Abstract
Background
Patients with type 2 diabetes (T2D) and coronary artery disease (CAD) have increased risk of cardiac dysfunction. The diabetic heart is characterized by increased fatty acid oxidation and reduced glucose uptake resulting in reduced cardiac efficiency. Glucagon-like peptide-1 (GLP-1) has shown to increase myocardial glucose uptake and to improve myocardial function. We examined the effect of the GLP-1 receptor agonist, liraglutide, on the systolic function of the left ventricle (LV) in patients with T2D and stable CAD.
Methods
In this placebo-controlled crossover study, 41 subjects with T2D and stable CAD were randomized to liraglutide or placebo and underwent dobutamine stress echocardiography (DSE) and exercise tolerance test at beginning and end of each intervention. The primary endpoint was changes in LV ejection fraction. Secondary endpoints were exercise capacity and other measures of systolic function: wall motion score index (WMSI), global longitudinal strain (GLS) and strain rate (GLSR).
Results
Liraglutide, when compared to placebo, did not improve LV ejection fraction at rest (+0.54 %; 95 % CI 2.38–3.45), at low stress (+0.03 %; 95 % CI 3.25–3.32), at peak stress (+1.12 %; 95 % CI 3.45–5.69), or at recovery (+4.06 %; 95 % CI 0.81–8.93). No significant changes in WMSI were observed at any stress levels. GLS and GLSR at rest did not improve. The maximal exercise capacity estimated by metabolic equivalents was not affected by liraglutide.
Conclusion
In conclusion, liraglutide did not improve the systolic function of the left ventricle during DSE or the exercise capacity in patients with T2D and stable CAD.
Clinical Trial Registrationhttp://www.clinicaltrials.gov (unique identifier: NCT01595789)
Electronic supplementary material
The online version of this article (doi:10.1186/s12933-016-0425-2) contains supplementary material, which is available to authorized users.
Keywords: GLP-1, Liraglutide, Coronary artery disease, Diabetes mellitus, Dobutamine stress echocardiography, Left ventricular ejection fraction
Background
Type 2 diabetes (T2D) and coronary artery disease (CAD) increases the risk of cardiac dysfunction [1]. Subclinical LV dysfunction is present in patients with T2D and is attributable to factors such as insulin resistance, microvascular disease and cardiac autonomic dysfunction [2]. Despite improved glycemic control in patients with T2D treated with glucose lowering agents, there is little evidence from clinical trials of reduced risk of heart failure, although the EMPA-REG Outcome Study recently has challenged this view [3]. Some glucose lowering agents have been associated with an increased risk of hospitalization for heart failure [4]. Consequently, investigations of safe anti-glycemic treatments that may improve or preserve cardiac function in patients with type T2D and CAD are warranted.
The diabetic heart is characterized by increased fatty acid (FA) oxidation and reduced glucose uptake resulting in a decreased cardiac efficiency as more oxygen is needed to generate ATP. This feature is undesirable in the ischemic setting where oxygen supply is limited [5]. The incretin hormone, glucagon-like peptide-1 (GLP-1), has shown to increase myocardial glucose uptake [6]. This observation has facilitated clinical studies where GLP-1 infusion improved cardiac function in patients with CAD and reduced [7, 8] or preserved systolic function [9]. However, the use of short-term continuous infusion of GLP-1 in these studies limits the clinical application of the findings. Thus, the effect on systolic function using a GLP-1 receptor agonist suitable for long-term treatment was evident. In particularly, this may be important in patients with T2D and CAD as it may affect the long term prognosis.
The long lasting GLP-1 RA liraglutide with a half-life of 13 h has in combination with the biguanide metformin shown to be a safe treatment option in patients with T2D [10]. We hypothesized that treatment with liraglutide added to a backbone therapy of metformin in patients with CAD and T2D would improve the systolic function of the left ventricle during dobutamine stress.
Methods
This is a randomized, double-blind, placebo-controlled 12 plus 12 week crossover study with a 2 week washout period. The outline of the trial visits, the inclusion criteria and the exclusion criteria have all been described in detail previously [11]. In short, patients with stable CAD, left ventricular ejection fraction (LVEF) >40 % and newly diagnosed T2D within 24 months were identified using patient files from selected hospitals in Copenhagen area and invited consecutively to participate in this study. Patients were enrolled from May 2012 until final data collection in October 2014. The subjects underwent dobutamine stress echocardiography, blood tests, anthropometric measurements, blood pressure measurements, and an exercise tolerance test at the beginning and end of each period (week 0, 12, 14, and 26). The allocation sequence was concealed until all subjects had completed the study and all the echocardiography analyses had been performed.
Primary and secondary endpoints
The primary endpoint was a change in LVEF assessed by Simpson’s biplane method at rest and during dobutamine stress echocardiography. Secondary endpoints were changes in exercise capacity and changes in other echocardiographic measures of systolic function including: global longitudinal strain (GLS) at rest, global longitudinal strain rate (GLSR) at rest, and wall motion score index (WMSI) at rest and during dobutamine stress echocardiography.
