Abstract
Background
Etanercept, a soluble tumor necrosis factor receptor, and acitretin have been shown to be effective in treating psoriasis. Acitretin is widely used in Korea. However, the combination of etanercept plus acitretin has not been evaluated among Korean patients with psoriasis. The objective of this study was to investigate the efficacy and safety of combination therapy with etanercept and acitretin in patients with moderate to severe plaque psoriasis.
Methods
Sixty patients with psoriasis were randomized to receive etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for 12 weeks (ETN-ETN); etanercept 25 mg BIW plus acitretin 10 mg twice daily (BID) for 24 weeks (ETN-ACT); or acitretin 10 mg BID for 24 weeks (ACT). The primary efficacy measurement was the proportion of patients achieving 75 % improvement in Psoriasis Area and Severity Index (PASI 75) at week 24. Secondary end points included 50 % improvement in PASI (PASI 50) at week 24 and clear/almost-clear by Physician Global Assessment (PGA) at each visit through week 24.
Results
The proportions of patients achieving PASI 75, PASI 50, and PGA clear/almost-clear at week 24 in the ETN-ETN (52.4, 71.4, and 52.4 %, respectively) and ETN-ACT groups (57.9, 84.2, and 52.6 %, respectively) were higher than in the ACT group (22.2, 44.4, and 16.7 %, respectively). The incidence of adverse events was similar across all arms. This was an open-label study with a small number of patients.
Conclusion
In Korean patients with moderate to severe plaque psoriasis, etanercept alone or in combination with acitretin was more effective than acitretin. All treatments were well tolerated throughout the study.
Trial registration
This study was registered on July 7, 2009 at ClinicalTrials.gov, NCT00936065.
Keywords: Psoriasis, Etanercept, Acitretin, Combination therapy, Efficacy, Safety, Korean patients
Background
Psoriasis is a chronic autoimmune condition that affects 1 to 3 % of the general population worldwide [1–3]. Psoriasis has been associated with an increased risk for arthritis [4, 5], diabetes [4, 6, 7], cardiovascular disease [4, 8], depression [4, 9], and poor quality of life (QoL) [4, 10]. Although there is as yet no cure [1–3], there are several effective treatments available to manage the disease [11–16].
In clinical trials, etanercept, a tumor necrosis factor-alpha (TNFα) inhibitor, has been shown to be effective in managing psoriasis including improvements in Psoriasis Area Severity Index (PASI) scores [17–19], Physician’s Global Assessment (PGA) [18, 19], and QoL [19, 20]. Acitretin, a systemic retinoid, is also effective for the management of psoriasis [21]. Acitretin is frequently used in combination with other agents (e.g., phototherapy and vitamin D), since acitretin monotherapy is often only moderately effective [21–24]. Since acitretin is not an immunosuppressive agent, combination treatment with etanercept may have a synergistic effect with a low risk of toxicity [21, 24]. This has been demonstrated in a pilot study in which the combination of acitretin and low-dose etanercept was as effective as high-dose etanercept, and both were significantly more effective than acitretin alone [25]. The purpose of the current study was to evaluate combination therapy of etanercept plus acitretin among Korean patients with psoriasis.
Methods
Patients
This study was reviewed and approved by local Institutional Review Boards (Appendix) and was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with Good Clinical Practice Guidelines. All patients provided signed informed consent.
Patients were eligible for study enrolment if they were at least 18 years of age and had active, clinically stable moderate to severe plaque psoriasis involving ≥10 % body surface area (BSA) or PASI ≥10. Exclusion criteria included evidence of skin conditions (e.g., eczema) other than psoriasis that would interfere with evaluations of the effect of study medication on psoriasis; any rheumatologic disease; prior exposure to biologic therapies, including etanercept, within 24 weeks of baseline visit; previous history of phototherapy or any systemic or topical therapy, including acitretin, for psoriasis within the previous 28 days before baseline visit; uncontrolled hypertension or diabetes mellitus; any severe hematologic, cardiovascular, renal, hepatic, or pulmonary disorders; known contraindication or hypersensitivity to etanercept or acitretin or their excipients; women who were pregnant, expecting to become pregnant, or breast-feeding during the study period; patients with any clinically relevant concurrent medical conditions such as active or chronic infections, including human immunodeficiency virus, hepatitis B virus and hepatitis C virus infections, and active or recent (within 2 years) tuberculosis; history of cancer (or carcinoma in situ) other than resected cutaneous basal cell or squamous cell carcinoma within the past 5 years before the screening visit; or patients who had received any investigational drug within 3 months of screening visit.
