Abstract
The polycyclic aromatic hydrocarbon pollutant benzo[a]pyrene (BaP) is a known developmental gonadotoxicant. However, the mechanism of BaP-induced germ cell death is unclear. We investigated whether exposure to BaP induces apoptotic germ cell death in the mouse fetal ovary or testis. Mouse fetal gonads were dissected at embryonic day 13.5 days postcoitum (dpc) and fixed immediately or cultured for 6, 24, 48, or 72 h with various concentrations of BaP (1–1000 ng/ml). Germ cells numbers, apoptosis, and proliferation were evaluated by immunostaining. Treatment of fetal ovaries with BaP for 72 h concentration-dependently depleted germ cells. Treatment with BaP elevated the expression of BAX protein at 6 h and activated downstream caspases-9 and -3 at 24 h in a concentration-dependent manner in germ cells of fetal ovaries. As a consequence, ovarian germ cell numbers were significantly and concentration-dependently decreased at 48 h. Pretreatment with z-VAD-fmk, a pan-caspase inhibitor, prior to exposure to 1000 ng/ml BaP prevented BaP-mediated ovarian germ cell death; there were no effects of BaP or z-VAD-fmk on germ cell proliferation. No significant effects of BaP exposure on caspase 3 activation or germ cell numbers were observed in fetal testes after 48 h of culture. Our findings show that BaP exposure increases caspase-dependent and BAX-associated germ cell apoptosis in the mouse fetal ovary, leading to germ cell depletion. In contrast, the cultured 13.5 dpc fetal testis is relatively resistant to BaP-induced germ cell death. This study provides a novel insight into molecular mechanisms by which BaP has direct gonadotoxicity in the mouse fetal ovary.
Keywords: benzo[a]pyrene, germ cells, apoptosis, ovary, testis, polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental toxicants generated during incomplete combustion of organic compounds. PAHs can induce a wide range of adverse health effects via the generation of reactive metabolites and reactive oxygen species (ROS) (Penning, 2004; Xue and Warshawsky, 2005). Some PAH metabolites are activating ligands for aryl hydrocarbon receptor (AhR), a transcription factor that mediates the cellular response to xenobiotic compounds, and elicit their toxic effects, at least in part, by the activation of AhR and subsequent up-regulation of cytochrome P450 enzymes that convert PAHs into reactive metabolites (Burczynski and Penning, 2000; Shimizu et al., 2000; Wilson and Safe, 1998). Benzo[a]pyrene (BaP), a carcinogenic PAH, is commonly found in cigarette smoke, gasoline and diesel exhaust, and in grilled and broiled foods (ATSDR, 1995). Maternal exposure to BaP causes extensive deleterious effects on fetal development and tumorigenesis in offspring (Shum et al., 1979; Turusov et al., 1990; Wislocki et al., 1986).
The mouse fetal ovary and testis are important targets for PAHs, including BaP. Maternal smoking, a primary route of human fetal exposure to BaP, disturbs human fetal ovarian development and endocrine signaling (Fowler et al., 2014), decreases fecundability (Weinberg et al., 1989) and advances menopause (Strohsnitter et al., 2008) in daughters. Both prenatal and postnatal BaP exposure reduce ovarian function and follicle numbers in female mice. Maternal smoking reduces sperm concentration and quality in sons in adulthood (Jensen et al., 2004), and both prenatal and postnatal exposure to BaP alter sperm morphology and decrease spermatogenesis in male mice (Nakamura et al., 2012). We have shown that BaP treatment dose-dependently decreases ovarian follicle numbers in F1 female mice exposed in utero to 2 or 10 mg/kg/day BaP from gestational days 7 to 16 compared with oil treated controls at 7.5 months of age (Lim et al., 2013). Moreover, the 10 mg/kg/day BaP dose destroys nearly all developing oogonia/oocytes and causes epithelial ovarian tumors in F1 offspring. Similarly, exposure to the same transplacental regimen of BaP exposure decreases testicular and epididymal sperm counts in the F1 male offspring, but in contrast to the ovary only minimal effects are observed at the lower, 2 mg/kg dose (Nakamura et al., 2012).
Apoptotic programmed cell death is responsible for germ cell loss in developing fetal gonads both in humans (Hartshorne et al., 2009) and mice (Alton and Taketo, 2007; Coucouvanis et al., 1993; De Felici et al., 1999; Robles et al., 2000). There are 2 major apoptotic pathways, the receptor-mediated or extrinsic pathway and the mitochondrial or intrinsic pathway, both of which initiate the activation of caspase cascades that converge on activation of caspase 3, resulting in cell death. AhR is expressed in mouse germ cells at all developing stages (Robles et al., 2000). Pro-apoptotic BCL2-family molecules such as BCL2-associated X protein (BAX) are major regulators of the mitochondrial pathway. In fetal and neonatal mouse ovaries, the model PAH 9,10-dimethylbenz[a]anthracene (DMBA) increases germ cell apoptosis and oocyte destruction via AhR-driven Bax expression (Matikainen et al., 2001a,). In vitro exposure of human fetal testis to DMBA increases BAX expression and germ cell apoptosis (Coutts et al., 2007). These studies suggest that BAX plays a critical role in apoptotic germ cell death in response to PAH exposure; however, DMBA is not an environmentally relevant PAH to which humans are exposed.
Several lines of evidence show that exposure to PAHs has negative impacts on the reproductive system in both fetuses and adults. Benzo[a]pyrene is an environmentally relevant PAH that we have shown destroys germ cells during prenatal development; however, the mechanism(s) by which prenatal BaP exposure destroys germ cells are not fully elucidated. Here, we hypothesized that exposure to BaP induces apoptotic germ cell death via activation of the mitochondrial apoptotic pathway in the mouse fetal gonad. To test this hypothesis, we examined the effects of BaP treatment on caspase activation and BAX protein expression in cultured fetal ovaries and testes.
MATERIALS AND METHODS
Animals. C57BL/6J mice were bred in our colony, housed in an American Association for the Accreditation of Laboratory Animal Care-accredited facility, with free access to deionized water and laboratory chow (Harlan Teklad 2919) on a 14:10 h light–dark cycle. Temperature was maintained at 69°F–75 °F. The experimental protocols were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine. The 10-week-old females were mated with males on the evening of proestrus, determined by vaginal cytology, and the morning after overnight mating was considered 0.5 day postcoitum (dpc). Pregnant mice were sacrificed by CO2 euthanasia on 13.5 dpc, and the fetuses were quickly removed from the uterus. Fetuses were dissected using a stereomicroscope, and the sex was determined by the morphology of the gonads and subsequently confirmed by Sry genotyping (Primers: F, TTGTCTAGAGAGCATGGAGGGCCATGACAA and R, CCACTCCTCTGTGACACTTTAGCCCTCCGA).
