Abstract
Accumulating evidence supports a role for T cells in behavioral stress responsiveness. Our laboratory previously reported that lymphocyte deficient Rag2−/− mice on a BALB/c background display resilience to maladaptive stress responses when compared with immune competent mice in the predator odor exposure (POE) paradigm while exhibiting similar behavior in a cued fear-conditioning (FC) paradigm. In the present study Rag2−/− mice on a C57BL/6 background were assessed in the same behavioral paradigms, as well additional tests of anxiety and depressive-like behavior. Furthermore, the effects of naïve CD4+ T cells were evaluated by adoptive transfer of functional cells from non-stressed, wild-type donors to Rag2−/− mice. Consistent with our prior results, Rag2−/− mice displayed an attenuated startle response after POE. Nevertheless, reconstitution of Rag2−/− mice with CD4+ T cells did not modify startle reactivity. Additionally, in contrast with our previous findings, Rag2−/− mice showed attenuated fear responses in the FC paradigm compared to wild type mice and reconstitution with CD4+ T cells promoted fear learning and memory. Notably, reconstitution with CD4+ T cells had anxiolytic and anti-depressant like effects in Rag2−/− mice that had not been previously stressed, but had no effect after POE. Taken together, our results support a role for CD4+ T cells in emotionality, but also indicate that they may promote fear responses by enhancing learning and memory processes.
Keywords: adaptive immunity, lymphocytes, anxiety, depression, fear memory, startle reactivity
Introduction
Extensive research has shown that T cells play an important role in modulating behavioral processes in a number of experimental paradigms of emotionality, stress responsiveness and memory (Schwartz and Kipnis, 2011; Kipnis et al., 2012; Brod et al., 2014). Several studies that have employed reconstitution of immune deficient mice with functional T cells to test the role of specific T cell subtypes have documented beneficial effects of naive CD4+ T cells on anxiety (Cohen et al., 2006; Lewitus et al., 2008), depressive-like behavior (Lewitus et al., 2009) and hippocampal-dependent memory (Brynskikh et al., 2008; Derecki et al., 2010; Radjavi et al., 2014). Moreover, it has been shown that inflammatory CD4+ T cells (TH17) impair stress responsiveness and promote depressive-like behavior (Beurel et al., 2013). However, some discrepancies exist for the role of regulatory CD4+ T cells in these functions, with one study reporting detrimental outcomes (Cohen et al., 2006) while another reported beneficial effects (Kim et al., 2012). Additionally, many of these studies use mice with the knockout of genes that are also expressed in the brain, including the recombination-activating gene 1 (Rag1) knockout mouse. Loss of function of this gene prevents V[D]J recombination processes of T and B cell receptors resulting in the lack of functional cells (Chun et al., 1991). However, Rag1 has known expression in the hippocampal formation and associated limbic areas (Chun et al., 1991), all of which are involved in modulation of behavior. This has led to the possibility that behavioral differences between immunocompetent and Rag1−/− mice may be dependent on potential RAG1 functions in the brain (McGowan et al., 2011; Rattazzi et al., 2013; Brachman et al., 2015).
There has been increasing interest in the use of the Rag2 knockout model of T cell deficiency to study behavioral processes due to the restricted expression of this gene to developing T cells and B cells (Shinkai et al., 1992), without detectable expression in the brain (Chun et al., 1991; Clark et al., 2014). Similar to RAG1, RAG2 function is required during the same T cell receptor rearrangement process initiated by RAG1, thus, Rag2−/− mice also fail to produce mature T cells and B cells. Using this model, recent work has confirmed a role for naive CD4+ T cells in spatial learning and memory (Radjavi et al., 2014). Furthermore, functional lymphocytes from stressed mice transferred to Rag2−/− mice have been shown to improve behavioral performance in tests of anxiety and depressive-like behavior (Brachman et al., 2015). In previous studies from our laboratory comparing immunocompetent wild-type (WT) with Rag2−/− mice on a BALB/c background (Clark et al., 2014; Clark, 2015), Rag2−/− mice displayed reduced basal anxiety-like behavior in an open field test (OFT) and attenuated startle reactivity following exposure to predator odor (POE) suggesting that the lack of T cells may result in a resilient phenotype. Nevertheless, our study did not test the effects of CD4+ T cells and our results may have been dependent on the background genotype of the Rag2−/− model employed. Thus, the objectives of the present study were to compare WT and Rag2−/− mice on a C57BL/6 background, using the same behavioral tests with the addition of the forced swim test (FST), and ascertain the effects of CD4+ T cells in this model. Results of our study confirm anxiolytic and anti-depressant like effects of CD4+ T cells and a role in fear learning and memory processes.
Methods
2.1 Animals
Male wild-type (WT) C57BL/6 (n = 46) and Rag2−/− mice (n = 89) on a C57BL/6 background (Taconic Farms, Inc.; Hudson, NY, USA) were age-matched (8-10 weeks) and group housed (2-4 mice per cage) under normal conditions (12h light/dark cycle) with access to food and water ad libitum. WT, Rag2−/− and Rag2−/− mice reconstituted with naïve CD4+ T cells from WT donors via adoptive transfer comprised independent cohorts of mice that underwent a single behavioral experiment or paradigm, as shown in Fig. 1A. All assessments were conducted between 10-14 weeks of age, with regular handling having begun several days prior, and occurred between 10 am and 3 pm, during the light cycle of the colony. Immune status was verified by flow cytometry in all reconstituted Rag2−/− mice after termination of experiments. All procedures were conducted in accordance with approved IACUC protocols and the established institutional guidelines at the University of Maryland, School of Medicine.
