Abstract
Objective:
Exsanguination due to coagulopathy and vascular injury is a common cause of death among trauma patients. Arterial injury can be treated either by angiography and embolization or by explorative laparotomy and surgical packing. The purpose of this study was to compare 30-day mortality and blood product consumption in trauma patients with active arterial haemorrhage in the abdominal and/or pelvic region treated with either angiography and embolization or explorative laparotomy and surgical packing.
Material and Methods:
From January 1st 2006 to December 31st 2011 2,173 patients with an ISS of >9 were admitted to the Trauma Centre of Copenhagen University Hospital, Rigshospitalet, Denmark. Of these, 66 patients met the inclusion criteria: age above 15 years and active arterial haemorrhage from the abdominal and/or pelvic region verified by a CT scan at admission. Gender, age, initial oxygen saturation, pulse rate and respiratory rate, mechanism of injury, ISS, Probability of Survival, treatment modality, 30-day mortality and number and type of blood products applied were retrieved from the TARN database, patient records and the Danish Civil Registration System.
Results:
Thirty-one patients received angiography and embolization, and 35 patients underwent exploratory laparotomy and surgical packing. Gender, age, initial oxygen saturation, pulse rate and respiratory rate, ISS and Probability of Survival were comparable in the two groups.
Conclusion:
A significant increased risk of 30-day mortality (P = 0.04) was found in patients with active bleeding treated with explorative laparotomy and surgical packing compared to angiography and embolization when data was adjusted for age and ISS. No statistical significant difference (P > 0.05) was found in number of transfused blood products applied in the two groups of patients.
Keywords: Embolization, outcome, surgical packing, uncontrolled bleeding
INTRODUCTION
Despite advances in trauma care, uncontrolled bleeding remains a major challenge, accounting for approximately 40% of trauma-related deaths, and it is usually caused by a combination of coagulopathy and vascular injury.[1,2,3,4] Two different treatment modalities have been advocated to address a persistent hemodynamic instability due to vascular injury. One modality is angiography with embolization controlling arterial haemorrhage. The other modality is exploratory laparotomy and surgical packing, which through indirect tamponade stops venous and, to some extent, arterial bleeding.
The overall frequency of simultaneous intra-abdominal injuries and unstable pelvic fracture is reported as high as 67%.[5,6,7] In patients with both retroperitoneal bleeding from pelvic fractures and free bleeding into the intraperitoneal space, the optimal treatment strategy remains controversial.[5,8,9,10,11,12,13] Moreover, it is difficult to compare the effectiveness of embolization and surgical packing, mainly because the published reports included patient cohorts with differences in the severity and complexity of injuries.
The purpose of this study was to compare 30-day mortality and blood product consumption in trauma patients with active arterial hemorrhage in the abdominal and/or pelvic region treated with either angiography and embolization or explorative laparotomy and surgical packing.
MATERIALS AND METHODS
This observational study consisted of a cohort of trauma patients admitted to the Level I Trauma Centre of Rigshospitalet Copenhagen University Hospital, Copenhagen, Denmark, from January 1, 2006, to December 31, 2011. Data were derived from patient records and the trauma audit and research network (TARN) database consisting of prospective data on trauma patients primarily admitted to the Trauma Centre or who had a total length of stay of at least 72 h, who were secondarily referred to the Trauma Centre from other hospitals, who were admitted to Intensive Care Unit regardless of length of stay, or who died in the hospital. Patients above 15 years of age in the TARN database were eligible for inclusion if the Injury Severity Score (ISS)[14] was 9 or more, abdominal and/or pelvic lesions with computerized tomography (CT) scan verified active arterial bleeding was present, and the patient underwent either arteriography and embolization or exploratory laparotomy and surgical packing. Patients without the Danish civil registration number were excluded because it was not possible to follow-up. Active arterial bleeding was defined as contrast blush beyond the borders of organs.
Routinely, trauma patients in our facility underwent clinical and radiographic examination on initial admission to the emergency room according to the advanced trauma life support guidelines.[15] Anteroposterior chest and pelvic radiographs were obtained and focused assessment with sonography for trauma (FAST) examination was performed. Unstable patients suffering from pelvic fracture had a pelvic sling applied before hospitalization or upon arrival to the emergency room. Volume resuscitation was applied according to the Copenhagen concept by Johansson,[16] involving an early administration of plasma and platelet concentrates (each pooled from four blood donors) in a balanced administration of blood products (unit-for-unit ratio) and goal-directed hemostatic control resuscitation based on the results of the thromboelastography analysis as early as possible. When hemodynamic stability permits radiographic workup, CT was performed using a specific trauma protocol on a multi-slice helical CT scanner. A nonionic iodine-based contrast agent in a dose of 100 ml (350 mg iodine/ml) was injected intravenously with a flow of 3 ml/s. The scan delay was 55 s aiming at the early venous phase. Axial, coronal, and sagittal soft-tissue reconstructions were performed with a slice thickness of 3 mm. Oral contrast agents were not used. Embolization was only possible from 8 a.m. to 11 p.m.