Study drug and dosage
Subjects had a minimum 2 week washout period for their glucose lowering therapy prior to the first baseline visit. The study drugs liraglutide/placebo subcutaneous injections and metformin tablets were titrated in an identical manner in both periods: 0.6 mg liraglutide/placebo od + 500 mg metformin bid was increased after 14 days to 1.2 mg od + (1000 mg + 500 mg) daily and to 1.8 mg od + 1000 mg bid after 28 days [11]. Efforts were made to give subjects the same dosage of study drug in both periods.
Ethics and dissemination
This study was approved by the Regional Committee on Biomedical Research Ethics of the Capital Region of Denmark and the Danish Medicines Agency. The study has been carried out in accordance with the ICH-GCP (International Conference on Harmonization-Good Clinical Practice) standards and was monitored by the GCP-unit for eastern Denmark. Written informed consent was obtained from each participant.
Dobutamine stress echocardiography
The details of echocardiography protocol have been described previously [11]. In short, two-dimensional echocardiography was performed at rest and during dobutamine infusion using a M5S transducer (Vivid E9, GE Vingmed Ultrasound, Horten, Norway) and analyzed off-line (GE EchoPAC V. 112). Dobutamine was administered an incremental regimen to reach target HR for each stress level [12]. LVEF was calculated using the Simpson biplane method [13] from contrast-enhanced images. Baseline images were used as reference for each stress level and for the examinations in the following visits in an attempt to get comparable visualization of the LV. The echocardiography examinations were performed by four investigators (AS, OWN, OK, and PK). Global longitudinal strain (GLS) and strain rate (GLSR) was assessed using 2D speckle tracking and calculated as the average of the peak systolic values for the apical 4-chamber, 2-chamber, and long-axis views. Wall motion score (WMS) was assessed using a 16-segment model of the left ventricle and graded by the following score: 1 = normal or hyperkinetic; 2 = hypokinetic; 3 = akinetic; 4 = dyskinetic or aneurysmal [13]. Wall motion score index (WMSI) was calculated as the sum of the scores divided by the number of segments visualized. An abnormal stress response was defined as stress induced regional wall motion abnormalities (RWMA), either because of increased WMS at peak stress compared to rest (ischemic response) or because of decreased WMS at low stress (biphasic) or peak stress (viable). LV mass index (2D method) and relative wall thickness (RWT) was calculated as recommended [13]. All echocardiography analyses were performed by one observer (PK). Consensus for LVEF was achieved by an average of the LVEF measurements between two observers in any questionable cases. WMS assessment was also reviewed by one senior cardiologist (AS or OWN). If the discrepancy between the observer (PK) and the senior cardiologist involved more than one segment classified as abnormal or more than two points in total WMS score, the images were also assessed by a second senior cardiologist and consensus was obtained.
Cycle ergometer exercise tolerance test
A standard cycle ergometer exercise tolerance test was performed with a workload appropriate for each subject: a starting work load of 25 W with an increasing work load of 25 or 50 W every 2 min. Subjects were encouraged to exercise until maximal exhaustion. The maximal exercise capacity was expressed as total exercise duration and as estimated metabolic equivalents (METs) [14]: METs = [12 × workload(watt)/weight(kg) + 3.5]/(3.5 ml/kg/min).
Statistical analyses
Power-calculation has been described previously [11]. A post hoc power calculation analysis was added based on the actual data. The SD for the difference between two values for the measurements in placebo period was used. At rest a SD of 5.0 % was observed, and with n = 30 patients a paired analysis provided 80 % power to detect a minimum detectable difference of 2.7 %. For low stress, peak stress and recovery a SD of 6.5, 7.3 and 9 % was observed and with n = 29, n = 24, and n = 29 this provided 80 % power to detect difference of 3.5, 4.4 and 4.9 %, respectively. A dropout rate of approximately 20 % was estimated. Continuous variables were summarized as the mean ± SD or medians with interquartile ranges, and categorical variables were summarized as percentages. The intention-to-treat (ITT) population was defined as subjects who completed minimum one measurement series in one period. The per-protocol population was defined as subjects who completed both measurement series in both intervention periods. A linear-mixed model with random effects for subjects and fixed effects for period and treatment was used in the analysis of the treatment effect on the primary endpoint in the ITT-population. The paired t test was used to compare the treatment effects of liraglutide and placebo for the per-protocol population. For non-normally distributed data Wilcoxon signed-rank test was used. Subgroup analyses were performed according to baseline DSE response. A two-sided p value of less than 0.05 was considered statistically significant. Reproducibility for LVEF was assessed in four randomly selected patients with four levels of stress (baseline, low stress, peak stress and recovery), totaling 16 measurements per observer. Interobserver variability for three observers (PK, OWN, AS) and intraobserver variability for one observer (PK) were calculated by obtaining variance estimates using two-way and one-way analysis of variance (ANOVA) and were expressed as a coefficient of variation (CoV) and a coefficient of repeatability (COR) [15, 16]. All statistical analyses were performed with Stata 13.1 (StataCorp, TX, USA).
Results
In total, 41 patients were randomized and assigned to an intervention. Two subjects declined to participate after randomization, leaving 39 subjects available for baseline visit with 19 subjects receiving liraglutide first and 20 subjects receiving placebo first. Subsequently, 9 subjects discontinued the study due to serious adverse events (n = 2), intolerance to medication (n = 3), and other reasons (n = 4). Thus, 30 subjects were available for per protocol analysis (Fig. 1).
