Abstract
Background
Emphysema on computed tomography (CT) is a risk factor for all-cause mortality in persons with and without airflow obstruction; however, causes of death associated with emphysema remain uncertain, particularly in the general population.
Aims
To test associations between quantitatively-assessed emphysema on CT and cause-of-death in persons with and without a substantial smoking history.
Methods
The Multi-Ethnic Study of Atherosclerosis recruited 6814 participants, age 45–84 years and without clinical cardiovascular disease, in 2000–02. Percent emphysema was defined on cardiac CT as percent of lung voxels <−950 Hounsfield Units; emphysema on CT was defined as percent emphysema above the upper limit of normal. Cause-of-death was classified by administrative codes. Proportional-hazards models were adjusted for age, race/ethnicity, gender, body mass index, smoking status, pack-years, coronary artery calcium, site, and education. Additional adjustment for lung function was made in a subset with spirometry from 2004–06.
Results
There were 1091 deaths over 12 years median follow-up. Emphysema on CT was strongly associated with increased mortality due to respiratory diseases (adjusted hazard ratio [HR] 2.94, 95% confidence interval [CI] 1.68–5.15), particularly chronic lower respiratory diseases (adjusted HR 9.54, 95% CI 4.70–19.35), and lung cancer (adjusted HR 1.84, 95% CI 1.09–3.12), but not cardiovascular disease. Associations persisted among participants with fewer than 10 pack-years and those without physician-diagnosed respiratory disease, and were similar after adjustment for airflow measures and in persons without airflow limitation.
Conclusions
Quantitatively-assessed emphysema on CT is associated with greater respiratory disease and lung cancer mortality, even among persons without traditional risk factors.
Keywords: Emphysema, Lung Cancer, Computed Tomography, Epidemiology, Cohort Studies
INTRODUCTION
Pulmonary emphysema is a common “incidental” finding on computed tomography (CT), occurring in 29% of smokers undergoing lung cancer screening,[1–3] and 4% of healthy adults undergoing cardiac scanning.[4] Emphysema is defined anatomically as destruction of lung parenchyma and loss of alveolar walls,[5] a definition distinct from that of chronic obstructive pulmonary disease (COPD), which is defined by airflow obstruction on spirometry that does not fully reverse with bronchodilators.[6] Emphysema is common in the absence of COPD and, conversely, approximately half of COPD patients do not have substantial emphysema.[3, 7–9] Further, although emphysema is especially common in smokers, autopsy studies demonstrate that 10% or more of never-smokers have some degree of emphysema.[10]
Important prognostic implications of emphysema have been demonstrated in high risk populations selected for lung cancer screening trials, independent of the airflow measures that define COPD. Visual CT emphysema scores improve prediction of lung cancer mortality and are associated positively with COPD deaths and all-cause mortality;[7, 11–13] associations of these outcomes with quantitative emphysema measures have been conflicting.[14]
In the general population, the clinical significance of emphysema remains inadequately characterized. Prior studies have been restricted to smokers with 10 or more pack-years, a group that comprises only 30% of the general population of older adults;[15] studies in never-smokers and ever-smokers with less than 10 pack-years remain infrequent, despite declining rates of current smoking and apparent shifts to low-smoking habits across diverse demographic groups.[16,17] We recently demonstrated that extent of emphysema-like lung (hereafter referred to as percent emphysema) on CT was associated with all-cause mortality in a population-based sample without airflow obstruction or COPD.[18] Power, however, was inadequate to address cause-of-death or to examine associations stratified by smoking history.
We therefore now report the associations between percent emphysema and mortality due to respiratory diseases and lung cancer over 12 years of follow up, in participants with and without substantial smoking histories, clinical lung disease, or airflow limitation, in order to elucidate the risk of emphysema in the general population.
METHODS
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6814 participants, ages 45 to 84 years, who self-reported White, African-American, Hispanic or Asian race/ethnicity in 2000–02.[19] Exclusion criteria were history of clinical cardiovascular disease, weight greater than 300 pounds, and impediments to long-term participation. Thirty participants were excluded for missing data (Figure E1).
Cause-of-death
Interviewers contacted participants or family members at intervals of 9 to 12 months, and the National Death Index (NDI) was reviewed to assure complete follow-up for mortality through December 31, 2013. Cause-specific mortality endpoints were defined according to International Classification of Diseases (ICD)-10 codes for underlying cause of death, as assigned by state vital statistics offices from the death certificate.[20] This approach was blinded to participant characteristics and consistent with population-based mortality surveillance and prior respiratory outcomes studies.[7, 21]
Main study endpoints were mortality due to respiratory diseases (J00–J99), lung cancer (C33–C34) and, combining these, all lung diseases. We specifically examined mortality due to chronic lower respiratory diseases (CLRD), defined as deaths with COPD, emphysema, chronic bronchitis or asthma (J40–47) as the underlying cause, or, in the context of pneumonia (J12–18) as the underlying cause, CLRD as a contributing cause.
Death certificates were available for 33 of 39 CLRD deaths (85%), family narratives were additionally available for 3 (8%), and discharge summaries for in-hospital deaths were available for 11 (28%). These records were independently reviewed by two physicians (E.C.O., R.G.B.) to confirm CLRD as the underlying cause-of-death according to the WHO definition.[20] Both reviewers classified CLRD as the underlying cause-of-death in 100% of available records. In one case, available records were ambiguous regarding whether CLRD predominated in causing fatal pneumonia, although CLRD was favored by both reviewers. All cases were retained for the main analyses.
Cardiovascular deaths adjudicated by MESA were examined as a secondary endpoint.
