Abstract
Nanoparticle (NP) association with macromolecules in a physiological environment forms a biocorona (BC), which alters NP distribution, activity, and toxicity. While BC formation is dependent on NP physicochemical properties, little information exists on the influence of the physiological environment. Obese individuals and those with cardiovascular disease exist with altered serum chemistry, which is expected to influence BC formation and NP toxicity. We hypothesize that a BC formed on NPs following incubation in hyperlipidemic serum will result in altered NP–BC protein content, cellular association, and toxicity compared to normal serum conditions. We utilized Fe3O4 NPs, which are being developed as MRI contrast and tumor targeting agents to test our hypothesis. We used rat aortic endothelial cells (RAECs) within a dynamic flow in vitro exposure system to more accurately depict the in vivo environment. A BC was formed on 20nm PVP-suspended Fe3O4 NPs following incubation in water, 10% normal or hyperlipidemic rat serum. Addition of BCs resulted in increased hydrodynamic size and decreased surface charge. More cholesterol associated with Fe3O4 NPs after incubation in hyperlipidemic as compared with normal serum. Using quantitative proteomics, we identified unique differences in BC protein components between the 2 serum types. Under flow conditions, formation of a BC from both serum types reduced RAECs association of Fe3O4 NPs. Addition of BCs was found to exacerbate RAECs inflammatory gene responses to Fe3O4 NPs (Fe3O4-hyperlipidemic > Fe3O4-normal > Fe3O4) including increased expression of IL-6, TNF-α, Cxcl-2, VCAM-1, and ICAM-1. Overall, these findings demonstrate that disease-induced variations in physiological environments have a significant impact NP-BC formation, cellular association, and cell response.
Keywords: nanotoxicology, endothelial cell, hyperlipidemia, iron oxide nanoparticles, darkfield microscopy, proteomics, protein corona.
The rapid growth of nanoparticle (NP)-based consumer products, medical devices, and drug delivery platforms in the last decade has significantly increased the risk of human exposure to nanomaterials (Dobrucki et al., 2015; Fan et al., 2015; Inturi et al., 2015; Ryan and Brayden, 2014). Many of the nanotherapeutics being developed will utilize intravenous delivery thereby leading to direct vascular exposure. However, it is becoming recognized that exposure to NPs in animal models induces a variety of vascular effects including exacerbation of atherosclerotic plaque formation, vascular inflammation, oxidative stress, and modified vascular reactivity (Gojova et al., 2009; Han et al., 2013; Mills et al., 2011; Rosas-Hernandez et al., 2009; Vesterdal et al., 2012; Wingard et al., 2011). In general, cardiovascular toxicity is of concern regarding the development of NPs and other pharmaceuticals as it is often difficult to identify in early toxicity screens and represents a major cause of new drugs failing phase II and III clinical studies. The improvement and development of in vitro approaches to screen NPs for cardiovascular toxicity prior to in vivo testing is, therefore, needed. A key to this improvement is the necessity that these in vitro approaches accurately depict the in vivo environment.
A large and growing portion of the population currently exists with underlying disease states. These underlying disease states, such as cardiopulmonary diseases, can modify how individuals respond to exposures resulting in exacerbated responses (Elder et al., 2004; Schwartz and Dockery, 1992; Schwartz and Morris, 1995). Hyperlipidemia is a significant underlying disease that is common in a significant portion of the U.S. population. Despite many common underlying disease states, such as hyperlipidemia within the population, the majority of studies assessing NP-induced toxicity are performed in healthy animal models and utilize in vitro models representing healthy physiological conditions. These healthy scenarios often do not represent the population in which nano-based therapeutics and diagnostic tools will be utilized. Many diseases such as hyperlipidemia result in altered expression of circulating macromolecules such as proteins, peptides, and lipids (Catalano et al., 1991; LaFramboise et al., 2012). Once NPs are introduced into a physiological environment they are rapidly coated with macromolecules forming a biocorona (BC) (Lundqvist et al., 2011; Monopoli et al., 2012). The addition of BC alters the interactive interface of the NPs and can thereby result in modified NP clearance, distribution, activity, and toxicity (Beduneau et al., 2009; Clift et al., 2010; Lartigue et al., 2012; Maiorano et al., 2010). Variations in the BC resulting from using dissimilar in vitro methods and the use of conditions that do not account for diseased physiological environments, which could modify serum content and ultimately BC, may explain many existing inconsistencies between in vitro toxicity screening and in vivo studies of NP safety (Joris et al., 2013). The formation of the BC is governed both by the physicochemical properties of the NP and by the physiological environment (Jedlovszky-Hajdu et al., 2012; Monopoli et al., 2011; Shannahan et al., 2013a; Shannahan et al., 2013b; Walkey and Chan, 2012; Walkey et al., 2014). To date, the examination of the toxicological impact of the BC has been performed only assessing the physicochemical properties of the NPs while ignoring the role of the physiological environment and disease related changes in the biological milieu.
Magnetite iron oxide (Fe3O4) NPs are of considerable interest for their biomedical applications, particularly for their use as MRI contrast agents due to their superparamagnetic properties (Babes et al., 1999). Furthermore, their surfaces are easily modified to allow for targeting of particular tissues, specifically for tumor targeting (Gupta and Gupta, 2005). Due to their emerging use in the biomedical field, evaluation of their toxicity is needed particularly in individuals with underlying disease states that may influence the formation of the BC. Additionally, the BC has been shown to influence the function of Fe3O4 NPs by interfering with their contrast abilities when used for MRI (Amiri et al., 2013). It is unclear how differential BC formation on the surface of Fe3O4 NPs due to disease-induced alterations in serum content may affect functionality and toxicity.
In our current study, we evaluated the formation of the BC on Fe3O4 NPs using normal/healthy and lipid-rich rat serum. This evaluation assessed changes in NP physicochemical properties due to the addition of varying BCs as well as utilized a proteomic approach to identify differences in the protein content of the BCs. Finally, we assessed the toxicological implications of these BCs on Fe3O4 NPs including cellular association, cytotoxicity, and cell activation. This toxicological assessment was performed in rat aortic endothelial cells (RAECs) and exposures occurred under flow conditions to more accurately depict the in vivo environment. Overall, this study enhances our understanding of nanotoxicity, as well as, the development of safe nano-therapeutics utilizing relevant exposure conditions, which more accurately portrays the in vivo environment.
MATERIALS AND METHODS
Isolation of normal and hyperlipidemic rat serum
Serum samples were kindly provided by Dr. Urmila Kodavanti at the U.S. Environmental Protection Agency. Briefly, healthy male Sprague Dawley rats (Charles River Laboratories Inc, Raleigh, North Carolina) received water ad libitum and were fed either a normal diet of standard (5001) Purina rat chow (Brentwood, Missouri) or a diet high in fructose (60%) (TD89247, Harlan Laboratories, Madison, Wisconsin). All rats were maintained in an isolated animal room in an Association for Assessment and Accreditation of Laboratory Care (AALAC) approved animal facility 21 ± 1° C, 50 ± 5% relative humidity, and 12 h light/dark cycle. Following 2 weeks on the diets animals were necropsied and blood was collected without anticoagulant present via abdominal aortic puncture. Following processing of serum, samples were pooled and aliquoted to be stored at −80 °C. Serum from rats fed a normal diet (normal serum) demonstrated a cholesterol content of 122.27 mg/dl whereas serum from rats fed a high fructose diet (lipid serum) demonstrated a cholesterol content of 253.11 mg/dl. The U.S. EPA NHEERL Institutional Animal Care and Use Committee (IACUC) approved the protocol. The animals were treated humanely and with regard for alleviation of suffering.