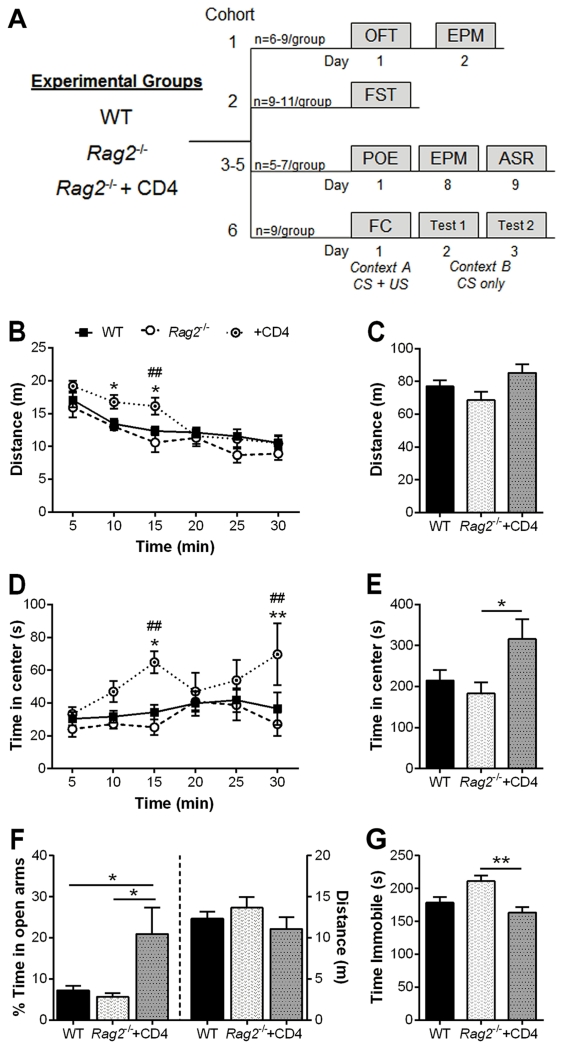
Figure 1. Open Field, Elevated Plus Maze and Forced Swim Tests.
A) Overview of the behavioral paradigms utilized for these experiments, showing timelines of tests conducted in independent cohorts of mice. B) Horizontal locomotor activity in the open field test (OFT) over the course of the test (n = 6 – 9/group). Rag2−/− mice reconstituted with CD4+ T cells show a significant increase in locomotion during the first 15 min, and habituate thereafter to wild-type (WT) and Rag2−/− non-reconstituted activity levels. Two-way repeated measures ANOVA: time: p < 0.0001, immune status: n.s., interaction: p = 0.013; Holm-Sidak post hoc. C) No significant difference in cumulative distance traveled was detected. D) Time spent in center of the arena over the course of the test. Reconstituted mice displayed reduced anxiety-like behavior compared to WT and non-reconstituted Rag2−/− mice. Two-way repeated measures ANOVA: time: p = 0.024, immune status: p = 0.039, interaction: n.s.; Holm-Sidak post hoc. E) Cumulative time in center. Reconstitution of Rag2−/− mice with CD4+ T cells significantly reduced anxiety-like behavior compared to non-reconstituted Rag2−/− mice. ANOVA: p = 0.045, Tukey post hoc. F) Reconstituted Rag2−/− mice display reduced anxiety-like behavior in the elevated plus maze (EPM) compared to WT and Rag2−/− non-reconstituted mice (n = 6 – 9/group; ANOVA: p = 0.009, Tukey post hoc). G) CD4+ T cells reduced depressive-like behavior in the forced swim test (FST) in reconstituted Rag2−/− mice (n = 9 –11/group; ANOVA: p = 0.004, Tukey post hoc). POE: predator odor exposure; ASR: acoustic startle response; FC: fear conditioning; CS: conditioned stimulus, US: unconditioned stimulus. Mean ± SEM. * p < 0.05, ** p < 0.01. ## p < 0.01 (Rag2−/− vs reconstituted Rag2−/−).
2.2 Adoptive Transfer of CD4+ T cells
Lymph nodes and spleens from donor WT mice were homogenized in complete RPMI medium and passed through a 40 um cell strainer to yield a single cell suspension. After centrifugation at 140 rpm for 10 minutes, the supernatant was removed and the cells were resuspended in medium. Naïve CD4+ T cells were isolated by negative selection using the EasySep T cell isolation kit (Stem Cell Technologies; Vancouver, BC, Canada) in accordance with the manufacturer’s guidelines for manual separation. Rag2−/− recipient mice were administered 8-10 million cells suspended in sterile PBS (200 μl) via tail vein injection. Control Rag2−/− mice were injected with 200 μL PBS. The reconstituted mice were left undisturbed for a minimum of two weeks before starting behavioral testing.