Emphysema
MESA participants underwent cardiac CT in 2000–02 using standardized protocols on either electron-beam or multidetector CT.[22] For each participant, two scans were performed at suspended full inspiration from the carina to the lung bases with transverse fields-of-view that captured the whole lung field. Scans captured on average 65% of the total lung volume.[23]
Image attenuation was assessed using a modified version of the Pulmonary Analysis Software Suite at a single reading center by trained readers blinded to other participant information. Percent emphysema was defined as the percentage of lung voxels with attenuation less than −950 Hounsfield units (HU) on the scan with higher air volume or, in the case of discordant quality scores, the higher quality scan.
Emphysema on CT was defined as percent emphysema greater than the upper limit of normal, defined by MESA reference equations.[24]
Percent emphysema was also calculated for upper-lobe and basilar regions, which were defined as the cephalad eighth and caudal third of the imaged lung, respectively. The area of lung with features suggestive of interstitial lung disease (ILD), hereafter referred to as high attenuation areas (HAA), was defined as volume of lung voxels with attenuation between −600 and −250 HU.[25] These measures were previously validated against those obtained from full lung scans in MESA.[23, 25]
Spirometry
Spirometry was attempted in 2004–06 for 3965 participants with baseline measurements of endothelial function, consent for genetic analyses, and an examination during the MESA Lung Study recruitment period (Figure E1). 3847 participants performed maneuvers in accordance with American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines on a dry rolling seal spirometer (Occupational Marketing);[26] results were reviewed by a single investigator. Restriction on spirometry was classified as forced vital capacity (FVC) less than 80% predicted with ratio of forced expiratory volume in one second (FEV1) to FVC greater than 0.7.[6]
Covariates
Age, sex, race/ethnicity, educational attainment, physician-diagnosed emphysema and asthma, and tobacco use were self-reported at baseline. Never smoking was defined as a lifetime smoking history of less than 100 cigarettes, and current smoking as cigarette use within the past 30 days. Urinary cotinine was measured for a subset of 3929 participants; 78 participants (2%) who denied current smoking but had urinary cotinine levels greater than 100 nanograms per milliliter were reclassified as current smokers. Pack-years were calculated as (cigarettes per day / 20) × years smoked.
Height and weight were measured using standard techniques, and body mass index (BMI) was calculated as kilograms/meters-squared. Phantom-adjusted coronary artery calcium Agatston scores were calculated from each cardiac CT and the mean of the two values was used.[27]
Statistical Analysis
Cause-specific mortality rates were computed per 10,000 person-years of observation. Survival time was calculated as age at death or, for non-deceased participants, age at last follow-up or the most recent NDI update, whichever was more recent, with left-truncation at age of study entry or, in analyses incorporating airflow measures, age at spirometry; survival time since enrollment was used in secondary analyses. For analyses regarding specific cause-of-death (e.g., lung cancer), participants dying from other causes (e.g., CLRD) were treated as censored. The proportional-hazards assumption was confirmed via interaction terms with time (P>0.10).
Hazard ratios (HRs) were reported for presence of emphysema on CT, as defined above. Since the distributions of continuous emphysema exposures were left-skewed, hazard ratios were reported per interquartile range (IQR). Analyses were adjusted for potential confounders and precision variables defined a priori based on prior literature: baseline age, sex, race/ethnicity, BMI, educational attainment, smoking status, pack-years, and Agatston score. The standard model was more parsimonious than in a prior publication given the smaller number of events in the present paper and the lack of appreciable confounding by these other factors, assessed by impact of sequential adjustment on the hazard ratio.[18] In the subset with spirometry, the standard model was additionally adjusted for the FEV1, FEV1/FVC ratio, and restriction on spirometry. To account for potential confounding by subclinical ILD, models were further adjusted for HAA.
Analyses were stratified by smoking history, baseline physician-diagnosed CLRD, and airflow limitation on study spirometry, defined as an FEV1/FVC ratio less than 0.70.[28], and multiplicative interaction terms were tested in fully-adjusted models.
Associations were compared to those for cardiovascular and non-lung cancer mortality, and also to effect estimates obtained from models treating cardiovascular and non-lung cancer mortality as competing risks.
Statistical analyses were performed in SAS version 9.3 (Cary, NC) or R (Vienna, Austria).
RESULTS
The 6784 participants in the primary analyses (Figure E1) had a mean age at CT scanning of 62 years, were 38.5% White, 27.7% African-American, 22.0% Hispanic and 11.8% Asian, and included 14.2% current-smokers, 40.5% former-smokers, and 45.3% never-smokers. The median value for percent emphysema was 2.9%, with an interquartile range of 4.5% (1.2%–5.7%). Percent emphysema was modestly inversely correlated with FEV1/FVC ratio (r=−0.37) and HAA (r=−0.14).
The 538 participants (8.6%) with emphysema on CT were slightly older, included a larger proportion of smokers, were more likely to have a prior diagnosis of emphysema or asthma, and demonstrated a lower FEV1/FVC ratio (Table 1).
Table 1.