The anesthesiologist was the team leader and decided, in consultation with the surgeons, whether to proceed with either arteriography and embolization or explorative laparotomy and surgical packing. This decision was made on an individual basis depending on which other injuries the patient might have. Standard endovascular techniques via arterial access to one of the two common femoral arteries were used for angiography and embolization. All patients underwent selective catheterization and digital subtraction angiography to identify the arterial bleeding sites. Embolization materials such as coils, particles, gelatin sponge, or vascular plugs were used on an individual basis. The laparotomies were performed using midline incisions and damage control principles.[17] The pelvic injury was stabilized with temporary external fixation with pins either supraacetabular or in the iliac wing, and pelvic packing performed through a Pfannenstiel incision or elongation of the laparotomy incision.
The following parameters were retrieved from TARN database and patients records: gender, age, initial oxygen saturation, pulse rate and respiratory rate in the emergency room, mechanism of injury, ISS, probability of survival (Ps),[18] treatment strategy (either arteriography and embolization or explorative laparotomy and surgical packing), initial arterial gas values from admission to the emergency room (pH, hemoglobin, pCO2, pO2, base deficit, and lactate), and units and type of blood products (packed red blood cells, fresh frozen plasma, and pooled thrombocytes) infused. Survival status was obtained from the Danish Civil Registration System. In Denmark, all citizens and foreign residents can be traced via their unique civil registration number.[19]
The primary outcome was 30-day mortality, and the secondary outcome measures were units of transfused blood products after 6 h, after 24 h, and the total amount during hospitalization.
The sample size was determined based on the comparison of the primary endpoint of death at 30 days between patients undergoing angiography and embolization or explorative laparotomy and surgical packing. Based on previously published data,[20,21] we estimated the 30-day mortality to be 33% in the surgical group and 10% in the embolization group. With a power of 80% at the 5% level of significance, a sample size of 50 patients in each subgroup was needed for the primary comparison of death at 30 days.
Ethics
The study was approved by the Danish Data Protection Agency (j. nr: 2007-58-0015) and the Danish Health and Medicines Authority (j. nr: 3-3013-861/1).
Statistics
Normality test, by probit plots, showed that age and ISS were normally distributed. Thus, data are presented as a mean with standard deviation in brackets. Arterial gas values, Ps, and units of blood products were not normally distributed; hence, they are presented as a median with range in brackets.
T-tests were used for continuous data that were normally distributed and Mann–Whitney test for nonparametric data. Chi-squared test was used for categorical data.
Since we found a marginal difference in 30-day mortality between patients receiving embolization and exploratory laparotomy, a logistic regression analysis was repeated adjusting for age and ISS.
All statistical analyses were performed with the STATA® Version 13.0 statistical software (StataCorp LP, College Station, TX, USA). A significance level of P < 0.05 was chosen for all comparisons.
RESULTS
In total, 2,107 of 2,173 trauma patients with an ISS >9 admitted during the study period were excluded, thus 66 patients met the inclusion criteria [Figure 1]. Of these, 31 received arteriography and embolization and 35 underwent exploratory laparotomy and surgical packing.. Most of the patients included suffered from a blunt trauma [Table 1].
Figure 1.
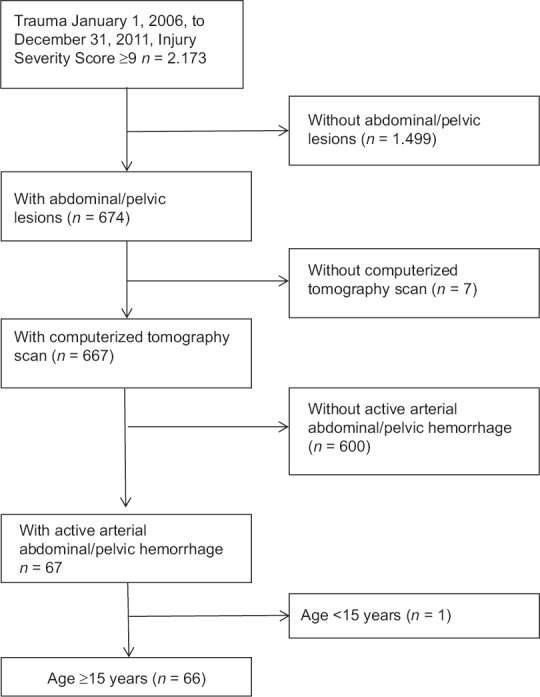
Patients above 15 years were eligible for inclusion if the Injury Severity Score was 9 or more, the patient had abdominal and/or pelvic lesions with computerized tomography scan verified active arterial bleeding and underwent either arteriography and embolization or exploratory laparotomy and surgical packing
Table 1.