2.3 Basal locomotor and anxiety levels: Open field test (OFT) and Elevated plus maze (EPM)
WT, Rag2−/− and Rag2−/− mice reconstituted with CD4+ T cells (n = 6-9/group; 2-3 weeks after adoptive transfer) were tested for locomotor activity and anxious behaviors under non-stressed conditions in the OFT and EPM (Fig. 1A). In the OFT, horizontal locomotor activity was assessed by placing mice into the corner of an open arena (50 × 50 cm) under dim light conditions (30 lux) and recording their activity for 30 minutes with an overhead camera. The total distance traveled and time spent in the center of the arena (interior 50%) were quantified with a video tracking system and automatic scoring program (TopScan; Cleversys; Reston, VA, USA).
The EPM consists of perpendicular, opposing open (39.5 × 5 cm) and closed arms (35 × 5 × 16 cm wall height). Mice were placed in the center (5 × 5 cm) of the EPM facing an open arm and allowed to explore the maze for 10 minutes while an overhead camera recorded their activity. Lights were dimmed to 30 lux for the duration of the test. TopScan (Cleversys) was used to analyze the distance traveled and the proportion of time spent in the open arms.
2.4 Forced Sim Test (FST)
Behavioral despair was assessed in a second cohort of mice in the FST (n = 9-11/group; Fig. 1A). Mice were gently placed into acrylic cylinders filled halfway with water at ~25° C and allowed to freely swim for six minutes. Swimming activity was recorded with a tripod-mounted camera level with the surface of the water. The last four minutes of the session was analyzed with a video tracking system (Forced Swim Scan; Cleversys); mice were scored as immobile if there was a lack of forward momentum initiated by limb movement. Total immobility time was quantified and used for analysis.
2.5 Predator Odor Exposure (POE) paradigm
Three independent cohorts of mice (n = 5-7/group) were evaluated in the POE paradigm (Fig. 1A) as previously described (Clark et al., 2014). Briefly, after transport and acclimation (minimum 30 min.) to a room sequestered from the colony, mice were exposed to soiled, sifted cat litter (Tidy Cat, Non-clumping, 24/7 Performance) for 10 min in a clear acrylic chamber (40 × 40 × 40 cm). Following exposure mice were returned to the colony room and left undisturbed for one week. Mice were then evaluated in the EPM as described above (Day 8) followed by acoustic startle response (ASR, day 9), twenty-four hours after later. Each mouse was tested in an enclosed startle chamber mounted on a piezoelectric accelerometer (SR-LAB, San Diego Instruments; San Diego, CA, USA). Following an acclimation period of 5 min, mice underwent 30 acoustic startle trials: 110 dB tone for 40 ms with an average inter-trial interval of 30 s. The response window for data collection was defined as the 65 ms following the start of the tone. The initial startle response (average of the maximum response for the first two trials) and the average maximum startle response across binned trials (5 trials/bin) were used for analysis.
2.6 Fear Conditioning (FC)
A cued-conditioning protocol was employed to evaluate fear learning and memory (Fig. 1A) in a final cohort of mice (n = 5/group). In brief, mice were initially exposed to a non-aversive conditioned stimulus (CS) paired with an aversive unconditioned stimulus (US) on one side of a shuttle box with a grid shock floor within a sound attenuating chamber (Coulbourn Instruments; Whitehall, PA, USA). The chamber has clear acrylic front and back walls with metallic side walls and lit by a house light (~12 lux) mounted in the ceiling. A speaker is mounted at the top corner of the side wall. During acquisition trials (day 1) mice were individually placed in the cage, allowed to acclimate for 3 min and then exposed to a single CS-US paired 30 s trial consisting of an auditory CS (75 dB, 4000 Hz) that co-terminated with a 2 s US foot shock (700 μA). Mice were immediately removed and returned to their home cage. Each chamber was cleaned with 70% ethanol to remove any scent traces between sessions. Control mice (n = 4/group) underwent the same protocol, but did not receive the US.
Fear responses and extinction were evaluated 24 h (Test 1) and 48 h (Test 2) after FC. Mice were individually placed in a novel test cage within an isolation chamber (Coulbourn Instruments). This new context consisted of four clear acrylic walls with a black and white striped background, a light mounted on the side of the chamber (~9 lux), a solid, white floor, and the addition of a drop of diluted almond extract (1:100 in water) in the drop pan. Each test session consisted of a 3 min habituation period followed by 30 trials of CS presentation only (75 dB tone, at 4000 Hz, for 30 s) with a 90 s inter-trial interval. Cages were cleaned with Vimoba between sessions to avoid olfaction cues imparted by the cleanser during the acquisition stage. All sessions were recorded with a camera mounted in the ceiling of each test cage and automatically scored with FreezeFrame (Actimetrics; Wilmette, IL, USA) to measure freezing during the habituation period and CS presentations.
2.7 Flow cytometry
Twenty four hours after completion of behavioral assessments animals were euthanized by cervical dislocation after they were deeply anesthetized with 5% isofluorane. Superficial cervical, axillary, brachial, mesenteric and inguinal lymph nodes were collected from each animal and placed into 5 ml complete media (10% FBS in RPMI + 1% penstrep + 1% L-Glutamate). Single cell suspensions were obtained as described above. The viability and number of cells in each suspension was then determined using a hemocytometer. Cell suspensions were centrifuged again and, after the supernatant was removed, brought up in FACs buffer (1× PBS + 2% FBS) to a final concentration of 1 × 106 cells/300 μl.