Study design
In this multicenter, randomized, open-label trial, patients were randomly assigned to one of three treatment groups: (a) etanercept 50 mg twice weekly (BIW) for 12 weeks followed by etanercept 25 mg BIW for a further 12 weeks (ETN–ETN); (b) etanercept 25 mg BIW and acitretin 10 mg twice daily (BID) for 24 weeks (ETN-ACT); (c) acitretin 10 mg BID for 24 weeks (ACT; Fig. 1). This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines and registered on ClinicalTrials.gov, identifier NCT00936065.
Fig. 1.
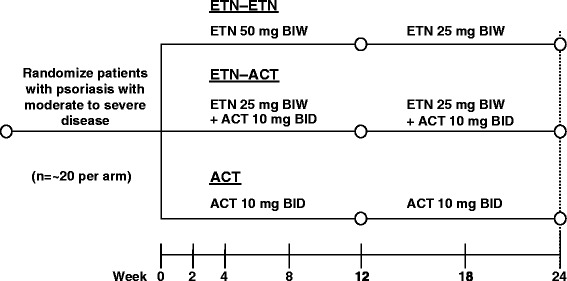
Study design. ACT, acitretin; BID, twice daily; BIW, twice weekly; ETN, etanercept
The primary endpoint was proportion of patients achieving ≥75 % improvement from baseline in PASI score (PASI 75) at week 24. Secondary endpoints included the proportion of patients achieving either PASI 75 or PASI 50 (≥50 % improvement from baseline) at each visit through week 24, PGA status of “clear/almost-clear”, and change in percent of BSA involvement from baseline over time.
Statistical analysis
The sample size was calculated assuming 20 patients per group with a response rate of 35–65 % which would yield a confidence interval below ± 21.9 %. Further assuming the response rate difference for primary efficacy endpoints is 20 % (i.e., 40 vs. 60 %), the 95 % confidence interval would be approximately ± 30 %.
Statistical analysis was performed using SAS software, version 9.13. Efficacy evaluation was performed on the modified intent-to-treat (mITT) and per protocol (PP) population sets. The mITT population included all randomly assigned patients who received at least one dose of test medication and had both baseline and on-therapy PASI evaluations. The PP population included those members of the mITT population who had no major protocol violations that could potentially alter the interpretation of the efficacy analysis.
For the outcomes at each visit, the proportions of responders and the 95 % confidence interval (CI) were determined and the differences between treatment groups were assessed using Fisher exact test or Chi-square test with multiple comparisons, if necessary. Kaplan-Meier estimations for time to first occurrence of each event were determined and the log-rank test was used for statistical testing. For these analyses, no imputation was applied and the patients who did not experience the event were censored at the time of last observation.
Basic descriptive statistics are presented for other measures and the statistical significance of the change from baseline within the treatment groups was assessed using the paired t-test or the Wilcoxon signed-rank test. Differences in change from baseline between the treatment groups were assessed using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test.
Results
All results of this study have been posted on ClinicalTrials.gov, NCT00936065.
Patients
Of the 60 patients enrolled in this study and randomized to the three treatment arms, 45 completed the study (Fig. 2). One patient withdrew from the study after randomization but before receiving any study medication. Reasons for study discontinuation included patient request (n = 6), protocol violation (n = 4), unsatisfactory response (n = 2), adverse event (AE; n = 2), and lost to follow-up (n = 1). The baseline demographics were similar across all treatment arms (Table 1).
Fig. 2.
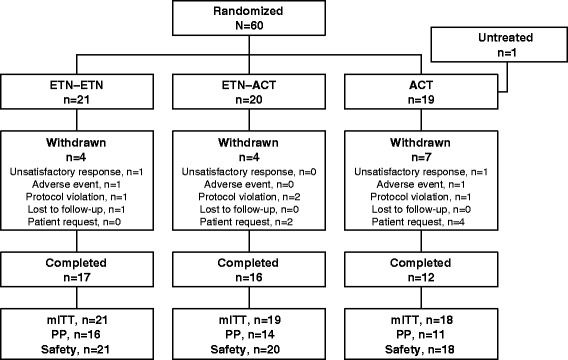
Patient disposition. ACT, acitretin; ETN, etanercept; mITT, modified intent-to-treat population; PP, per-protocol population
Table 1.