Gonad culture and BaP treatment. Fetal gonads were dissected with mesonephros intact and either fixed immediately (0 h) or cultured as described previously (Lambrot et al., 2006; Livera et al., 2006) for 6, 24, 48, or 72 h in media with 0.005% dimethyl sulfoxide (DMSO) alone or with various concentrations of BaP (1, 5, 10, 100, 500, or 1000 ng/ml) in Dulbecco’s modified Eagle’s medium/Ham F12 (1:1) (Gibco, Grand Island, New York) supplemented with 0.1% bovine serum albumin, 100 μg/ml streptomycin, and 100 IU/ml penicillin G. Benzo[a]pyrene (Supelco, Bellefonte, Pennsylvania) was dissolved in DMSO and a fresh aliquot of stock solution (20 ng/ml) was used to make the BaP treatment media for each experimental run. Gonads were placed on 0.4 µm Millicell-CM Biopore membranes (Millipore, Billerica, Massachusetts) floating on 400 μl culture medium in tissue culture dishes and cultured at 37 °C in a humidified atmosphere containing 95% air and 5% carbon dioxide. In preliminary experiments (72 h), we cultured fetal ovaries with or without 0.005% DMSO and with or without additional growth factors (stem cell factor/KitL, leukemia inhibitory factor, and insulin-like growth factor) and observed no effects on germ cell numbers (data not shown). Therefore, the additional growth factors were omitted for subsequent experiments. At the end of the culture, gonads were fixed in Bouin’s solution overnight at 4 °C and embedded in OCT before being stored at −80 °C. The embedded gonads were sectioned at 5 μm for immunostaining.
Immunofluorescence for germ cell counting. Complete serial sections were cut for every gonad and mounted so that there were 4 sets of slides that contained 4 sections each, which were separated by 3 intervening sections. One complete set of slides, containing every fourth section through the entire gonad was immunostained with TRA98 antibody for germ cell counts, and the other sets were used for immunostaining with other antibodies as described below. We performed immunofluorescence assays for germ cell-specific antigen (TRA98) using a rat anti-TRA98 monoclonal IgG antibody (1:200; Abcam ab82527, Cambridge, Massachusetts). We confirmed that this antibody stained germ cells by double staining with mouse Vasa homolog antibody (data not shown; Baltus et al., 2006). The germ cells were identified by their distinctive morphology with large size and spherical shape. We counted germ cells in every fourth section and multiplied the sum of the values obtained for the observed sections of 1 gonad by 4 to obtain a total count of germ cells per gonad. All counts were carried out blind to treatment on images captured using a Retiga 2000R digital camera with an Olympus BX60 microscope equipped with fluorescence filters using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Immunohistochemistry. Slides were thawed and heated for 15 min in a 10 mM citrate buffer (pH 6.0). The primary antibodies—rabbit anticleaved caspase-3 Asp 175 (1:100; Cell Signaling #9664, Beverly, Massachusetts), rabbit anticleaved caspase-9 Asp 353 (1:100; Cell Signaling #9509), rabbit anti-BAX (1:200; Santa Cruz Biotechnology sc-6236, Santa Cruz, California), and rabbit anti-Ki67 (1:200; Abcam #15580)—were detected using biotinylated goat antirabbit secondary antibodies in 5% or 10% normal goat serum and avidin–biotin–peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, California). Peroxidase activity was visualized using 3,3′-diaminobenzidine substrate. Slides were counterstained with hematoxylin. Germ cells that stained positively and negatively for each apoptotic marker were counted and quantities expressed as fractions were used for statistical analyses. Negative controls (primary antibody replaced with nonimmune IgG, primary antibody without secondary antibody, or secondary antibody without primary antibody) were included in every experiment. To confirm the specificity of cleaved caspase-9 immunostaining, we included adult mouse ovaries as positive controls. As anticipated, caspase-9 was activated in the granulosa cells of atretic ovarian follicles (data not shown). We previously validated the cleaved caspase-3 antibody in a similar manner (Lim et al., 2015). In addition, our results showed similar patterns of activated caspase-9 immunostaining in both nuclei and cytoplasm of cells as have been reported by others (Angenard et al., 2010; Eckle et al., 2004; Hanoux et al., 2007).
Pan-caspase inhibitor treatment
Fetal ovaries were treated with (1) 0.005% DMSO (vehicle control); (2) pretreated with 50 μM z-VAD-fmk (R&D Systems FMK001, Minneapolis, Minnesota), a pan-caspase inhibitor, for 1 h and then incubated in fresh media with 0.005% DMSO (inhibitor control); 3) 1000 ng/ml BaP alone for 48 h; or 4) pretreated with 50 μM z-VAD-fmk for 1h and then incubated with 1000 ng/ml BaP for 48 h. At the end of the culture, fetal ovaries were processed for TRA98 immunofluorescence or for Ki67 immunostaining and TRA98- or Ki67-positive germ cells were counted, as described above.
Statistical analysis
All values are presented as mean ± SEM in figures. For studies on apoptosis, quantities expressed as fractions were subjected to arcsine square root transformation prior to analysis followed by 1-way analysis of variance and then LSD post hoc test. For germ cell numbers, we used linear regression to examine the effect of BaP concentration followed by pairwise comparisons using Fisher’s LSD test. Statistical analyses were performed using SPSS 20.0 for Mac OS X (IBM Software).
