Abstract
Background:
Hypertensive individuals are known to exhibit greater increases in blood pressure during an isometric handgrip exercise (IHE) than their normotensive counterparts.
Aim:
This study tests the hypothesis that, compared to normotensive individuals, prehypertensive individuals exhibit an exaggerated response to IHE.
Materials and Methods:
In this study, the effects of IHE were compared in matched prehypertensive vs. normotensive healthy African-American females. Six healthy young adult African–American female university students were screened in a physician's office for blood pressure in the range of prehypertension, systolic blood pressure (SBP) 120–139 mmHg and diastolic blood pressure (DBP) 80–89 mmHg. Six young adult African–American women were also recruited to serve as a healthy normotensive control group with SBP ≤119 mmHg and DBP ≤79 mmHg. Cardiovascular fitness was determined by peak oxygen uptake (VO2 peak) measured during a progressive exercise test.
Results:
During the handgrip exercise, the prehypertensive group exhibited greater increases in SBP (from 139 ± 6 to 205 ± 11 mmHg, +48%) than the controls (from 132 ± 3 to 145 ± 3 mmHg, +10%); intergroup difference P < 0.001. The prehypertensive group also exhibited greater increases in DBP (from 77 ± 2 to 112 ± 5 mmHg, +46%) compared to the controls (from 72 ± 3 to 78 ± 4 mmHg, +8%); intergroup difference P < 0.001. The increase in systemic vascular resistance was also greater in the prehypertensive group (from 1713 ± 91 to 2807 ± 370 dyne.s.cm-5, +64%) than in the controls (from 1668 ± 80 to 1812 ± 169 dyne.s.cm-5, +9%); intergroup difference P < 0.05.
Conclusion:
These results suggest that blood pressure measurements performed during IHE may be a useful screening tool in evaluating prehypertensive individuals for antihypertensive treatments.
Keywords: Arterial compliance, blood pressure, heart rate, myocardial oxygen demand, systemic vascular resistance
Introduction
The incidence of hypertension and prehypertension has been reported to be 19 and 62%, respectively among young adult college football players studied between 1999 and 2012 in the US.[1] In India, prehypertension was found in 38.1% of women.[2] Prehypertension is a prodrome of hypertension, which evolves over many years, is resistant to treatment, and is a costly burden to individuals as well as society.[3] Prehypertension is characterized by systolic blood pressure of 120–139 mmHg and diastolic blood pressure of 80–89 mmHg, measured at rest.[4] High total peripheral resistance is the most commonly reported mechanism for the mildly increased blood pressure in hypertension, which is often accompanied by decreased arterial compliance.[5] It is unclear whether these changes occur in prehypertension.[6]
Aerobic exercise and mental stress have been used as provocation tests to identify individuals with prehypertension.[7,8,9] However, such exercise testing is often not amenable to routine screening in a primary care physician's office. Isometric handgrip exercise has been used to demonstrate that heathy young adult offspring of hypertensive parents had higher resting and postexercise systolic and diastolic blood pressures than offspring of normotensive parents.[10] African–American women are reported to have one of the highest prevalence of hypertension in the world with higher blood pressures than their Caucasian–American counterparts.[11] Compared with healthy young men, healthy young women appear to be protected from high blood pressure by the vasodilator activity of estrogens; women are also reported to possess less sympathetic influence on their cardiovascular systems than men.[12,13]
These findings suggest that young adult African–American women are particularly vulnerable to having their blood pressures go undetected when they are mildly elevated, thereby putting them at risk for developing hypertension. These women are also likely to have morbidity and mortality from cardiovascular disease as middle and elderly aged adults. The present study was, therefore, designed to determine whether an isometric handgrip exercise is useful for identifying women with prehypertension and exaggerated vasopressor responses who may be at high risk for developing cardiovascular disease.
Materials and Methods
Six healthy young adult African–American female university students volunteered as study participants and were screened in a physician's office for blood pressure in the range of prehypertension, systolic pressure 120–139 mmHg, and diastolic pressure 80–89 mmHg. Six young adult African–American women were also recruited to serve as a healthy normotensive control group with systolic blood pressure ≤119 mmHg and diastolic blood pressure ≤79 mmHg. All individuals were nonsmokers and physically inactive. A sub-screening of the participants consisted of age, body weight, body composition, and cardiovascular fitness. The study was approved by the Human Participants Institutional Review Board of Howard University, and written informed consent was obtained from all participants.