Baseline characteristics of participants in the Multi-Ethnic Study of Atherosclerosis, by presence of emphysema on computed tomography, 2000–2002.
| Emphysema on computed tomography | ||
|---|---|---|
| Absent | Present | |
| Number of participants | 6233 | 538 |
| Age, years | 62.1 (10.3) | 63.1 (10.0) |
| Males | 2902 (47%) | 290 (54%) |
| Race | ||
| White | 2365 (38%) | 241 (45%) |
| African-American | 1703 (27%) | 169 (31%) |
| Hispanic/Latino | 1407 (23%) | 83 (15%) |
| Asian | 758 (12%) | 45 (8%) |
| Smoking status | ||
| Never | 2915 (47%) | 157 (29%) |
| Former | 2459 (39%) | 287 (53%) |
| Current | 859 (14%) | 94 (17%) |
| Pack-years | 14 (3, 32) | 19 (6, 42) |
| Body mass index, kg/m2 | 28.2 (5.3) | 29.9 (7.3) |
| Education | ||
| Less than high school | 1147 (18%) | 75 (14%) |
| High school | 1139 (18%) | 95 (18%) |
| Some college but no degree | 1003 (16%) | 102 (19%) |
| Associate/technical degree | 770 (12%) | 53 (10%) |
| Bachelor’s degree | 1062 (17%) | 107 (20%) |
| Graduate degree | 1112 (18%) | 106 (20%) |
| Site | ||
| Forsyth County, North Carolina | 994 (16%) | 75 (14%) |
| Upper Manhattan and the Bronx, New York | 1014 (16%) | 86 (16%) |
| Baltimore County, Maryland | 929 (15%) | 133 (25%) |
| Minneapolis, Minnesota | 963 (15%) | 99 (18%) |
| Chicago, Illinois | 1074 (17%) | 88 (16%) |
| Los Angeles County, California | 1259 (20%) | 57 (11%) |
| Physician diagnosis of emphysema | 69 (1%) | 34 (6%) |
| Physician diagnosis of asthma | 561 (9%) | 99 (18%) |
| Pre-bronchodilator airflow measures | ||
| FEV1, L | 2.38 (0.72) | 2.38 (0.86) |
| FEV1/FVC | 0.76 (0.08) | 0.68 (0.13) |
| Restrictive ventilatory defect | 309 (9%) | 12 (4%) |
| Agatston score | 0 (0, 82) | 11 (0, 149) |
| Percent emphysema | 2.6 (1.1, 4.8) | 12.2 (8.9, 15.7) |
Emphysema on computed tomography defined as percent emphysema greater than the upper limit of normal according to reference equations. Data are n, n/N (%), mean (SD), or median (IQR). Percentages may not sum to 100% due to rounding. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. Airflow obstruction defined as FEV1/FVC > 0.7. Pack-years enumerated among ever-smokers only. Pre-bronchodilator airflow measures are calculated only among the 3,830 participants who underwent spirometry in 2004–06. For 13 participants with current smoking, reference equations for the upper limit of normal could not be calculated due to missing smoking intensity data; nonetheless, these participants were included in analyses using the exposure of continuous percent emphysema.
Mortality attributed to lung disease
Telephone follow-up was completed for 96.2% of participants; deaths of participants lost to follow-up were obtained via NDI, hence vital status was known for 100%.
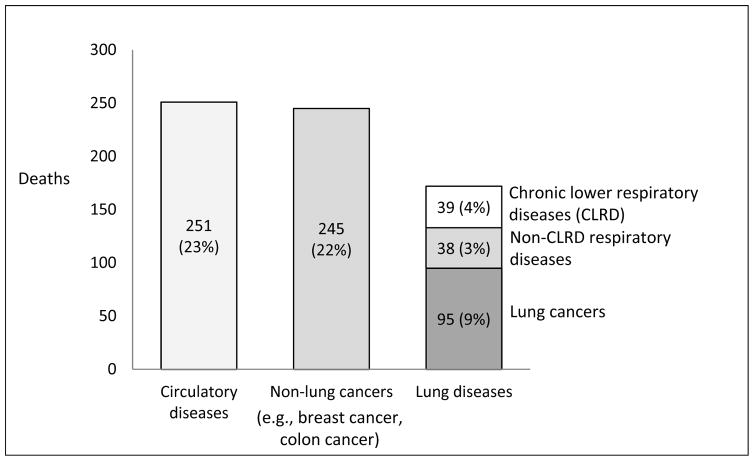
There were 1091 deaths among 6784 participants over a median of 12 years of observation, corresponding to a mortality rate of 132 per 10,000 person-years. Underlying cause-of-death was defined for 89% and attributed to diseases of the respiratory system or lung cancer in 77 and 95 cases, respectively, making lung disease the third leading cause-of-death, following circulatory diseases and non-lung (e.g., breast, colon) cancers (Figure 1).
Figure 1.
Top three leading causes of death in the Multi-Ethnic Study of Atherosclerosis (MESA), 2000–2013.
Half (51%) of respiratory disease mortality was attributed to CLRD, followed by pneumonia (31%) and ILD (18%) (Table E1).
Emphysema and lung disease mortality
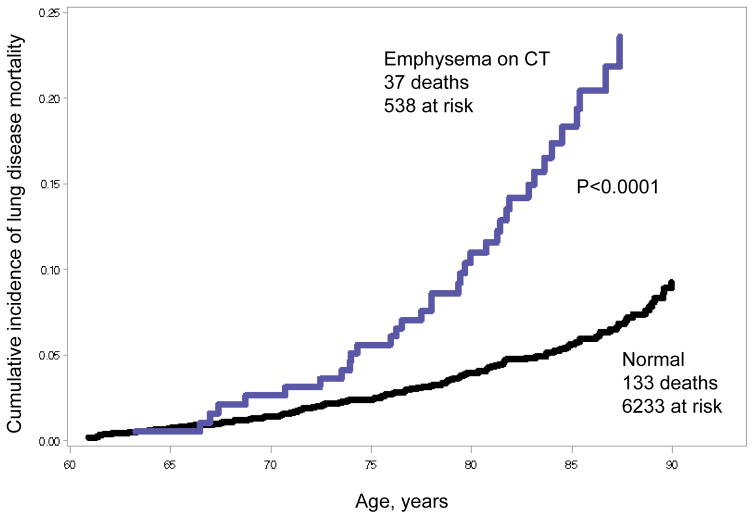
Cumulative incidence of mortality due to respiratory disease and lung cancer was significantly higher among persons with emphysema on CT (log-rank p<0.001, Figure 2).