Formation of Fe3O4 nanoparticle–biocorona
Spherical 20 nm Fe3O4 NPs suspended in PVP at a concentration of 20 mg/ml were purchased from Nanocomposix (San Diego, California). Thermal gravimetric analysis showed that dried Fe3O4 NPs were 55–57% weight of Fe suggesting that the remaining 43–45% weight of the sample is composed of PVP in accordance with the data provided by the manufacturer (Nanocomposix). PVP coating of Fe3O4 NPs was further confirmed via Fourier transform infrared (FTIR) spectroscopy measurements (Supplemental Figure 1). BCs were formed on Fe3O4 NPs as described in our recent publications (Shannahan et al., 2013a,b,2015b,c). Fe3O4 NPs were diluted in water to a concentration of 1 mg/ml incubated for 8 h at 4 °C in distilled (DI) water (control), 10% normal serum, or 10% lipid-rich serum while being mixed constantly. Specifically, 250 μl of Fe3O4 NP (1 mg/ml), 650 μl of water, and 100 μl of serum were combined in a 1.5 ml tube. Following incubation Fe3O4 NPs were then pelleted via centrifugation 14 000 rpm (20 817 × g) 10 min and washed with PBS. Following centrifugation, the supernatant was removed and Fe3O4 NPs were resuspended with 250 μl of water to its initial concentration of 1 mg/ml. The BC formed following incubation of Fe3O4 NPs in normal serum is referred to as Fe3O4 NP – normal BC whereas the BC form following incubation in lipid-rich serum is referred to as Fe3O4 NP – lipid BC.
Characterization of Fe3O4 nanoparticle–biocorona
The hydrodynamic size and zeta potentials (ZetaSizer Nano, Malvern) of uncoated Fe3O4 NPs, and Fe3O4 NPs with BCs were characterized in DI water with Fe3O4 NPs at a concentration of 50 μg/ml (n = 3/particle). Cholesterol content of the BCs formed on the surface of Fe3O4 NPs was evaluated via a commercially available colorimetric total cholesterol assay kit (Cell Biolabs, San Diego, CA) following instructions of the manufacturer. Briefly, 50 μl of Fe3O4 NPs without or with BCs were loaded into a 96-well plate in duplicate and then were treated with a cholesterol reaction reagent that includes cholesterol esterase, cholesterol oxidase, and a colorimetric probe. The cholesterol esterase causes the hydrolysis of cholesterol esters from the cholesterol bound to the Fe3O4 NPs allowing for their determination. Finally, the pH of the Fe3O4 NPs solution was not altered following addition of the different biocoronas.
Transmission electron microscopy
Transmission electron microscopy images were obtained using a Hitachi H7600 microscope. For TEM studies, Fe3O4 NPs, Fe3O4 NP – normal BC and Fe3O4 NP – lipid BC samples were stained by incubating them in 0.1% OsO4 solution for 30 min. The samples were washed with double-distilled water to remove unbound OsO4 after which the samples were drop casted on a 400 mesh Cu grid and dried overnight. Z-contrast images were also acquired using Hitachi HD2000 microscope without staining the samples. The high Z-contrast between Fe3O4 NPs and BC allowed us to distinguish the 2 phases (i.e. NPs and BC).
Evaluation of protein components of Fe3O4 NP–biocorona
Utilizing a proteomics approach, individual protein components of the BCs were identified similar to our previous studies evaluating the BC (Shannahan et al., 2013a,b). Briefly, following the addition of BCs, Fe3O4 NPs were washed and pelleted 3 times with PBS (10 min 14 000 rpm/20 817 × g). Proteins were then solubilized using 8 M urea and 10 mM dithiothreitol (DTT) for 45 min at 60 °C. Fe3O4 NPs were then pelleted via centrifugation at 15 000 × g for 10 min and supernatant containing solubilized proteins was collected. Protein samples were then treated with 30 mM iodoacetamide for 30 min in the dark at room temp followed by the addition of 10 mM DTT in 50 mM ammonium bicarbonate (ABC) for 30 min at room temperature. Finally, 50 mM ABC was used to dilute remaining urea to less than 1 M. Samples were then proteolyzed using porcine trypsin (0.2 ng/μl) overnight at 37 °C, heated to 90 °C to deactivate trypsin, and concentrated to dryness. Samples were then resuspended in 0.1% trifluoroacetic acid in water, purified via Ziptip Pipette Tips (Millipore, Billerica, Massachusetts), and concentrated to dryness. Samples were then resuspended in 0.1% Formic Acid in HPLC-grade water and injected onto a nHPLC utilizing a flow rate of 800 nL/min with a gradient of 5–50% 0.1% formic acid in acetonitrile (ACN) over 30 min on C18 trapping (20×0.1 mm2) and analytical columns (150×0.1 mm2). The nLC was coupled to a nano-ESI source and Impact HD Q-TOF mass spectrometer (Bruker Daltonics, Inc., Billerica, MA). The acquired data were searched using a MASCOT (ver. 2.4.1) search engine with a peptide cutoff of 10 ppm and a minimum peptide score of 20 and a protein score of 40. The false discovery rate for samples was < 1% and the significance threshold was P < .05. To determine differences in common protein components of the BCs, ratios of individual proteins were assessed utilizing ProteinScape and ProfileAnalysis software packages (Bruker Daltonic, Billerica, Massachusetts). Four samples of each BC were analyzed individually and injected 4 separate times. Peptides to be considered for evaluation had to be found within 5 of 8 samples with a minimum of 2 per BC type. Only peptides determined to be statistically significant were utilized to calculate ratios and more than one peptide had to be identified for a protein for that individual protein to be quantified.
Endothelial cell culture
RAECs were cultured in rat endothelial cell growth media (Cell Applications Inc., San Diego, CA). Cells were maintained in flasks under standard conditions at 37 °C and 5% CO2. For the assessment of the BC and its role in cellular responses, all experiments were performed utilizing serum-free media as done in our previous experiments (Shannahan et al., 2015b,c). The removal of serum from media allows for the evaluation of the intentionally formed BC without the addition of a secondary BC within the cell culture system. The use of serum-free media limits the study of the BCs and the cellular responses to acute time points.
Assessment of Fe3O4 NP cytotoxicity
RAECs were grown to 90% confluency in 96 well plates (Costar) and were exposed to increasing concentrations of Fe3O4 NPs (0, 2.5, 5, 10, 20, 40, and 80 μg/ml) with or without BCs for 2 h and 6 h in serum-free media in a static system. Cells were exposed in this static system due the high number of concentrations evaluated and to screen for overt cytotoxicity. Due to the use of serum-free media, later time points were not evaluated. The concentrations of Fe3O4 NPs utilized were selected based on the possible human exposures as well as previous in vitro examination of Fe3O4 NPs and other NPs (Shannahan et al., 2015b,c,d; Xia et al., 2013). Briefly, concentrations of Fe3O4 NPs were determined based on the levels used for human MRI analysis utilizing Fe3O4 NPs as a contrast agent, which typically range from 5 to 10 μg/ml blood volume, considering a male of 90 kg (Fukuda et al., 2006; Wang, 2011). Changes in cell viability were assessed via the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxmethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, Wisconsin) via instructions of the manufacturer using a spectrophotometer (BioTek Synergy HT, BioTek, Winooski, Vermont). No overt cytotoxicity was identified, therefore, a concentration of 20 μg/ml was utilized for all subsequent experimentation of RAEC association and activation (Supplementary Figure 3). This concentration of 20 μg/ml was also selected due to it being within the range of a human relevant concentration.