Fig. 1.
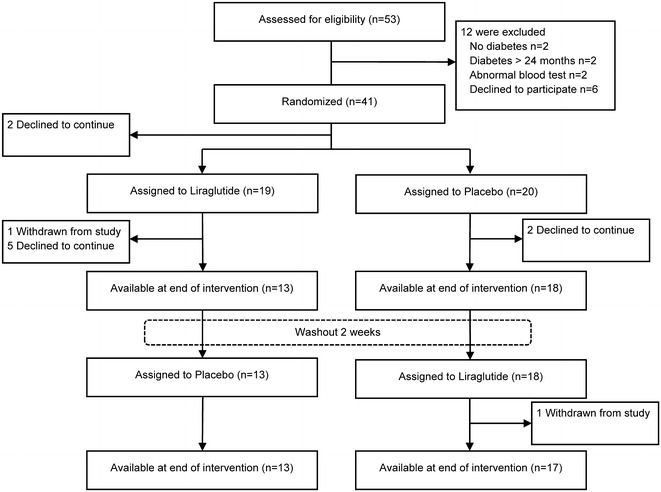
Screening, enrollment, and follow-up of the study population
Table 1 shows the baseline characteristics for the study population. Subjects had normal LVEF and normal LV geometry. Prior to enrolment in the study, 24 patients were not receiving diabetes medication and were being treated with diet and life style therapy. Fifteen patients were on metformin, and one patient was also receiving sulfonylurea therapy. All subjects had CAD defined by one or more of the following conditions: previous myocardial infarction (MI) (n = 23), previous CABG (n = 13), previous PCI (n = 25) or stenosis >50 % of a major coronary artery (n = 2) (Table 1).
Table 1.
Baseline characteristics of the study population
| Characteristics | Total (n = 39) |
|---|---|
| Clinical characteristics | |
| Age, years | 61.8 (7.6) |
| Male sex, n (%) | 31 (79) |
| Weight, kg | 96.9 (17.1) |
| BMI, kg/m2 | 31.6 (4.8) |
| Waist, cm | 110.4 (11.2) |
| Systolic blood pressure, mmHg | 139.3 (19.4) |
| Diastolic blood pressure, mmHg | 80.2 (10.1) |
| Heart rate, bpm | 71.7 (12.1) |
| Risk factors | |
| Smoker, n (%) | 14 (36) |
| Hypertension, n (%) | 29 (74) |
| Coronary artery disease | |
| Previous MI, n (%) | 23 (59) |
| Previous CABG, n (%) | 13 (33) |
| Previous PCI, n (%) | 25 (64) |
| Coronary stenosis, medical therapy only, n (%) | 2 (5) |
| Biochemistry | |
| Fasting blood glucose, mmol/L | 6.5 (1.4) |
| HbA1C, % | 6.4 (0.5) |
| LDL-cholesterol, mmol/L | 2.3 (0.7) |
| eGFR, ml/min | 80.5 (11) |
| HOMA IR, median (IQR) | 4.02 (2.96, 7.49) |
| f-Insulin, median (IQR), pmol/L | 93 (64, 155) |
| Medication | |
| Beta blockers, n (%) | 24 (62) |
| Calcium antagonists, n (%) | 21 (54) |
| ACE-I, ARB, n (%) | 26 (67) |
| Statins, n (%) | 37 (95) |
| Ivabradine, n (%) | 1 (3) |
| Diuretics, n (%) | 11 (28) |
| Nitrate, n (%) | 11 (28) |
| Aspirin, n (%) | 37 (95) |
| Pre-study diabetes medication | |
| Biguanide (metformin), n (%) | 15 (38) |
| Sulfonylurea, n (%) | 1 (3) |
| Diet and lifestyle therapy only, n (%) | 24 (62) |
| Echocardiographic measures | |
| LVEF, % | 58.9 (7.6) |
| LVmass index, g/m2 | 81.9 (21.4) |
| RWT, cm | 0.33 (0.09) |
Data are expressed as the mean (SD), n (%) or median (quartiles 1–3)
ACE-I angiotensin converting enzyme inhibitor, ARB angiotensin receptor blocker, BMI body mass index, bpm beats per minute, CABG coronary artery bypass grafting, eGFR estimated glomerular filtration rate, HbA1C glycated hemoglobin, HOMA-IR homeostasis model analysis of insulin resistance, MI myocardial infarction, LDL low-density lipoprotein, LVEF left ventricular ejection fraction, RWT relative wall thickness, PCI percutaneous coronary intervention
Effect of liraglutide on systolic function
Table 2 shows the treatment effect of liraglutide and placebo on systolic function for patients with a complete measurement series at each stress level. Changes in LVEF during liraglutide period were not significantly different from changes during placebo period at any stress level. No improvement in WMSI at any stress level was observed. No significant changes were evident for GLS and GLSR. ITT analysis revealed a near-significant change in LVEF at recovery (coefficient 4.10, 95 % CI 0.01–8.20). No significant changes were observed in the other stress levels (Additional file 1: Table S1).
Table 2.