Figure 2.
Cumulative incidence of mortality due to lung diseases according to presence or absence of emphysema on computed tomography (CT) over 12 years of follow-up in the Multi-Ethnic Study of Atherosclerosis (MESA), 2000–2013.
Emphysema on CT conferred an almost three-fold increased risk of respiratory disease mortality in adjusted models (Tables 2, E2).
Table 2.
Associations between emphysema on computed tomography, percent emphysema, and mortality due to lung disease in the Multi-Ethnic Study of Atherosclerosis, 2000–2013.
| Underlying cause of death | Emphysema on computed tomography (dichotomous) | Percent emphysema (continuous) | ||||
|---|---|---|---|---|---|---|
| Absent (N=6,233) | Present (N=538) | P-value | All (N=6,784) | P-value | ||
| Respiratory diseases | ||||||
| Deaths | 57 | 18 | 77 | |||
| Person-years | 71,845 | 6,078 | 77,750 | |||
| Mortality rate per 10,000 person-years | 7.9 | 29.6 | 9.9 | |||
| Hazard Ratiocrude (95% CI) | 1.00 (referent) | 3.67 (2.16–6.25) | <0.001 | 1.48 (1.35–1.63) | <0.001 | |
| Hazard Ratioadjusted (95% CI) | 1.00 (referent) | 2.94 (1.68–5.15) | <0.001 | 1.51 (1.35–1.69) | <0.001 | |
| Chronic lower respiratory diseases | ||||||
| Deaths | 20 | 17 | 39 | |||
| Person-years | 71,845 | 6,078 | 77,750 | |||
| Mortality rate per 10,000 person-years | 2.8 | 28.0 | 5.0 | |||
| Hazard Ratiocrude (95% CI) | 1.00 (referent) | 10.11 (5.25–19.46) | <0.001 | 1.71 (1.55–1.88) | <0.001 | |
| Hazard Ratioadjusted (95% CI) | 1.00 (referent) | 9.54 (4.70–19.35) | <0.001 | 1.78 (1.57–2.03) | <0.001 | |
| Lung cancer | ||||||
| Deaths | 76 | 19 | 95 | |||
| Person-years | 71,845 | 6,078 | 77,750 | |||
| Mortality rate per 10,000 person-years | 10.6 | 31.3 | 12.2 | |||
| Hazard Ratiocrude (95% CI) | 1.00 (referent) | 2.81 (1.70–4.65) | <0.001 | 1.35 (1.20–1.52) | <0.001 | |
| Hazard Ratioadjusted (95% CI) | 1.00 (referent) | 1.84 (1.09–3.12) | 0.023 | 1.21 (1.06–1.38) | 0.006 | |
| All lung diseases | ||||||
| Deaths | 133 | 37 | 172 | |||
| Person-years | 71,845 | 6,078 | 77,750 | |||
| Mortality rate per 10,000 person-years | 18.5 | 60.9 | 22.1 | |||
| Hazard Ratiocrude (95% CI) | 1.00 (referent) | 3.16 (2.20–4.56) | <0.001 | 1.42 (1.32–1.53) | <0.001 | |
| Hazard Ratioadjusted (95% CI) | 1.00 (referent) | 2.25 (1.54–3.30) | <0.001 | 1.34 (1.23–1.46) | <0.001 | |
The endpoints were defined by an underlying cause of death of respiratory disease (J00–J99), lung cancer (C33–C34) and, combining these, all lung disease. We also specifically examined mortality due to chronic lower respiratory disease (CLRD; J40–47), which was defined as deaths with COPD, emphysema, chronic bronchitis or asthma as the underlying cause, or, in the context of pneumonia as the underlying cause (J12–18), with these diseases (J40–47) recorded as a contributing cause.
For percent emphysema, hazard ratios reported per interquartile range (4.5%), which is equivalent to the difference between the third quartile (5.7%) and the first quartile (1.2%) of percent emphysema. Models adjusted for baseline age, sex, race/ethnicity, body mass index, site, smoking status, pack-years of smoking, coronary artery calcium score, and educational attainment. Upper limit of normal for percent emphysema defined by reference equations.
This association was driven by the subset of CLRD deaths, for which there was an almost ten-fold higher risk. This association was unchanged after exclusion of seven CLRD deaths with missing (N=6) or ambiguous (N=1) records. Conversely, emphysema on CT demonstrated non-significant inverse associations with non-CLRD respiratory disease mortality. With respect to lung cancer, emphysema on CT was associated with an almost two-fold increase in risk. Overall, emphysema on CT was associated with an adjusted HR of 2.25 (95% CI 1.54–3.30) for the combined endpoint of death due to all lung diseases.
Results were similar after additional adjustment (Table E2, Figure E2), in models specifying survival time as time since enrollment, and for upper-lobe and basilar emphysema (Table E3).