Cellular association of Fe3O4 NPs
In order to more accurately depict the in vivo environment, RAEC association of Fe3O4 NPs was assessed utilizing a Quasi Vivo system (Kirkstall, Rotherham, UK) that pumps media over the surface of the cells. RAECs were grown on coverslips and placed into an individual chamber. Serum-free media (control), serum-free media containing Fe3O4 NPs – normal BC, or serum-free media contain Fe3O4 NPs – lipid BC at a concentration of 20 μg/ml was loaded into the reservoir. A flow rate of 1 ml/min of fluid was utilized during the 2 h exposure. The NP concentration and time point were selected for the evaluation of cellular association based on the previous research examining the association of other NPs by RAECs (Shannahan et al., 2015b) and possible human blood concentrations of Fe3O4 NPs when utilized as an MRI contrast agent. The flow rate of 1 ml/min was estimated to result in a shear stress of 100 × 10−3 Pa. The Quasi Vivo system as manufactured can produce a shear stress of 2 × 10−5 Pa at a flow rate of 1 ml/min (Mazzei et al., 2010), however, by inserting silicone glue into the base of the chamber thereby raising the cells nearer to the inlet of media a higher shear stress can be achieved. For comparison, a shear stress of 1.5–2.0 Pa occurs in human arteries while the higher diameter post-capillary venules have a shear stress at 300 × 10−3 Pa (Grabinski et al., 2015; Koutsiaris et al., 2007). A shear stress of 100 × 10−3 Pa is below the necessary 200 × 10−3 – 5 Pa need to induce cell alignment and tight junctions in vitro (Seebach et al., 2000). Therefore, this shear stress utilized in this study will not induce biological responses by itself. Following exposure, coverslips were removed from the chamber and placed into a 24 well plate. Cells were then detached with 250 μl of trypsin and neutralized with an equal volume of RAEC media. Cells were then washed 3 times with PBS and differences in cellular association were determined by ICP-MS analysis of Fe content. These washes were performed to remove non-cell associated Fe3O4 NPs. All samples were dissolved in 6 ml of 2% HNO3. The Fe concentration was determined with ICP-MS (X series II, Thermo Scientific) using an internal standard containing Li, Y, and In with a detection limit of 3 ppb. Qualitative assessment of Fe3O4 NP uptake and cellular association was performed by darkfield microscopy (Cytoviva, Auburn, Alabama). RAECs underwent the same exposure protocol, were fixed with 2% paraformaldehyde, and had their nucleuses stained with DAPI. Focusing on the nucleus allowed for the assessment of internalized Fe3O4 NPs. A mean spectral profile of Fe3O4 NPs was produced via hyperspectral microscopy (Cytoviva, Auburn, Alabama) and then this spectral profile was used to map Fe3O4 NPs against exposed RAECs to identify cell associated Fe3O4 NPs.
Fe3O4 NP – induced cellular activation
RAEC activation by Fe3O4 NPs without or with BCs was assessed utilizing a Quasi Vivo system (Kirkstall, Rotherham, UK) at a flow rate of 1 ml/min of fluid. RAECs were grown on coverslips and placed into an individual chamber. Serum-free media (control), serum-free media containing Fe3O4 NPs – normal BC, or serum-free media contain Fe3O4 NPs – lipid BC at a concentration of 20 μg/ml was loaded into the reservoir and cells were exposed for 1 h. Total RNA was isolated from cells using Direct-zol RNA MiniPrep (Zymo Research Corp., Irvine, California) via instructions of the manufacturer and quantified via nanodrop (Nanodrop 2000c Spectrophotometer, Thermo Scientific). Total RNA was reverse transcribed to cDNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California). A rat endothelial cell biology PCR array was utilized to identify markers of endothelial activation following exposure to Fe3O4 NPs without or with BCs (RT2 Profiler PCR Array Systems, Cat# PARN-015Z, SABiosciences; Frederick, MD). This PCR array identified 84 genes related to angiogenesis, vasoconstriction/dilation, inflammatory response, apoptosis, cell adhesion, coagulation, and platelet activation. Plates were prepared according to instructions of the manufacturer and using a StepOnePlue Real-Time PCR System (ABI, Foster City, California). Data analysis on SaBiosciences web-based PCR array expression analysis suite utilized the ΔΔCt method with normalization to β-actin (housekeeping gene) and considering serum-free media exposed cells as controls. Experiments were performed in triplicate and data from all genes is available within Supplementary Table 2.
To confirm PCR array results mRNA expression of VCAM-1 was assessed. RAECs were grown in 24 well plates and exposed to 20 μg/ml of Fe3O4 NPs without or with BCs in static conditions. In addition, RAECs were exposed to control serum samples that had undergone BC processing absent of Fe3O4 NPs. These samples were created by adding 100 μl of serum to 900 μl of water without NPs present and processing them as before. These samples were added to insure that free protein was not being carried over and resulting in cell activation. mRNA was isolated and cDNA synthesized as stated previously. Quantitative real-time PCR was performed for VCAM-1 and β-actin (control) using SsoAdvancedTM SYBR Green Supermix (Bio-Rad) and QuantiTect primer assays (Qiagen, Balencia, California). Relative mRNA fold changes were calculated considering serum-free media exposed cells as control and normalized to β-actin as the internal reference.
VCAM-1 expression
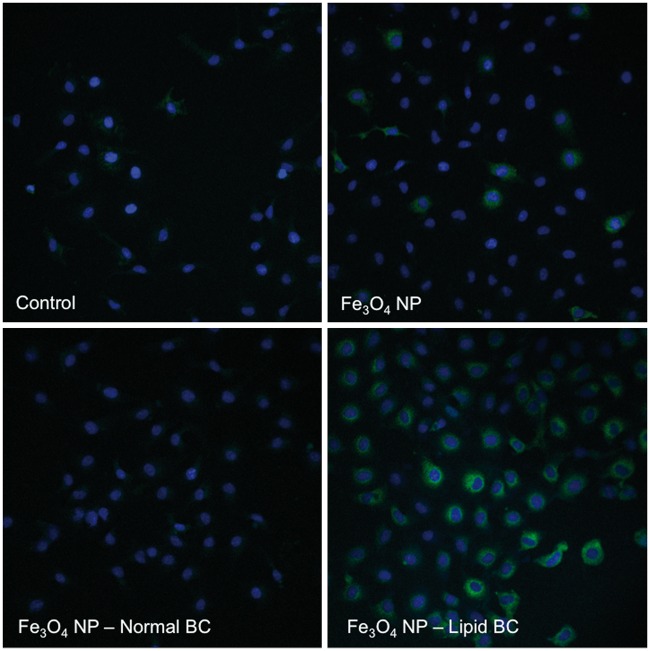
RAEC surface expression of VCAM-1 was evaluated following a 1 h exposure under flow conditions (1 ml/min) to 20 μg/ml of Fe3O4 NPs without or with BCs by fluorescent microscopy. Following exposure, coverslips were removed from the exposure chambers, washed with PBS and fixed with 2% paraformaldehyde. After undergoing a PBS wash cells were incubated for 30 min with a VCAM-1 antibody (1:200) (Cat# 13-1060-81, eBioscience, San Diego, California) followed by the incubation for 30 min with FITC-labeled streptavidin (1:200) (Cat# 11-4317-87, eBioscience) at room temperature. Cells were then washed with PBS and allowed to dry before being stained with DAPI to stain the nucleus and coverslipped. Alterations in cell surface expression of VCAM-1 were then qualitatively examined by fluorescent microscopy (Nikon Eclipse TE 2000-E, Tokoyo, Japan). Nuclei were visualized as blue whereas VCAM-1 was visualized as green.
Statistical analysis
All data are presented as mean ± SEM consist of 3–6 experiments. Data were analyzed by one-way ANOVA, with differences between groups assessed by Tukey’s post hoc tests. All graphs and analysis were performed using GraphPad Prism 6 software (GraphPad, San Diego, California). Statistical significance was determined when P was found to be less than or equal to .05 between groups.