Prior to enrollment into the study, the participants underwent blood pressure screening in a physician's office at the Howard University Hospital to determine the presence or absence of prehypertension. Blood pressure measurements were performed on three separate visits during similar times of the day and the participants underwent three blood pressure measurements per visit. After 5 min of rest, blood pressure measurements were performed on the left arm in a seated position using a standard sphygmomanometer blood pressure cuff and stethoscope. Manual blood pressure recordings were performed by a single research investigator using the same sphygmomanometer, cuff, and stethoscope. Participants were assigned to the prehypertension group when resting systolic arterial pressure was between 120 and 139 mmHg and diastolic pressure was between 80 and 89 mmHg, as defined by The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC7).[4] Changes in blood pressure have been reported in normotensive and mildly hypertensive women during the different phases of the menstrual cycle.[14] Thus, all screenings and testing conditions of the study were performed during the luteal phase of the participants’ menstrual cycle.
After meeting the screening criteria, the participants visited the laboratory on a separate day for sub-screening measurements of body height and weight, body composition, and peak oxygen uptake (VO2 peak). Physical activity was assessed using the Godin Leisure-Time Exercise Questionnaire.[15] Body height and weight were measured using standard laboratory procedures. Measurement of body composition included fat mass measured by a scanner using dual energy X-ray absorptiometry (DEXA, Hologic QDR 4500 DXA System, Hologic, Waltham, MA). Cardiovascular fitness was determined by VO2 peak measured during a progressive exercise test. After screenings, on a separate day, participants entered the laboratory with prior instructions to abstain from food for 3 h and from exercise for 12 h prior to testing. The participants then performed an isometric handgrip dynamometer exercise test. The procedure of the isometric handgrip exercise test was explained to all the study participants. Before the test, they were allowed to rest for 10 min. The participants were then instructed to perform the isometric handgrip exercise under supervision. They were asked to hold a Smedley handgrip spring dynamometer (Independent Living Products, Peoria, AZ) in their left hand to get a full grip of it and compress the handles of the dynamometer by exerting maximal effort for a few seconds. The performance of maximal handgrip contraction was performed in triplicate, with the mean of three readings recorded as the maximal isometric tension. The participants were then instrumented for the following physiological outcome measurements, that is, blood pressure, cardiac output, and heart rate variability.
Cardiac output was monitored using a noninvasive impedance cardiography device (Sorba Model CIC-1000, Sorba Medical Systems, Inc., Milwaukee, WI). In addition, hemodynamic variables of stroke volume, heart rate, and systemic vascular resistance (SVR) were derived from the cardiac impedance monitor.
Beat-by-beat, noninvasive measurements of blood pressure were performed using the Vasotrac APM205A system (Medwave®, Inc., St. Paul, MN). The blood pressure tonometer sensor was placed on the radial artery of the right wrist. This method of measuring arterial pressure is reported to be highly correlated with indwelling arterial line blood pressure (r = 0.74).[16] The area under the diastolic pressure waveform of the radial artery was used to estimate arterial compliance.[17] In a prior study, we have reported arterial compliance measurements using a similar contour analysis method.[18] Specifically, the analysis included two successive 10-beat radial blood pressure recordings. Total arterial compliance was estimated using the ratio of stroke volume to pulse pressure.[19] SVR was derived from the cardiac output and mean arterial pressure values and expressed as dynes‧s‧cm−5. Heart rate, represented by its reciprocal value (RR interval), was monitored beat-to-beat using a Biopac MP100 electrocardiogram data acquisition system (Biopac Systems, Inc, Goleta, CA) with a sampling rate of 1000 Hz. Heart rate variability measurements were performed in the time domain via specialized heart rate variability software (Nevrokard version 11.0.2, Izola, Slovenia). Selected time domain parameter of the square root of the mean squared difference of successive RR intervals (RMSSD) was computed from the raw RR intervals.