RESULTS
Prenatal BaP Exposure Increases Female Germ Cell Death
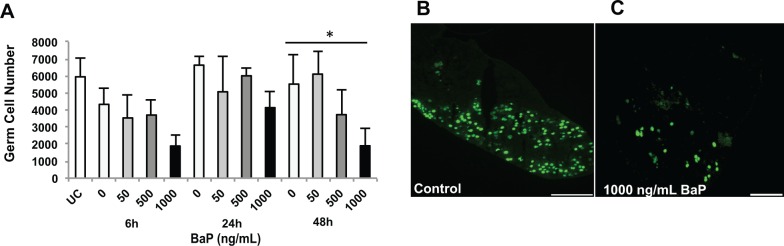
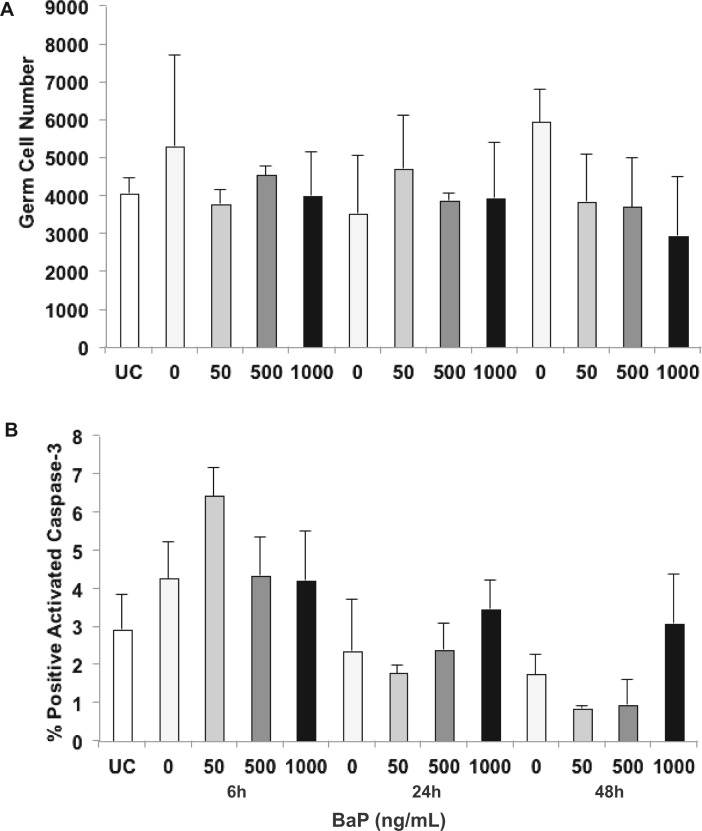
In an initial concentration–response experiment, dpc 13.5 fetal ovaries were cultured for 72 h with 0, 1, 5, 10, 100, 500, or 1000 ng/ml BaP in 0.005% DMSO. We observed a concentration-dependent depletion of germ cells, with the 1000 ng/ml group differing significantly from control (Supplementary Figure 1). In subsequent experiments, 13.5 dpc fetal ovaries were cultured with 0, 50, 500, or 1000 ng/ml BaP in 0.005% DMSO. At 13.5 dpc, uncultured ovaries (0 h) contained 5948 ± 1077 germ cells per ovary (Figure 1). In fetal ovaries cultured for 48h, the mean number of total germ cells per ovary (5520 ± 1694) in control gonads was maintained at about 92% of 0h ovaries, which is similar to germ cell number (5158 ± 468) observed in the same mouse strain (C57BL/6) at 15.5 dpc (Rodrigues et al., 2009). Germ cell number concentration- and time-dependently decreased with BaP treatment, and the effect of BaP concentration was statistically significant at 48 h (P = .025, effect of concentration by linear regression; Figure 1).
FIG. 1.
Benzo[a]pyrene (BaP) induces germ cell loss in mouse fetal ovaries. Total number of germ cells was counted as described in Materials and Methods section in 13.5 dpc ovaries cultured for 0, 6, 24, or 48 h with 0, 50, 500, or 1000 ng/ml respectively, BaP in 0.005% DMSO. A, Mean ± SEM total number of germ cells per fetal ovary. Representative images of germ cells in control (B) and BaP-treated (C) ovaries at 48 h identified by TRA98 immunofluorescence. *P = .025, effect of concentration by linear regression. n = 3 − 5/group. Scale bars, 100 µm. UC, uncultured (0 h).
Increased Apoptosis in the Mouse Fetal Ovary After BaP Exposure Correlated With Female Germ Cell Loss
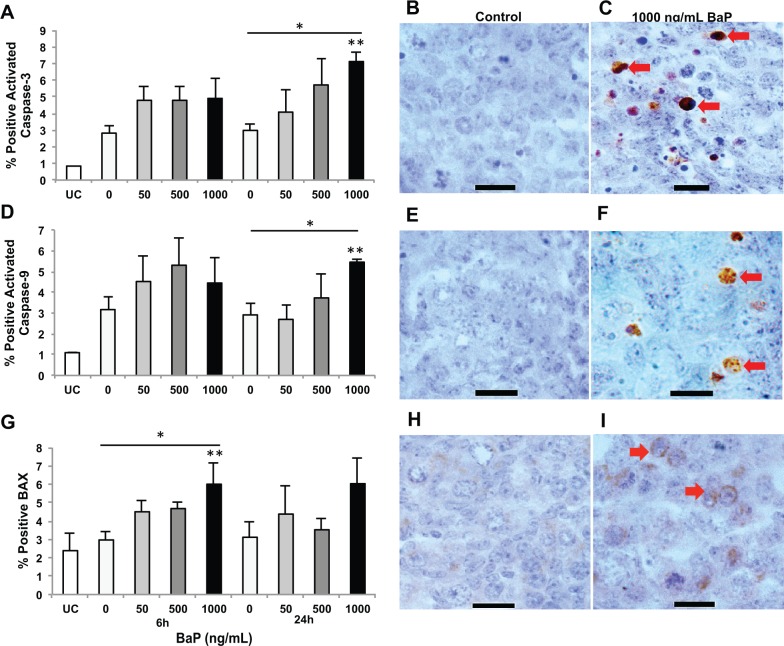
Based on the concentration-dependent difference in germ cell numbers at 48 h, we examined 3 key markers of apoptosis at 6 and 24 h by immunohistochemistry. Caspase-3, the executioner caspase, is activated by both the intrinsic and extrinsic apoptotic pathways. Few apoptotic germ cells were detected by cleaved caspase-3 staining in uncultured (0 h) mouse fetal ovaries (< 1% of total germ cells). About 3% of germ cells were cleaved caspase-3 positive in control ovaries at both 6 and 24 h. After 24 h in culture, treatment with 1000 ng/ml BaP significantly increased the percentage of caspase-3–positive germ cells (7.1% ± 0.6%) compared with controls (3.0% ± 0.4%; P = .018), and treatment with increasing concentrations of BaP at 24 h induced caspase-3 activation in a concentration-dependent manner (P = .007; Figure 2A–C). However, no statistically significant change was observed at 6 h. Caspase-9 is the initiator caspase in the intrinsic or mitochondrial apoptotic pathway. Similar to activated caspase-3, 3% of germ cells were cleaved caspase-9 positive in control ovaries at both 6 and 24h. Treatment with increasing concentrations of BaP-induced caspase-9 activation at 24 h in a concentration-dependent manner (P = .007; Figure 2D–F). Intergroup comparisons showed significant differences between control (2.9% ± 0.6%) and 1000 ng/ml BaP (5.5% ± 0.1%) groups at 24 h (P = .036). Like cleaved caspase-3, no statistically significant change was observed at 6 h.
FIG. 2.