RESULTS
Characterization of Fe3O4 Nanoparticle Biocorona
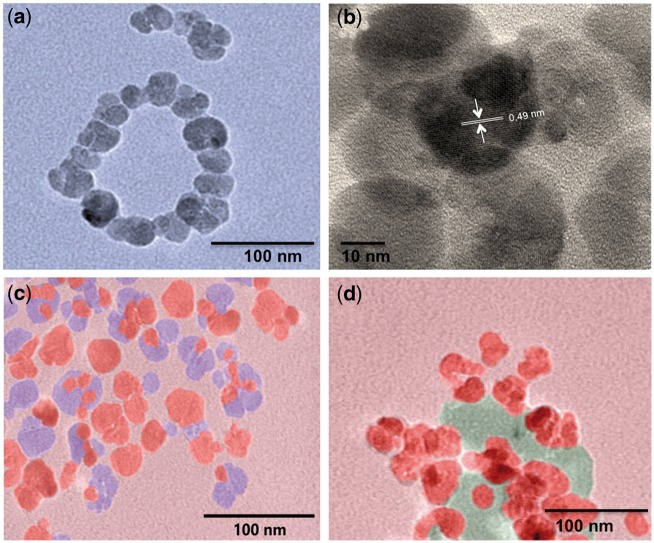
Following incubation of Fe3O4 NPs in 10% normal or lipid-rich serum and formation of the biocorona (BC), hydrodynamic size and zeta potential were assessed (Table 1 and Supplementary Figure 2). Formation of either BC slightly increased the hydrodynamic size of the Fe3O4 NPs (Table 1, Supplementary Figure 2). Evaluation of polydispersity index demonstrated a stable suspension of Fe3O4 NPs with slight agglomeration (Table 1). However, the formation of both the normal BC and lipid BC on Fe3O4 NP resulted in a significant decrease in zeta potential (Table 1). High-resolution TEM measurements revealed Fe3O4 NPs to have a mean diameter of 25 ± 2.6 nm (Figure 1A and Supplementary Figure 3). This is significantly smaller than the hydrodynamic size (>100 nm) suggesting that there may be some agglomeration of Fe3O4 NPs without a BC in solution. High-resolution TEM suggests agglomerates of approximately 3–5 Fe3O4 NPs with an interlayer spacing of 0.49 nm (Figure 1B). Additionally, TEM images showed that the Fe3O4 NPs are not completely spherical (Figure 1A and B). We found that both Fe3O4 – normal BC and Fe3O4 – lipid BC contained Fe3O4 NPs embedded within the BC (Figure 1C and D). Interestingly, we also found the presence some liposomes in Fe3O4 – lipid BC, which were confirmed by OsO4 staining and Z-contrast studies.
TABLE 1.
Fe3O4 NP-Biocorona Characterization (n = 3)
| Nanoparticle | Hydrodynamic Size (nm) | Polydispersity Index | Zeta Potential (mV) | Cholesterol Content (μM) |
|---|---|---|---|---|
| Fe3O4 | 103.0 ± 2.3 | 0.30 ± 0.04 | −34.1 ± 0.6 | – |
| Fe3O4 – normal BC | 117.3 ± 5.5 | 0.35 ± 0.09 | −24.1 ± 0.4* | 7.2 ± 0.3 |
| Fe3O4 – lipid BC | 107.4 ± 5.5 | 0.29 ± 0.03 | −23.0 ± 1.4* | 62.2 ± 13.1# |
*Statistical difference from Fe3O4 without BC (P ≤ .05).
#Statistical difference from Fe3O4 – normal BC (P ≤ .05).
FIG. 1.
False-colored transmission electron micrographs for (a) Fe3O4 NPs without a BC, (b) high-resolution micrograph of Fe3O4 NPs without a BC showing interlayer spacing of ∼0.49 nm matching [111] plane, (c) Fe3O4 – normal BC, and (d) Fe3O4 – lipid BC. Biocorona is shown in blue (c) and green colors (d). The NPs were observed to be embedded in the BCs. Please see online version to view figure in color.
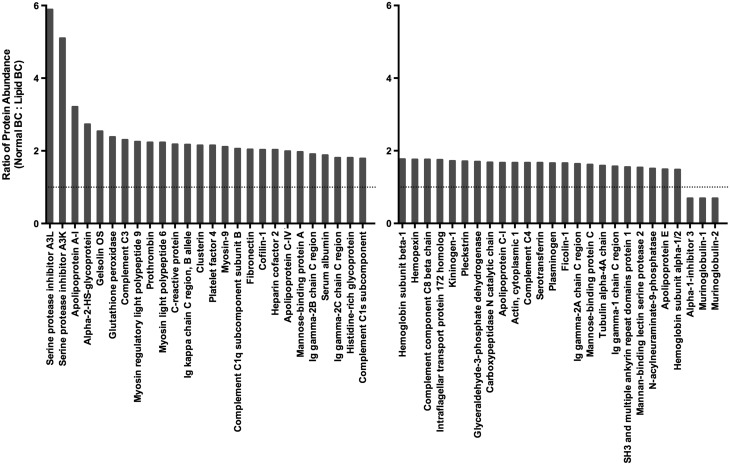
Variations in the cholesterol content and protein components of the BCs formed on Fe3O4 NPs in the differing physiological environments were evaluated. Assessment of the cholesterol content of each BC demonstrated cholesterol as a component of both BCs (Table 1). As expected, the lipid BC had a greater amount of cholesterol associated with it compared with the normal BC (Table 1). Label-free quantitative proteomics was utilized to evaluate differences in protein components of the BCs. The BCs on Fe3O4 NPs incubated in either normal or lipid-rich serum were found to consist of 103 or 121 individual proteins (Figure 2). In common, between the 2 BCs were 92 proteins including complement C3, prothrombin, serum albumin, serotransferrin, alpha-2-HS glycoprotein, apolipoprotein A-II, apolipoprotein E, and apolipoprotein C-I (Figure 1 and Supplementary Table 1). Fe3O4 NP with a normal serum BC were determined to bind 11 unique proteins when compared to Fe3O4 NP with a lipid-rich BC (Figure 2). These 11 unique proteins included superoxide dismutase, T-kininogen, and tropomyosin alpha-3 chain (Supplementary Table 1). Fe3O4 NP with a lipid-rich BC were found to bind 29 unique proteins when compared to Fe3O4 NP with a normal BC (Figure 1). These 29 unique proteins were composed of alpha-1 antiproteinase, fibrinogen alpha chain, metalloproteinase inhibitor 3, and heparanase (Supplementary Table 2). A complete list of all proteins found to associate with Fe3O4 NP following incubation in the different physiological environments is found in Supplementary Table 1. Of the 92 proteins found to associate with Fe3O4 NPs in common following incubation in both normal and hyperlipidemic serum, relative quantitation was possible on 56 (Figure 3). For each protein, multiple peptides of sufficient abundance are required for quantitation. Therefore, only 60% of identified proteins were quantifiable. In general, more of these common proteins were found to associate with Fe3O4 NPs following incubation in normal serum compared with hyperlipidemic serum (Figure 3). Of these, commonly associated proteins only alpha-1-inhibitor 3, murinoglobulin-1, and murinoglobulin-2 were found to be more abundant in Fe3O4 NPs incubated in hyperlipidemic serum compared with normal serum (Figure 3).
FIG. 2.