Effect of liraglutide versus placebo on systolic function at each stress level
| Before liraglutide | After liraglutide | Before placebo | After placebo | N | Treatment effect | Difference (95 % CI) | p value | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | Liraglutide | Placebo | ||||
| LVEF, % | |||||||||
| Rest | 59.46 (7.44) | 60.13 (9.07) | 59.27 (7.92) | 59.41 (7.92) | 30 | 0.67 (6.30) | 0.13 (4.95) | 0.54 (−2.38 to 3.45) | 0.710 |
| Low stress | 70.73 (9.58) | 71.03 (10.7) | 71.31 (9.51) | 71.44 (8.43) | 29 | 0.28 (6.27) | 0.25 (6.56) | 0.03 (−3.25 to 3.32) | 0.984 |
| Peak stress | 75.72 (9.52) | 76.92 (9.14) | 76.26 (8.72) | 76.02 (8.12) | 24 | 0.87 (6.91) | −0.25 (7.26) | 1.12 (−3.45 to 5.69) | 0.618 |
| Recovery | 60.65 (10.38) | 64.91 (10.44) | 62.52 (10.20) | 62.84 (9.88) | 29 | 4.25 (7.13) | 0.20 (8.97) | 4.06 (−0.81 to 8.93) | 0.099 |
| WMSI | |||||||||
| Rest | 1.088 (0.155) | 1.084 (0.163) | 1.088 (0.144) | 1.075 (0.140) | 30 | −0.003 (0.065) | −0.013 (0.055) | 0.009 (−0.018 to 0.037) | 0.492 |
| Low stress | 1.078 (0.202) | 1.077 (0.171) | 1.059 (0.143) | 1.048 (0.128) | 29 | 0.004 (0.097) | −0.011 (0.056) | 0.007 (−0.035 to 0.049) | 0.725 |
| Peak stress | 1.079 (0.235) | 1.052 (0.160) | 1.058 (0.149) | 1.060 (0.213) | 24 | −0.03 (0.10) | 0.01 (0.08) | −0.038 (−0.112 to 0.035) | 0.292 |
| Recovery | 1.090 (0.198) | 1.096 (0.212) | 1.081 (0.157) | 1.090 (0.192) | 29 | 0.007 (0.07) | 0.006 (0.11) | 0.001 (−0.045 to 0.047) | 0.963 |
| GLS | |||||||||
| Rest | 16.26 (2.60) | 15.53 (2.72) | 16.44 (3.07) | 16.34 (2.90) | 30 | −0.73 (1.87) | −0.10 (1.87) | −0.63 (−0.42 to 1.67) | 0.231 |
| GLSR, s−1 | |||||||||
| Rest | 0.90 (0.18) | 0.91 (0.18) | 0.86 (0.17) | 0.88 (0.2)18 | 30 | 0.01 (0.15) | 0.02 (0.14) | −0.01 (−0.05 to 0.08) | 0.713 |
Data are expressed as the mean (SD)
N is the number of subjects for each treatment phase with valid measurements in each stress level, GLS global longitudinal strain, GLSR global longitudinal strain rate, WMSI wall motion score index, LVEF left ventricular ejection fraction
Liraglutide did not affect any significant changes in LV end-diastolic and end-systolic volume (Additional file 1: Table S2). Nine subjects in the per-protocol population had abnormal stress response at baseline and sub-group analyses did not reveal any significant changes in LVEF at any stress level (Additional file 1: Table S3).
Effect of liraglutide on exercise test performance
Exercise tolerance test results were available for 21 subjects for all 4 visits. No significant differences in the maximal achieved METS was observed between the liraglutide and placebo treatment period (METS −0.13 ± 0.6 vs. −0.42 ± 0.87; 95 % CI −0.22 to 0.80; p = 0.244). The total exercise time was slightly reduced at the end of both treatment periods, but with no difference in the treatment effect (−25 vs. −24 s; p = 0.980, respectively).
Effect of liraglutide on metabolic and hemodynamic parameters
Liraglutide induced a significant weight loss (−3.2 kg; 95 % CI −4.8 to −1.6; p < 0.001), reduction in waist ratio (−2.2 cm; 95 % CI −4.1 to −0.3; p = 0.026), and reduction in HbA1C (−0.4 %; 95 % CI −0.6 to −0.2; p < 0.001) in comparison to placebo. No significant changes in LDL-cholesterol, triglyceride, HOMA IR, insulin or fasting blood glucose were observed (Table 3).
Table 3.