Subgroup Analyses
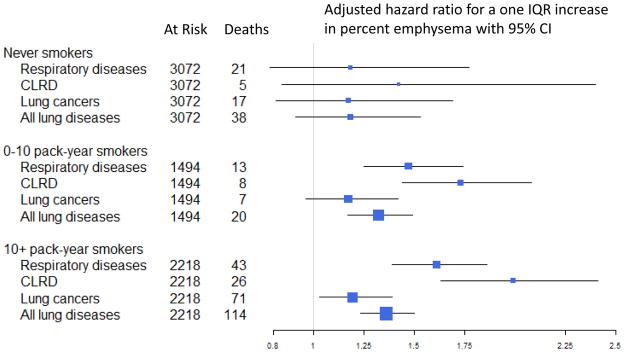
Multiplicative interaction terms with smoking status and pack-years did not attain statistical significance (P>0.25). In adjusted models, the risks of lung disease mortality associated with a one IQR increase in percent emphysema were 1.23 (95% CI 1.03–1.48) and 1.36 (95% CI 1.23–1.50) in persons with less than and more than 10-pack years, respectively. Analyses restricted to never-smokers – among whom the lung disease mortality rate was markedly lower – were imprecise: emphysema on CT was associated with an approximate doubling of lung disease mortality (Figure E2), yet effect estimates per IQR of percent emphysema were lower, and none of these associations attained statistical significance (Figures 3, E2; Table E4). In ever-smokers with less than ten pack-years, statistically-significant associations were similar to those observed in persons with ten or more pack-years.
Figure 3.
Emphysema on computed tomography and mortality due to lung diseases, stratified by smoking history.
Exclusion of participants with physician-diagnosed emphysema (N=104) or asthma (N=622) at baseline only slightly attenuated results, which mainly retained statistical significance (Table E5).
Models incorporating spirometry measures required restriction to persons with valid spirometry at four years following study entry, reducing the sample to 3,835 participants and decreasing person-years of observation by 61%. The fully-adjusted model yielded similar effect estimates as in the main analysis, albeit with wider confidence intervals (Table E6). Further adjustment for airflow parameters did not substantially change effect estimates, but the adjusted results were not statistically significant unless they were additionally adjusted for HAA (Tables E2, E6).
There was no consistent evidence for effect measure modification by presence or absence of airflow limitation (Table 3). Multiplicative interaction terms were not statistically significant. In stratified analyses, point estimates were particularly similar for respiratory disease mortality, but confidence intervals were wide and overlapping for all endpoints. Extended models adjusting for spirometry measures and HAA yielded statistically significant associations in persons both with and without airflow limitation.
Table 3.
Associations between percent emphysema and mortality due to lung disease in the Multi-Ethnic Study of Atherosclerosis, stratified by airflow limitation, 2000–2013.
| Underlying cause of death | Percent emphysema as continuous exposure in persons with valid spirometry measures | P-interaction for airflow limitation | ||||
|---|---|---|---|---|---|---|
| Airflow limitation (N=863) | P-value | No airflow limitation (N=2,965) | P-value | |||
| Respiratory diseases | ||||||
| Deaths | 8 | 12 | 0.597 | |||
| Person-years | 6,418 | 22,843 | ||||
| Mortality rate per 10,000 person-years | 12.5 | 5.3 | ||||
| Hazard Ratio (95% CI) | ||||||
| Hazard Ratiocrude (95% CI) | 1.38 (0.87–2.19) | 0.171 | 1.41 (0.79–2.50) | 0.244 | ||
| Hazard Ratioadjusted (95% CI) | 1.32 (0.98–1.78) | 0.069 | 1.30 (0.58–2.94) | 0.525 | ||
| Hazard Ratioextended (95% CI) | 1.40 (1.02–1.93) | 0.038 | 2.38 (1.01–5.60) | 0.047 | ||
| Lung cancer | ||||||
| Deaths | 18 | 18 | 0.593 | |||
| Person-years | 6,418 | 22,843 | ||||
| Mortality rate per 10,000 person-years | 28.1 | 7.9 | ||||
| Hazard Ratio (95% CI) | ||||||
| Hazard Ratiocrude (95% CI) | 1.39 (1.03–1.88) | 0.031 | 1.03 (0.58–1.85) | 0.920 | ||
| Hazard Ratioadjusted (95% CI) | 1.31 (0.89–1.93) | 0.170 | 1.14 (0.58–2.24) | 0.709 | ||
| Hazard Ratioextended (95% CI) | 1.52 (1.01–2.28) | 0.046 | 1.56 (0.82–2.96) | 0.172 | ||
| All lung diseases | ||||||
| Deaths | 26 | 30 | 0.930 | |||
| Person-years | 6,418 | 22,843 | ||||
| Mortality rate per 10,000 person-years | 40.5 | 13.1 | ||||
| Hazard Ratio (95% CI) | ||||||
| Hazard Ratiocrude (95% CI) | 1.39 (1.08–1.78) | 0.011 | 1.18 (0.79–1.78) | 0.413 | ||
| Hazard Ratioadjusted (95% CI) | 1.32 (0.98–1.78) | 0.071 | 1.16 (0.71–1.90) | 0.542 | ||
| Hazard Ratioextended (95% CI) | 1.38 (1.01–1.90) | 0.044 | 1.63 (1.02–2.61) | 0.043 | ||
The endpoints were defined by an underlying cause of death of respiratory disease (J00–J99), lung cancer (C33–C34) and, combining these, all lung disease. We do not report mortality due to chronic lower respiratory disease (CLRD; J40–47) due to very low event rates in this group (N=6 and N=2 in persons with and without airflow limitation, respectively).
Hazard ratios reported per interquartile range (4.5%), which is equivalent to the difference between the third quartile (5.7%) and the first quartile (1.2%) of percent emphysema. Models adjusted for baseline age, sex, race/ethnicity, body mass index, site, smoking status, pack-years of smoking, coronary artery calcium score, and educational attainment. Interaction terms are reported for this adjusted model. The extend model is additionally adjusted for the forced expiratory volume in one second (FEV1), absence/presence of restriction on spirometry, and log-transformed volume of high attenuation areas.