Trauma patients (n=66) with active abdominal and/or pelvic arterial hemorrhage receiving either arteriography and embolization or explorative laparotomy and surgical packing
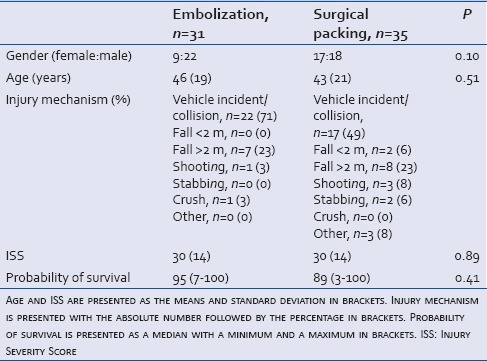
Gender, age, ISS, and Ps are shown in Table 1. There was no statistically significant difference between the two subgroups regarding initial oxygen saturation, pulse rate, and respiratory rate in the emergency room (data not shown), demonstrating comparable patients group.
An overview of injuries and ISS in the two subgroups is presented in Tables 2 and 3. Twenty-seven of 31 patients (87%) who underwent embolization and 29 of 35 (83%) who underwent surgical packing were severely injured, with an ISS >15.[14]
Table 2.
Overview of patients undergoing angiography and embolization. Abdominal and pelvic lesions are specified as well as additional injuries’ Injury Severity Score are shown
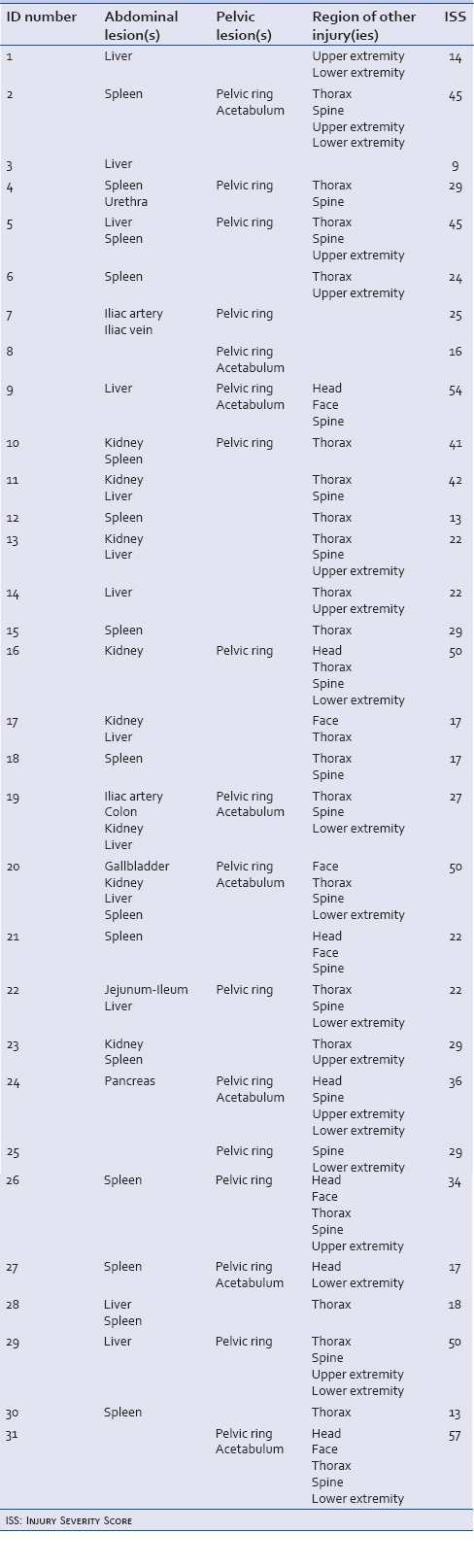
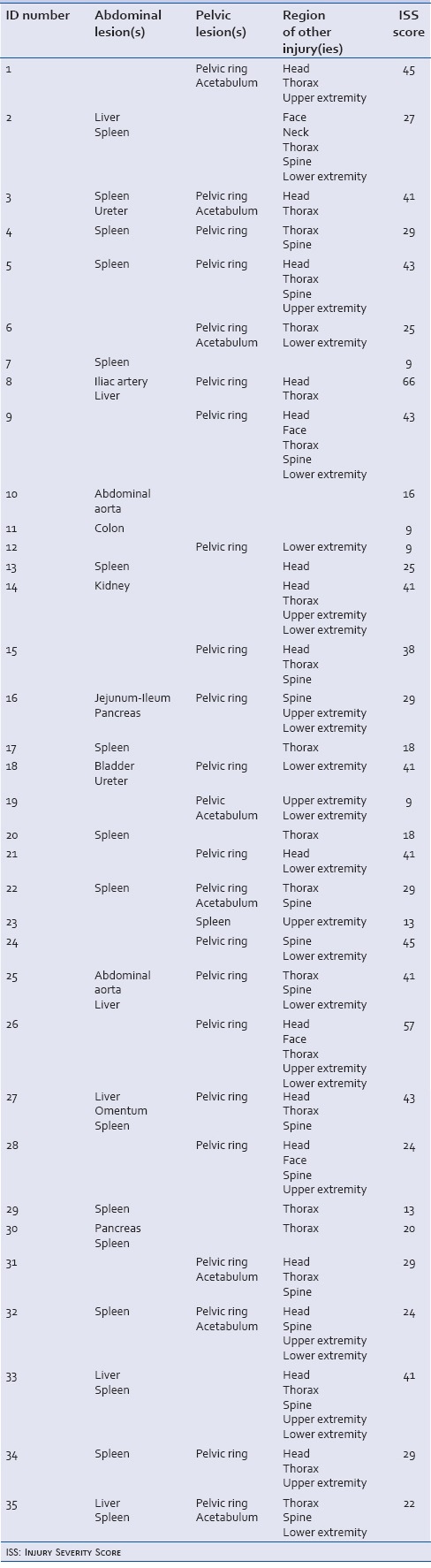
Table 3.
Overview of patients undergoing exploratory laparotomy and surgical packing. Abdominal and pelvic lesions are specified as well as additional injuries’ Injury Severity Score are shown
The 30-day mortality rate of patients undergoing embolization was 10% (3/31) compared with 26% (9/35) for patients being packed (P = 0.09). A logistic regression analysis adjusting for age and ISS showed a more than 9-fold increased risk of mortality in patients undergoing surgical packing compared to embolization (P = 0.04, odds ratio 9.4, 95% confidence interval 1.2.–75.7).