Baseline demographics for the mITT population
| Characteristic | ETN 50 mg–ETN 25 mg (n = 21) | ETN 25 mg–ACT 10 mg (n = 19) | ACT 10 mg (n = 18) | Total (N = 58) |
|---|---|---|---|---|
| Mean age, y (SD) | 38.6 (9.5) | 35.5 (8.8) | 42.4 (12.0) | 38.8 (10.3) |
| Mean height, cm (SD) | 169.5 (9.3) | 171.7 (5.5) | 170.9 (6.8) | 170.6 (7.4) |
| Mean weight, kg (SD) | 74.1 (16.0) | 74.0 (11.6) | 74.2 (9.8) | 74.1 (12.7) |
| Gender, n (%) | ||||
| Male | 16 (76.2 %) | 17 (89.5 %) | 15 (83.3 %) | 48 (82.8 %) |
| Cigarette status, n (%) | ||||
| Prior cigarette usage | 5 (23.8 %) | 5 (26.3 %) | 3 (16.7 %) | 13 (22.4 %) |
| Current cigarette usage | 10 (47.6 %) | 11 (57.9 %) | 10 (55.6 %) | 31 (53.5 %) |
| No | 6 (28.6 %) | 3 (15.8 %) | 5 (27.8 %) | 14 (24.1 %) |
| Alcohol status, n (%) | ||||
| Ex-drinker | 2 (9.5 %) | 2 (10.5 %) | 2 (11.1 %) | 6 (10.3 %) |
| Current-drinker | 13 (61.9 %) | 13 (68.4 %) | 11 (61.1 %) | 37 (63.8 %) |
| No | 6 (28.6 %) | 4 (21.1 %) | 5 (27.8 %) | 15 (25.9 %) |
| Prior therapies for psoriasis, n (%) | ||||
| Methotrexate | 2 (9.5 %) | 1 (5.3 %) | 0 | 3 (5.2 %) |
| Cyclosporine | 2 (9.5 %) | 2 (10.5 %) | 1 (5.6 %) | 5 (8.6 %) |
| PUVA | 0 | 3 (15.8 %) | 0 | 3 (5.2 %) |
| Othera | 12 (57.1 %) | 12 (63.2 %) | 10 (55.6 %) | 34 (58.6 %) |
Abbreviations: ACT acitretin, CS clinically significant, ETN etanercept, mITT modified intent-to-treat population, NCS not clinically significant, PUVA psoralen plus ultraviolet A radiation therapy, SD standard deviation
aIncludes systemic antimycobacterials, medication for treating alimentary tract and metabolism conditions, cardiovascular drugs, respiratory drugs, dermatologicals, and systemic hormonal preparations
Efficacy
The proportion of patients achieving PASI 75 by week 24 in the ETN–ETN and the ETN-ACT groups was numerically more than twice that observed for the ACT group (Fig. 3); however, only the pairwise comparison between the ETN-ACT and ACT groups showed statistical significance (p = 0.0448).
Fig. 3.
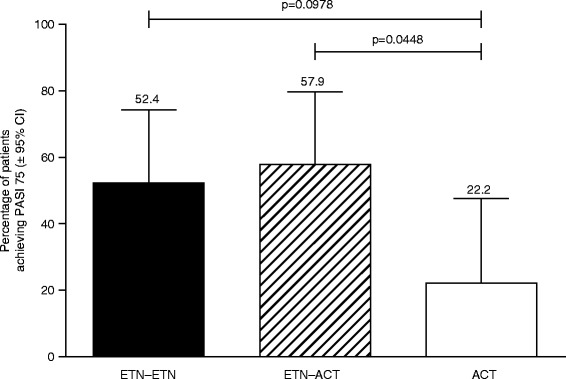
Proportion of patients achieving PASI 75 at week 24. ACT, acitretin; ETN, etanercept; PASI, Psoriasis Area Severity Index
The proportion of patients achieving PASI 75 increased at each visit for all treatment arms (Fig. 4a). Of the three treatment arms, patients in the ETN–ETN group demonstrated the greatest PASI 75 response during the first 18 weeks, after which their response was comparable to that observed for the ETN-ACT group. The PASI 75 responses demonstrated by both of these groups was numerically greater than double that observed for the ACT group. However, statistically significant treatment difference was not shown at any time point.