Benzo[a]pyrene (BaP) exposure activates caspases-3 and -9, and increases germ cell expression of BAX protein in fetal ovaries. Germ cell apoptosis was assessed by cleaved caspase-3, -9, and BAX immunostaining in 13.5 dpc ovaries cultured for 0, 6, or 24 with 0, 50, 500, or 1000 ng/ml, respectively, BaP in 0.005% DMSO. A, Mean ± SEM percentage of cleaved caspase-3 positive germ cells at 6 and 24 h (*P = .007 effect of concentration by linear regression, **P = .018 vs control). B–C, Representative images of cleaved caspase-3 immunostaining in fetal ovaries at 24 h. D, Mean ± SEM percentage of cleaved caspase-9 positive germ cells at 6 and 24 h (*P = .007 effect of concentration by linear regression, **P < .05 vs control and 50 ng/ml BaP). E and F, Representative images of cleaved caspase-9 immunostaining in fetal ovaries at 24 h. G, Mean ± SEM percentage of BAX-positive germ cells at 6 and 24h (*P = .036 effect of concentration by linear regression, **P = .024 vs control). H and I, Representative images of fetal ovaries identified by BAX immunostaining at 6 h. Arrows point to caspase-positive germ cells (C and F) or BAX-positive germ cells (I). n = 3–4/group. Scale bars, 15 µm. UC, uncultured (0 h).
BAX plays an important role in apoptosis, acting as an upstream regulator of caspase-mediated cell death in the mitochondrial apoptotic pathway. In female gonads cultured with or without BaP, germ cells, and to a lesser degree somatic, pre-granulosa cells, were immunopositive for BAX (Figure 2G–I). Treatment with BaP increased the number of BAX-positive germ cells in a concentration-dependent manner at 6 h (P = .036), whereas no significant differences were observed at 24 h. Intergroup comparisons showed that the number of BAX-positive germ cells was significantly higher in the 1000 ng/ml BaP group compared with controls (6.0% ± 1.2% vs 3.0% ± .4%; P = .024).
Inhibition of Caspases Normalizes the Number of Germ Cells in BaP Exposed Fetal Ovaries
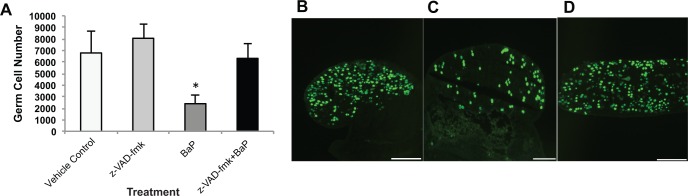
To confirm that BaP-mediated germ cell death requires caspase activation, we used in vitro pretreatment with 50 µM z-VAD-fmk, a pan-caspase inhibitor. Treatment with 1000 ng/ml BaP alone significantly reduced the number of germ cells compared with vehicle control (2393 ± 764 vs 6782 ± 1850; P = .038) and inhibitor alone (2393 ± 764 vs 8053 ± 1254; P = .007), respectively (Figure 3). Pretreatment with z-VAD-fmk prior to exposure to 1000 ng/ml BaP completely prevented the depletion of germ cells (6312 ± 1305 in BaP plus z-VAD-fmk vs 6782 ± 1850 in vehicle controls).
FIG. 3.
Benzo[a]pyrene (BaP)-mediated germ cell death in fetal ovaries requires caspase activation. Germ cells were counted as described in Materials and Methods section in 13.5 dpc ovaries cultured for 48 h with 0.005% DMSO alone, 50 μM of z-VAD-fmk pretreatment followed by control media, 1000 ng/ml of BaP alone, or z-VAD-fmk pretreatment followed by BaP. A, Mean ± SEM total number of germ cells per ovary. Representative images of germ cells identified by TRA98 immunodetection in fetal ovaries cultured with z-VAD-fmk (B); 1000 ng/ml BaP (C); z-VAD-fmk and 1000 ng/ml BaP cotreatment (D). n = 6–7/group. Scale bars, 100 µm. *P < .05 compared with vehicle and z-VAD-fmk control. UC, uncultured (0 h).
BaP Treatment Does Not Alter Germ Cell Proliferation in Cultured Fetal Ovaries
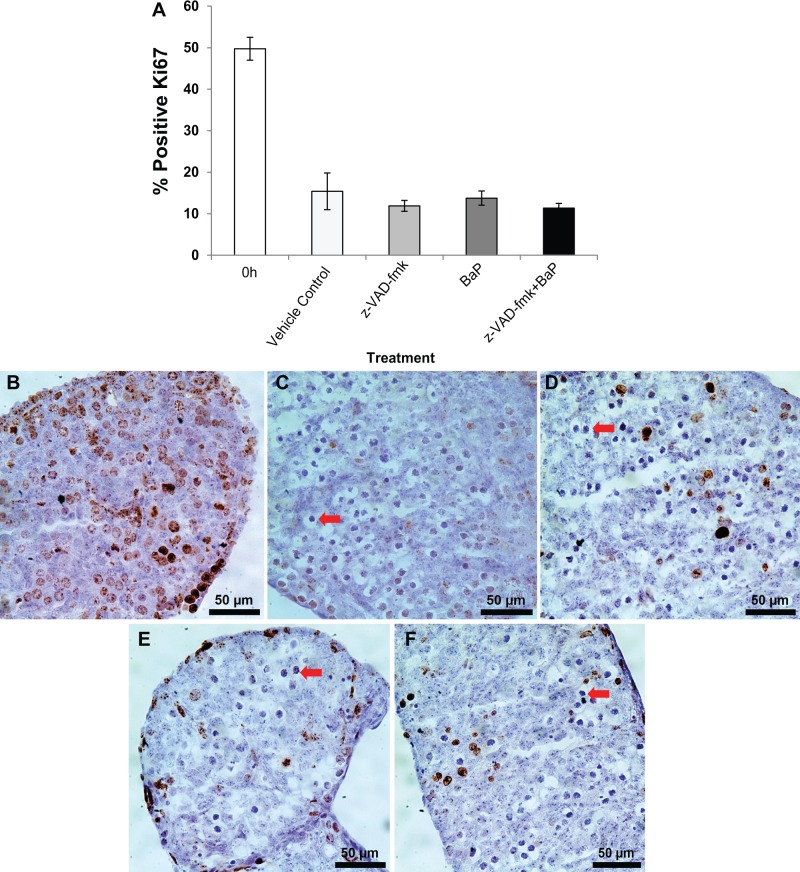
We also assessed the effects of BaP treatment on germ cell proliferation in the ovaries from the caspase inhibition experiment by immunostaining for the mitosis-specific protein, Ki67 (Scholzen and Gerdes, 2000). We observed that about half of the germ cells were Ki67 positive in uncultured E13.5 ovaries, whereas only 10%–15% were Ki67 positive in all 4 experimental groups cultured for 48 h, consistent with the large majority of germ cells having entered meiosis (Figure 4 and Supplementary Figure 2). There were no statistically significant differences in the percentages of Ki67-positive cells among the vehicle control, z-VAD-fmk, BaP, and z-VAD-fmk plus BaP groups.