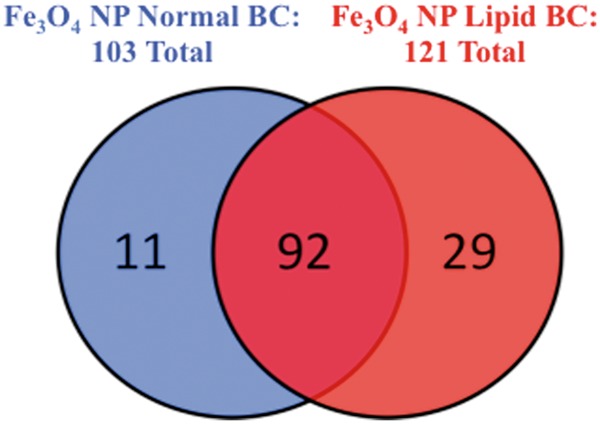
Venn diagram representing the distribution of protein components found to associate with 20 nm Fe3O4 NPs following incubation in 10% normal (blue) or hyperlipidemic (red) serum. Please see online version to view figure in color.
FIG. 3.
Ratios of common protein components found to associate with 20 nm Fe3O4 NPs following incubation in both 10% normal or hyperlipidemic serum. Data are expressed as ratio of protein abundance in normal serum BC compared with hyperlidemic BC.
Influence of Biocorona on Fe3O4 nanoparticle-Induced Endothelial Cell Cytotoxicity and Cellular Association
To determine possible differences in cytotoxicity due to the addition of BCs, RAECs were exposed to Fe3O4 NPs without or with BCs at concentrations of 2.5, 5, 10, 20, 40, or 80 μg/ml for 2 h or 6 h and examined for differences in viability. This initial assessment of cytotoxicity was performed in a static cell culture system due to the number of concentrations examined. Due to the removal of serum from the cell culture media, only acute time points were evaluated. None of the concentrations utilized were found to induce significant cytotoxicity (Supplementary Figure 4). Furthermore, addition of BCs was not found to alter cytotoxicity (Supplementary Figure 4). Based on these data, previous work evaluating NP toxicity in RAEC, and relevant human concentrations a concentration of 20 μg/ml was utilized for all experimentation investigating disease-induced BC toxicity (Shannahan et al., 2015b; Fukuda et al., 2006; Wang, 2011).
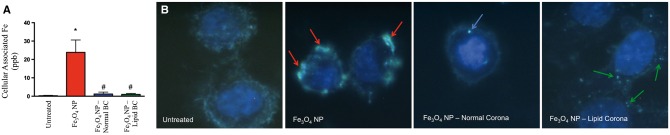
BC-induced alterations in RAEC association were evaluated by ICP-MS and darkfield microscopy (Figure 4). RAECs were exposed to 20 μg/ml of Fe3O4 NPs without or with BCs for 2 h under a flow rate of 1 ml/min. RAECs were found to readily associate Fe3O4 NPs without a BC; however, addition of both BCs resulted in reduced association as evaluated by ICP-MS (Figure 4A). These results were qualitatively confirmed via darkfield microscopy (Figure 4B). Fe3O4 NPs without a BC were internalized (red arrows) to a greater degree than Fe3O4 NPs with a normal BC (blue arrows) or Fe3O4 NPs with a lipid BC (green arrows) (Figure 4B). Hyperspectral analysis was utilized to produce a mean spectral profile of Fe3O4 NPs (Supplementary Figure 5A) and used to confirm the identity of internalized Fe3O4 NPs within RAECs (red labeled pixels) (Supplementary Figure 5B).
FIG. 4.
Measurement of 20 nm Fe3O4 NP association by ICP-MS and darkfield microscopy. Rat aortic endothelial cells (RAEC) were exposed to serum-free media (control), Fe3O4 NPs without a biocorona (BC), or Fe3O4 NPs with either a normal serum or lipid serum BC. Cells were exposed to a concentration of 20 μg/ml for 2 h under flow conditions of 1 ml/min. (A) Following exposure cells were assessed by ICP-MS for Fe3O4 NP association. Values are expressed as mean ± SEM (n = 3/group). *Significant difference from controls (P < .05). (B) Alterations in RAEC association of Fe3O4 NPs due to addition of BCs were qualitatively evaluated via darkfield microscopy. Fe3O4 NPs without a BC are identified with red arrows, Fe3O4 NP-normal BC with blue arrows, and Fe3O4 NP-lipid BC with green arrows. Please see online version to view figure in color.
Variations in Endothelial Cell Activation Due to Differences in the Fe3O4 Nanoparticle Biocorona
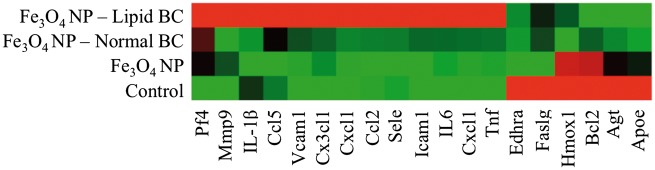
To determine differences in Fe3O4 NP-induced RAEC activation due to addition of the BCs, mRNA gene expression was examined. RAECs were exposed for 1 h to 20 μg/ml of Fe3O4 NPs without or with BCs under a flow rate of 1 ml/min. A PCR array specific for rat endothelial cell biology was utilized which evaluated mRNA expression changes in 85 genes related to a variety of processes. These processes included angiogenesis, vasoconstriction/dilation, inflammatory response, apoptosis, cell adhesion, coagulation, and platelet activation. Alterations in selected genes are depicted in a heat map (Figure 5), while data from 85 genes are included in Supplementary Table 2. In summary, RAECs exposed to Fe3O4 NPs without a BC demonstrated a significant (P ≤ .05) upregulation of 4 genes and downregulation of 10, while cells exposed to Fe3O4 NPs with a normal BC had 7 genes upregulated and 18 downregulated and those exposed to Fe3O4 NPs with a lipid BC had 10 genes upregulated and 8 downregulated (Supplementary Table 2). Exposure to all Fe3O4 NPs (without and with BCs) significantly altered the expression of a subset of genes in common including the upregulation of Cx3cl1, Cxcl1, Selp, and Vcam1 and the downregulation of Ednra, Hif1a, and Sod1 (Supplementary Table 2). Inflammatory genes such as Il-6, Cxcl1, Cxcl2, and Tnf were induced to a greater degree upon RAEC exposure to Fe3O4 NPs with BCs compared with the absence of a BC (Figure 5). The induction of these inflammatory genes was greatest in RAECs exposed to Fe3O4 NPs with a lipid BC. Genes related to cell adhesion such as Vcam1 and Icam1 were more intensely up-regulated when RAECs were exposed to Fe3O4 NPs with a BC as compared to RAECs exposed to Fe3O4 NPs without a BC (Fe3O4 NP lipid BC > Fe3O4 NP normal BC) (Figure 5). Verification of Vcam1 mRNA expression seen in the PCR array through real-time PCR demonstrated similar gene induction (Supplementary Figure 6). Additional controls were added to this experiment to ensure that changes seen in mRNA expression were not related to carry over of free serum proteins (proteins not associated with Fe3O4 NP BCs). These control groups demonstrated no changes in Vcam1 mRNA expression (Supplementary Figure 6). To further assess activation of RAEC by Fe3O4 NPs without or with BCs, cell surface expression of VCAM-1 was evaluated by immunofluorescence (Figure 6). Cells were exposed under flow conditions of 1 ml/min to Fe3O4 NPs without or with BCs (20 μg/ml) for 1 h and stained for VCAM-1 expression. Fe3O4 NPs without a BC and Fe3O4 NPs with a normal BC did not demonstrate differences in VCAM-1 expression compared to controls (Figure 6). Exposure to Fe3O4 NPs with a lipid BC, however, did demonstrate increased cell surface expression of VCAM-1 (Figure 5). This alteration in surface VCAM-1 expression verified results examining mRNA expression.
FIG. 5.
Heat map of selected endothelial cell cytokine and inflammatory genes following exposure to Fe3O4 NPs. Rat aortic endothelial cells (RAECs) were exposed to serum-free media (control), Fe3O4 NPs without a BC or Fe3O4 NPs with either a normal serum or lipid serum BC. Cells were exposed to a concentration of 20 μg/ml for 1 h under flow conditions of 1 ml/min. Red indicates genes that have high expression values, green indicates genes that have low expression values across all groups, and black indicates median expression. Fold change values and P values are included for all 85 assessed genes in Supplementary Table 2. Please see online version to view figure in color.