After the participants were instrumented with the aforementioned monitoring devices, they rested for 10 min in the seated position. Baseline measures of blood pressure, heart rate, cardiac output, and heart rate variability were recorded during the last 3 min of the rest period. Then, each participant was asked to perform the isometric handgrip exercise at 30% of maximal isometric tension for 3 min. During the test, blood pressure was recorded from the nonexercised arm, and each participant was instructed to perform normal respiration to limit any Valsalva effect. During the isometric handgrip exercise, the blood pressure, heart rate, cardiac output, and heart rate variability were again measured for 3 min. After the isometric handgrip exercise, the participants rested for 10 min (recovery) and the blood pressure, heart rate, cardiac output, and heart rate variability were recorded during the last 3 min of exercise recovery.
Statistical analysis
The dependent cardiovascular response variables of interest were identified as systolic and diastolic blood pressures, cardiac output, systemic vascular resistance, heart rate, rate-pressure product, arterial compliance stroke volume/pulse pressure quotient, and root mean square of the standard deviation of normal-normal ECG RR interbeat intervals (RMSSD). Statistical significance of differences between each of these dependent variables was evaluated across the independent categorical variables, with study participants designated as normotensive (control) or prehypertensive (comparison) groups. The assumption of normality in the distribution of least-square procedure residuals was met by virtue of skewness and kurtosis values between −1.0 and +1.0 and statistical significance of the intergroup differences was determined by one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSS) version 22 software (IBM Corp., Armonk, NY). Results are expressed as mean ± SE, and the significance level was set at P < 0.05.
Results
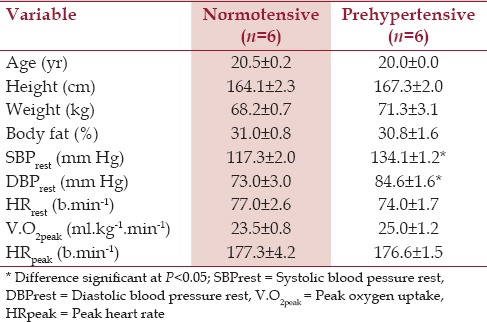
The demographic and physiological characteristics of the two study groups’ physician's office blood pressure measurements were greater in the prehypertensive participants as shown in Table 1.
Table 1.
Characteristics of study participants