Emphysema and other major causes of death
Percent emphysema was not significantly associated with circulatory disease mortality, whether this was ICD-defined (adjusted HR 1.00, 95% CI 0.88–1.14) or adjudicated (adjusted HRs 1.03–1.11, p-values >0.25; Table E7). The association between emphysema on CT and lung disease mortality was not attenuated in competing risk regression (HR 3.31, 95% CI 2.30–4.77).
DISCUSSION
Emphysema on CT was associated with a two-fold increase in mortality due to respiratory diseases and lung cancer in a large, population-based, multiethnic cohort. The increase in mortality risk was specific to lung diseases, particularly CLRD, without evidence for increased mortality due to cardiovascular disease and non-lung cancers. These associations did not differ consistently across smoking strata, and persisted among ever-smokers with less than 10 pack-years of smoking. Associations were similar after adjustment for airflow measures and in persons without airflow limitation. These findings suggest that emphysematous changes may be clinically relevant among persons not traditionally considered at high risk of mortality due to CRLD or lung cancer.
The prognostic significance of emphysema on CT has been mainly explored in high-risk smokers undergoing lung cancer screening and persons with alpha-1 antitrypsin deficiency (AATD), among whom emphysema has been linked to all-cause and COPD mortality, independent of airflow limitation.[2, 7, 29–31] Studies of emphysema remain infrequent in persons without substantial smoking or COPD, among whom historical pathological series and contemporary chest CT audits have shown emphysema to be relatively common.[1, 2, 4, 32] Smoking histories and spirometry were not available in a recent study of persons undergoing chest CT for non-pulmonary indications, which showed that radiologist-scored emphysema was specifically associated with increased mortality rates from COPD.[33] In a lung cancer screening cohort, visually-assessed emphysema was associated with the same increase in lung cancer risk in both never-smokers and smokers.[34] MESA is one of the few resources available to study the prognostic significance of quantitative CT measures in persons without COPD or heavy smoking histories. In MESA, quantitatively-assessed percent emphysema has been associated with incident dyspnea and hospitalization for COPD, independent of airflow measures.[35, 36] Furthermore, we recently demonstrated that percent emphysema was associated with all-cause mortality among persons without airflow obstruction on spirometry.[18] The present study builds upon this prior work by leveraging additional accrual of events in the cohort to examine associations between percent emphysema and cause-specific mortality, including in “low risk” subgroups.
Our results demonstrate strong associations between percent emphysema and lung disease mortality that did not differ consistently across strata of smoking, clinical disease, or airflow limitation, and there was no statistical evidence for effect measure modification by these factors. In ever-smokers with less than ten pack-years, statistically-significant associations were of similar magnitude as among heavy smokers. In never-smokers, associations for percent emphysema were attenuated compared to ever-smokers, yet the presence emphysema on CT was associated with a doubling of lung disease mortality; however, event rates in never-smokers were less than half of those in smokers, and results did not attain statistical significance. In the context of a modest number of events, we were also unable to confirm that associations between emphysema on CT and lung disease mortality were independent of airflow measures, but effect estimates were only modestly attenuated after adjustment. In persons without airflow limitation, effect estimates were similar and attained statistical significance with additional adjustment for airflow measures and HAA, a potential radiologic confounder. Hence, longer follow-up may be required to confirm associations, but the cautious interpretation is that emphysema is a potential risk factor for lung disease mortality, even in persons without substantial smoking history or spirometrically-defined COPD.
The strong associations demonstrated between quantitatively-assessed emphysema and lung cancer contrast with some prior literature, which paradoxically found inverse associations, even though visually-assessed emphysema has shown consistent direct associations with lung cancer.[14] One explanation has been that visually-assessed emphysema was superior for detecting centrilobular emphysema (CLE),[37] which is mainly smoking-related,[10] whereas quantitatively-assessed emphysema may preferentially detect panlobular emphysema (PLE), which is equally common in smokers and never-smokers.[14] In MESA, percent emphysema as a measure of PLE is supported by associations with gene variants relating to alpha-1 antitrypsin[38] and measurement on cardiac CT, which excludes the major apical location of CLE. Our findings in participants with less than 10 pack-years – as well as robust associations for lower-lobe emphysema, where PLE is predominantly located – may suggest that PLE is an important prognostic factor for both respiratory disease and lung cancer mortality, even in the absence of smoking-related CLE.
Emphysema may contribute to lung disease mortality via several mechanisms. Loss of lung parenchyma and vasculature reduces area for gas exchange and results in untethering of airways, contributing to hypoxemia and airflow obstruction.[39] Obstruction may be intermittent, for example only during respiratory infection, for which emphysema may increase susceptibility.[40] Emphysema also reduces cardiac output.[41] Altogether, these effects of emphysema may result in poor exercise capacity and reduced functional status.[42] Lung cancer may be increased in the presence of emphysema due to shared causes, such as smoking, enzymatic imbalances and inflammatory responses hypothesized to cause both emphysema and tumorigenesis.[43] Emphysema may also lead to scarring and repair processes that directly result in cancer.
There was no increased cardiovascular mortality risk related to emphysema on CT. The fact that MESA excluded persons with clinical cardiovascular disease at baseline is unlikely to account for this finding, as cardiovascular mortality was still frequent, and prior work has demonstrated that both standard and novel risk factors predict cardiovascular events in this cohort [44]. Our results are consistent with the lack of association of percent emphysema with coronary artery calcium in this cohort,[45] yet seemingly inconsistent with extensive prior work demonstrating high rates of comorbidity and cardiovascular mortality in COPD,[46] and associations between cardiovascular outcomes and smoking, the major risk factor for emphysema. Our data therefore suggest that percent emphysema on CT is not merely a radiologic correlate of smoking exposure or airflow obstruction; further support for this includes limited correlations between percent emphysema and spirometric measures, only modest attenuation of associations after adjustment for spirometry, and persistence of associations among persons without clinical CLRD.