FIG. 4.
Benzo[a]pyrene (BaP) does not affect germ cell proliferation in mouse fetal ovaries. Germ cell proliferation was assessed by Ki67 immunostaing in the same ovaries as for Figure 3. A, Mean ± SEM percentage of Ki67 positive germ cells at 0 and 48 h. Representative images of germ cells identified by Ki67 immunodetection in fetal ovaries: 0 h uncultured (B); 0.005% DMSO (C); z-VAD-fmk (D); 1000 ng/ml BaP (E); z-VAD-fmk and 1000 ng/ml BaP cotreatment (F). n = 4–5/group. Ki67 positive cells are dark brown in online version. Arrows point to examples of meiotic germ cells typical of the leptotene stage.
Effect of BaP on Male Germ Cells
We cultured mouse fetal testes from male littermates of the female fetuses used for the fetal ovary experiments using the same experimental protocol. A total of 13.5 dpc uncultured testes (0 h) contained about 4000 germ cells per testis. The germ cell number was increased to about 130% of 0 h levels in fetal testes cultured with control media for 48 h, but there were no statistically significant effects of BaP treatment on germ cell numbers (Figure 5A). We observed a nonsignificant 2% increase in the percentage of cleaved caspase 3 positive germ cells at 6 h of 50 ng/ml BaP treatment compared with controls. Culture with 50, 500, or 1000 ng/ml BaP for up to 48 h did not increase the percentage of germ cells positive for activated caspase-3 (Figure 5B).
FIG. 5.
Benzo[a]pyrene (BaP) does not deplete germ cells in cultured 13.5 dpc mouse fetal testes. A, Germ cells were counted as described in Materials and Methods section in 13.5 dpc testes cultured for 0, 6, 24, or 48 h with 0, 50, 500, or 1000 ng/ml, respectively, BaP in 0.005% DMSO. Mean ± SEM total number of germ cells identified by TRA98 immunodetection. B, Mean ± SEM percentage of apoptotic germ cells identified by immunohistochemical staining of cleaved caspase-3. n = 3–5/group. UC, uncultured (0 h).
DISCUSSION
Several PAHs have demonstrated developmental gonadotoxicity in males and females (Coutts et al., 2007; Lim et al., 2013; MacKenzie and Angevine, 1981; Nakamura et al., 2012), but the mechanism of fetal gonadotoxicity of BaP, a known carcinogenic PAH and environmental toxicant, remains unclear. We investigated for the first time the direct effects of BaP on cultured mouse fetal gonads. Our study demonstrates that exposure of mouse fetal gonads to BaP at 13.5 dpc increases apoptosis of female germ cells without affecting their proliferation, resulting in decreased germ cell number in the mouse fetal ovary, whereas the same concentrations have no effect on the fetal testis. Our findings also show that the loss of female germ cells requires activation of pro-apoptotic caspases in the mouse fetal ovary and that BAX may be involved in BaP-induced caspase activation.
In humans, maternal cigarette smoking is a major route of BaP exposure to the developing fetus and is also associated with various adverse health effects in exposed offspring (Chen et al. 2006; Harvey et al. 2007), including decreased fecundability in females and decreased semen quality in males (Jensen et al., 2004; Ramlau-Hansen et al., 2007; Weinberg et al., 1989). Concentrations of BaP have been reported to be 20–40 ng BaP/cigarette (Lodovici et al., 2004; Shopland et al., 2001) in mainstream cigarette smoke and 1.32 ± 0.68 ng/ml in ovarian follicular fluid of women who smoke (Neal et al., 2008). The exposure levels of BaP used in this study are 1–1000 ng/ml (4 nM to 4 µM). Thus, the 2 lowest concentrations of BaP tested bracket BaP concentrations in human follicular fluid. Whereas these concentrations of BaP did not significantly affect germ cell number in the present study, humans are simultaneously exposed to dozens of PAHs, and total exposure to PAHs in highly exposed humans (smokers who live in an urban area and consume grilled or smoked foods) is in the neighborhood of 2 µg/kg/day (ATSDR, 1995; Lodovici et al., 2004; Menzie et al., 1992; Shopland et al., 2001). At concentrations of 50–1000 ng/ml BaP, we observed that fetal ovaries undergo concentration-dependent apoptotic germ cell death, resulting in significantly decreased germ cell numbers (Figure 1). In cultured rat secondary follicles, 1.5 ng/ml of BaP significantly inhibited follicle growth (Neal et al., 2007). The difference in BaP concentrations inducing toxic effects may be associated with the different species studied, the different endpoints examined, or the culture system. Isolated follicles in culture have direct contact with BaP, whereas germ cells in intact fetal ovaries cultured in our experiments are surrounded by somatic cells and epithelial membrane. Similarly, in a study in which postnatal day 4 mouse ovaries were cultured with increasing concentrations (1–10 000 ng/ml) of BaP, no increase in pro-apoptotic markers was observed, but the antiapoptotic protein BCL-2 was increased at 100 ng/ml BaP (Tuttle et al., 2009). Because apoptosis and necrosis can occur simultaneously and caspase activation is also an early event associated with necrosis (Zeiss, 2003), we cannot definitively rule out the possibility that germ cells may be destroyed by necrosis by BaP exposure. Cigarette smoke exposure decreases primordial follicle counts and induces autophagy in ovarian granulosa cells in mice (Furlong et al., 2015; Gannon et al., 2012; Tuttle et al., 2009). Because cigarette smoke is a primary source of exposure to BaP, autophagic germ cell death could be another pathway involved in BaP-induced germ cell loss observed in the present study.