To verify the immune status of each animal, 300 μl of each cell suspension were transferred to clean 5 ml FACS tubes and blocked for 15 min using purified rat anti-mouse CD16/CD32 (mouse BD Fc Block; BD Biosciences, San Jose, CA, USA). One μl each PE conjugated anti-mouse CD3 (BD Biosciences), Pacific Blue conjugated anti-mouse CD4 (BD Biosciences) and FITC anti-mouse CD8 (BD Biosciences) was then added to each tube and allowed to incubate for 30 min at 4° in the dark. Cells were then washed 3 times by spinning them down at 15000 rpm for 5 min and adding 1 ml FACs buffer. Samples were analyzed on a BD LSRII flow cytometer (BD Biosciences). The lymphocyte population was identified by size (forward scatter, FSC-A) and granularity (side scatter, SSC-A) and then gated for CD3 expression. CD4 and CD8 expression was then analyzed within the CD3 gate for each sample and data evaluated using FlowJo version X(10) software (Tree Star; Ashland, OR, USA).
2.8 Statistical Analysis
Group comparisons for the OFT, EPM and FST, were analyzed by one-way ANOVA followed by a Tukey post hoc as needed. For data with skewed values, the non-parametric Kruskal-Wallis ANOVA with the Dunn’s post hoc test was used as noted. Trial binned data, as well as test day comparisons, were analyzed by repeated measure two-way ANOVA followed by the Holm-Sidak post hoc test for multiple comparisons when a main effect or interaction between factors was detected. All outliers (± 2 standard deviations) were removed and data are presented as the mean ± standard error of the mean (SEM). Analysis was conducted with GraphPad Prism 6.01 (GraphPad Software, Inc.; La Jolla, CA, USA).
Results
3.1 CD4+ T cells reduce anxiety-like and depressive-like behavior
Immune competent WT, immune deficient Rag2−/− and Rag2−/− mice reconstituted with CD4+ T cells were evaluated in the OFT and EPM to assess anxiety-like behavior and FST to measure behavioral despair (Fig. 1A). Analysis of 5 min binned data in the OFT showed that reconstituted Rag2−/− mice had increased horizontal locomotor activity over WT and Rag2−/− non-reconstituted mice during the first 15 min of the session (time: F (5, 90) = 51.41, p < 0.0001, immune status: n.s., interaction: F (10, 90) = 2.94, p = 0.013; Fig. 1B). However, their locomotor activity habituated to WT and Rag2−/− levels and the total distance traveled was not significantly different among groups (Fig. 1C). Rag2−/− mice reconstituted with CD4+ T cells spent significantly more time in the center of the arena over the course of the session compared to WT and Rag2−/− non-reconstituted mice (time: F (5, 90) = 2.73, p = 0.024, immune status: F (2, 18) = 3.88, p = 0.039, interaction: n.s.; Fig. 1D). This difference was also significant in the total time spent in the center of the arena (F (2, 16) = 3.78, p = 0.045; Fig. 1E) indicating an anxiolytic effect of CD4+ cells in reconstituted Rag2−/− mice. Likewise, reconstitution of Rag2−/− mice with CD4+ T cells resulted in a reduction in anxiety-like behavior in the EPM. Reconstituted mice had a significant increase in the percentage of time spent in the open arms of the EPM compared to WT and Rag2−/− non-reconstituted mice (F (2, 18) = 6.051, p = 0.01; Fig. 1F) with no difference in locomotor activity (Fig. 1F). Finally, CD4+ T cells significantly reduced immobility time in the FST in reconstituted Rag2−/− versus non-reconstituted Rag2−/− mice (F (2, 26) = 6.871, p = 0.004; Fig. 1G). No significant differences in immobility time were detected between WT and reconstituted Rag2−/− mice. Notably, immune deficiency did not result in anxiety-like or depressive-like behavior, as indicated by a lack of difference between WT and Rag2−/− mice; nevertheless, the addition of CD4+ T cells had evident anxiolytic and anti-depressive-like effects, supporting a role for T cells in modulating behavioral responses in these paradigms.