Since percent emphysema was shown not to be significantly associated with other leading causes of death in MESA, associations with lung disease mortality are unlikely to be substantially biased by informative censoring or competing risks effects. Results were essentially unchanged in competing risks regression.
There are nonetheless several limitations that should be considered. Percent emphysema was calculated from cardiac CT scans, which did not include the apices, yet these measures show high correlation with measures from full lung scans in MESA.[23] We defined emphysema on CT based upon reference equations derived from healthy never-smokers in MESA to account for known differences in percent emphysema by age, sex, race/ethnicity, and body size, using an approach similar to the one used for spirometry.[24, 47] While this approach is valid within the cohort and consistent with ATS/ERS recommendations, it limits generalization: MESA reference equations for percent emphysema on full-lung scans are likely to apply in cohorts using the same CT protocol, yet there is no evidence to date that they apply in cohorts or clinical practice using different protocols.
Cause-of-death was defined by administrative coding, which has the potential for both over- and under-diagnosis.[48] Standardized protocols for adjudicating CLRD events are lacking, and validation studies for ICD-coded COPD endpoints have generally used spirometric airflow obstruction as the reference standard, even though this is frequently absent in emphysema, chronic bronchitis, and asthma. We performed independent two-physician review of available records for CLRD deaths to confirm the underlying cause-of-death, and found that censoring of the small number of ambiguous or unavailable records did not alter results. Neither participants nor their physicians were informed regarding results for percent emphysema on study CTs, avoiding diagnostic suspicion bias. Since we ascertained mortality rather than incidence, participants with non-fatal pulmonary outcomes, such as CLRD exacerbations, might be analyzed as censored with respect to lung disease mortality if they were still alive or if they died from a competing cause. However, limiting endpoints according to underlying cause-of-death was intended to minimize false positive misclassification, which is more likely to introduce bias than false negatives.[49]
Spirometry was only available for a subset, was acquired after baseline and hence at a different time than CT and other covariate measures, and did not include post-bronchodilator measures. Restricting to persons without airflow limitation on pre-bronchodilator spirometry conservatively excludes COPD, but follow-up and events in this subgroup were limited.
Modest event rates for CLRD mortality and in never-smoking and no-airflow-limitation subgroups limited the precision and stability of results in related analyses, and the number of sub-analyses could raise concern for type I error. Nonetheless, the high level of statistical significance for our main findings, buttressed by their consistency in sequentially-adjusted and subgroup analyses, as well as with the prior literature in high-risk samples, supports the interpretation that emphysema is associated with elevated risk of lung disease mortality in the general population.
In conclusion, emphysema on CT was strongly associated with increased respiratory and lung cancer mortality in a multiethnic population-based sample. Results persisted among participants without substantial smoking histories or clinical disease, and were similar in persons without airflow limitation. From a clinical standpoint, these findings suggest that emphysema on CT is not a benign incidental finding. Even in never- or light-smokers, detection of emphysema on chest radiography should prompt physicians to review potentially modifiable risk factors for lung disease mortality and to consider spirometry testing and, if indicated, medical therapy for COPD. Further investigation into mechanisms of and specific therapies for emphysema detected on CT is warranted.
Supplementary Material
What is the key question?
Is increased pulmonary emphysema on computed tomography (CT) associated with increased risks of respiratory and lung cancer mortality in the general population?
What is the bottom line?
The results show that percent emphysema on CT greater than the upper limit of normal, as defined by reference equations, was associated with 2- to 10-fold higher rates of mortality from chronic lower respiratory disease and lung cancer in the general population, without evidence for substantial effect modification by smoking history or the presence or absence of airflow limitation on spirometry.
Why read on?
This study describes the prognostic significance of emphysema assessed quantitatively on CT in the general population and supports the conclusion that emphysema may be clinically relevant among persons not traditionally considered at high risk of mortality due to chronic lower respiratory disease or lung cancer.
Acknowledgments
Funding: NIH/NHLBI R01-HL077612, RC1-100543, N01-HC-95159 through -95169, and UL1-TR000040.
MESA is supported by the National Heart, Lung, and Blood Institute (NHLBI) and was designed and conducted by the MESA investigators in collaboration with NHLBI staff. The information contained herein was derived in part from data provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene. NHLBI staff routinely monitored study performance and participated in the internal review of this manuscript prior to submission. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Contributors: Dr Oelsner was primarily responsible for data analysis and drafting of the manuscript, and vouches for the integrity of the data and analyses presented. All authors were involved in the conception, design, and drafting of the manuscript for important intellectual content, and all have approved the final manuscript.
Ethics approval: The study was approved by the National Heart, Lung and Blood Institute as well as institutional review boards of all collaborating institutions. All participants gave written informed consent.