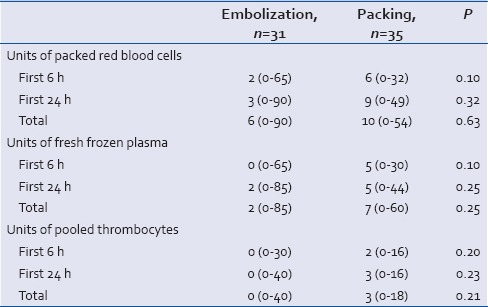
In patients who underwent surgical packing, more units of packed red blood cells, fresh frozen plasma, and pooled thrombocytes were transfused within the first 6 h, first 24 h, and the total amount during their hospital stay compared with patients embolized. However, these differences were not statistically significant [Table 4].
Table 4.
The number of blood products applied in the first 6 h, first 24 h, and the total amount during hospitalization is presented as a median with a minimum and a maximum in brackets. Each unit of thrombocytes was pooled from four blood donors
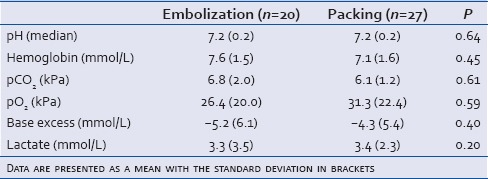
Initial arterial gas values were available in a subgroup of 20 patients undergoing embolization and 27 patients undergoing surgical packing. There was no significant difference in gender, age, ISS, or Ps between the two subgroups (data not shown) or we did not find any significant differences in initial pH, hemoglobin, pCO2, pO2, base deficit, and lactate values between the two subgroups [Table 5]. In both groups, pH and base deficit were decreased and lactate, pCO2, and pO2 were increased compared to normal values. There was no statistically significant difference between the two subgroups regarding the number of blood products applied or mortality (data not shown).