FIG. 6.
Assessment of Fe3O4 NP-BC alterations in cell surface expression of VCAM-1 by immunofluorescent staining. Rat aortic endothelial cells (RAECs) were exposed to serum-free media (control), Fe3O4 NPs without a BC or Fe3O4 NPs with either a normal serum or lipid serum BC. Cells were exposed to a concentration of 20 μg/ml for 1 h under flow conditions of 1 ml/min. Cells were then immunofluorescently stained with DAPI to identify the nucleus (Blue) and with a VCAM-1 antibody to identify endothelial cell surface expression of VCAM-1 (Green). Please see online version to view figure in color.
DISCUSSION
Due to the rapid production of NPs for an ever-growing number of applications, it is necessary to utilize in vitro toxicity assessments for the evaluation of toxicity. However, these in vitro toxicity assessments of NPs often do not correlate with in vivo studies thus decreasing their usefulness. Therefore, this study utilized a variety of techniques in an attempt to more accurately depict the in vivo environment in an in vitro setting. This study incorporated the use of serum from both normal and hyperlipidemic rats in order to simulate differing human populations. Further we investigated a human relevant exposure to Fe3O4 NPs that are being developed for theranostic applications, such as drug delivery and MRI. To date, the majority of the research investigating formation of the NP–BC has focused on the role of NP properties. In the present study, we addressed the role of disease-induced modifications in serum chemistry, which facilitated differential NP–BC formation resulting in altered biological activity. We also evaluated the responses of endothelial cells, which represent a primary cell type that would encounter NPs through clinical applications utilizing intravenous delivery. To date, many studies have ignored the contribution of the BC in the toxicity evaluation of NPs. Differences in the formation of the BC may explain a significant portion of the variability seen between laboratories examining the same NPs and differing responses observed between in vitro and in vivo studies or across cell types that utilize a variety of media. Finally, we utilized a flow system to expose endothelial cells to NPs under conditions that would occur in vivo. In summary, our study demonstrated that different BCs associate with 20 nm PVP-suspended Fe3O4 NPs depending on serum chemistry (e.g. normal or hyperlipidemic serum). Specifically, the BC that formed under hyperlipidemic conditions consisted of substantially more cholesterol and more unique proteins compared with a normal serum BC. The additions of these different BCs reduced endothelial cell association of Fe3O4 NPs whereas the BC that formed under hyperlipidemic conditions caused an exacerbation of endothelial cell activation and inflammatory responses. Taken together, these findings demonstrate the importance of the physiological environment in the formation of the NP–BC and more importantly its impact on biological responses.
Previously, we have investigated alterations in the protein content of the BC due to NP physicochemical properties (Shannahan et al., 2013a,b). In these studies, we utilized a variety of surface functionalized carbon nanotubes and silver NPs (AgNPs) of differing surface coatings and diameters incubated in fetal bovine serum. In our current study, we focused on one NP, but varied the physiological environment (e.g. normal or hyperlipidemic rat serum) and found similarities and differences in comparison with the BC of our previous studies utilizing fetal bovine serum. Previously, we demonstrated that 20 nm PVP suspended AgNPs associated 48 proteins whereas our current assessment of 20 nm PVP suspended Fe3O4 NPs identified a total of 103 proteins for particles incubated in normal serum and 121 proteins for those incubated in hyperlipidemic serum (Shannahan et al., 2013b). An evaluation of associated proteins that form a BC on AgNPs (20 nm PVP or citrate suspended and 110 nm PVP or citrate suspended) and the Fe3O4 NPs utilized in this study demonstrated commonalities. All were found to associate 5 common proteins apolipoprotein A-I, apolipoprotein A-II, alpha-1-antiproteinase, alpha-2-HS glycoprotein, and serum albumin. Further, our evaluation of the BC on multiwalled carbon nanotubes of various surface modifications demonstrated that these same 5 proteins were also present (Shannahan et al., 2013a). This suggests that these proteins consistently associate with a wide range of NPs when introduced into both fetal bovine and rat serum.
Our study demonstrates that a common underlying disease state, hyperlipidemia, can alter the formation of the BC on Fe3O4 NPs demonstrating the importance of the physiological environment. Specifically, Fe3O4 NPs introduced into this hyperlipidemic environment demonstrated substantially more cholesterol present compared with those in a normal serum environment. In general, quantitative assessment of common protein components of the normal and hyperlipidemic BCs demonstrated that the normal BC had more of these proteins (e.g. fibronectin, albumin, C-reactive protein, complement C3, and apolipoproteins A-I, C1, and E) present compared with the hyperlipidemic BC. It is likely that the abundance of cholesterol present in the hyperlipidemic BC that forms on Fe3O4 NPs reduces the ability of the protein constituents to associate. This inhibition of protein association with NPs due to the presence of cholesterol may be due to differential binding affinities and/or the association of cholesterol reducing the available NP surface area for protein binding. Even though the majority of proteins that associated with Fe3O4 NPs following incubation in either serum were shared, the unique components (proteins and cholesterol) may play a pivotal role in Fe3O4 NP function and toxicity. Serum albumin has been shown in other studies to be one of the most abundant protein constituents of the BC on NPs (Shannahan et al., 2013a,b). Recently, we have assessed its contribution to toxicity by comparing the BC that forms on AgNPs following incubation in cell culture media (e.g. 10% fetal bovine serum) to AgNPs with a BC consisting of only bovine serum albumin (BSA) (Shannahan et al., 2015c). This study demonstrated that although BSA is the most abundant protein within the BC it does not solely contribute to BC-induced alterations in AgNP cellular responses and toxicity. This suggests that these unique individual components, although sometime minor in terms of relative quantity, may play a significant role in mediating cellular-NP interactions and toxic responses. Our investigation of single protein BCs has further demonstrated the importance individual proteins have on BC-induced cellular responses (Shannahan et al., 2015b). BCs made of either BSA, human serum albumin, or HDL were determined to result in variations in RAEC association as well as inflammatory response. These differential responses demonstrate that all proteins are not equal in stimulating cellular interactions and that the identities of the proteins are important.
To date the majority of the evaluation of the BC has focused on the protein constituents of the BC while ignoring other components such as the cholesterol content. Cholesterol content is likely an important component as serum content of cholesterol is variable within our population with many individuals exhibiting high levels. Increased cholesterol content of the BC may facilitate cell interactions and responses thereby influencing NP cellular association and uptake, biodistribution, and inflammatory response. Future studies will investigate the lipidomic profiles of the NP–BC in varied physiologically relevant serum models and is expected to provide additional mechanistic data regarding NP responses. Modifications in these NP-induced biological responses will have implications for their use in biomedical applications due to unexpected toxicity and alterations in NP functionality. Alterations in NP toxicity may also occur between individuals due to differences in serum content of biomolecules resulting in differential BC formation. In the current study, addition of either BC resulted in decreased association with endothelial cells as compared with Fe3O4 NPs without a BC. These results illustrate the importance of understanding BC formation for in vitro NP cellular dosimetry studies for biomedical applications and prediction of in vivo biodistribution.