The systolic and diastolic blood pressures before (baseline), during (exercise), and immediately after (recovery) an isometric handgrip exercise test are depicted in Figure 1. The prehypertension group exhibited greater increases in systolic blood pressure (from 139 ± 6 to 205 ± 11 mmHg, +48%) than the normotensive control group (from 132 ± 3 to 145 ± 3 mmHg, +10%); intergroup difference P < 0.001. The prehypertension group also exhibited greater increases in diastolic blood pressure (from 77 ± 2 to 112 ± 5 mmHg, +46%) compared to the normotensive control group from 72 ± 3 to 78 ± 4 mmHg, +8%); intergroup difference P < 0.001. During recovery, systolic, diastolic, and mean blood pressures were also significantly greater in the prehypertension group.
Figure 1.
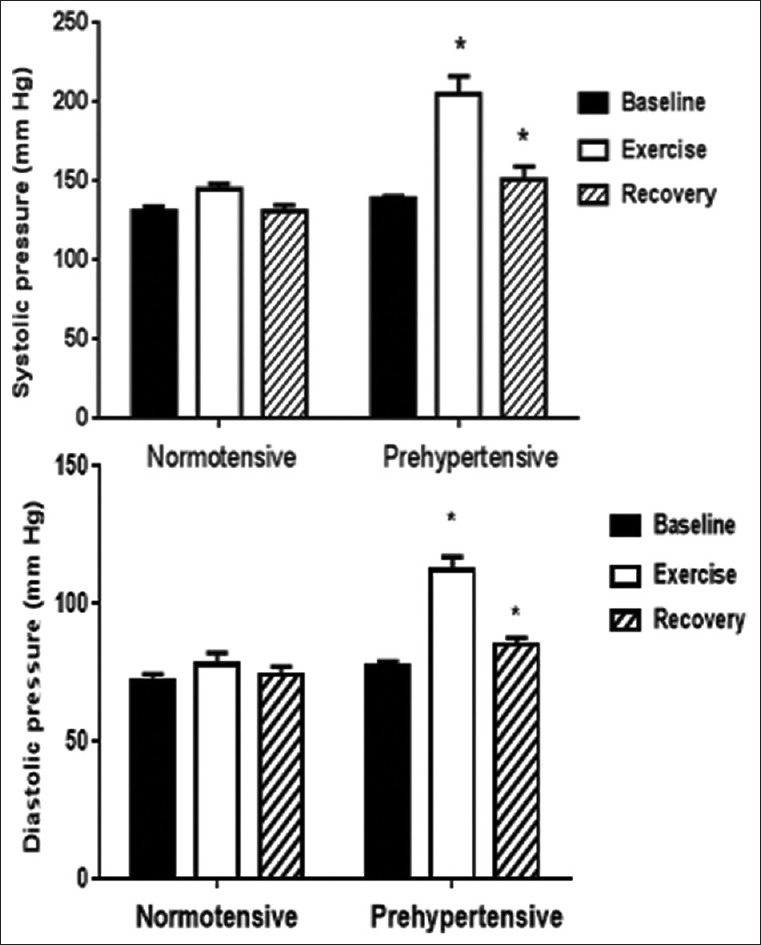
Effects of handgrip exercise on blood pressure. Mean ± SE measurements of systolic and diastolic BP before (baseline), during (exercise) and after (recovery) performance of an isometric handgrip exercise in groups of healthy female control subjects compared to subjects with prehypertension. *Intergroup difference significant at P<0.001
The prehypertension group exhibited greater increases in systemic vascular resistance (from 1713 ± 91 to 2807 ± 370 dyne· s· cm−5, +64%) than the normotensive control group (from 1668 ± 80 to 1812 ± 169 dyne·s·cm−5, +9%); intergroup difference P < 0.05, as shown in Figure 2. The prehypertension group also had greater increases in the rate-pressure product than the control group (P < 0.05). The rate-pressure product was also greater in the prehypertension group during recovery.
Figure 2.
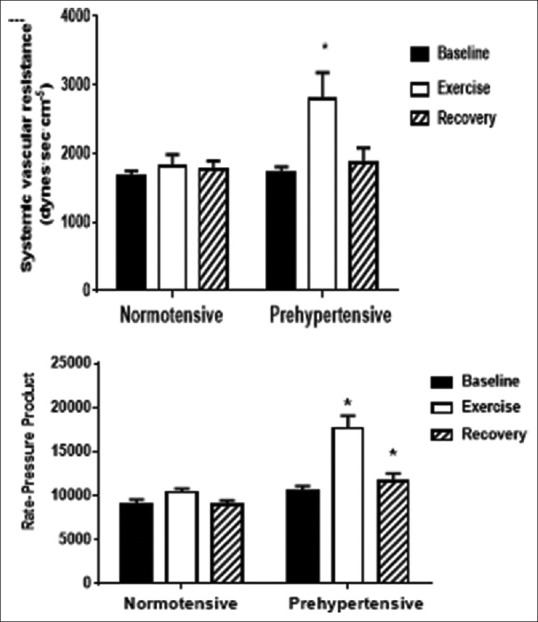
Effects of handgrip exercise on rate-pressure product and systemic resistance. Mean ± SE measurements of systolic and diastolic BP before (baseline), during (exercise) and after (recovery) performance of an isometric handgrip exercise in groups of healthy female control subjects compared to subjects with prehypertension. *Intergroup difference significant at P<0.001
The pulse-pressure/stroke-volume quotient and arterial compliance before, during, and after the handgrip exercise are represented in Figure 3. The pulse pressure was increased and the pulse-pressure/stroke-volume quotient was decreased during the handgrip exercise in both groups, with greater decrements in the prehypertension group (P < 0.05).
Figure 3.