Table 5.
For 47 patients, arterial gas values from the emergency room were present
DISCUSSION
This study found a statistical significant increased risk of 30-day mortality in patients with active bleeding treated with explorative laparotomy and surgical packing compared to angiography and embolization when data were adjusted for age and ISS. No statistically significant difference was found in number of transfused blood products applied in the two groups of patients.
The management of solid organ injuries has shifted during the last 30 years from operative to nonoperative management strategies.[22] Nowadays, embolization is widely accepted as an option for hemodynamically stable patients with organ injuries and evidence of active contrast extravasation on CT scan as long as peritonitis or other indications for laparotomy do not exist.[23] However, experienced trauma centers are broadening their criteria to include patients who are also hypotensive.[7,24] In pelvic fractures, the presacral venous plexus, cancellous surfaces of the fractures pelvic bones, and ruptured branches of the iliac vessels, in any combination, constitute the main source of intrapelvic hemorrhage.[8] Exsanguination is the most common cause of death among trauma patients and whether the optimal treatment strategy is arteriography and embolization or laparotomy and surgical packing is still debated. Some authors consider embolization to be the optimal method for controlling arterial hemorrhage, thereby allowing the tamponade of the hematoma to control venous bleeding,[8,9,10,11] whereas others prefer laparotomy and packing.[5,12,13] Recent studies have been unable to measure a difference in mortality between surgical packing and angiographic intervention.[20,21,25]
Active hemorrhage can be diagnosed by CT scan on the basis of increased radiodensity compared with surrounding tissue, resulting from the extravasation of intravascular contrast agent. This has proven an accurate indicator of on-going arterial hemorrhage.[26,27] Velmahos et al.[28] reported angiographic embolization effective in 95% of patients with major pelvic fractures or solid visceral organ injuries. This is supported by previous studies finding arterial hemorrhage arising from pelvic fracture[8,20,29,30,31,32,33,34] and solid organ injuries[22,35,36,37,38,39,40] amenable to management with angiography and embolization. Disadvantages to angiography are the transportation of a severely injured patient to the angiography suite and the availability of a skilled interventional radiologist and associated technical staff. However, hybrid suites are increasingly implemented in trauma centers to avoid transportation for endovascular procedures. Moreover, a previous study of patients suffering from abdominal and pelvic trauma[41] described the incidence of contrast-induced nephropathy to be 24% after angiography and embolization, 11% of the patients developed renal failure, 19% had organ-specific complications, and 15% had rebleeding.
Compared with angiography, surgical packing is a more invasive procedure. However, some prefer laparotomy and surgical packing because it is ideal when angiography is unavailable. In addition, it is up to four times faster than angiography and embolization[13] and the patient can rapidly undergo additional procedures in the operating room such as external fixation of extremity fractures.[5,12,13] However, up to 60% of patients suffer from postoperative infection, abscess, hernia, and fistula after acute laparotomy.[42,43]
In the present study, 30-day mortality was chosen as an endpoint because this has proved to be an appropriate period analyzing trauma-related death.[44] The overall 30-day mortality rate of our study was 18% (12/66), which is in accordance with previous studies.[21,45,46] Adjusting for age and ISS, an increased risk of 30-day mortality was found (P = 0.04) in patients undergoing surgical packing compared with patients undergoing embolization.
In patients who underwent surgical packing, more units of packed red blood cells, fresh frozen plasma, and pooled thrombocytes were transfused within the first 6 h, first 24 h, and the total amount during the hospital stay compared with patients embolized. However, the difference was not statistically significant. This was in contrast to Osborn et al.,[21] who found a decreased need for blood transfusion in a group of patients with pelvic ring injuries undergoing surgical packing compared to a group undergoing angiography. In a study by Osborn et al.,[21] unstable patients with abdominal bleeding confirmed by either diagnostic lavage or FAST underwent laparotomy and surgical packing whereas unstable patients without bleeding confirmed by FAST had angiography. Ten of twenty patients in Osborn’s study underwent embolization for bleeding pelvic arterial lesions and three underwent embolization for nonbleeding pelvic arteries. In the remaining seven cases, no active pelvic arterial bleeding was identified and embolization was not performed. Hence, patients in the two groups might have been biased. In the present study, only patients with active arterial bleeding verified by CT were included. Moreover, all patients evaluated by angiography were also embolized. This may explain the different conclusions.