The extensive production of ROS during BaP metabolism may play a critical role in initiating and propagating BaP-induced germ cell death. Biotransformation of BaP to reactive metabolic intermediates occurs via several metabolic pathways, and these metabolites are largely responsible for BaP toxicity. The most well-known pathway, which is catalyzed by cytochrome P450 (CYP) and microsomal epoxide hydrolase enzymes, promotes the formation of BaP-7,8-dihydrodiol 9,10-epoxide (BPDE), a major reactive metabolite that forms mutagenic DNA adducts (Xue and Warshawsky, 2005). Another pathway involves enzymatic dehydrogenation of dihydrodiol metabolites to generate BaP quinones with the concomitant production of ROS that directly attack DNA (Penning, 2004; Penning et al., 1996). Prostaglandin-endoperoxide synthases are important enzymes involved in bioactivation of PAHs in the developing embryo (Rich and Boobis, 1997; Wells et al., 2009). Prostaglandin-endoperoxide synthases oxidize PAHs to free radical intermediates, which also initiate ROS generation (Wells et al., 2009). Due to relative lack of adequate CYP metabolic capacity in the fetus (Miller et al., 1996), the latter ROS-generating pathway may be dominant in fetal gonads in response to BaP exposure. Therefore, the germ cell death observed in the present study may be initiated via oxidative stress-induced activation of pro-apoptotic caspases. In human keratinocytes, a strong enhancement of CD95 (Fas)-mediated apoptosis was observed with BaP at concentrations activating the AhR, but BPDE failed to enhance CD95-mediated cell death, indicating that the pro-apoptotic effect of BaP is neither associated with BPDE-mediated DNA adduct nor BPDE-related signaling (Stolpmann et al., 2012). In cultured mouse zygotes exposure to 5 and 50 nM BaP increased the production of ROS and apoptotic cell death (Zhan et al., 2015). In this context, it seems very likely that BaP could be metabolized to gonadotoxic intermediates with the concurrent production of ROS that are responsible for apoptotic germ cell death in the fetal ovary. Because the role of ROS in germ cell apoptosis in the fetal ovary remains to be determined, further investigations are required to verify the involvement of ROS in germ cell loss during development.
In response to stress activation, BAX forms a homodimer and promotes of the release of cytochrome c from the mitochondria, which results in the activation of caspase-9 and subsequent activation of caspase-3. We observed that BAX protein is expressed in both germ and somatic cells of the fetal ovary, with intense staining in germ cells (Figure 2G–I). This distribution of BAX immunostaining agrees well with the previously reported distribution of BAX protein in mouse fetal ovaries (Alton and Taketo, 2007) and neonatal ovaries (Matikainen et al., 2001a). Our findings suggest that BAX may be involved in the activation of downstream pro-apoptotic caspases-9 and -3 following exposure of fetal ovaries to BaP (Figure 2). We further demonstrated that fetal ovarian germ cell destruction by BaP requires caspase activation by showing that pre-treatment with a pan-caspase inhibitor completely prevented the BaP-induced depletion of germ cells (Figure 3). Whereas the z-VAD-fmk-pretreated ovaries appeared morphologically healthy, it is possible that they sustained BaP-induced damage that may persist and have deleterious effects later in life. Interestingly, culture of postnatal day 4 mouse ovaries with similar concentrations of BaP (1–10 000 ng/ml) for 6 or 24 h, did not increase BAX, BCL2, caspase-3, caspase-8, or Fas ligand protein levels (Tuttle et al., 2009), suggesting that the fetal ovary is much more sensitive to BaP-induced germ cell destruction than the neonatal ovary. Our findings are in good agreement with previously published studies demonstrating that exposure of cultured 13.5 dpc fetal mouse ovaries to the DMBA metabolite DMBA-3,4-dihydrodiol for 72 h causes BAX-dependent oocyte loss (Matikainen et al., 2002), and that BAX regulates mouse germ cell survival and apoptosis during fetal life (Rucker et al., 2000).
To our knowledge, this is the first study to report on Ki67 immunostaining in 13.5 dpc ovaries and in cultured fetal ovaries at this stage of development. We observed that about half of the germ cells were Ki67 positive in uncultured 13.5 dpc ovaries. A previous report described “almost all” germ cells as Ki67 positive in 12.5 dpc ovaries (Atchison et al., 2003). A 50% decline in the percentage of Ki67-positive germ cells in the fetal ovary between 12.5 and 13.5 dpc is consistent with the onset of entry into meiosis on 13.5 dpc (Baltus et al., 2006; Menke et al., 2003). Moreover, after 48 h of culture in our system, we clearly observe progression to leptotene and zygotene stages of meiotic prophase I in the control ovaries, based on the characteristic nuclear morphology (Supplementary Figure 2), as has been described for uncultured ovaries at 14.5–16.5 dpc (Baltus et al., 2006). Notably, BaP treatment had no effect on the percentages of Ki67-positive cells.
We previously showed that prenatal exposure to BaP decreases sperm counts in the F1 male offspring (Nakamura et al., 2012). Interestingly, our immunohistologic analysis of fetal testes in the present study showed no significant effect of BaP on either the total number of germ cells per testis (Figure 4A) or on the rate of apoptosis (Figure 4B). The developmental window from 13.5 to 16.5 dpc is critically important in the development of the murine ovary and the testis. The transition from mitosis to meiosis of primordial germ cells occurs between 13.5 and 15.5 dpc in the ovary (Borum, 1961; Menke et al., 2003), whereas in the testis, primordial germ cells proliferate until 12.5 dpc and then gradually enter the quiescence phase in an unsynchronized manner between 12.5 and 14.5 dpc (Western et al., 2008). In our prior in vivo study, we observed dose-dependent declines in spermatogenesis with BaP dosing of the pregnant dam daily from 6.5 to 16.5 dpc, spanning both the period of testicular germ cell mitosis and the onset of quiescence (Nakamura et al., 2012). Similar to the present in vitro results, the developing ovary was more sensitive to BaP than the developing testis of littermate male siblings (Lim et al., 2013; Nakamura et al., 2012). Taken together our in vivo and in vitro studies suggest that the critical window for the fetal testicular germ cell toxicity of BaP is during the window from 6.5 to 12.5 dpc, prior to the cessation of mitotic proliferation. Other possible explanations for the apparently lower sensitivity of the fetal testis to BaP include less bioactivation of BaP and/or greater detoxification or antioxidant production in the fetal testis compared with the fetal ovary. We plan to explore these possibilities in future studies.
Our results show that culture of fetal mouse ovaries with BaP concentration-dependently induces caspase-dependent germ cell death, which is preceded by increased germ cell expression of the pro-apoptotic BCL-2 family protein BAX. The results are consistent with BAX-mediated activation of the proteolytic caspase cascade. In contrast, cultured fetal testes at the developmental stage studied are resistant to the induction of germ cell death by the same concentrations of BaP. Together with our prior studies showing that transplacental ovarian toxicity was more severe than testicular toxicity in male littermates at the same maternal dose of BaP, the present study provides additional evidence that the fetal ovary is more sensitive to BaP-induced germ cell death than the fetal testis.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors thank Dr Melissa Pepling for help establishing the gonad culture system and Laura Ortiz, Christine Pham, Angelica del Rosario, Jennifer Welch, and Chau Tran for help with vaginal cytology. Conflict of interest: None declared.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health (grant number R01ES020454 to U.L.); by a University of California Irvine Summer Undergraduate Research Program fellowship (to M.L.); and by the University of California Irvine Center for Occupational and Environmental Health.