Competing Interests: The authors have no relevant disclosures with the following exceptions. Eric A. Hoffman is a founder and shareholder of VIDA Diagnostics, a company commercializing lung imaging analysis software developed, in part, at the University of Iowa. He is also a non-paid member of Siemens Medical Imaging CT advisory board. Dr. Lederer reports serving as a consultant to Gilead, Intermune, Boehinger-Ingelheim, Immuneworks, and XVIVO therapeutics related to clinical trials in IPF and lung transplantation. Dr. Kawut reports personal fees from Insmed, non-financial support from ACCP, non-financial support from ATS, personal fees from European Respiratory Journal, grants from Actelion, grants from United Therapeutics, grants from Gilead, grants from Lung Biotech, grants from Pfizer, grants from Ikaria, grants from Pulmonary Hypertension Association, grants from Actelion, grants from Gilead, grants from Merck, grants from GeNO, grants from Bayer, all outside the submitted work. Dr. Lima reports grants from Toshiba Medical Systems, outside the submitted work. Dr. Barr reports personal fees from UpToDate, non-financial support from COPD Foundation, both outside the submitted work.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsushima K, Sone S, Fujimoto K, et al. Identification of occult parechymal disease such as emphysema or airway disease using screening computed tomography. COPD. 2010;7(2):117–125. doi: 10.3109/15412551003631717. [DOI] [PubMed] [Google Scholar]
- 4.Burt JR, Iribarren C, Fair JM, et al. Incidental findings on cardiac multidetector row computed tomography among healthy older adults: prevalence and clinical correlates. Arch Intern Med. 2008;168(7):756–761. doi: 10.1001/archinte.168.7.756. [DOI] [PubMed] [Google Scholar]
- 5.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 7.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 8.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med. 2015;175(9):1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh SE, Travers J, Weatherall M, et al. Proportional classifications of COPD phenotypes. Thorax. 2008;63(9):761–767. doi: 10.1136/thx.2007.089193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson AE, Jr, Hernandez JA, Eckert P, et al. Emphysema in Lung Macrosections Correlated with Smoking Habits. Science. 1964;144(3621):1025–1026. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Salcedo P, Wilson DO, de-Torres JP, et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med. 2015;191(8):924–931. doi: 10.1164/rccm.201410-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med. 2015;191(3):285–291. doi: 10.1164/rccm.201407-1210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith BM, Pinto L, Ezer N, et al. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control. National Health and Nutrition Examination Survey Data, 2009–10. National Center for Health Statistics; Hyattsville, MD: [Google Scholar]
- 16.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, et al. Current Cigarette Smoking Among Adults - United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–40. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 17.Navas-Nacher EL, Kelley MA, Birnbaum-Weitzman O, Gonzalez P, Ghiachello AL, Kaplan RC, et al. Association between exposure to household cigarette smoking behavior and cigarette smoking in Hispanic adults: Findings from the Hispanic Community Health Study/Study of Latinos. Prev Med. 2015;77:35–40. doi: 10.1016/j.ypmed.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oelsner EC, Hoffman EA, Folsom AR, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161(12):863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. International Classification of Diseases. 10. Geneva, Switzerland: 2010. [Google Scholar]
- 21.Ford ES, Croft JB, Mannino DM, et al. COPD surveillance--United States, 1999–2011. Chest. 2013;144(1):284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman EA, Ahmed FS, Baumhauer H, et al. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11(6):898–907. doi: 10.1513/AnnalsATS.201310-364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 28.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173(12):1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 31.Dawkins PA, Dowson LJ, Guest PJ, Stockley RA. Predictors of mortality in alpha1-antitrypsin deficiency. Thorax. 2003;58(12):1020–6. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auerbach O, Hammond EC, Garfinkel L, et al. Relation of smoking and age to emphysema. Whole-lung section study. N Engl J Med. 1972;286(16):853–857. doi: 10.1056/NEJM197204202861601. [DOI] [PubMed] [Google Scholar]
- 33.Jairam PM, van der Graaf Y, Lammers JW, et al. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax. 2015;70(8):725–731. doi: 10.1136/thoraxjnl-2014-206160. [DOI] [PubMed] [Google Scholar]
- 34.Henschke CI, Yip R, Boffetta P, Markowitz S, Miller A, Hanaoka T, et al. CT screening for lung cancer: Importance of emphysema for never smokers and smokers. Lung Cancer. 2015;88(1):42–7. doi: 10.1016/j.lungcan.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Oelsner EC, Lima JA, Kawut SM, et al. Noninvasive tests for the diagnostic evaluation of dyspnea among outpatients: the Multi-Ethnic Study of Atherosclerosis lung study. Am J Med. 2015;128(2):171–180. e175. doi: 10.1016/j.amjmed.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister DA, Ahmed FS, Austin JH, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PloS one. 2014;9(4):e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Copley SJ, Wells AU, Muller NL, et al. Thin-section CT in obstructive pulmonary disease: discriminatory value. Radiology. 2002;223(3):812–819. doi: 10.1148/radiol.2233010760. [DOI] [PubMed] [Google Scholar]
- 38.Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-Wide Study of Percent Emphysema on Computed Tomography in the General Population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saetta M, Finkelstein R, Cosio MG. Morphological and cellular basis for airflow limitation in smokers. Eur Respir J. 1994;7(8):1505–1515. doi: 10.1183/09031936.94.07081505. [DOI] [PubMed] [Google Scholar]
- 40.Beasley V, Joshi PV, Singanayagam A, et al. Lung microbiology and exacerbations in COPD. Int J COPD. 2012;7:555–569. doi: 10.2147/COPD.S28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estepar RS, Kinney GL, Black-Shinn JL, et al. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5(3):182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 44.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;348(13):1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 45.Barr RG, Ahmed FS, Carr JJ, et al. Subclinical atherosclerosis, airflow obstruction and emphysema: the MESA Lung Study. Eur Respir J. 2012;39(4):846–854. doi: 10.1183/09031936.00165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sin DD, Anthonisen NR, Soriano JB, et al. Mortality in COPD: Role of comorbidities. Eur Respir J. 2006;28(6):1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 47.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jensen HH, Godtfredsen NS, Lange P, et al. Potential misclassification of causes of death from COPD. Eur Respir J. 2006;28(4):781–785. doi: 10.1183/09031936.06.00152205. [DOI] [PubMed] [Google Scholar]
- 49.Barr RG, Herbstman J, Speizer FE, et al. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am J Epidemiol. 2002;155(10):965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.