In this study, we utilized a flow chamber to better mimic the in vivo environment. The use of a flow chamber has been shown to decrease the deposition and interactions of NPs with cells as compared with the commonly used static systems (Grabinski et al., 2015; Klingberg et al., 2015). This decreased deposition is due to differences in NP kinetics between the 2 systems, whereas in a static system, sedimentation and diffusion forces are present while only diffusion occurs in a flow system (Grabinski et al., 2015). Under flow conditions, there is less agglomeration and polydispersity of NPs compared with static systems (Grabinski et al., 2015). The removal of sedimentation forces by utilizing a flow system may normalize differences in dosimetry that may occur in static systems due to addition of the BC thereby making variations in cellular responses more comparable. The assessment of silver NP toxicity in HUVEC cells comparing static and flow systems has demonstrated increased cytotoxicity and inflammatory response under flow conditions (Ucciferri et al., 2014). Further, in static systems, cytokines that are produced are trapped near the cells of production. The addition of flow also removes NP-induced cytokines, which are produced from the endothelial cells, as would occur in vivo. Our goal in this current study was to utilize the most relevant exposure system to investigate the influence of disease-induced variations in the BC on cellular responses. There remain numerous other parameters that can be modified that could make the in vitro assessment of NP toxicity more accurate, however, in our current study we have addressed a variety of factors, which are often not taken into account including flow and the assessment of relevant BCs.
Importantly, our study demonstrates that the addition of a lipid-rich BC on Fe3O4 NPs enhances activation and inflammatory response by endothelial cells even though cellular association was diminished. Endothelial cell uptake and activation/inflammatory responses do not appear to be linked and seem to occur via independent mechanisms. In our previous study, we demonstrated that the addition of a BC on AgNPs consisting of only high-density lipoprotein resulted in decreased internalization by rat lung epithelial cells while exacerbating the inflammatory response as compared to AgNPs without a BC (Shannahan et al., 2015b). These findings together suggest that the characteristics of the BC, which influence cellular association, are complex and include a variety of factors. Properties that are responsible for the BC-dependent reduction in RAEC association of Fe3O4 NPs seen in our current study likely involve reductions in NP zeta potential due to BC formation and the associated protein components. Whereas the components of the BC and, specifically in our study, the cholesterol content of the BC may be responsible for selective cell surface receptor activation resulting in an enhanced inflammatory response. Our previous study and others have shown scavenger receptors are involved in cellular internalization and the inflammatory response following NP exposures (Aldossari et al., 2015a,b; Orr et al., 2011; Shannahan et al., 2015b; Singh and Ramarao, 2012). Scavenger receptors are also responsible for the interactions of endothelial cells with cholesterol in the circulation (Rigotti et al., 1997; Shannahan et al., 2015a; Uittenbogaard et al., 2000). Association of cholesterol within the BC on Fe3O4 NPs in our current study may enhance the activation of cell surface receptors such as scavenger receptors resulting in increased endothelial cell activation and inflammatory response. These differences in cellular activation and inflammatory response due to the addition of the lipid BC on Fe3O4 NPs demonstrate how individuals with underlying disease states may exhibit distinct toxicities. These adverse responses may not be readily identified in traditional toxicological studies due to the use of healthy animal models and in vitro evaluations that do not take into account variations in serum biomolecule content and particularly cholesterol.
Overall our study demonstrates the impact of underlying disease states on the formation of the BC on Fe3O4 NPs and its implications for the safe development of NPs for biomedical applications. Although numerous studies have demonstrated that the physicochemical properties of NPs result in differential BC formation, few have examined the role of the physiological environment in terms of variations due to underlying disease states. The addition of the lipid BC was determined to influence cellular association and activation, thereby having consequences for the safe utilization of NPs. In the current study, we investigated a common human disease state, hyperlipidemia; however, numerous other conditions are known to alter physiological environments, which may influence the formation of the NP–BC and toxicity thereby requiring additional investigation. There are also many other biological fluids other than serum that NPs may come into contact with due to their routes of exposure such as bronchoalveolar fluid, gastrointestinal fluid, and others. In order for the safe and effective use of NPs for biomedical applications, it is necessary to evaluate the formation of the BC in different environments and assess its impact on NP function and toxicity.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Drs Urmila Kodavanti and Samantha Snow of the United States Environmental Protection Agency for providing the rat serum samples utilized in this study. Further the authors acknowledge the assistance from the University of Colorado Denver Anschutz Medical Campus Department of Pharmaceutical Sciences Mass Spectroscopy Core and Dr Nichole Reisdorph for identification and quantification of biocorona protein components.
FUNDING
This work was supported by the Society of Toxicology Colgate-Palmolive Post-Doctoral Fellowship Award (JHS), NIEHS Grant K99 ES024392 (JHS) and R01 ES019311 (JMB). Any opinions, findings, conclusions, or recommendations expressed herein are those of the authors and do not necessarily reflect the views of the Society of Toxicology or the National Institute of Environmental Health Sciences/NIH.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Aldossari A. A., Shannahan J. H., Podila R., Brown J. M. (2015a). Influence of physicochemical properties of silver nanoparticles on mast cell activation and degranulation. Toxicol in Vitro 29, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldossari A. A., Shannahan J. H., Podila R., Brown J. M. (2015b). Scavenger receptor B1 facilitates macrophage uptake of silver nanoparticles and cellular activation. J Nanopart Res 17, 313. [Google Scholar]
- Amiri H., Bordonali L., Lascialfari A., Wan S., Monopoli M. P., Lynch I., Laurent S., Mahmoudi M. (2013). Protein corona affects the relaxivity and MRI contrast efficiency of magnetic nanoparticles. Nanoscale 5, 8656–8665. [DOI] [PubMed] [Google Scholar]
- Babes L., Denizot B., Tanguy G., Le Jeune J. J., Jallet P. (1999). Synthesis of iron oxide nanoparticles used as MRI contrast agents: a parametric study. J Colloid Interface Sci. 212, 474–482. [DOI] [PubMed] [Google Scholar]
- Beduneau A., Ma Z., Grotepas C. B., Kabanov A., Rabinow B. E., Gong N., Mosley R. L., Dou H., Boska M. D., Gendelman H. E. (2009). Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS One 4, e4343–e4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano M., Aronica A., Carzaniga G., Seregni R., Libretti A. (1991). Serum lipids and apolipoproteins in patients with essential hypertension. Atherosclerosis 87, 17–22. [DOI] [PubMed] [Google Scholar]
- Clift M. J., Bhattacharjee S., Brown D. M., Stone V. (2010). The effects of serum on the toxicity of manufactured nanoparticles. Toxicol Lett. 198, 358–365. [DOI] [PubMed] [Google Scholar]
- Dobrucki L. W., Pan D., Smith A. M. (2015). Multiscale imaging of nanoparticle drug delivery. Curr Drug Targets 16, 560–570. [DOI] [PubMed] [Google Scholar]
- Elder A. C., Gelein R., Azadniv M., Frampton M., Finkelstein J., Oberdorster G. (2004). Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhal Toxicol. 16, 461–471. [DOI] [PubMed] [Google Scholar]
- Fan J., He N., He Q., Liu Y., Ma Y., Fu X., Huang P., Chen X. (2015). A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale. 7, 20055–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Ando K., Ishikura R., Kotoura N., Tsuda N., Kato N., Yoshiya S., Nakao N. (2006). Superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging: differentiating bone metastasis and osteomyelitis. Magn Reson Med Sci. 5, 191–196. [DOI] [PubMed] [Google Scholar]