Effect of liraglutide versus placebo on anthropometric and biochemical variables
| Treatment effect | Difference | 95 % CI | p value | ||
|---|---|---|---|---|---|
| Liraglutide | Placebo | ||||
| Weight, kg | −4.17 (3.49) | −0.98 (2.62) | −3.18 (4.31) | −4.79 to −1.57 | <0.001 |
| Waist, cm | −2.80 (4.11) | −0.57 (2.52) | −2.22 (4.89) | −4.16 to −0.29 | 0.026 |
| BMI, kg/m2 | −1.35 (1.10) | −0.31 (0.85) | −1.04 (1.34) | −1.54 to −0.54 | <0.001 |
| Systolic blood pressure, mmHg | −8.10 (17.27) | −3.17 (16.07) | −4.93 (23.68) | −13.78 to 3.91 | 0.263 |
| Diastolic blood pressure, mmHg | −3.13 (12.11) | −3.83 (8.75) | 0.70 (17.06) | −5.67 to 7.07 | 0.826 |
| HbA1C, % | −0.42 (0.34) | −0.04 (0.43) | −0.37 (0.54) | −0.57 to −0.17 | <0.001 |
| LDL-cholesterol, mmol/L | −0.25 (0.72) | −0.17 (0.63) | −0.08 (0.96) | −0.47 to 0.30 | 0.657 |
| HOMA IR, pmol/L | −1.35 (3.18) | −0.57 (2.41) | −0.78 (3.24) | −2.01 to 0.45 | 0.336 |
| Fasting plasma insulin, pmol/L | −11.73 (57.54) | −1.69 (43.79) | −10.04 (68.27) | −36.01 to 15.93 | 0.469 |
| Fasting blood glucose, mmol/L | −0.99 (1.11) | −0.62 (0.96) | −0.36 (1.06) | −0.76 to 0.03 | 0.125 |
Data are expressed as the mean (SD)
BMI body mass index, HbA1C glycated hemoglobin, LDL low-density lipoprotein, HOMA IR homeostasis model analysis of insulin resistance
Systolic and diastolic blood pressure measurements at study visits or during dobutamine stress did not change significantly; however, a significant increase in the resting heart rate was observed (6.2 beats per minute (bpm); 95 % CI 0.8–11.5; p = 0.001). Heart rate was also increased after liraglutide treatment at low stress (10.3 bpm; 95 % CI 0.2–20.4; p = 0.046) and peak stress levels (5.3 bpm; 95 % CI 1.2–9.5; p = 0.014) (Additional file 1: Table S4).
Reproducibility
For LVEF measurement the interobserver and intraobserver variability were, SD 3.4 and 2.2 %; CoV 4.4 and 2.9 %; COR 6.6 and 6.1 %, respectively.
Safety and compliance
The frequency of adverse events was higher in the liraglutide treatment period and was predominantly due to gastrointestinal side effects: nausea (20 %), anorexia (13 %), and gastroesophageal reflux disease (9 %) (Table 4; Additional file 1: Table S5). A total of 9 serious adverse events (SAE) were observed in the study period (Additional file 1: Table S6). Compliance to liraglutide and placebo was high, and there was no difference between treatment periods (94.4 and 93.6 %; p = 0.66). No difference in compliance to metformin was observed (94.4 and 90.9 %; p = 0.07).
Table 4.
Adverse events in each period by event category
| Event category | Liraglutide, n (%) | Placebo, n (%) | Washout, n (%) |
|---|---|---|---|
| Abnormal blood test | 4 (4.4) | 1 (2.6) | 1 (7.7) |
| Cardiac | 8 (8.8) | 6 (15.8) | 4 (30.8) |
| Gastrointestinal | 56 (61.5) | 15 (39.5) | 2 (15.4) |
| Infection | 4 (4.4) | 5 (13.2) | 3 (23.1) |
| Muscle | 0 (0) | 1 (2.6) | 1 (7.7) |
| Neurological | 11 (12.1) | 6 (15.8) | 2 (15.4) |
| Renal, urine | 1 (1.1) | 1 (2.6) | 0 (0) |
| Skin | 3 (3.3) | 1 (2.6) | 0 (0) |
| Vascular | 2 (2.2) | 2 (5.3) | 0 (0) |
| Other | 2 (2.2) | 0 (0) | 0 (0) |
| Total adverse events | 91 | 38 | 13 |
Data are expressed as n (%)
Discussion
In this double-blind, placebo controlled study we showed that 12 weeks of liraglutide treatment did not improve LVEF during dobutamine stress in patients with stable CAD, preserved LVEF and newly diagnosed T2D. Furthermore, no significant changes in WMSI or exercise capacity were observed. GS and GSR parameters at rest did not improve either.
Early studies of GLP-1 infusion were performed in patients with heart failure and showed an improvement in LVEF after both short term and long term GLP-1 infusion [7, 17]. However, these findings could not be confirmed in randomized placebo-controlled studies. Two days of GLP-1 infusion did not improve LVEF in non-diabetic patients with chronic compensated heart failure [18]. Recently, a randomized study showed no effect of 12 weeks of treatment with the GLP-1 RA albiglutide on cardiac function in patients with heart failure [19]. Although the subjects in the two latter studies did not have T2D and had reduced LVEF, the findings are consistent with results in the present study.
In patients with preserved LVEF, GLP-1 infusion during coronary artery bypass grafting did not improve postoperative LVEF in a cohort of diabetic and non-diabetic patients [20]. However, 1 week of subcutaneous liraglutide treatment after MI in subjects with and without diabetes showed an improvement in LVEF at 3 months follow-up [21, 22]. In contrast to our study, these studies were performed in a setting of acute MI and thus under stress-induced hyperglycemia and inflammatory response, that might be subject to a particular beneficial effect of GLP-1 RA treatment [23].