3.2 Immune status affects stress responses in the POE paradigm
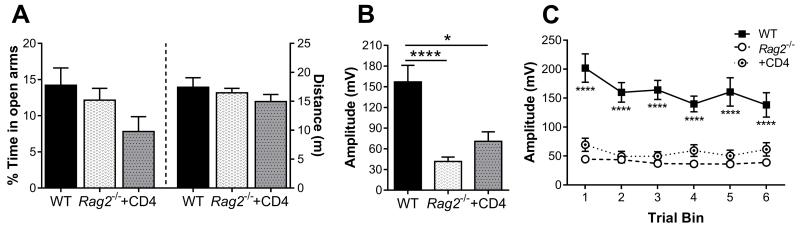
Anxiety-like behavior and startle reactivity in response to exposure to predator odor were compared in WT, Rag2−/− and Rag2−/− mice reconstituted with CD4+ T cells (Fig. 1A) as described in Clark, et al. (2014), modified from Cohen, et al. (2006). No significant differences in anxiety-like behavior were detected in the EPM (p = 0.069, Fig 2A). Nevertheless, there was a significant difference in startle reactivity in the ASR, with analysis of the initial startle response revealing that WT mice displayed significantly greater startle reactivity than Rag2−/− mice regardless of reconstitution status (Kruskal Wallis non-parametric ANOVA, p < 0.0001; Fig. 2B). This difference in startle responsiveness persisted throughout the entire 30 trial session (Fig. 2C) with analysis of binned trails indicating main effects of immune status (F (2, 55) = 42.26, p < 0.0001) and time (F (5, 275) = 4.729, p = 0.0004), with an interaction between factors (F (10, 275) = 2.481, p = 0.007). Although reconstituted Rag2−/− mice showed a slight increase in startle reactivity compared to Rag2−/− non-reconstituted mice, this difference was not significant. To verify that each group of mice was displaying a startle response and not freezing in reaction to the tone, the mean baseline movement at the beginning of each trial (first 5 ms) was compared to the mean max response for each mouse. On average, WT mice showed a greater than 10 fold increase (13.3 ± 6.055 SD) over baseline, whereas Rag2−/− mice displayed an increase of approximately 2.6 fold (± 1.58 SD) while Rag2−/− mice with CD4+ T cells had a 1.7 fold (± 0.79 SD) increase (data not shown). These results indicate that each group of mice did indeed display a startle response, though it was greatly attenuated in Rag2−/− mice regardless of reconstitution status. These findings suggest that CD4+ T cells have no effect on behavior following stress exposure in the POE paradigm and that there is an inherent difference in startle reactivity between WT and Rag2−/− mice that cannot be modified by reconstitution with CD4+ T cells.
Figure 2. Behavioral responses following exposure to predator odor.
A) No significant difference in anxiety-like behavior was detected in the EPM after POE (Kruskal-Wallis non-parametric ANOVA: p = 0.069). B) The average initial startle response is significantly higher in wild-type (WT) mice versus Rag2−/− and Rag2−/− mice reconstituted with CD4+ T cells (Kruskal-Wallis non-parametric ANOVA: p < 0.001, Dunn’s post hoc). C) Trial-binned data of startle reactivity over the course of the test. Startle responses of WT mice remain significantly elevated above those of Rag2−/− mice regardless of reconstitution status (Two-way repeated measures ANOVA: time: p = 0.0004, immune status: p < 0.0001, interaction: p = 0.007). N = 16 – 21/group (3 independent cohorts). Mean ± SEM. * p < 0.05, **** p < 0.0001.
3.3 CD4+ T cells contribute to the establishment of fear memory
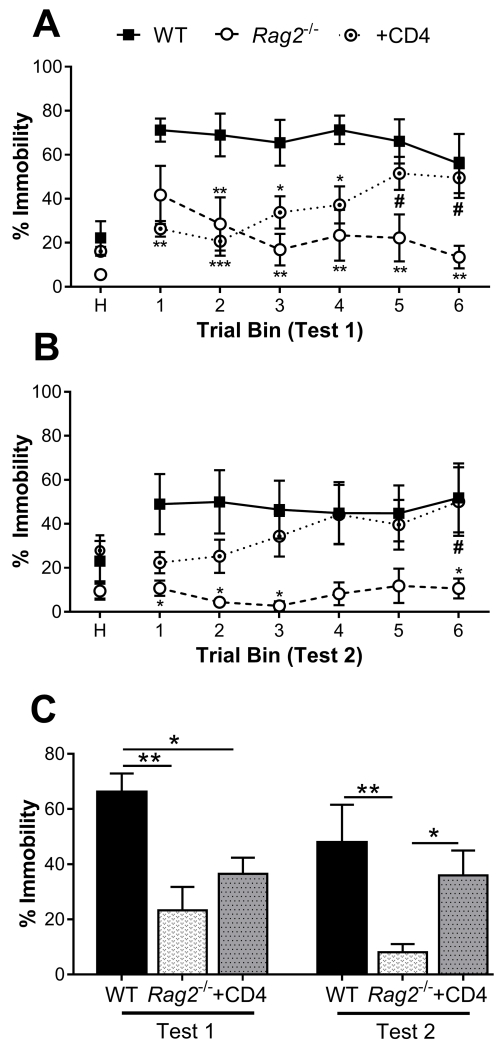
A cued fear conditioning paradigm was used to evaluate differences in the establishment and extinction of fear memory in WT, Rag2−/− and Rag2−/− mice reconstituted with CD4+ T cells (Fig. 1A). Mice were initially conditioned to a tone by co-terminating it with a 2 s foot shock in one context. The following 2 days they were assessed for fear memory by measuring freezing behavior in a novel context. Two-way repeated measures ANOVA analysis of freezing during Test 1 (Fig. 3A) revealed a significant interaction between immune status and time (F (10, 65) = 3.27, p = 0.002) with a main effect of immune status (F (2, 13) = 9.19, p = 0.003), but not time. Post hoc analysis indicated that while WT mice displayed a robust response to tone presentations, which was significantly greater than that of Rag2−/− mice, reconstitution of Rag2−/− mice with CD4+ T cells reversed this difference over the course of the test. Furthermore, there was a significant reduction in freezing between the first and last trial bins for Rag2−/− mice with no difference for WT mice. In contrast, Rag2−/− mice that received CD4+ T cells had a significant increase in freezing from the second trial bin to the last. Thus, Rag2−/− mice appear to show extinction of freezing behavior, whereas those that received CD4+ T cells became more sensitized to the tone over multiple presentations. This difference in fear memory was reinforced during Test 2 (Fig. 3B) with statistical analysis indicating a main effect of immune status (F (2, 13) = 4.27, p = 0.038) with no effect of time, nor interaction between factors. Control mice exposed to tone only during the conditioning phase displayed relatively low freezing behavior compared to experimental mice indicating that each group of mice exhibited indications of associative learning (data not shown). Analysis of the average freezing behavior from Test 1 to Test 2 revealed main effects of test day (time: F (1, 13) = 5.99, p = 0.029) and immune status (F (2, 13) = 3.39), p = 0.007), with no interaction between factors (Fig. 3C). These results indicate that CD4+ T cells may contribute to the formation of fear learning and memory.