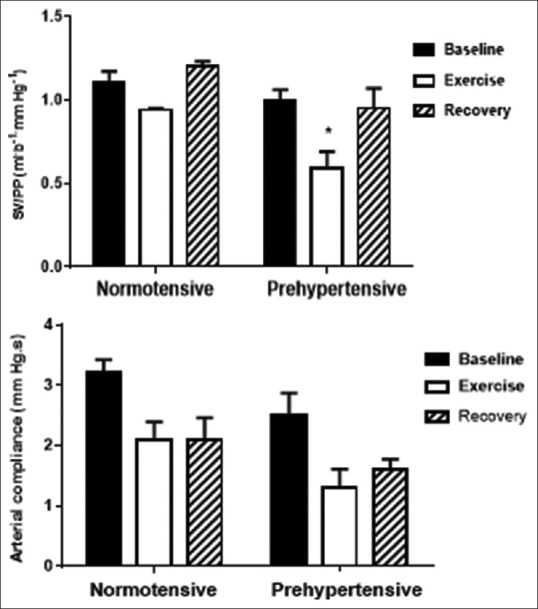
Effects of handgrip exercise on pulse-pressure/stroke-volume quotient and arterial compliance. Mean ± SE measurements of systolic and diastolic BP before (baseline), during (exercise) and after (recovery) performance of an isometric handgrip exercise in groups of healthy female control subjects compared to subjects with prehypertension. *Intergroup difference significant at P<0.001
Discussion
This pilot study demonstrates that healthy young adult African–American women with routine blood pressure measurements in the range of prehypertension may exhibit augmented cardiovascular responses that can be elicited by an isometric handgrip exercise. This handgrip exercise induced greater rise in systolic, diastolic, pulse pressures, systemic (arterial) resistance, and heart rate-pressure product (index of myocardial oxygen demand) in the prehypertensive subjects. The decrement in stroke-volume/pulse-pressure quotient (index of arterial compliance) was also greater in the prehypertensive subjects.
Despite the fact that the physician's office blood pressure of the two study groups was significantly different, the two groups were indistinguishable by baseline measurements of their cardiovascular variables. This might be attributed to white-coat anxiety and/or laboratory testing stress produced by instruments and instructing the subjects, as well as by the unfamiliar environment of a research facility. Stress-induced increases in cardiac output and blood pressure are common responses to stress in normotensive individuals by the mechanism of beta-adrenergic stimulation.[20]
Effect of isometric handgrip exercise on blood pressure
Aerobic exercise, such as an exercise stress test, is the most commonly used experimental intervention to elicit physiological increase in blood pressure.[21] Aerobic exercise has also been used to differentiate normotensive from hypertensive patients,[22] and, infrequently, to differentiate individuals with prehypertensive blood pressure.[23] Aerobic exercise has also been used as a treatment modality to decrease blood pressure in prehypertensive individuals.[24] Isometric handgrip exercise has also been shown to be effective for decreasing blood pressure in prehypertensive individuals.[25] Augmented cardiovascular responses to isometric handgrip exercise can also differentiate individuals with hypertensive parents from individuals with normotensive parents.[26]
The results of our study, showing an augmented blood pressure response in prehypertensive subjects, is seemingly paradoxical to reports that isometric handgrip exercise training acts as an antihypertensive treatment strategy.[25] This can be explained by the methodological differences between the results of our study because the exaggerated blood pressure response was to a single bout of isometric handgrip exercise, not to exercise training per se. The studies demonstrating a significant reduction in blood pressure resulting from an isometric handgrip exercise training regimen were usually of at least 4 weeks duration.[25]
The present study is unique because it employed an isometric handgrip exercise to elicit increase in blood pressure in prehypertensive subjects. Isometric handgrip exercise is reported to increase both aortic and brachial artery pressures whereas ergometer-bicycle exercise only increases brachial artery pressure.[27] The isometric handgrip exercise used in the present study showed that a group of healthy young adult African-American women with routine blood pressures in the prehypertensive range had greater increases than their normotensive counterparts in radial artery systolic, diastolic, and pulse pressures contralateral to the exercising hand. Overall, these findings imply that the increase in peripheral (radial) pressure reflected generalized arterial vasoconstriction in our study and that our use of isometric handgrip exercise may have increased cardiac afterload. Afterload is a major long-term predictor of cardiovascular risk.[27]
Vasoconstrictor responses to exercise and to other environmental stressors are physiological indicators of predilections for cardiovascular disease. The renin-angiotensin system (RAS) is a mediator of sympathetic vasoconstrictor responses. A high plasma angiotensin level mediates cardiac myocyte hypertrophy and cardiac remodeling, leading to cardiovascular disease.[28] In one study, angiotensin receptor blockade did not attenuate the sympathetic response to isometric handgrip exercise[29] but alpha-adrenergic receptor blockade did.[30] Isometric handgrip exercise after angiotensin receptor blockade with losartan did increase heart rate and firing of sympathetic nerves.[29] The vasopressor responses to isometric handgrip exercise in the present study were, therefore, probably mediated by sympathetic release of norepinephrine and activation of alpha-adrenergic receptors. This issue can be addressed by measuring forearm plasma norepinephrine concentrations. A greater increase in forearm norepinephrine during isometric handgrip exercise would suggest sympathetic overactivity as a mechanism for the exaggerated blood pressure responsiveness observed in this study of prehypertensive subjects.
