) | (n = 17) | (n = 7) | |||
| Full-scale IQ | –0.26** | 0.05 | –0.45*** | .015 | 0.09 | –0.23 | –0.66** | .072 | –0.84* |
| Verbal Comprehension Index | –0.23** | –0.06 | –0.36* | .259 | –0.05 | –0.19 | –0.57* | .375 | –0.58 |
| Perceptual Reasoning Index | –0.20* | 0.09 | –0.41** | .013 | 0.09 | –0.17 | –0.60* | .010 | –0.77* |
| Working Memory Index | –0.13 | 0.18 | –0.32* | .007 | 0.34** | 0.00 | –0.70** | .001 | –0.89** |
| Processing Speed Index | –0.20* | 0.06 | –0.35* | .029 | 0.13 | –0.27 | –0.40 | .141 | 0.67 |
| CVLT-C | (n = 75) | (n = 46) | (n = 62) | (n = 27) | (n = 14) | (n = 6) | |||
| Total correct | –0.27** | –0.21† | –0.21 | .885 | –0.20 | –0.03 | –0.54* | .087 | –0.70 |
| Recognition discrimination | –0.30*** | –0.15 | –0.34* | .157 | –0.14 | –0.26 | –0.49† | .838 | –0.46 |
| Delis-Kaplan Verbal Fluency | (n = 75) | (n = 46) | (n = 62) | (n = 26) | (n = 14) | (n = 6) | |||
| Letters | –0.13 | 0.08 | –0.28† | .079 | 0.11 | –0.09 | –0.39 | .051 | –0.56 |
| Categories | –0.15† | –0.04 | –0.25† | .296 | –0.05 | –0.13 | –0.50† | .085 | 0.33 |
| Category switching | –0.24** | 0.05 | –0.45** | .008 | 0.12 | –0.42* | –0.61* | .035 | 0.53 |
Values are Pearson r. CVLT-C, California Verbal Learning Test-Children’s Version; JSAIS, Junior South African Individual Scales; WISC-IV, Wechsler Intelligence Scale for Children IV.
Excluding children with late-onset stunting.
From multiple regression analyses including AA/day, SGA (yes/no), and the interaction term (AA/day × SGA [yes/no]).
From multiple regression analyses including AA/day, a binary variable comparing children who were born SGA without catch-up growth to all other children, and the interaction term (AA/day × SGA without catch-up growth [yes/no]).
P ≤ .05.
P ≤ .01.
P ≤ .001.
P ≤ .10.
Table 4 also presents the analyses stratified by prenatal and postnatal growth trajectory category. No consistent pattern of fetal alcohol-related adverse effects was seen in the children born AGA with normal postnatal growth. By contrast, modest correlations between PAE and poorer IQ, recognition memory, and executive function (category switching) were seen in the children born SGA who exhibited catch-up growth, although most of these low-to-moderate size effects fell short of statistical significance, presumably due to small sample size. The strongest effects were seen in the children born SGA who did not exhibit postnatal catch-up growth. Regression analyses including an interaction term for AA per day × SGA with no catch-up growth (yes/no) indicated that the effects of alcohol exposure on 7 of the 11 neurocognitive measures were significantly stronger among children born SGA who failed to catch up. Moreover, when children with FAS were excluded, most of the Pearson rs for the children born SGA without catch-up growth were the same or greater in magnitude, indicating that these findings were not seen only in children with FAS and are not attributable to an FAS diagnosis.
Discussion
In this prenatally recruited longitudinal cohort, we found a linear dose-response relation between maternal alcohol consumption during pregnancy and persistence of growth restriction. In other fetal growth restriction models, such as prenatal nicotine and amphetamine exposure, most children exhibit catch-up growth during the first year of life.10,11 By contrast, our data show that a large proportion of children with heavy PAE exhibit growth restriction in utero that persists postnatally. Moreover, these data are the first to demonstrate that a child’s growth trajectory pattern predicts severity of fetal alcohol-related neurocognitive impairment: the strongest effects were seen in children with both fetal and long-term postnatal growth restriction, more moderate effects in those with fetal growth restriction and postnatal catch-up growth, and the weakest effects in those without any growth restriction. The effects on IQ, learning and memory, and cognitive flexibility for the subgroup with both prenatal and postnatal growth restriction, even after exclusion of those without full FAS, are among the strongest reported in the literature.