Figure 3. Fear learning and memory in a cued-fear conditioning paradigm.
Trial-binned data of the cued fear response during A) Test 1 and B) Test 2, 24 h and 48 h after FC respectively. Rag2−/− mice have a reduced fear response that extinguishes over time compared to wild-type (WT) mice. Reconstitution of Rag2−/− mice with CD4+ T cells reverses this behavior, such that the fear response of reconstituted mice fails to extinguish and reaches levels comparable to WT mice by the end of each session. (Test 1: immune status: p = 0.003, time: n.s., interaction: p = 0.002; Test 2: immune status: p = 0.038, time and interaction: n.s.). C) Analysis of average percent immobility across test days indicates that Rag2−/− mice exhibit significantly attenuated fear responses compared to WT mice on Test 1 and to WT and reconstituted Rag2−/− mice on Test 2 (immune status: p = 0.007, test: p = 0.029, interaction: n.s.). n = 5 – 6/group. Two-way repeated measures ANOVA with Holm-Sidak post hoc. Mean ± SEM. H: 3 min habituation period. * p < 0.05, ** p < 0.01, *** p < 0.001. # p < 0.05 (Rag2−/− vs reconstituted Rag2−/−).
3.4 Analysis of CD4+ T cell reconstitution
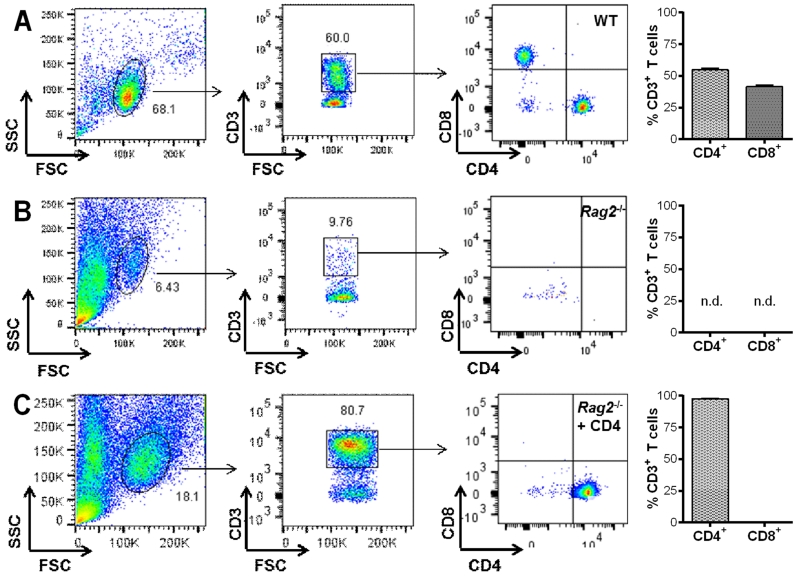
Immune status and the proportion of T cell populations in the lymph nodes were verified at the conclusion of the behavioral experiments by flow cytometry analysis. Figure 4 shows representative dot plots displaying the percentages of CD3+/CD4+ and CD3+/CD8+ T cells within the lymphocyte population of WT mice (Fig. 4A) and the absence of these cells in Rag2−/− mice (Fig. 4B). Reconstituted Rag2−/− mice had a highly pure population of CD4+ T cells (97.67% ± 0.38% SEM) reflecting the efficacy for the reconstitution strategy employed (Fig. 4C).
Figure 4. Analysis of reconstitution by flow cytometry.
Representative dot plots with quantification of CD4+ and CD8+ T cells within the CD3+ lymphocyte population derived from the lymph nodes of A) wild-type (WT), B) Rag2−/− non-reconstituted mice and C) Rag2−/− mice reconstituted with CD4+ T cells.
Discussion
The present study provides supportive evidence of a role for CD4+ T cells as modulators of behavior in a number of functions involving emotional and memory processing in reconstituted Rag2−/− mice indicating that the effects are independent of genetic deletion of shared genes between the immune and central nervous system (CNS). In the FC paradigm, the presence of CD4+ T cells enhanced fear responses, promoting fear learning and memory. In contrast, under non-stressful conditions, CD4+ T cells were anxiolytic in the OFT and EPM and reduced behavioral despair in the FST. Nevertheless, they had no effect on behavior following predator odor stress exposure. Indeed, the lack of an effect of CD4+ T cells on startle reactivity in the ASR indicates that some behaviors cannot be rescued by temporarily restoring T cell function, suggesting the possibility for an organizational role for T cells during development of the CNS leading to permanent alterations in behavior.