Fig. 4.
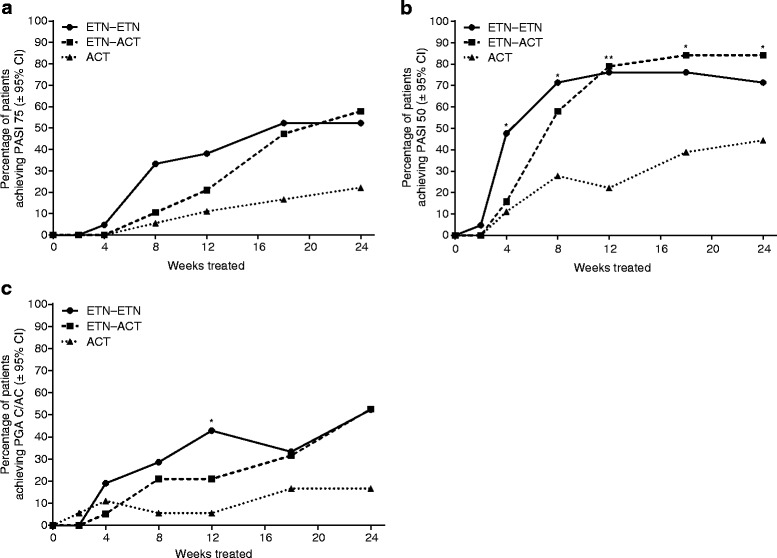
Proportion of patients achieving (a) PASI 75, (b) PASI 50, and (c) PGA clear/almost-clear at each visit. ACT, acitretin; ETN, etanercept; PASI, Psoriasis Area Severity Index; PGA, Physician’s Global Assessment. *p < 0.05; **p < 0.0005
The proportion of patients achieving PASI 50 increased for all treatment arms (Fig. 4b). The change from baseline was statistically significant for all treatment arms. A greater proportion of patients in the ETN–ETN and ETN-ACT treatment arms achieved PASI 50 at all visits than did patients in the ACT arm.
The proportion of patients achieving PGA clear/almost-clear increased for all treatment arms (Fig. 4c). Patients in the ETN–ETN treatment arm demonstrated the greatest improvements up to week 12, after which improvements were similar to those of patients in the ETN-ACT treatment arm. The proportion of patients achieving PGA clear/almost-clear in the ETN–ETN and ETN-ACT arms was more than triple that for the ACT arm.
The median time to achieve PASI 75 for patients in the ETN–ETN arm was 126 days compared with 146 days for patients in the ETN-ACT arm (Table 2). The median time to achieve PASI 50 was the same for patients in the ETN–ETN and the ETN-ACT arms (56 days) and much shorter than for patients in the ACT arm (126 days). The difference was statistically significant among the three treatment arms (PASI 75: p = 0.0448 and PASI 50: p = 0.0033). Additionally, the median time to achieve PGA clear/almost-clear was comparable between the ETN–ETN and ETN-ACT treatment arms (167 and 165 days, respectively). The median time to achieve PASI 75 and PGA clear/almost-clear could not be determined for patients in the ACT arm.
Table 2.
Median time to response
| Response | Median time to response, days (95 % CI) | p value | ||
|---|---|---|---|---|
| ETN 50 mg–ETN 25 mg (n = 21) | ETN 25 mg–ACT 10 mg (n = 19) | ACT 10 mg (n = 18) | ||
| PASI 75 | 126 (56, 146) | 146 (124, NA) | NA (127, NA) | 0.0448 |
| PASI 50 | 56 (28, 56) | 56 (54, 84) | 126 (56, NA) | 0.0033 |
| PGA clear/almost-clear | 167 (55, 172) | 165 (59, NA) | NA (87, NA) | 0.3536 |
Abbreviations: ACT acitretin, CI confidence interval, ETN etanercept, NA not available, PASI Psoriasis Area Severity Index, PGA Physician’s Global Assessment
After 24 weeks of treatment, mean reduction in percent BSA involvement from baseline was greatest in the ETN–ETN treatment arm (−17.5 %) compared with the ETN-ACT treatment arm (−16.9 %) and the ACT treatment arm (−10.3 %) at all visits (Fig. 5). The treatment difference was statistically significant at weeks 4, 8, 12, and 18, whereas the biggest reduction from baseline was at week 24 in all three treatment groups.