REFERENCES
- Alton M., Taketo T. (2007). Switch from BAX-dependent to BAX-independent germ cell loss during the development of fetal mouse ovaries. J. Cell Sci. 130, 417–424. [DOI] [PubMed] [Google Scholar]
- Angenard G., Muczynski V., Coffigny H., Pairault C., Duquenne C., Frydman R., Habert R., Rouiller-Fabre V., Livera G. (2010). Cadmium increases human fetal germ cell apoptosis. Environ. Health Perspect. 118, 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison F. W., Capel B., Means A. R. (2003). Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development 130, 3579–3586. [DOI] [PubMed] [Google Scholar]
- ATSDR (1995). Toxicological Profile for Polycyclic Aromatic Hydrocarbons. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA. [PubMed] [Google Scholar]
- Baltus A. E., Menke D. B., Hu Y. C., Goodheart M. L., Carpenter A. E., De Rooij D. G., Page D. C. (2006). In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat. Genet. 38, 1430–1434. [DOI] [PubMed] [Google Scholar]
- Borum K. (1961). Oogenesis in the mouse. A study of the meiotic prophase. Exp. Cell Res. 24, 495–507. [DOI] [PubMed] [Google Scholar]
- Burczynski M. E., Penning T. M. (2000). Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 60, 908–915. [PubMed] [Google Scholar]
- Chen A., Pennell M. L., Klebanoff M. A., Rogan W. J., Longnecker M. P. (2006). Maternal smoking during pregnancy in relation to child overweight: follow-up to age 8 years. Int. J. Epidemiol. 35, 121–130. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E., Sherwood S. W., Carswell-Crumpton C., Spack E. G., Jones P. P. (1993). Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp. Cell Res. 209, 238–247. [DOI] [PubMed] [Google Scholar]
- Coutts S. M., Fulton N., Anderson R. A. (2007). Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum. Reprod. 22, 2912–2918. [DOI] [PubMed] [Google Scholar]
- De Felici M., Di Carlo A., Pesce M., Iona S., Farrace M. G., Piacentini M. (1999). Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death Differ. 6, 908–915. [DOI] [PubMed] [Google Scholar]
- Eckle V. S., Buchmann A., Bursch W., Schulte-Hermann R., Schwarz M. (2004). Immunohistochemical detection of activated caspases in apoptotic hepatocytes in rat liver. Toxicol. Pathol. 32, 9–15. [DOI] [PubMed] [Google Scholar]
- Fowler P. A., Childs A. J., Courant F., MacKenzie A., Rhind S. M., Antignac J. P., Le Bizec B., Filis P., Evans F., Flannigan S., et al. (2014). In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. Hum. Reprod. 29, 1471–1489. [DOI] [PubMed] [Google Scholar]
- Furlong H. C., Stämpfli M. R., Gannon A. M., Foster W. G. (2015). Cigarette smoke exposure triggers the autophagic cascade via activation of the AMPK pathway in mice. Biol. Reprod. 93, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon A. M., Stämpfli M. R., Foster W. G. (2012). Cigarette smoke exposure leads to follicle loss via an alternative ovarian cell death pathway in a mouse model. Toxicol. Sci. 125, 274–284. [DOI] [PubMed] [Google Scholar]
- Hanoux V., Pairault C., Bakalska M., Habert R., Livera G. (2007). Caspase-2 involvement during ionizing radiation-induced oocyte death in the mouse ovary. Cell Death Differ. 14, 671–681. [DOI] [PubMed] [Google Scholar]
- Hartshorne G. M., Lyrakou S., Hamoda H., Oloto E., Ghafari F. (2009). Oogenesis and cell death in human prenatal ovaries: What are the criteria for oocyte selection? Mol. Hum. Reprod. 15, 805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey N. C., Poole J. R., Javaid M. K., Dennison E. M., Robinson S., Inskip H. M., Godfrey K. M., Cooper C., Aihie Sayer A. and SWS Study Group (2007). Parental determinants of neonatal body composition. J. Clin. Endocrinol. Metab. 92, 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. K., Jørgensen N., Punab M., Haugen T. B., Suominen J., Zilaitiene B., Horte A., Anderson G. A., Carlsen E., Toppari J., Skakkebaek N. E. (2004). Association of in utero exposure to maternal smoking with reduced semen quality and testis size in adulthood: A cross-sectional study of 1,770 young men from the general population in five European countries. Am. J. Epidemiol. 159, 49–58. [DOI] [PubMed] [Google Scholar]
- Lambrot R., Coffigny H., Pairault C., Donnadieu A. C., Frydman R., Habert R., Rouiller-Fabre V. (2006). Use of organ culture to study the human fetal testis development: effect of retinoic acid. J. Clin. Endocrinol. Metab. 91, 2696–2703. [DOI] [PubMed] [Google Scholar]
- Lim J., Lawson G. W., Nakamura B. N., Ortiz L., Hur J. A., Kavanagh T. J., Luderer U. (2013). Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. Cancer Res. 73, 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Nakamura B. N., Mohar I., Kavanagh T. J., Luderer U. (2015). Glutamate cysteine ligase modifier subunit (Gclm) null mice have increased ovarian oxidative stress and accelerated age-related ovarian failure. Endocrinology 156, 3329–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livera G., Delbes G., Pairault C., Rouiller-Fabre V., Habert R. (2006). Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res. 324, 507–521. [DOI] [PubMed] [Google Scholar]
- Lodovici M., Akpan V., Evangelisti C., Dolara P. (2004). Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J. Appl. Toxicol. 24, 277–281. [DOI] [PubMed] [Google Scholar]
- MacKenzie K. M., Angevine D. M. (1981). Infertility in mice exposed in utero to benzo(a)pyrene. Biol. Reprod. 24, 183–191. [DOI] [PubMed] [Google Scholar]
- Matikainen T., Moriyama T., Morita Y., Perez G. I., Korsmayer S. J., Sherr D. H., Tilly J. L. (2002). Ligand activation of the fetal aromatic hydrocarbon receptor transcription factor drives bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology 143, 615–620. [DOI] [PubMed] [Google Scholar]
- Matikainen T., Perez G. I., Jurisicova A., Pru J. K., Schlezinger J. J., Ryu H. Y., Laine J., Sakai T., Korsmeyer S. J., Casper R. F., et al. (2001a). Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 28, 355–360. [DOI] [PubMed] [Google Scholar]
- Matikainen T., Perez G. I., Zheng T. S., Kluzak T. R., Rueda B. R., Flavell R. A., Tilly J. L. (2001b). Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology 142, 2468–2479. [DOI] [PubMed] [Google Scholar]