In comparing WT and Rag2−/− mice on a BALB/c background we previously found reduced startle reactivity in response to POE in Rag2−/− mice with respect to WT animals (Clark et al., 2014), an effect that was reproduced in Rag2−/− mice of a C57BL/6 background. Since the Rag2 gene is not functionally expressed in the CNS, it strongly suggests that startle reactivity in response to a prior stressor is affected by the absence of T cells. Nevertheless, the fact that CD4+ T cells were unable to modify the behavioral phenotype in Rag2−/− mice presents a number of possible scenarios. First, T cells may affect the development of the CNS leading to stable phenotypes, as was demonstrated in T cell receptor beta-delta chain knock-out mice (TCRβ-/∂-) (Rilett et al., 2015). In this study the authors showed a role for functional T cells during brain development, including changes in the volume of specific brain structures with a loss of sexual dimorphism, two stable changes in the CNS. Secondly, since Rag2−/− mice are also deficient in B cell function, startle reactivity may be dependent on these cells. However, many studies have shown that behavioral effects of lymphocytes are independent of B cell function (Radjavi et al., 2014; Rilett et al., 2015). Finally, the lack of effect of CD4+ T cells may be related to a floor effect in which startle reactivity cannot be modified below the threshold displayed by non-reconstituted Rag2−/− mice. While the first possibility has more support from the literature, the specific effects of lymphocytes and T cells on startle reactivity is a matter for future studies.
A potential influence of the background genome on T cell function during stress reactivity was revealed by the differential effects in the FC paradigm between WT and Rag2−/− mice of the BALB/c (Clark et al., 2014) and C57BL/6 backgrounds. In contrast to our findings in mice of the BALB/c background, WT and Rag2−/− mice on a C57BL/6 background display significant differences in cued fear memory following FC, with Rag2−/− mice showing reduced fear responses that extinguish more rapidly. However, the presence of CD4+ T cells in Rag2−/− mice abrogated this effect, resulting in an enhanced fear response in reconstituted Rag2−/− mice approaching the same level as WT with no signs of extinction. This effect was more evident during the second extinction test suggesting that both learning and memory processes are likely involved in this effect. A plausible interpretation is related to the memory enhancing effects of CD4+ T cells reported in other tests such as the Morris water maze (Brynskikh et al., 2008; Radjavi et al., 2014). Thus, it is possible that an improved memory function mediated by CD4+ T cells during conditioning leads to enhanced fear responses during successive exposure to the CS, as occurs in WT mice. Subsequently, although Rag2−/− mice learned quicker to dissociate the CS from US in the new context and performed better than the WT mice, reconstitution with CD4+ T cells enhanced fear responses, either by reinforcing the memory (with some delay), or by inhibiting extinction. Further investigation into the specific components of learning and memory will be required to fully assess the role of CD4+ T cells that may help clarify the role of these cells in the stress response.
A number of studies in immune deficient mice have reported several deficits in behaviors when compared with WT animals (Cushman et al., 2003; Kipnis et al., 2004; Cohen et al., 2006; McGowan et al., 2011; Kim et al., 2012; Rattazzi et al., 2013; Radjavi et al., 2014). For example, studies in Rag1−/− mice on various genetic backgrounds suggest that deletion of this gene has significant effects on locomotor activity, anxiety-like behavior, and social interaction (Cushman et al., 2003; McGowan et al., 2011; Rattazzi et al., 2013). As previously discussed, this gene, as well as others producing T cell deficiency, such as the protein kinase, DNA-activated, catalytic polypeptide (Prkdc) gene, are expressed in the CNS during development and throughout adulthood. Though their precise impact on neuronal function is not known, it is possible that deficits in basal behavior in these models are due to lack of function of these genes in the CNS. In contrast, and consistent across studies using Rag2−/− mice (McGowan et al., 2011; Clark et al., 2014; Brachman et al., 2015), the present results confirmed that lack of T cell function does not contribute to basal behavioral deficits. For example, behavioral performance and anxiety is comparable between WT and Rag2−/− in the OFT and EPM. Interestingly, reconstitution with CD4+ T cells in Rag2−/− mice conferred anxiolytic effects in these tests corroborating the conclusions about beneficial effects of naive CD4+ T cells on anxiety (Schwartz and Kipnis, 2011; Brod et al., 2014). Thus, it is likely that CD4+ T cells contribute to the maintenance of brain homeostasis, improving overall brain function as proposed (Schwartz and Kipnis, 2011), resulting in improved emotional regulation and memory processing. By the same token, when given to T cell deficient mice, they may enhance memory association with adverse events leading to impaired fear responses when re-exposed to stress, as was observed in the POE and FC. This may explain why two very different results were obtained for the EPM under basal conditions and following POE. Specifically, CD4+ T cells may contribute to enhanced fear memory during exposure to predator odor stress, which would be sufficient to elicit marked alterations in behavior in the EPM. In contrast, under non-stressed conditions, CD4+ T cells may improve homeostasis and motivational states resulting in anxiolytic effects in the same test. This is further supported by the results observed in the FST, a test used to screen the efficacy of antidepressant compounds in preclinical studies, wherein a beneficial effect of CD4+ T cells in mice not previously exposed to a stressor was also observed. This effect provides additional support for a beneficial role of CD4+ T cells in emotionality. Nevertheless, whether the behavioral performance of CD4+ reconstituted Rag2−/− mice may be altered in this test when a prior acute intense stressor is applied was not investigated in the present study.