Vorhees proposed a conceptual model of dose-response in teratogenesis, in which neurobehavioral function is hypothesized to be most sensitive to a neurotoxic prenatal exposure, followed by growth, congenital malformations, and embryolethality, which are manifest at successively higher doses.28 Our findings extend this model in the case of PAE to demonstrate distinct patterns of effects on fetal and postnatal growth. Fetal growth restriction is more sensitive in that it was seen at lower levels of exposure than postnatal growth restriction. In a new, recently recruited Cape Town cohort, we found that maternal alcohol consumption is associated with reductions in placental weight.29 Given the critical role of the placenta in fetal growth, alcohol-related reductions in placental size may mediate the effects of alcohol exposure on growth in utero. By contrast, the postnatal growth restriction seen in children with the heaviest exposure may reflect different mechanisms, such as alterations in fetal programming, endocrinologic development, postnatal metabolism, and/or feeding behaviors.
In this study, we examined effects on IQ, learning and memory, and executive function, 3 neurocognitive domains that we and others have shown to be affected by PAE.23,30–35 The significant interactions between PAE and growth trajectory suggest that growth trajectory may provide a biomarker of which heavily exposed children are at greatest risk for neurocognitive deficits. Of note, these findings were not attributable to FAS diagnosis alone. Because most children who exhibited catch-up growth did so by age 12 months, growth trajectory may be a clinically useful biomarker as early as 12 months.
Most diagnostic schemes for FASD treat prenatal and postnatal growth restriction as equivalent. For example, in the Hoyme et al revised Institute of Medicine diagnostic criteria, prenatal or postnatal growth retardation is a criterion used in diagnosing FAS and PFAS.12 Our findings are the first to demonstrate that these 2 periods of growth restriction are not equivalent markers of severity of impairment. Children with fetal and postnatal growth restriction are at greater risk of intellectual impairment than children with fetal growth restriction who exhibit catch-up growth, and both groups are at greater risk than children with normal prenatal and postnatal growth.
These findings validate the inclusion of growth restriction as a diagnostic criterion for FAS and PFAS and suggest that the consistent finding that neurocognitive deficits are worse in syndromal than nonsyndromal children is partly due to the inclusion of growth restriction as a diagnostic criterion. The recently revised Canadian guidelines36 include reduced head circumference but not height or weight. Our data suggest that length/height restriction may be the most sensitive indicator of intellectual deficit. In the current study, among children with both prenatal and postnatal growth restriction, the group most severely affected neurobehaviorally, 66.7% did not meet the Canadian criterion for reduced head circumference (<10th percentile). It is important to emphasize that although cognitive deficits are more readily detectable in prenatally exposed children with growth restriction, our sample size apparently limited our ability to detect cognitive deficits found in larger sample studies of alcohol-exposed children with normal growth.30,37,38
This study had limitations common to other longitudinal studies of prenatal exposures. Some children were unable to attend all visits. However, attrition was low, and growth data were available from at least 4 of 6 time points for 92.5% of children. Individual variation in maternal and fetal alcohol metabolism due to factors such as body size, metabolic activity, hydration, and genetic influences may affect the accuracy and precision of estimates of the amount of alcohol that reaches the fetus. Differences between true and estimated exposure are likely small, however, given the validation of our pregnancy alcohol ascertainment protocol in relation to levels of fatty acid ethyl esters in meconium samples in this community17 and the predictive validity of the timeline follow-back interviewing techniques we used.15 Measures of postnatal diet and nutrition were not available. Although nutrition and SES may have an impact on growth in this disadvantaged population, PAE was not related to risk of late-onset postnatal stunting, and SES was not different among growth trajectory groups (all Ps > .10). The current study provided a unique opportunity to assess growth trajectory in a relatively homogenous population with the unusually high prevalence of FAS and PFAS and heavy exposure. Because this cohort comprised mothers and children from a socioeconomically disadvantaged Cape Coloured community, replication using cohorts from other ethnic backgrounds and economic strata is warranted.