Fig. 5.

Change from baseline in percent BSA involvement of psoriasis at each visit. ANCOVA, analysis of covariance; ACT, acitretin; BSA, body surface area; ETN, etanercept. *p < 0.05; **p < 0.005 (treatment difference tested by ANCOVA)
Safety
Safety was evaluated on all patients who received at least one dose of study medication. Across the three treatment arms, 38 (64.4 %) patients reported 85 AEs, and 27 (45.8 %) patients reported 46 treatment-related AEs during the study. The overall incidence of AEs was similar in the three treatment arms (Table 3). The most common AEs reported across any treatment arm (Table 4) were pruritus (n = 6, 10.2 %), alopecia (n = 5, 8.5 %), and dry lips (n = 5, 8.5 %). One patient in the ACT arm reported a serious AE (severe back pain), which was determined by the investigator not to be related to the study treatment. No life-threatening treatment-emergent AEs occurred during the study nor were there any changes in laboratory tests, vital signs, or physical observations that were considered clinically important (Table 3).
Table 3.
Summary of treatment-emergent adverse events
| All Causality TEAEs | Treatment-Related AE | |||||||
|---|---|---|---|---|---|---|---|---|
| ETN–ETN (n = 21) | ETN–ACT (n = 20) | ACT (n = 18) | Total (N = 59) | ETN–ETN (n = 21) | ETN–ACT (n = 20) | ACT (n = 18) | Total (N = 59) | |
| Patients with TEAEs, n (%) | 14 (66.7 %) | 14 (70.0 %) | 10 (55.6 %) | 38 (64.4 %) | 9 (42.9 %) | 10 (50.0 %) | 8 (44.4 %) | 27 (45.8 %) |
| Total number of TEAEs | 22 | 38 | 25 | 85 | 11 | 22 | 13 | 46 |
| Patients with SAEs, n (%) | 0 (0.0 %) | 0 (0.0 %) | 1 (5.6 %) | 1 (1.7 %) | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) |
| Total number of SAEs | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Permanent discontinuation due to AE, n (%) | 1 (4.8 %) | 0 | 1 (5.6 %) | 2 (3.4 %) | – | – | – | – |
Abbreviations: ACT acitretin, AE adverse event, ETN etanercept, SAE serious adverse event, TEAE treatment-emergent adverse event
Table 4.
Incidence of TEAEs in ≥10 % of patients in any treatment arm
| System organ class | ETN 50 mg–ETN 25 mg (n = 21) | ETN 25 mg–ACT 10 mg (n = 20) | ACT 10 mg (n = 18) | Total (N = 59) |
|---|---|---|---|---|
| Skin and subcutaneous tissue disorders | ||||
| Pruritus | 3 (14.3 %) | 2 (10.0 %) | 1 (5.6 %) | 6 (10.2 %) |
| Alopecia | – | 4 (20.0 %) | 1 (5.6 %) | 5 (8.5 %) |
| Skin exfoliation | – | 2 (10.0 %) | 1 (5.6 %) | 3 (5.1 %) |
| Gastrointestinal disorders | ||||
| Dry lip | – | 3 (15.0 %) | 2 (11.1 %) | 5 (8.5 %) |
| Cheilitis | – | 2 (10.0 %) | 2 (11.1 %) | 4 (6.8 %) |
| Chapped lips | – | 1 (5.0 %) | 2 (11.1 %) | 3 (5.1 %) |
| Investigations | ||||
| Alanine aminotransferase increased | 1 (4.8 %) | 2 (10.0 %) | – | 3 (5.1 %) |
| Blood bilirubin increased | – | 2 (10.0 %) | – | 2 (3.4 %) |
| Musculoskeletal and connective tissue disorders | ||||
| Myalgia | – | – | 2 (11.1 %) | 2 (3.4 %) |
| Vascular disorders | ||||
| Hypertension | – | 2 (10.0 %) | – | 2 (3.4 %) |
Abbreviations: ACT acitretin, ETN etanercept, TEAE treatment-emergent adverse event
Discussion
Psoriasis is a chronic autoimmune disease and is often associated with joint inflammation in psoriatic arthritis, a comorbidity that affects between 10 and 30 % of all people with psoriasis [2, 3, 26]. Severe cases of psoriasis have been shown to affect health-related QoL to an extent similar to the effects of other chronic diseases such as depression, hypertension, congestive heart failure, or type 2 diabetes [27].