Effect of isometric handgrip exercise on arterial resistance and compliance
The present study appears to be the first to demonstrate greater SVR in prehypertensive than in normotensive subjects during an isometric handgrip exercise. Increased SVR is not considered to be a normal sympathetic response to static exercise[31] and pregnant women with and without hypertension did not have increased SVR after isometric handgrip exercise.[32] Nevertheless, increased SVR has been demonstrated during isometric handgrip exercise in some hypertensive individuals.[33] This finding suggests that our participants with prehypertension were probably exhibiting sympathetic vasopressor responses during the isometric handgrip exercises, quite similar to what has been reported for patients diagnosed with hypertension.
Decreased arterial compliance, measured as the stroke-volume/end-systolic pressure quotient, has been demonstrated following isometric handgrip exercise in patients diagnosed with hypertension.[34] We computed arterial compliance by measuring the area under the curve for the decay in aortic pressure during diastole, which showed a marginally greater decrease during the handgrip exercise in the prehypertension group (P = 0.07). We also computed arterial compliance as the stroke-volume/pulse-pressure quotient. The change in stroke-volume/pulse-pressure quotient was statistically more significant than the diastolic pressure decay during the handgrip exercise in the group with prehypertension (P = 0.018). It is likely, therefore, that arterial stiffness was increased during the handgrip exercise, with greater stiffness in the prehypertensive than in the normotensive subjects. Arterial stiffness is a component of increased SVR, as well as a structural property that permits storage of energy to buffer sudden increases in arterial pressure. The capacity for energy storage is very low in a rigid structure such as a lead pipe. Both SVR and stiffness of the arterial system were, therefore, increasing, becoming more like a lead pipe, in the prehypertensive than in the normotensive subjects during the handgrip exercise.
Effect of the isometric handgrip exercise on myocardial oxygen demand
The greater increases in blood pressure and SVR observed in the group with prehypertension during the handgrip exercise were associated with greater increases in the rate-pressure product. Increased rate-pressure product has been reported in healthy subjects during isometric handgrip exercise.[35] Increased myocardial oxygen demand is a key factor in myocardial ischemia and infarction, for which hypertension is a known risk factor.[35] Our findings suggest that the greater blood pressure and SVR increases in the prehypertension group in this study were likely to be clinically significant. Increased myocardial oxygen demand during the handgrip exercise may, therefore, reveal those individuals who could benefit from the early introduction of antihypertensive treatments.
Study limitations
The major limitation of this pilot study is the small sample size. The highly significant P values for the intergroup differences in the dependent cardiovascular response variables of interest suggest a very low probability of a type 1 statistical error. However, the small sample size cannot rule out the probability of a type 2 statistical error, which requires further investigation with a larger number of subjects. The results described here should, therefore, be considered as a preliminary report.
Conclusion
This preliminary report suggests that a single bout of isometric handgrip exercise may reveal exaggerated blood pressure responses in a group of healthy young adult African–American females with physician's office blood pressure in the range of prehypertension. Despite the limitation of small sample size, the differences in blood pressure responses between normotensive controls and the prehypertension comparison group were highly significant, similar to previously discussed differences between groups of normotensive and hypertensive subjects. These findings seem to suggest that isometric handgrip exercise may be a useful technique for identifying subpopulations of prehypertensive subjects for whom the risk of developing chronic hypertension and cardiovascular disease may be abated by early employment of antihypertensive treatments.