Read et al. [9] performed DSE in 14 subjects with preserved left ventricular function awaiting coronary revascularization and showed a significant improvement in LVEF at peak stress during GLP-1 infusion and an attenuation of post-ischemic dysfunction. Accordingly, we would have expected some GLP-1 RA mediated improvement in the systolic parameters in our study cohort during DSE. However, in the study by Read et al. the beneficial effect of GLP-1 was predominantly in ischemic segments. Our subgroup analysis did not show any distinct effect on LVEF in subjects with abnormal stress response. Notably, the group accounted for just nine subjects. Our study probably reflects a clinical relevant situation where most patients with diabetes and CAD have undergone revascularization leading to a low frequency of abnormal stress response. Although microcirculation may still be compromised in these patients, we did not observe any positive effects on LV systolic function by liraglutide treatment. Furthermore, dobutamin stress would increase the myocardial oxygen demand through its inotropic and chronotropic effects mediated by myocardial beta-receptors [24]. The combination of the increased oxygen demand and the less energy efficient FA oxidation, which is increased in the diabetic heart, is expected to reduce the cardiac efficiency [5]. Thus, a shift towards increased glucose-oxidation mediated by liraglutide would potentially improve LV performance. However, this was not evident in our study.
Interestingly, the largest improvement in LVEF in the present study was observed during recovery period after peak stress. In addition to increased myocardial glucose uptake, GLP-1 may activate pro-survival intracellular signaling pathways that may have beneficial effects in particularly in post-ischemic myocardial function [25]. Furthermore, additional pathways such as cyclic adenosine monophosphate mediated activation of protein kinase A [26] may also be involved in the infarct-size reducing effects of GLP–1 RAs as demonstrated in studies using exenatide infusion [27, 28].
GLS assessed by 2D speckle-tracking allows for an angle-independent assessment of systolic function and has shown to be a very sensitive marker for early detection of myocardial disease [29]. Despite preserved LVEF our study population had a reduced GLS compared to a previous suggested reference value of −19.7 % thus reflecting a subclinical systolic dysfunction that characterizes the T2D and CAD population [30, 31].
We observed a significant reduction in HbA1C and a net body weight loss that was comparable to what has been reported in previous studies on liraglutide [32]. It could be contemplated that the newly diagnosed T2D in our study population may have limited the potential effect of GLP-1 agonism on the cardiac function. However, previous studies have shown improvement in cardiac function in cohorts consisting of both T2D and non-T2D patients [7, 9, 21]. Furthermore, myocardial insulin resistance have shown to be an inherent feature of both CAD and T2D [33], and thus providing a treatment target for liraglutide.
GLP-1 infusion has shown to improve remodeling in pre-clinical studies [34]. This was also demonstrated in a retrospective study assessing remodeling by cardiac MRI in patients treated with liraglutide [35]. However, consistent with our study, no improvement in LVEF was observed after 6 months.
Previous reporting of elevated heart rate associated with liraglutide treatment was also observed in our study [36]. In particularly, this has been of concern due to the association between elevated HR and increased risk of LV dysfunction and heart failure [37]. Despite a significant increased resting HR during 12 weeks of liraglutide treatment, we did not find any significant worsening of LV function at rest or during DSE. Interestingly, HR was also increased during low-stress and peak-stress stages of DSE after liraglutide. The chronotropic effect of GLP-1 RAs is believed to be mediated via GLP-1 receptors located to the sinoatrial node [38]. However, studies have suggested that GLP-1 may exhibit an inhibitory effect on sympathovagal balance as well [39].
Exercise capacity is an independent predictor of all-cause mortality and CV-mortality [40]. An improvement in exercise capacity assessed by a 6 min walk test was found after 5 weeks of GLP-1 infusion in patients with heart failure [8], but could not be confirmed in a randomized study where cardiopulmonary exercise testing was performed after 48 h GLP-1 infusion [18] or after 12 weeks of albiglutide treatment [19]. Despite the significant weight loss during liraglutide treatment in our study, we did not observe any improvement in exercise capacity assessed by METS or the total exercise time.
Safety and adverse events
In general the study drugs were well-tolerated as only three subjects discontinued the study due to intolerance to medication. Two patients experienced MI during the study and were subsequently withdrawn from the study. Both of these events were deemed by the investigators to be unrelated to treatment. One patient experienced an allergic reaction during the DSE at baseline visit. This patient subsequently underwent ergometer stress echocardiography without contrast during all four visits.
Strengths and limitations
Although comparable DSE scans were aimed for at all four levels of stress and between visits by using reference images from the baseline visit, we cannot exclude that some differences in the scanning positions may have contributed to the overall variation. However, the use of contrast-enhanced 2D echo has shown to increase the accuracy and reproducibility echocardiographic examinations [41]. The homogeneity of our study populations limits the application of our results to other patient groups such as patients with heart failure or patients with longer duration of diabetes and poor glycemic control.
Perspectives
The presence of subclinical LV dysfunction and increased risk of heart failure in subjects with T2D and CAD necessities exploration of glucose lowering medication that may improve and prevent further deterioration of cardiac function. In this context, an understanding of how GLP-1 RAs affect the myocardial function during conditions of myocardial stress is of great importance. Any improvement in systolic function during stress may prevent the long-term deterioration of LV performance and progression to heart failure. Although, no significant improvement in systolic function was observed in our study, we did not observe any deterioration in cardiac function. Thus, liraglutide may be a safe treatment option in patients with cardiac risk factors and preserved LVEF. The recently published LEADER trial showed improved effect on CV mortality after liraglutide treatment [42]. Notably, no increased risk of hospitalization for heart failure was observed. We believe that our study adds to the further understanding of the results from the various long-term GLP-1 RA trials.