Conclusions
To our knowledge, this is the first study to demonstrate that fetal growth restriction is more sensitive to PAE than postnatal growth restriction and the first to demonstrate that a child’s growth trajectory provides a biological indicator of severity of FASD. These findings validate the use of growth restriction as a clinical diagnostic criterion for FASD. Furthermore, the relatively strong effect sizes (among the strongest in the literature to date) found among children born SGA with persistent postnatal growth restriction demonstrate that this growth trajectory pattern identifies children at particularly high risk for fetal alcohol-related neurocognitive disabilities who therefore warrant targeting for additional developmental screening and intervention programs.
Acknowledgments
We acknowledge the contribution of the late Andrea Hay, who conducted the cognitive assessments at the 5-year visit. We thank Denis Viljoen, MD, who collaborated on recruitment of the Cape Town Longitudinal Cohort; Maggie September, for her work on recruitment and maintenance of the cohort and her contribution to the organization and coordination of the dysmorphology clinics; our research staff at University of Cape Town including Anna-Susan Marais, Julie Croxford, Mandy Cronje, Mariska Pienaar, Nadine Lindinger, and Catherine Lewis; and our Wayne State University research staff, Renee Sun, and Douglas Fuller. We also thank the dysmorphologists H. Eugene Hoyme, Luther Robinson, and Nathaniel Khaole for their work in examining the children during the dysmorphology clinics. We greatly appreciate the contribution of the mothers and children for their long-term participation in the Cape Town cohort.
Recruitment of the Cape Town Longitudinal Cohort was funded by two administrative supplements to National Institutes of Health (NIH)/National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant R01 AA09524 (S. Jacobson, Principal Investigator [PI]), NIH Office of Research on Minority Health, and Foundation for Alcohol Related Research, Cape Town, South Africa. The 5-year follow-up assessment was funded by grant U01 AA014790 (S. Jacobson, PI) in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorder (CIFASD); the 9-year assessments by grant R01 AA016781 (S. Jacobson, PI). The Cape Town FASD dysmorphology clinics were funded in part by grants U01 AA014790 (S. Jacobson, PI), U24 AA014815 (K.L. Jones, PI), and U01 AA014809 (T. Foroud, PI) from CIFASD. RCC’s participation was supported by NIAAA K23 AA020516 (R.C. Carter, PI). This research received supplemental funding from the Lycaki-Young Fund from the State of Michigan (S. Jacobson and J. Jacobson).
Glossary
- AA
absolute alcohol
- AGA
appropriate for gestational age
- FAS
fetal alcohol syndrome
- FASD
fetal alcohol spectrum disorders
- PAE
prenatal alcohol exposure
- PFAS
partial fetal alcohol syndrome
- SES
socioeconomic status
- SGA
small for gestational age
Footnotes
Dr Carter conceptualized the analytic approach, conducted the data analyses, and wrote the initial manuscript; Drs S. Jacobson and J. Jacobson designed the original cohort study, recruited and conducted the follow-up of the cohort, contributed to the design of the analytical approach used in this study, and critically reviewed and edited the manuscript; Dr Molteno coordinated and supervised data collection and critically reviewed the manuscript; Dr Dodge assisted with data management and statistical analyses and reviewed the manuscript; Dr Meintjes assisted with coordination of data collection and reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism grants: R01 AA09524; R01 AA016781; U01 AA014790; U24 AA014815; U01 AA014809; K23 AA020516; and Lycaki-Young Fund from the State of Michigan. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.May PA, Gossage JP, Kalberg WO, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192 [DOI] [PubMed] [Google Scholar]
- 2.May PA, Blankenship J, Marais A-S, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37(5):818–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127C(1):42–50 [DOI] [PubMed] [Google Scholar]
- 4.Greene T, Ernhart CB, Sokol RJ, et al. Prenatal alcohol exposure and preschool physical growth: a longitudinal analysis. Alcohol Clin Exp Res. 1991;15(6):905–913 [DOI] [PubMed] [Google Scholar]
- 5.Streissguth AP, Martin DC, Martin JC, Barr HM. The Seattle longitudinal prospective study on alcohol and pregnancy. Neurobehav Toxicol Teratol. 1981;3(2):223–233 [PubMed] [Google Scholar]
- 6.Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Shankaran S. Effects of alcohol use, smoking, and illicit drug use on fetal growth in black infants. J Pediatr. 1994;124(5 pt 1):757–764 [DOI] [PubMed] [Google Scholar]