In multiple trials, etanercept has been shown to be effective in improving the disease severity of psoriasis and patient QoL [17–20]. However, treatment with high doses of etanercept can be expensive [28]. In this context, even though acitretin monotherapy is often only moderately effective [21–24], acitretin is widely used to treat psoriasis and is considered a standard of care in Korea. Furthermore, acitretin has been reported to have malignancy chemoprevention characteristics [21, 29]. Since acitretin is not an immunosuppressive agent and may act synergistically with biologic agents (e.g., etanercept), with a low risk of toxicity, a combination of etanercept and acitretin could be a viable treatment option for patients with psoriasis while keeping treatment affordable and potentially having additional beneficial effects. The results of a pilot study [25], a case series [30], and a posthoc review of clinical treatment practice [24] have suggested that the combination of etanercept and acitretin is effective and safe for the treatment of psoriasis. However, the efficacy and safety of this combination to treat Korean patients with psoriasis have not been determined until now.
The data presented here demonstrate that the combination of etanercept 25 mg BIW + acitretin 10 mg BID appears to be as effective for the treatment of psoriasis in Korean patients as etanercept 50 mg BIW for 12 weeks followed by etanercept 25 mg BIW. Furthermore, based on the numerical proportion of patients achieving efficacy endpoints (i.e., PASI 75, PASI 50, PGA clear/almost-clear, and reduction in disease-related BSA), and time to achieve these efficacy endpoints (i.e., time to achieve PASI 75, PASI 50, and PGA clear/almost-clear), both of these treatments appear to be more effective than acitretin 10 mg BID alone. In particular, the PASI 75 response rate at week 24 for the combined treatment arm (57.9 %) and the etanercept 50 mg–etanercept 25 mg arm (52.4 %) was numerically more than twice the rate of the acitretin 10 mg arm (22.2 %), although the differences were not statistically significant. However, unadjusted pairwise comparison showed a significant difference between the combined treatment group and acitretin 10 mg group (p = 0.0448). Furthermore, the proportion of patients achieving PGA clear/almost-clear in the ETN–ETN and ETN-ACT arms was more than triple that for the ACT arm. It is possible that due to the small number of patients, statistical significance was not achieved between the ETN–ETN and ETN-ACT arms and the ACT arm. Our results are consistent with an earlier Italian, randomized, controlled, pilot trial that showed PASI 75 response at week 24 was achieved by 45 % of the patients in the etanercept 25 mg BIW group, 44 % of the patients treated with etanercept 25 mg + acitretin, and 30 % of the patients in the acitretin 0.4 mg-kg−1 daily group. However, etanercept 25 mg BIW is not commonly effective in treating plaque psoriasis. Consequently, in our study, we treated patients at a higher initial dose of etanercept (50 mg BIW) and adjusted the dose downward to 25 mg BIW as the patients improved – a treatment regimen that we believe better reflects the real-world conditions.
These data demonstrate that the combination of etanercept with acitretin is just as effective as etanercept alone at week 24. However, patients treated with etanercept alone achieved the efficacy outcome endpoints faster than did patients receiving the combination. Treatment with etanercept alone may be preferred by patients wanting a more rapid resolution of their disease whereas treatment with the combination of these two drugs may be preferable for others without sacrificing efficacy or safety. The treating physician will need to consider these options in consultation with the patient to determine the optimum treatment regimen.
All treatments appeared to be safe and well tolerated by the patients. The incidence and severity of AEs appeared to be similar across all treatment arms. No life-threatening AEs were reported and no patient-reported SAEs that were related to study treatment. For patients with skin diseases, these results are important in terms of satisfaction with treatment and improvement of signs and symptoms of the disease, and could potentially affect their QoL.
Conclusions
In this study, the treatment of psoriasis with the combination therapy of etanercept and acitretin substantially reduced the severity of disease in Korean patients over a period of 24 weeks. These results suggest that etanercept could be added to acitretin to treat Korean patients with psoriasis, especially when the disease is resistant or responding inadequately to topical treatment or phototherapy. Combination therapy of etanercept and acitretin, and etanercept alone, are both effective and well tolerated; however, the choice of treatment may depend on finding a balance between the costs of the treatment versus the rapidity with which patients desire to experience the benefits of the treatment.