Financial support and sponsorship
This work supported in part by NIH/NCRR/RCMI Grant No. 2G12RR003048 to Howard University.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Karpinos AR, Roumie CL, Nian H, Diamond AB, Rothman RL. High prevalence of hypertension among collegiate football athletes. Cir Cardiovasc Qual Outcomes. 2013;6:716–23. doi: 10.1161/CIRCOUTCOMES.113.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari P, Pemminati S, Pathak R, Kotian MS, Ullal S. Prevalence of hypertention in boloor diabetes study (BDS-II) and its risk factors. J Clin Diagn Res. 2015;9:IC01–4. doi: 10.7860/JCDR/2015/16509.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagogo-Jack S, Egbuonu N, Edeoga C. Principles and practice of nonpharmacological interventions to reduce cardiometabolic risk. Med Princ Pract. 2010;19:167–75. doi: 10.1159/000285280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 5.Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, et al. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–5. doi: 10.1016/s0735-1097(02)01717-5. [DOI] [PubMed] [Google Scholar]
- 6.Kingwell BA. Large artery stiffness: Implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol. 2002;29:214–7. doi: 10.1046/j.1440-1681.2002.03622.x. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros RF, Silva BM, Neves FJ, Rocha NG, Sales AR, Nobrega AC. Impaired hemodynamic response to mental stress in subjects with prehypertension is improved after a single bout of maximal dynamic exercise. Clinics. 2011;66:1523–9. doi: 10.1590/S1807-59322011000900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz MG, Hare JL, Marwick TH, Stowasser M, Sharman JE. Masked hypertension is “unmasked” by low-intensity exercise blood pressure. Blood Press. 2011;20:284–9. doi: 10.3109/08037051.2011.566251. [DOI] [PubMed] [Google Scholar]
- 9.Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: A systematic review and meta-analysis. Am J Hypertens. 2013;26:357–66. doi: 10.1093/ajh/hps053. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Malhotra V, Dhar U, Tripathi Y. The isometric handgrip exercise as a test for unmasking hypertension in the offspring of hypertensive parents. J Clin Diagn Res. 2013;7:996–9. doi: 10.7860/JCDR/2013/5094.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein KM, Melnyk SD, Zullig LL, Stechuchak KM, Oddone E, Bastian LA, et al. Heart matters: Gender and racial differences cardiovascular disease risk factor control among veterans. Womens Health Issues. 2014;24:477–83. doi: 10.1016/j.whi.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: Implications for human blood pressure regulation. Hypertension. 2009;53:571–6. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyner MJ, Barnes JN, Hart EC, Wallin BG, Charkoudian N. Neural control of the circulation: How sex and age differences interact in humans. Compr Physiol. 2015;5:193–215. doi: 10.1002/cphy.c140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne FP, Barry DG, Ferriss JB, Grealy G, Murphy D. Changes in blood pressure during the normal menstrual cycle. Clin Sci. 1991;81:515–8. doi: 10.1042/cs0810515. [DOI] [PubMed] [Google Scholar]
- 15.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 16.Hager H, Mandadi G, Pulley D, Eagon JC, Mascha E, Nutter B, Kurz A. A comparison of noninvasive blood pressure measurement on the wrist with invasive arterial blood pressure monitoring in patients undergoing bariatric surgery. Obes Surg. 2009;19:717–24. doi: 10.1007/s11695-008-9607-7. [DOI] [PubMed] [Google Scholar]
- 17.Liang YL, Teede H, Kotsopoulos D, Shiel L, Cameron JD, Dart AM, McGrath BP. Non-invasive measurement of arterial structure and function: Repeatability, interrelationships and trial sample size. Clin Sci. 1998;95:669–79. doi: 10.1042/cs0950669. [DOI] [PubMed] [Google Scholar]
- 18.Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, et al. Low arterial compliance in young African-American males. Am J Physiol Heart Circ Physiol. 2003;285:H457–62. doi: 10.1152/ajpheart.00497.2002. [DOI] [PubMed] [Google Scholar]
- 19.Stergiopulos N, Meister JJ, Westerhof N. Evaluation of methods for estimation of total arterial compliance. Am J Physiol. 1995;268:H1540–8. doi: 10.1152/ajpheart.1995.268.4.H1540. [DOI] [PubMed] [Google Scholar]