- 7.Carter RC, Jacobson JL, Molteno CD, et al. Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol Clin Exp Res. 2012;36(11):1973–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter RC, Jacobson JL, Sokol RJ, Avison MJ, Jacobson SW. Fetal alcohol-related growth restriction from birth through young adulthood and moderating effects of maternal prepregnancy weight. Alcohol Clin Exp Res. 2013;37(3):452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–1591 [DOI] [PubMed] [Google Scholar]
- 10.Lumeng JC, Cabral HJ, Gannon K, Heeren T, Frank DA. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29(4):446–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JL, Jacobson SW, Sokol RJ. Effects of prenatal exposure to alcohol, smoking, and illicit drugs on postpartum somatic growth. Alcohol Clin Exp Res. 1994;18(2):317–323 [DOI] [PubMed] [Google Scholar]
- 12.Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: National Academy Press; 1996 [Google Scholar]
- 14.Jacobson SW, Stanton ME, Molteno CD, et al. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–372 [DOI] [PubMed] [Google Scholar]
- 15.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–825 [DOI] [PubMed] [Google Scholar]
- 16.Bowman RS, Stein LI, Newton JR. Measurement and interpretation of drinking behavior. I. On measuring patterns of alcohol consumption. II. Relationships between drinking behavior and social adjustment in a sample of problem drinkers. J Stud Alcohol. 1975;36(9):1154–1172 [DOI] [PubMed] [Google Scholar]
- 17.Bearer CF, Jacobson JL, Jacobson SW, et al. Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143(4):463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Astley SJ, Clarren SK. Measuring the facial phenotype of individuals with prenatal alcohol exposure: correlations with brain dysfunction. Alcohol Alcohol. 2001;36(2):147–159 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual. Atlanta, GA: CDC; 2007 [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002; (246):1–190 [PubMed] [Google Scholar]
- 21.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3(6):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madge EM, van den Berg AR, Robinson M, Landman J. Junior South African Individual Scales. Pretoria, South Africa: Human Sciences Research Council; 1981 [Google Scholar]
- 23.Lewis CE, Thomas KGF, Dodge NC, et al. Verbal learning and memory impairment in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2015;39(4):724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindinger NM, Malcolm-Smith S, Dodge NC, et al. Theory of mind in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2016;40(2):367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effects. Psychol Bull. 1993;114(2):376–390 [DOI] [PubMed] [Google Scholar]
- 26.Selvin S. Statistical Analysis of Epidemiologic Data. 3rd ed. Oxford, United Kingdom: Oxford University Press; 2004 [Google Scholar]
- 27.Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51 [Google Scholar]
- 28.Vorhees CV. Principles of Behavioral Teratology. In: Riley EP, Vorhees CV, eds. Handbook of Behavioral Teratology. New York, NY: Plenum Press; 1986:23–48 [Google Scholar]
- 29.Carter RC, Wainwright H, Molteno CD, et al. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol Clin Exp Res. 2016;40(4):753–764 [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28(11):1732–1745 [DOI] [PubMed] [Google Scholar]
- 31.Mattson SN, Riley EP, Jernigan TL, et al. Fetal alcohol syndrome: a case report of neuropsychological, MRI and EEG assessment of two children. Alcohol Clin Exp Res. 1992;16(5):1001–1003 [DOI] [PubMed] [Google Scholar]
- 32.Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12(1):146–153 [DOI] [PubMed] [Google Scholar]
- 33.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21(2):81–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26(6):875–882 [PubMed] [Google Scholar]
- 35.Kodituwakku PW, Handmaker NS, Cutler SK, Weathersby EK, Handmaker SD. Specific impairments in self-regulation in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1995;19(6):1558–1564 [DOI] [PubMed] [Google Scholar]
- 36.Cook JL, Green CR, Lilley CM, et al. ; Canada Fetal Alcohol Spectrum Disorder Research Network . Fetal alcohol spectrum disorder: a guideline for diagnosis across the lifespan. CMAJ. 2016;188(3):191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29(3):443–452 [DOI] [PubMed] [Google Scholar]
- 38.Goldschmidt L, Richardson GA, Stoffer DS, Geva D, Day NL. Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol Clin Exp Res. 1996;20(4):763–770 [DOI] [PubMed] [Google Scholar]