Abbreviations
ACT, acitretin; AE, adverse event; ANCOVA, analysis of covariance; ANOVA, analysis of variance; BID, twice daily; BIW, twice weekly; BSA, body surface area; CI, confidence interval; ETN, etanercept; mITT, modified intent-to-treat population; NA, not available; PASI, Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment; PP, per-protocol population; PUVA, psoralen plus ultraviolet A radiation therapy; QoL, quality of life; SAE, serious adverse event; SD, standard deviation; TEAE, treatment-emergent adverse event; TNFα, tumor necrosis factor alpha
Acknowledgements
Medical writing support was provided by Mukund Nori, PhD, MBA, CMPP, of Engage Scientific Solutions and was funded by Pfizer.
Funding
This study was funded by Pfizer Pharmaceuticals Korea Limited; etanercept is a product of Pfizer.
Availability of data and material
The dataset supporting the conclusions of this article are included within the article and available at https://clinicaltrials.gov/ct2/show/results/NCT00936065.
Authors’ contributions
All authors participated in study design, data collection, data interpretation, development, review, and final approval of the manuscript.
Competing interests
Hyun-Jeong Yoo is an employee of Pfizer Pharmaceuticals Korea Limited; etanercept is a product of Pfizer. All other authors report no competing interests.
Ethics approval and consent to participate
This study was reviewed and approved by local Institutional Review Boards (Appendix) and was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with Good Clinical Practice Guidelines. All patients provided signed informed consent.
Appendix
| Site ID | Institution | PI | IRB No. |
| 1002 | Hallym University Medical Center | Kwang-Joong Kim | 2010-S003 |
| 1003 | The Catholic University of Korea Seoul St. Mary’s Hospital | Tae-Yoon Kim | KC09MSMV0185 |
| 1004 | Samsung Medical Center | Joo-Heung Lee | 2009-08-101 |
| 1005 | Asian Medical Center | JeeHo Choi | 2009-0645 |
| 1006 | Bucheon St. Mary’s Hospital | ChulJong Park | HC09BSMV0091 |
| 1007 | Konkuk University Hospital | YongBeom Choe | KUH1120010 |
| 1008 | Korea University Medical Center, Guro Hospital | HaeJun Song | GR09163-001 |
| 1009 | Kyung Hee University Medical Center | NackIn Kim | KMC IRB 0922-05 |
| 1010 | Seoul National University Hospital | Jai-Il Yoon | H-0911-041-301 |
| 1011 | Chungnam National University Hospital | JeungHoon Lee | 0912-128 |
Contributor Information
Joo-Heung Lee, Phone: 82-2-3410-3542, Email: joosoo@skku.edu.
Jai-Il Youn, Email: jaiil@snu.ac.kr.
Tae-Yoon Kim, Email: tykimder@catholic.ac.kr.
Jee-Ho Choi, Email: hchoy@amc.seoul.kr.
Chul-Jong Park, Email: cjpark777smp@gmail.com.
Yong-Beom Choe, Email: cyb@kuh.ac.kr.
Hae-Jun Song, Email: hjsongmd@gmail.com.
Nack-In Kim, Email: nikim@khmc.or.kr.
Kwang-Joong Kim, Email: kkj51818@hallym.or.kr.
Jeung-Hoon Lee, Email: jhoon@cnu.ac.kr.
Hyun-Jeong Yoo, Email: Hyun-Jeong.Yoo@pfizer.com.
References
- 1.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Science of Psoriasis: Statistics [https://www.psoriasis.org/sites/default/files/psoriasis_fact_sheet.pdf].
- 3.Facts about psoriasis [http://www.worldpsoriasisday.com/web/page.aspx?refid=130].