Conclusions
In patient with preserved LVEF, T2D, and stable CAD, the addition of liraglutide to the backbone therapy of metformin did not improve the systolic function of the LV or the exercise capacity.
Authors’ contributions
AS and SBH conceived and designed the study. PK, CA, OPK, OW and AS acquired the data. PK, OW and AS performed the statistical analyses. PK drafted the manuscript. PK, CA, OW, OPK, JM, SM, SBH and AS analyzed and interpreted the data and revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the patients who participated in this study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Source of funding
This study was supported by a unrestricted grant for investigator-initiated studies from Novo Nordisk A/S. Additional funding was provided by The AP Møller Foundation, The Bispebjerg Hospital Research Foundation, The Danish Heart Foundation, Department of Internal Medicine Amager Hospital and Clinical Research Centre Hvidovre Hospital.
Disclosures
SBH has received funding of educational and research tasks from Novo Nordisk, Abbott, Eli Lilly, Pfizer, Boehringer-Ingelheim, Bristol-Meyers Squibb and Merck, Sharp & Dome. OWN has received funding of educational and research tasks from Resmed and participated in advisory boards for Novartis Pharma. SM has during the past 3 years participated in advisory boards for Novartis Pharma, Novo Nordisk, Merck, Sharp & Dome, Sanofi-Aventis, AstraZeneca, Johnson & Johnson, Roche, Mankind, Boehringer-Ingelheim, Zeeland, Eli Lilly, Intarcia Therapeutics and has received honorary for lectures from Novo Nordisk, Merck, Sharp & Dome, AstraZeneca, Johnson and Johnson, Roche, Shering-Ploug, Sanofi-Aventis, Novartis Pharma, Eli Lilly and Bristol-Meyers Squibb. PK, CA, OK, JM and AS have nothing to disclose.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CoV
coefficient of variation
- COR
coefficient of repeatability
- CV
cardiovascular
- DSE
dobutamin stress echocardiography
- eGFR
estimated glomerular filtration rate
- GLP-1
glucagon-like peptide-1
- GLS
global longitudinal strain
- GLSR
global longitudinal strain rate
- HbA1C
glycated hemoglobin
- HR
heart rate
- ICH-GCP
International Conference on Harmonization-Good Clinical Practice
- ITT
intention-to-treat
- LDL
low-density lipoprotein
- LVEF
left ventricular ejection fraction
- METs
estimated metabolic equivalents
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- RWT
relative wall thickness
- T2D
type 2 diabetes
- WMSI
wall motion score index
Additional file
10.1186/s12933-016-0425-2 Additional tables.
Contributor Information
Preman Kumarathurai, Phone: +45 35313531, Email: preman.kumarathurai@dadlnet.dk.
Christian Anholm, Email: canholm@youmail.dk.
Olav W. Nielsen, Email: own@dadlnet.dk
Ole P. Kristiansen, Email: Ole.Peter.Kristiansen@regionh.dk
Jens Mølvig, Email: jens.molvig@gmail.com.
Sten Madsbad, Email: Sten.Madsbad@regionh.dk.
Steen B. Haugaard, Email: d299057@dadlnet.dk
Ahmad Sajadieh, Email: asajadieh@yahoo.com.
References
- 1.Authors/Task Force Members. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes H-P, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen M-R, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, ESC Committee for Practice Guidelines (CPG) Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboratio. Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 2.Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, Rosamond WD, Heiss G. The population burden of heart failure attributable to modifiable risk factors: the ARIC (atherosclerosis risk in communities) study. J Am Coll Cardiol. 2012;60:1640–1646. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME investigators: empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL. SAVOR-TIMI 53 Steering Committee and Investigators*: heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 5.Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–2090. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen Y-T, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 8.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 9.Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–413. doi: 10.1136/hrt.2010.219345. [DOI] [PubMed] [Google Scholar]
- 10.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. LEAD-2 Study Group: efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anholm C, Kumarathurai P, Klit MS, Kristiansen OP, Nielsen OW, Ladelund S, Madsbad S, Sajadieh A, Haugaard SB. Adding liraglutide to the backbone therapy of biguanide in patients with coronary artery disease and newly diagnosed type-2 diabetes (the AddHope2 study): a randomised controlled study protocol. BMJ Open. 2014;4:e005942. doi: 10.1136/bmjopen-2014-005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becher H, Chambers J, Fox K, Jones R, Leech GJ, Masani N, Monaghan M, More R, Nihoyannopoulos P, Rimington H, Senior R, Warton G. British Society of Echocardiography Policy Committee: BSE procedure guidelines for the clinical application of stress echocardiography, recommendations for performance and interpretation of stress echocardiography: a report of the British Society of Echocardiography Policy Committee. Heart. 2004;90(Suppl 6):23–30. doi: 10.1136/hrt.2004.047985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 14.Saunamäki K, Egstrup K, Krusell L, Mickley H, Nielsen JR, Schnohr P: Vejledende Retningslinier for Klinisk Arbejdstest I Relation Til Iskæmisk Hjertesygdom (Dansk Cardiologisk Selskab); 2002.