There are several strengths of our study. First, all patients included had active extravasation on CT and had a standardized resuscitation regimen[16] applied throughout the study period. Second, the amount and type of blood products applied for each patient were known, as well as the point of time the products were used. Third, TARN data are collected prospectively, and finally, we were able to follow-up all patients due to the unique civil registration number. The weaknesses of our study include the small number of patients, which may limit the findings and increase the potential risk of a Type II error. In a post hoc analysis of our data (mortality 26% vs. 10%), we found that more than 180 patients would have been needed to detect a significant difference, which was more than twice the number of patients in our study (66 patients). Different anesthesiologists and surgeons were responsible for deciding which treatment the patient should undergo, and access to embolization was limited. Hence, treatment might be biased. However, there were no differences in gender, age, initial oxygen saturation, pulse rate and respiratory rate, ISS, Ps, and initial arterial gas values between the group undergoing arteriography and embolization compared with the group undergoing laparotomy and surgical packing. Thus, it seems that the patients included in this study were comparable between the two groups according to gender, age, and severity of trauma. According to Tay et al.,[47] 4% of ISS scores are misclassified; hence, we might have excluded patients who met the inclusion criteria and vice versa. Moreover, this study is observational and causality cannot be determined as in a randomized controlled trial.
CONCLUSION
A significant increased risk of 30-day mortality (P = 0.04) was found in patients with active bleeding treated with explorative laparotomy and surgical packing compared to angiography and embolization when data were adjusted for age and ISS. No statistically significant difference was found in number of transfused blood products applied in the two groups of patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Cosgriff N, Moore EE, Sauaia A, Kenny-Moynihan M, Burch JM, Galloway B. Predicting life-threatening coagulopathy in the massively transfused trauma patient: Hypothermia and acidoses revisited. J Trauma. 1997;42:857–61. doi: 10.1097/00005373-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB. Methods for improved hemorrhage control. Crit Care. 2004;8(Suppl 2):S57–60. doi: 10.1186/cc2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyt DB. A clinical review of bleeding dilemmas in trauma. Semin Hematol. 2004;41(1 Suppl 1):40–3. doi: 10.1053/j.seminhematol.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Ruchholtz S, Waydhas C, Lewan U, Pehle B, Taeger G, Kühne C, et al. Free abdominal fluid on ultrasound in unstable pelvic ring fracture: Is laparotomy always necessary? J Trauma. 2004;57:278–85. doi: 10.1097/01.ta.0000133840.44265.ca. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Menachem Y, Coldwell DM, Young JW, Burgess AR. Hemorrhage associated with pelvic fractures: Causes, diagnosis, and emergent management. AJR Am J Roentgenol. 1991;157:1005–14. doi: 10.2214/ajr.157.5.1927786. [DOI] [PubMed] [Google Scholar]
- 7.Fu CY, Wang YC, Wu SC, Chen RJ, Hsieh CH, Huang HC, et al. Angioembolization provides benefits in patients with concomitant unstable pelvic fracture and unstable hemodynamics. Am J Emerg Med. 2012;30:207–13. doi: 10.1016/j.ajem.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Papakostidis C, Giannoudis PV. Pelvic ring injuries with haemodynamic instability: Efficacy of pelvic packing, a systematic review. Injury. 2009;40(Suppl 4):S53–61. doi: 10.1016/j.injury.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF., 3rd Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997;43:395–9. doi: 10.1097/00005373-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gruen GS, Leit ME, Gruen RJ, Peitzman AB. The acute management of hemodynamically unstable multiple trauma patients with pelvic ring fractures. J Trauma. 1994;36:706–11. doi: 10.1097/00005373-199405000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Mucha P, Jr, Farnell MB. Analysis of pelvic fracture management. J Trauma. 1984;24:379–86. doi: 10.1097/00005373-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Eastridge BJ, Starr A, Minei JP, O’Keefe GE, Scalea TM. The importance of fracture pattern in guiding therapeutic decision-making in patients with hemorrhagic shock and pelvic ring disruptions. J Trauma. 2002;53:446–50. doi: 10.1097/00005373-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Cothren CC, Osborn PM, Moore EE, Morgan SJ, Johnson JL, Smith WR. Preperitonal pelvic packing for hemodynamically unstable pelvic fractures: A paradigm shift. J Trauma. 2007;62:834–9. doi: 10.1097/TA.0b013e31803c7632. [DOI] [PubMed] [Google Scholar]