While a more consistent picture on the behavioral effects of T cells beyond genetic differences and across different studies is starting to emerge, the mechanisms mediating these effects remain poorly understood. Initially, it was proposed that CD4+ T cells promote the production of BDNF in response to behavioral tasks or exposure to a stressor helping to maintain optimal functioning of neuronal circuitries modulating behavior (Lewitus and Schwartz, 2009). Nevertheless BDNF levels in the hippocampus of Rag2 knockout mice on a BALB/c background were found comparable or even higher than WT (Clark et al., 2014). Likewise, it has been proposed that CD4+ T cells promote neurogenesis, maintaining optimal hippocampal function and associated behavioral tasks (Ziv et al., 2006; Wolf et al., 2009), although a recent study in Rag2−/− mice failed to confirm an effect of naïve T cells on hippocampal neurogenesis, as only mice that had received stressed T cells showed increased proliferation (Brachman et al., 2015). Another proposed mechanism relates to the effects of T cells in modulating inflammatory factors and cytokines. For example, it has been proposed that T cells counteract the pro-inflammatory effects of innate immune cells in the brain and thus, help to maintain neuroinflammatory homeostasis during demands imposed by behavioral processes and stress responsiveness (Derecki, et al. 2010; Ron-Harel et al., 2011). The sparse existing evidence for these proposed mechanisms appear to favor the latter, although they are not necessarily mutually exclusive processes.
In summary, these results in Rag2−/− mice on a C57BL/6 background provide additional support of a role for CD4+ T cells in modulating behavioral stress responsiveness. While the evidence for naive CD4+ T cells overwhelmingly favors a protective function, it is also possible that they may participate in mechanisms necessary during the development of pathological stress responses, such as fear learning, memory formation and consolidation. Confirming biological processes across different murine genetic models and behavioral paradigms strengthens the value of this research for translation to the human condition. Due to the established association between stress-related disorders and inflammatory pathology in humans, further research is warranted to define the mechanisms underlying the actions of T cells, and CD4+ T cells in particular, in CNS function and behavior.
Acknowledgments
This work was supported by Department of Veterans Affairs (VA Merit Review Award BX00935) and the NIMH (OppNet R01MH097676) to LHT.
Footnotes
Declaration of Interests
The authors have no conflicts of interest to disclose and are solely responsible for the content and writing of this manuscript.
References
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73(7):622–30. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J Neurosci. 2015;35(4):1530–8. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod S, Rattazzi L, Piras G, D’Acquisto F. ‘As above, so below’ examining the interplay between emotion and the immune system. Immunology. 2014;143(3):311–8. doi: 10.1111/imm.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav Immun. 2008;22(6):861–9. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64(1):189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, Tonelli LH. Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2(−/−) mice. Behav Brain Res. 2015;278:74–82. doi: 10.1016/j.bbr.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, Kusnecov A, Gould TD, Tonelli LH. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain Behav Immun. 2014 May;38:192–201. doi: 10.1016/j.bbi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol. 2006;66(6):552–63. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Cushman J, Lo J, Huang Z, Wasserfall C, Petitto JM. Neurobehavioral changes resulting from recombinase activation gene 1 deletion. Clin Diagn Lab Immunol. 2003;10(1):13–8. doi: 10.1128/CDLI.10.1.13-18.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207(5):1067–80. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, Bae H. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PloS one. 2012;7(7):e42054. doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101(21):8180–5. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12(9):663–9. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22(7):1108–14. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Schwartz M. Behavioral immunization: immunity to self-antigens contributes to psychological stress resilience. Mol Psych. 2009;14(5):532–6. doi: 10.1038/mp.2008.103. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Wilf-Yarkoni A, Ziv Y, Shabat-Simon M, Gersner R, Zangen A, Schwartz M. Vaccination as a novel approach for treating depressive behavior. Biol Psych. 2009;65(4):283–8. doi: 10.1016/j.biopsych.2008.07.014. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain Res. 2011;1383:187–95. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain Behav Immun. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psych. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilett KC, Friedel M, Ellegood J, MacKenzie RN, Lerch JP, Foster JA. Loss of T cells influences sex differences in behavior and brain structure. Brain Behav Immun. 2015;46:249–60. doi: 10.1016/j.bbi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Ron-Harel N, Cardon M, Schwartz M. Brain homeostasis is maintained by “danger” signals stimulating a supportive immune response within the brain’s borders. Brain Behav Immun. 2011;25(5):1036–43. doi: 10.1016/j.bbi.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun. 2011;25(5):817–9. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68(5):855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182(7):3979–84. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268–75. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]