- Gojova A., Lee J. T., Jung H. S., Guo B., Barakat A. I., Kennedy I. M. (2009). Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal Toxicol. 21, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinski C., Sharma M., Maurer E., Sulentic C., Mohan Sankaran R., Hussain S. (2015). The effect of shear flow on nanoparticle agglomeration and deposition in in vitro dynamic flow models. Nanotoxicology 1–10. [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Gupta M. (2005). Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021. [DOI] [PubMed] [Google Scholar]
- Han S. G., Newsome B., Hennig B. (2013). Titanium dioxide nanoparticles increase inflammatory responses in vascular endothelial cells. Toxicology 306, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturi S., Wang G., Chen F., Banda N. K., Holers V. M., Wu L., Moghimi S. M., Simberg D. (2015). Modulatory role of surface coating of superparamagnetic iron oxide nanoworms in complement opsonization and leukocyte uptake. ACS Nano 9, 10758–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlovszky-Hajdu A., Bombelli F. B., Monopoli M. P., Tombacz E., Dawson K. A. (2012). Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir 28, 14983–14991. [DOI] [PubMed] [Google Scholar]
- Joris F., Manshian B.B., Peynshaert K., De Smedt S.C., Braeckman K., Soenen S.J. (2013). Assessing nanoparticle toxicity in cell based assays: influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem Soc Rev 42(21), 8339–8359. [DOI] [PubMed] [Google Scholar]
- Klingberg H., Loft S., Oddershede L. B., Moller P. (2015). The influence of flow, shear stress and adhesion molecule targeting on gold nanoparticle uptake in human endothelial cells. Nanoscale 7, 11409–11419. [DOI] [PubMed] [Google Scholar]
- Koutsiaris A. G., Tachmitzi S. V., Batis N., Kotoula M. G., Karabatsas C. H., Tsironi E., Chatzoulis D. Z. (2007). Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 44, 375–386. [PubMed] [Google Scholar]
- LaFramboise W. A., Dhir R., Kelly L. A., Petrosko P., Krill-Burger J. M., Sciulli C. M., Lyons-Weiler M. A., Chandran U. R., Lomakin A., Masterson R. V., et al. (2012). Serum protein profiles predict coronary artery disease in symptomatic patients referred for coronary angiography. BMC Med. 10, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartigue L., Wilhelm C., Servais J., Factor C., Dencausse A., Bacri J. C., Luciani N., Gazeau F. (2012). Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: impact on macrophage uptake. ACS Nano 6, 2665–2678. [DOI] [PubMed] [Google Scholar]
- Lundqvist M., Stigler J., Cedervall T., Berggard T., Flanagan M. B., Lynch I., Elia G., Dawson K. (2011). The evolution of the protein corona around nanoparticles: a test study. ACS Nano 5, 7503–7509. [DOI] [PubMed] [Google Scholar]
- Maiorano G., Sabella S., Sorce B., Brunetti V., Malvindi M. A., Cingolani R., Pompa P. P. (2010). Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 4, 7481–7491. [DOI] [PubMed] [Google Scholar]
- Mazzei D., Guzzardi M. A., Giusti S., Ahluwalia A. (2010). A low shear stress modular bioreactor for connected cell culture under high flow rates. Biotechnol Bioeng. 106, 127–137. [DOI] [PubMed] [Google Scholar]
- Mills N. L., Miller M. R., Lucking A. J., Beveridge J., Flint L., Boere A. J., Fokkens P. H., Boon N. A., Sandstrom T., Blomberg A., et al. (2011). Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur Heart J. 32, 2660–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli M. P., Aberg C., Salvati A., Dawson K. A. (2012). Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 7, 779–786. [DOI] [PubMed] [Google Scholar]
- Monopoli M. P., Walczyk D., Campbell A., Elia G., Lynch I., Bombelli F. B., Dawson K. A. (2011). Physical–chemical aspects of protein corona: relevance to in vitro and in vivo biological impacts of nanoparticles. J Am Chem Soc 133, 2525–2534. [DOI] [PubMed] [Google Scholar]
- Orr G. A., Chrisler W. B., Cassens K. J., Tan R., Tarasevich B. J., Markillie L. M., Zangar R. C., Thrall B. D. (2011). Cellular recognition and trafficking of amorphous silica nanoparticles by macrophage scavenger receptor A. Nanotoxicology 5, 296–311. [DOI] [PubMed] [Google Scholar]
- Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. (1997). A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 94, 12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Hernandez H., Jimenez-Badillo S., Martinez-Cuevas P. P., Gracia-Espino E., Terrones H., Terrones M., Hussain S. M., Ali S. F., Gonzalez C. (2009). Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicol Lett. 191, 305–313. [DOI] [PubMed] [Google Scholar]
- Ryan S. M., Brayden D. J. (2014). Progress in the delivery of nanoparticle constructs: towards clinical translation. Curr Opin Pharmacol. 18, 120–128. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Dockery D. W. (1992). Increased mortality in Philadelphia associated with daily air pollution concentrations. Am Rev Respir Dis. 145, 600–604. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Morris R. (1995). Air pollution and hospital admissions for cardiovascular disease in Detroit, Michigan. Am J Epidemiol. 142, 23–35. [DOI] [PubMed] [Google Scholar]
- Seebach J., Dieterich P., Luo F., Schillers H., Vestweber D., Oberleithner H., Galla H. J., Schnittler H. J. (2000). Endothelial barrier function under laminar fluid shear stress. Lab Invest. 80, 1819–1831. [DOI] [PubMed] [Google Scholar]
- Shannahan J. H., Bai W., Brown J. M. (2015a). Implications of scavenger receptors in the safe development of nanotherapeutics. Receptors Clin Investig. 2, e811–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Brown J. M., Chen R., Ke P. C., Lai X., Mitra S., Witzmann F. A. (2013a). Comparison of nanotube-protein corona composition in cell culture media. Small 9, 2171–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Lai X., Ke P. C., Podila R., Brown J. M., Witzmann F. A. (2013b). Silver nanoparticle protein corona composition in cell culture media. PLoS One 8, e74001–e74010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Podila R., Aldossari A. A., Emerson H., Powell B. A., Ke P. C., Rao A. M., Brown J. M. (2015b). Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol Sci. 143, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Podila R., Brown J. M. (2015c). A hyperspectral and toxicological analysis of protein corona impact on silver nanoparticle properties, intracellular modifications, and macrophage activation. Int J Nanomed. 10, 6509–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannahan J. H., Sowrirajan H., Persaud I R. P., Brown J., (2015d). Impact of silver and iron nanoparticle exposure on choelsterol uptake by macrophages. J. Nanomater. 2215, Article ID 127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Ramarao P. (2012). Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol Lett. 213, 249–259. [DOI] [PubMed] [Google Scholar]
- Ucciferri N., Collnot E. M., Gaiser B. K., Tirella A., Stone V., Domenici C., Lehr C. M., Ahluwalia A. (2014). In vitro toxicological screening of nanoparticles on primary human endothelial cells and the role of flow in modulating cell response. Nanotoxicology 8, 697–708. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A., Shaul P. W., Yuhanna I. S., Blair A., Smart E. J. (2000). High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 275, 11278–11283. [DOI] [PubMed] [Google Scholar]
- Vesterdal L. K., Mikkelsen L., Folkmann J. K., Sheykhzade M., Cao Y., Roursgaard M., Loft S., Moller P. (2012). Carbon black nanoparticles and vascular dysfunction in cultured endothelial cells and artery segments. Toxicol Lett. 214, 19–26. [DOI] [PubMed] [Google Scholar]
- Walkey C. D., Chan W. C. (2012). Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 41, 2780–2799. [DOI] [PubMed] [Google Scholar]
- Walkey C. D., Olsen J. B., Song F., Liu R., Guo H., Olsen D. W., Cohen Y., Emili A., Chan W. C. (2014). Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455. [DOI] [PubMed] [Google Scholar]
- Wang Y. X. (2011). Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg. 1, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard C. J., Walters D. M., Cathey B. L., Hilderbrand S. C., Katwa P., Lin S., Ke P. C., Podila R., Rao A., Lust R. M., and., et al. (2011). Mast cells contribute to altered vascular reactivity and ischemia–reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology 5, 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T., Hamilton R. F., Bonner J. C., Crandall E. D., Elder A., Fazlollahi F., Girtsman T. A., Kim K., Mitra S., Ntim S. A., et al. (2013). Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 121, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.