- 4.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim G, Waxman R, Helliwell PS. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Rheum. 2009;61(10):1373–1378. doi: 10.1002/art.24608. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf N, Quaranta M, Prescott NJ, Allen M, Smith R, Burden AD, Worthington J, Griffiths CE, Mathew CG, Barker JN, et al. Psoriasis is associated with pleiotropic susceptibility loci identified in type II diabetes and Crohn disease. J Med Genet. 2008;45(2):114–116. doi: 10.1136/jmg.2007.053595. [DOI] [PubMed] [Google Scholar]
- 8.Ahlehoff O. Psoriasis and Cardiovascular Disease: epidemiological studies. Dan Med Bull. 2011;58(11):B4347. [PubMed] [Google Scholar]
- 9.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubertret L, Mrowietz U, Ranki A, van de Kerkhof PC, Chimenti S, Lotti T, Schäfer G. European patient perspectives on the impact of psoriasis: the EUROPSO patient membership survey. Br J Dermatol. 2006;155(4):729–736. doi: 10.1111/j.1365-2133.2006.07405.x. [DOI] [PubMed] [Google Scholar]
- 11.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb A, Korman NJ, Gordon KB, Feldman SR, Lebwohl M, Koo JY, Van Voorhees AS, Elmets CA, Leonardi CL, Beutner KR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 13.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Lim HW, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb AB, Koo JY, Lebwohl M, Lim HW, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Lim HW, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Leonardi CL, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 17.Papp KA, Tyring S, Lahfa M, Prinz J, Griffiths CE, Nakanishi AM, Zitnik R, van de Kerkhof PC, Melvin L. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 18.van de Kerkhof PC, Segaert S, Lahfa M, Luger TA, Karolyi Z, Kaszuba A, Leigheb G, Camacho FM, Forsea D, Zang C, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x. [DOI] [PubMed] [Google Scholar]
- 19.Strohal R, Puig L, Chouela E, Tsai TF, Melin J, Freundlich B, Molta CT, Fuiman J, Pedersen R, Robertson D. The efficacy and safety of etanercept when used with as-needed adjunctive topical therapy in a randomised, double-blind study in subjects with moderate-to-severe psoriasis (the PRISTINE trial) J Dermatolog Treat. 2013;24(3):169–178. doi: 10.3109/09546634.2012.658015. [DOI] [PubMed] [Google Scholar]
- 20.Thaci D, Galimberti R, Amaya-Guerra M, Rosenbach T, Robertson D, Pedersen R, Yang S, Kuligowski M, Boggs R. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2013;28(7):900–906. doi: 10.1111/jdv.12207. [DOI] [PubMed] [Google Scholar]
- 21.Dunn LK, Gaar LR, Yentzer BA, O’Neill JL, Feldman SR. Acitretin in dermatology: a review. J Drugs Dermatol. 2011;10(7):772–782. [PubMed] [Google Scholar]
- 22.Claes C, Kulp W, Greiner W, von der Schulenburg JM, Werfel T. Therapy of moderate and severe psoriasis. GMS Health Technol Assess. 2006;2:Doc07. [PMC free article] [PubMed] [Google Scholar]
- 23.Monfrecola G, Baldo A. Retinoids and phototherapy for psoriasis. J Rheumatol Suppl. 2009;83:71–72. doi: 10.3899/jrheum.090231. [DOI] [PubMed] [Google Scholar]
- 24.Smith EC, Riddle C, Menter MA, Lebwohl M. Combining systemic retinoids with biologic agents for moderate to severe psoriasis. Int J Dermatol. 2008;47(5):514–518. doi: 10.1111/j.1365-4632.2008.03470.x. [DOI] [PubMed] [Google Scholar]
- 25.Gisondi P, Del Giglio M, Cotena C, Girolomoni G. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158(6):1345–1349. doi: 10.1111/j.1365-2133.2008.08564.x. [DOI] [PubMed] [Google Scholar]
- 26.Committee for Medicinal Products for Human Use (CHMP) Guideline on clinical investigation of medicinal products indicated for the treatment of psoriasis. London: European Medicines Agency; 2004. [Google Scholar]
- 27.Sampogna F, Chren MM, Melchi CF, Pasquini P, Tabolli S, Abeni D. Age, gender, quality of life and psychological distress in patients hospitalized with psoriasis. Br J Dermatol. 2006;154(2):325–331. doi: 10.1111/j.1365-2133.2005.06909.x. [DOI] [PubMed] [Google Scholar]
- 28.D’Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015;72(4):589–598. doi: 10.1016/j.jaad.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Bettoli V, Zauli S, Virgili A. Retinoids in the chemoprevention of non-melanoma skin cancers: why, when and how. J Dermatolog Treat. 2013;24(3):235–237. doi: 10.3109/09546634.2012.746634. [DOI] [PubMed] [Google Scholar]
- 30.Conley J, Nanton J, Dhawan S, Pearce DJ, Feldman SR. Novel combination regimens: biologics and acitretin for the treatment of psoriasis-- a case series. J Dermatolog Treat. 2006;17(2):86–89. doi: 10.1080/09546630500482928. [DOI] [PubMed] [Google Scholar]