- 20.Balanos GM, Phillips AC, Frenneaux MP, McIntyre D, Lykidis C, Griffin HS, et al. Metabolically exaggerated cardiac reactions to acute psychological stress: The effects of resting blood pressure status and possible underlying mechanisms. Biol Psychol. 2010:85:104–11. doi: 10.1016/j.biopsycho.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation. 2013:128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 22.Jae SY, Franklin BA, Choo J, Choi YH, Fernhall B. Exaggerated exercise blood pressure response during treadmill testing as a predictor of future hypertension in men: A longitudinal study. Am J Hypertens. 2015;28:1362–7. doi: 10.1093/ajh/hpv036. [DOI] [PubMed] [Google Scholar]
- 23.Aneni E, Roberson LL, Shaharyar S, Blaha MJ, Agatston AA, Blumenthal RS, et al. Delayed heart rate recovery is strongly associated with early and late-stage prehypertension during exercise stress testing. Am J Hypertens. 2014;27:514–21. doi: 10.1093/ajh/hpt173. [DOI] [PubMed] [Google Scholar]
- 24.Karoline de Morais P, Sales MM, Alves de Almeida J, Motta-Santos D, Victor de Sousa C, Simões HG. Effects of aerobic exercise intensity on 24-h ambulatory blood pressure in individuals with type 2 diabetes and prehypertension. J Phys Ther Sci. 2015;27:51–6. doi: 10.1589/jpts.27.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, et al. Beyond medications and diet: Alternative approaches to lowering blood pressure: A scientific statement from the American Heart Association. Hypertension. 2013;61:1360–83. doi: 10.1161/HYP.0b013e318293645f. [DOI] [PubMed] [Google Scholar]
- 26.Lopes HF, Consolim-Colombo FM, Barreto-Filho JA, Riccio GM, Negrão CE, Krieger EM. Increased sympathetic activity in normotensive offspring of malignant hypertensive parents compared to offspring of normotensive parents. Braz J Med Biol Res. 2008;41:849–53. doi: 10.1590/s0100-879x2008005000042. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Sugiura T, Yamashita S, Dohi Y, Kimura G, Ohte N. Differential response of central blood pressure to isometric and isotonic exercises. Sci Rep. 2014;25:5439. doi: 10.1038/srep05439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos L, Bader M, Baltatu OC. Brain renin angiotensin system in cardiac hypertrophy and failure. Front Physiol. 2012;3:115. doi: 10.3389/fphys.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGowan CL, Notarius CF, McReynolds A, Morris BL, Kimmerly DS, Picton PE, et al. Effect of angiotensin AT1 receptor blockade on sympathetic responses to handgrip in healthy men. Am J Hypertens. 2011;24:537–43. doi: 10.1038/ajh.2011.14. [DOI] [PubMed] [Google Scholar]
- 30.Hamada M, Kazatani Y, Shigematsu Y, Ito T, Kokubu T, Ishise S. Enhanced blood pressure response to isometric handgrip exercise in patients with essential hypertension: Effects of propranolol and prazosin. J Hypertens. 1987;5:305–9. doi: 10.1097/00004872-198706000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Alexander T, Friedman DB, Levine BD, Pawelczyk JA, Mitchell JH. Cardiovascular responses during static exercise. Studies in patients with complete heart block and dual chamber pacemakers. Circulation. 1994;89:1643–7. doi: 10.1161/01.cir.89.4.1643. [DOI] [PubMed] [Google Scholar]
- 32.Nisell H, Hjemdahl P, Linde B, Lunell NO. Cardiovascular responses to isometric handgrip exercise: An invasive study in pregnancy-induced hypertension. Obstet Gynecol. 1987;70:339–43. [PubMed] [Google Scholar]
- 33.Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, et al. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H320–30. doi: 10.1152/ajpheart.00334.2009. [DOI] [PubMed] [Google Scholar]
- 34.Kuznetsova T, D’hooge J, Kloch-Badelek M, Sakiewicz W, Thijs L, Staessen JA. Impact of hypertension on ventricular-arterial coupling and regional myocardial work at rest and during isometric exercise. J Am Soc Echocardiogr. 2012;25:882–90. doi: 10.1016/j.echo.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, Sinoway LI. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol. 2012;302:H1737–46. doi: 10.1152/ajpheart.01195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