- 14.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 15.Kortbeek JB, Al Turki SA, Ali J, Antoine JA, Bouillon B, Brasel K, et al. Advanced trauma life support, 8th edition, the evidence for change. J Trauma. 2008;64:1638–50. doi: 10.1097/TA.0b013e3181744b03. [DOI] [PubMed] [Google Scholar]
- 16.Johansson PI. Goal-directed hemostatic resuscitation for massively bleeding patients: The Copenhagen concept. Transfus Apher Sci. 2010;43:401–5. doi: 10.1016/j.transci.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Rotondo MF, Schwab CW, McGonigal MD, Phillips GR, 3rd, Fruchterman TM, Kauder DR, et al. 'Damage control': An approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–82. [PubMed] [Google Scholar]
- 18.Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: The TRISS method. Trauma score and the injury severity score. J Trauma. 1987;27:370–8. [PubMed] [Google Scholar]
- 19.Frank L. Epidemiology. When an entire country is a cohort. Science. 2000;287:2398–9. doi: 10.1126/science.287.5462.2398. [DOI] [PubMed] [Google Scholar]
- 20.Thorson CM, Ryan ML, Otero CA, Vu T, Borja MJ, Jose J, et al. Operating room or angiography suite for hemodynamically unstable pelvic fractures? J Trauma Acute Care Surg. 2012;72:364–70. doi: 10.1097/TA.0b013e318243da10. [DOI] [PubMed] [Google Scholar]
- 21.Osborn PM, Smith WR, Moore EE, Cothren CC, Morgan SJ, Williams AE, et al. Direct retroperitoneal pelvic packing versus pelvic angiography: A comparison of two management protocols for haemodynamically unstable pelvic fractures. Injury. 2009;40:54–60. doi: 10.1016/j.injury.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 22.Salcedo ES, Brown IE, Corwin MT, Galante JM. Angioembolization for solid organ injury: A brief review. Int J Surg. 2015 doi: 10.1016/j.ijsu.2015.10.030. pii: S1743-919101295-9. [DOI] [PubMed] [Google Scholar]
- 23.Olthof DC, van der Vlies CH, Joosse P, van Delden OM, Jurkovich GJ, Goslings JC PYTHIA Collaboration Group. Consensus strategies for the nonoperative management of patients with blunt splenic injury: A Delphi study. J Trauma Acute Care Surg. 2013;74:1567–74. doi: 10.1097/TA.0b013e3182921627. [DOI] [PubMed] [Google Scholar]
- 24.Hagiwara A, Murata A, Matsuda T, Matsuda H, Shimazaki S. The usefulness of transcatheter arterial embolization for patients with blunt polytrauma showing transient response to fluid resuscitation. J Trauma. 2004;57:271–6. doi: 10.1097/01.ta.0000131198.79153.3c. [DOI] [PubMed] [Google Scholar]
- 25.Katsura M, Yamazaki S, Fukuma S, Matsushima K, Yamashiro T, Fukuhara S. Comparison between laparotomy first versus angiographic embolization first in patients with pelvic fracture and hemoperitoneum: A nationwide observational study from the Japan Trauma Data Bank. Scand J Trauma Resusc Emerg Med. 2013;21:82. doi: 10.1186/1757-7241-21-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira SJ, O’Brien DP, Luchette FA, Choe KA, Lim E, Davis K, Jr, et al. Dynamic helical computed tomography scan accurately detects hemorrhage in patients with pelvic fracture. Surgery. 2000;128:678–85. doi: 10.1067/msy.2000.108219. [DOI] [PubMed] [Google Scholar]
- 27.Yoon W, Kim JK, Jeong YY, Seo JJ, Park JG, Kang HK. Pelvic arterial hemorrhage in patients with pelvic fractures: Detection with contrast-enhanced CT. Radiographics. 2004;24:1591–605. doi: 10.1148/rg.246045028. [DOI] [PubMed] [Google Scholar]
- 28.Velmahos GC, Toutouzas KG, Vassiliu P, Sarkisyan G, Chan LS, Hanks SH, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002;53:303–8. doi: 10.1097/00005373-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Tanizaki S, Maeda S, Hayashi H, Matano H, Ishida H, Yoshikawa J, et al. Early embolization without external fixation in pelvic trauma. Am J Emerg Med. 2012;30:342–6. doi: 10.1016/j.ajem.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 30.Papakostidis C, Kanakaris N, Dimitriou R, Giannoudis PV. The role of arterial embolization in controlling pelvic fracture haemorrhage: A systematic review of the literature. Eur J Radiol. 2012;81:897–904. doi: 10.1016/j.ejrad.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 31.Niola R, Pinto A, Sparano A, Ignarra R, Romano L, Maglione F. Arterial bleeding in pelvic trauma: Priorities in angiographic embolization. Curr Probl Diagn Radiol. 2012;41:93–101. doi: 10.1067/j.cpradiol.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Bozeman MC, Cannon RM, Trombold JM, Smith JW, Franklin GA, Miller FB, et al. Use of computed tomography findings and contrast extravasation in predicting the need for embolization with pelvic fractures. Am Surg. 2012;78:825–30. [PubMed] [Google Scholar]
- 33.Arvieux C, Thony F, Broux C, Ageron FX, Rancurel E, Abba J, et al. Current management of severe pelvic and perineal trauma. J Visc Surg. 2012;149:e227–38. doi: 10.1016/j.jviscsurg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture – Update and systematic review. J Trauma. 2011;71:1850–68. doi: 10.1097/TA.0b013e31823dca9a. [DOI] [PubMed] [Google Scholar]
- 35.Morris CS, Cho KJ. Interventional Radiology for Vascular and Solid Organ Trauma. [Last accessed on 2015 Nov 20]. Available from: http://www.emedicine.medscape.com/article/423295-overview .