- 15.Jones M, Dobson A, O’Brian S. A graphical method for assessing agreement with the mean between multiple observers using continuous measures. Int J Epidemiol. 2011;40:1308–1313. doi: 10.1093/ije/dyr109. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31:466–475. doi: 10.1002/uog.5256. [DOI] [PubMed] [Google Scholar]
- 17.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 18.Halbirk M, Nørrelund H, Møller N, Holst JJ, Schmitz O, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Bøtker HE, Wiggers H. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1096–H1102. doi: 10.1152/ajpheart.00930.2009. [DOI] [PubMed] [Google Scholar]
- 19.Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, Fossler M, Davila-Roman VG, Russell SD, Gropler RJ. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016;4:559. doi: 10.1016/j.jchf.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Maher TD, Dean DA, Bailey SH, Marrone G, Benckart DH, Elahi D, Shannon RP. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, Yang JJ, Wang ZF, Tian F, Ning QX. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170:845–854. doi: 10.1016/j.ahj.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Chen W-R, Shen X-Q, Zhang Y, Chen Y-D, Hu S-Y, Qian G, Wang J, Yang J-J, Wang Z-F, Tian F. Effects of liraglutide on left ventricular function in patients with non-ST-segment elevation myocardial infarction. Endocrine. 2016;52:516–526. doi: 10.1007/s12020-015-0798-0. [DOI] [PubMed] [Google Scholar]
- 23.McCormick LM, Heck PM, Ring LS, Kydd AC, Clarke SJ, Hoole SP, Dutka DP. Glucagon-like peptide-1 protects against ischemic left ventricular dysfunction during hyperglycemia in patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc Diabetol. 2015;14:102. doi: 10.1186/s12933-015-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geleijnse ML, Fioretti PM, Roelandt JR. Methodology, feasibility, safety and diagnostic accuracy of dobutamine stress echocardiography. J Am Coll Cardiol. 1997;30:595–606. doi: 10.1016/S0735-1097(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 25.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 26.Clarke SJ, McCormick LM, Dutka DP. Optimising cardioprotection during myocardial ischaemia: targeting potential intracellular pathways with glucagon-like peptide-1. Cardiovasc Diabetol. 2014;13:12. doi: 10.1186/1475-2840-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Køber L, Treiman M, Holst JJ, Engstrøm T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 28.Woo JS, Kim W, Ha SJ, Kim JB, Kim S-J, Kim W-S, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 29.Holland DJ, Marwick TH, Haluska BA, Leano R, Hordern MD, Hare JL, Fang ZY, Prins JB, Stanton T. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–1066. doi: 10.1136/heartjnl-2014-307391. [DOI] [PubMed] [Google Scholar]
- 30.Leung M, Wong VW, Hudson M, Leung DY. Impact of improved glycemic control on cardiac function in type 2 diabetes mellitus. Circ Cardiovasc Imaging. 2016;9:e003643. doi: 10.1161/CIRCIMAGING.115.003643. [DOI] [PubMed] [Google Scholar]
- 31.Yingchoncharoen T, Agarwal S, Popović ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, Düring M, Matthews DR. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iozzo P, Chareonthaitawee P, Dutka D, Betteridge DJ, Ferrannini E, Camici PG. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. doi: 10.2337/diabetes.51.10.3020. [DOI] [PubMed] [Google Scholar]
- 34.Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295–H304. doi: 10.1152/ajpheart.00990.2012. [DOI] [PubMed] [Google Scholar]
- 35.Nozue T, Yamada M, Tsunoda T, Katoh H, Ito S, Iwaki T, Michishita I: Effects of liraglutide, a glucagon-like peptide-1 analog, on left ventricular remodeling assessed by cardiac magnetic resonance imaging in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels; 2015. [DOI] [PubMed]
- 36.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:e001986–e001986. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opdahl A, Ambale Venkatesh B, Fernandes VRS, Wu CO, Nasir K, Choi EY, Almeida ALC, Rosen B, Carvalho B, Edvardsen T, Bluemke DA, Lima JAC. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:1182–1189. doi: 10.1016/j.jacc.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 39.Griffioen KJ, Wan R, Okun E, Wang X, Lovett-Barr MR, Li Y, Mughal MR, Mendelowitz D, Mattson MP. GLP-1 receptor stimulation depresses heart rate variability and inhibits neurotransmission to cardiac vagal neurons. Cardiovasc Res. 2011;89:72–78. doi: 10.1093/cvr/cvq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris CK, Ueshima K, Kawaguchi T, Hideg A, Froelicher VF. The prognostic value of exercise capacity: a review of the literature. Am Heart J. 1991;122:1423–1431. doi: 10.1016/0002-8703(91)90586-7. [DOI] [PubMed] [Google Scholar]
- 41.Malm S, Frigstad S, Sagberg E, Larsson H, Skjaerpe T. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance imaging. J Am Coll Cardiol. 2004;44:1030–1035. doi: 10.1016/j.jacc.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 42.Marso SP, Daniels GH, BrownFrandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee on behalf of the LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