- Menke D. B., Koubova J., Page D. C. (2003). Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 262, 303–312. [DOI] [PubMed] [Google Scholar]
- Menzie C. A., Potocki B. B., Santodonato J. (1992). Ambient concentrations and exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 26, 1278–1284. [Google Scholar]
- Miller M. S., Juchau M. R., Guengerich F. P., Nebert D. W., Raucy J. L. (1996). Drug metabolic enzymes in developmental toxicology. Fundam. Appl. Toxicol. 34, 165–175. [DOI] [PubMed] [Google Scholar]
- Nakamura B. N., Mohar I., Lawson G. W., Hoang Y. D., Cortés M. M., Ortiz L., Patel R., Rau B. R., McConnachie L., Kavanagh T. J., et al. (2012). Increased sensitivity to testicular toxicity of transplacental benzo[a]pyrene exposure in male glutamate cysteine ligase modifier subunit knockout (Gclm-/-) mice. Toxicol. Sci. 126, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. S., Zhu J., Foster W. G. (2008). Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 25, 100–106. [DOI] [PubMed] [Google Scholar]
- Neal M. S., Zhu J., Holloway A. C., Foster W. G. (2007). Follicle growth is inhibited by benzo[a]pyrene, at concentrations representative of human exposure, in an isolated rat follicle culture assay. Hum. Reprod. 22, 961–967. [DOI] [PubMed] [Google Scholar]
- NRC (1996). Guide for the Care and Use of Laboratory Animals. National Research Council, National Academy of Sciences, Washington, DC. [Google Scholar]
- Penning T. M. (2004). Aldo-keto reductases and formation of polycyclic aromatic hydrocarbon o-quinones. Methods Enzymol. 378, 31–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning T. M., Ohnishi S. T., Ohnishi T., Harvey R. G. (1996). Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem. Res. Toxicol. 9, 84–92. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen C. H., Thulstrup A. M., Storgaard L., Toft G J. O., Bonde J. P. (2007). Is prenatal exposure to tobacco smoking a cause of poor semen quality? Am. J. Epidemiol. 165, 1372–1379. [DOI] [PubMed] [Google Scholar]
- Rich K. J., Boobis A. R. (1997). Expression and inducibility of P450 enzymes during liver ontogeny. Microsc. Res. Tech. 39, 424–435. [DOI] [PubMed] [Google Scholar]
- Robles R., Morita Y., Mann K. K., Perez G. I., Yang S., Matikainen T., Sherr D. H., Tilly J. L. (2000). The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology 141, 450–453. [DOI] [PubMed] [Google Scholar]
- Rodrigues P., Limback D., McGinnis L. K., Plancha C. E., Albertini D. F. (2009). Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction 137, 709–720. [DOI] [PubMed] [Google Scholar]
- Rucker E. B., III, Dierisseau P., Wagner K. U., Garrett L., Wynshaw-Boris A., Flaws J. A., Hennighausen L. (2000). Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol. Endocrinol. 14, 1038–1052. [DOI] [PubMed] [Google Scholar]
- Scholzen T., Gerdes J. (2000). The Ki-67 protein: From the known to the unknown. J. Cell. Physiol. 182, 311–322. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Nakatsuru Y., Ichinose M., Takahashi Y., Kume H., Mimura J., Fujii-Kuriyama Y., Ishikawa T. (2000). Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 97, 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shopland D. R., Burns D. M., Benowitz N. L., Amacher R. H. (2001). Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine In Smoking and Tobacco Control Monographs, Vol. 13, pp. 1–236. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute. [Google Scholar]
- Shum S., Jensen N. M., Nebert D. W. (1979). The murine Ah locus: In utero toxicity and teratogenesis associated with genetic differences in benzo[a]pyrene metabolism. Teratology 20, 365–376. [DOI] [PubMed] [Google Scholar]
- Stolpmann K., Brinkmann J., Salzmann S., Genkinger D., Fritsche E., Hutzler C., Wajant H., Luch A., Henkler F. (2012). Activation of the aryl hydrocarbon receptor sensitises human keratinocytes for CD95L- and TRAIL-induced apoptosis. Cell Death Dis. 3, e388.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohsnitter W. C., Hatch E. E., Hyer M., Troisi R., Kaufman R. H., Robboy S. J., Palmer J. R., Titus-Ernstoff L., Anderson D., Hoover R. N., et al. (2008). The association between in utero cigarette smoke exposure and age at menopause. Am. J. Epidemiol. 167, 727–733. [DOI] [PubMed] [Google Scholar]
- Turusov V. S., Nikonova T. V., Parfenov Yu D. (1990). Increased multiplicity of lung adenomas in five generations of mice treated with benz(a)pyrene when pregnant. Cancer Lett. 55, 227–231. [DOI] [PubMed] [Google Scholar]
- Tuttle A. M., Stampfli M., Foster W. G. (2009). Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum. Reprod. 24, 1452–1459. [DOI] [PubMed] [Google Scholar]
- Weinberg C. R., Wilcox A. J., Baird D. D. (1989). Reduced fecundability in women with prenatal exposure to cigarette smoke. Am. J. Epidemiol. 129, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Wells P. G., McCallum G. P., Chen C. S., Henderson J. T., Lee C. J. J., Perstin J., Preston T. J., Wiley M. J., Wong A. W. (2009). Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol. Sci. 108, 4–18. [DOI] [PubMed] [Google Scholar]
- Western P. S., Miles D. C., van den Bergen J. A., Burton M., Sinclair A. H. (2008). Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 26, 339–347. [DOI] [PubMed] [Google Scholar]
- Wilson C. L., Safe S. (1998). Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses. Toxicol. Pathol. 26, 657–671. [DOI] [PubMed] [Google Scholar]
- Wislocki P. G., Bagan E. S., Lu A. Y., Dooley K. L., Fu P. P., Han-Hsu H., Beland F. A., Kadlubar F. F. (1986). Tumorigenicity of nitrated derivatives of pyrene, benz[a]anthracene, chrysene and benzo[a]pyrene in the newborn mouse assay. Carcinogenesis 7, 1317–1322. [DOI] [PubMed] [Google Scholar]
- Xue W., Warshawsky D. (2005). Metabolic activation of polycyclic aromatic hydrocarbon and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 206, 73–93. [DOI] [PubMed] [Google Scholar]
- Zeiss C. J. (2003). The apoptosis-necrosis continuum: Insights from genetically altered mice. Vet. Pathol. 40, 481–495. [DOI] [PubMed] [Google Scholar]
- Zhan S., Zhang X., Cao S., Huang J. (2015). Benzo(a)pyrene disrupts mouse preimplantation embryo development. Fertil. Steril. 103, 815–825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.