- 36.Zealley IA, Chakraverty S. The role of interventional radiology in trauma. BMJ. 2010;340:c497. doi: 10.1136/bmj.c497. [DOI] [PubMed] [Google Scholar]
- 37.Glorsky SL, Wonderlich DA, Goei AD. Evaluation and management of the trauma patient for the interventional radiologist. Semin Intervent Radiol. 2010;27:29–37. doi: 10.1055/s-0030-1247886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gould JE, Vedantham S. The role of interventional radiology in trauma. Semin Intervent Radiol. 2006;23:270–8. doi: 10.1055/s-2006-948766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wilden GM, Velmahos GC, Emhoff T, Brancato S, Adams C, Georgakis G, et al. Successful nonoperative management of the most severe blunt liver injuries: A multicenter study of the research consortium of new England centers for trauma. Arch Surg. 2012;147:423–8. doi: 10.1001/archsurg.2012.147. [DOI] [PubMed] [Google Scholar]
- 40.Skattum J, Naess PA, Eken T, Gaarder C. Refining the role of splenic angiographic embolization in high-grade splenic injuries. J Trauma Acute Care Surg. 2013;74:100–3. doi: 10.1097/TA.0b013e31827890b2. [DOI] [PubMed] [Google Scholar]
- 41.van der Vlies CH, Saltzherr TP, Reekers JA, Ponsen KJ, van Delden OM, Goslings JC. Failure rate and complications of angiography and embolization for abdominal and pelvic trauma. J Trauma Acute Care Surg. 2012;73:1208–12. doi: 10.1097/TA.0b013e318265ca9f. [DOI] [PubMed] [Google Scholar]
- 42.Brenner M, Bochicchio G, Bochicchio K, Ilahi O, Rodriguez E, Henry S, et al. Long-term impact of damage control laparotomy: A prospective study. Arch Surg. 2011;146:395–9. doi: 10.1001/archsurg.2010.284. [DOI] [PubMed] [Google Scholar]
- 43.Fox N, Crutchfield M, LaChant M, Ross SE, Seamon MJ. Early abdominal closure improves long-term outcomes after damage-control laparotomy. J Trauma Acute Care Surg. 2013;75:854–8. doi: 10.1097/TA.0b013e3182a8fe6b. [DOI] [PubMed] [Google Scholar]
- 44.Skaga NO, Eken T, Jones JM, Steen PA. Different definitions of patient outcome: Consequences for performance analysis in trauma. Injury. 2008;39:612–22. doi: 10.1016/j.injury.2007.11.426. [DOI] [PubMed] [Google Scholar]
- 45.Hauschild O, Aghayev E, von Heyden J, Strohm PC, Culemann U, Pohlemann T, et al. Angioembolization for pelvic hemorrhage control: Results from the German pelvic injury register. J Trauma Acute Care Surg. 2012;73:679–84. doi: 10.1097/TA.0b013e318253b5ba. [DOI] [PubMed] [Google Scholar]
- 46.Ordoñez C, Pino L, Badiel M, Sanchez A, Loaiza J, Ramirez O, et al. The 1-2-3 approach to abdominal packing. World J Surg. 2012;36:2761–6. doi: 10.1007/s00268-012-1745-3. [DOI] [PubMed] [Google Scholar]
- 47.Tay SY, Sloan EP, Zun L, Zaret P. Comparison of the new injury severity score and the injury severity score. J Trauma. 2004;56:162–4. doi: 10.1097/01.TA.0000058311.67607.07. [DOI] [PubMed] [Google Scholar]


